Characterization and Biological Activities of Seed Oil Extracted from Berberis dasystachya Maxim. by the Supercritical Carbon Dioxide Extraction Method
Abstract
1. Introduction
2. Results and Discussion
2.1. Single-Factor Experiments
2.2. Optimal Extraction Conditions Obtained by Central Composite Design
2.2.1. Optimal SC-CO2 Conditions
2.2.2. Validation of Models
2.2.3. Model Analysis
2.3. Seed Oil Yield
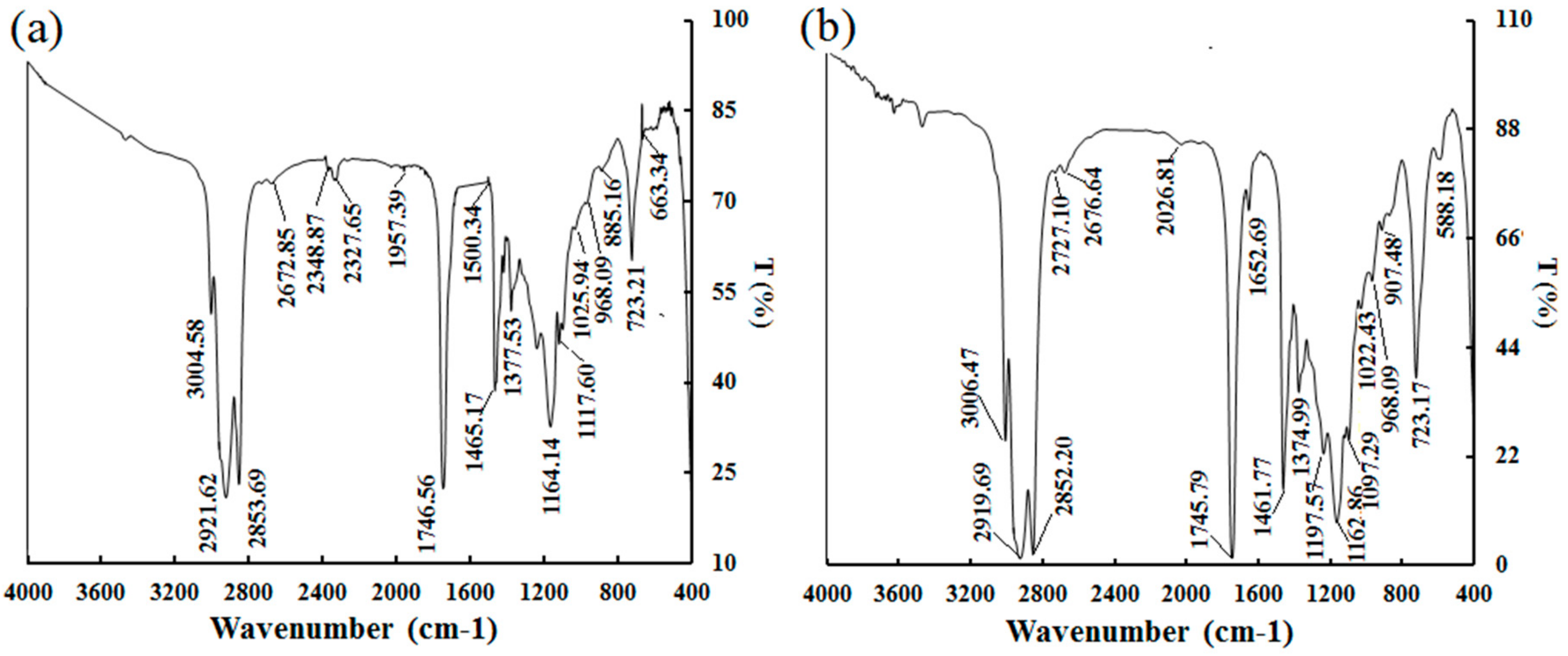
2.4. FT-IR Spectra
2.5. Fatty Acid Composition
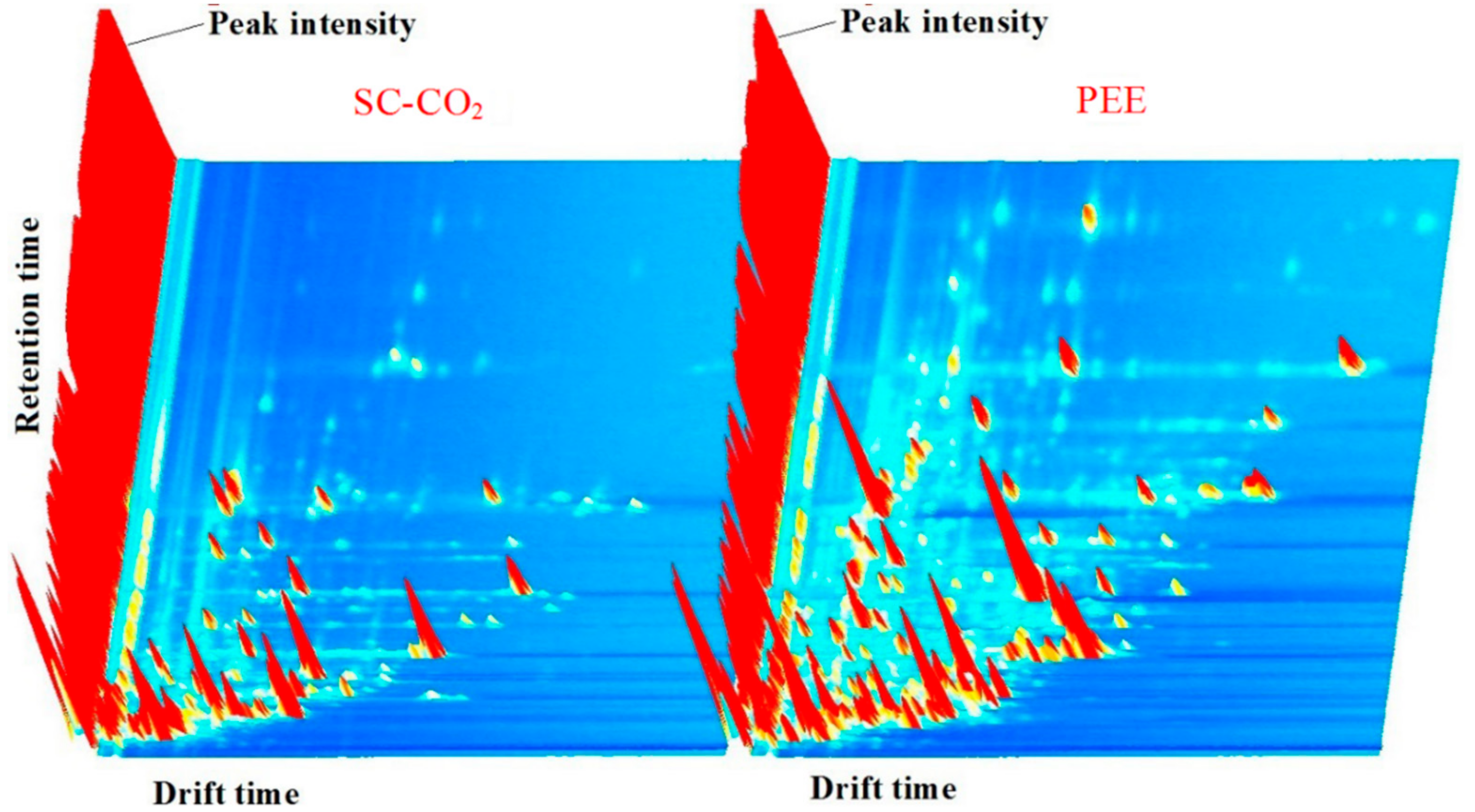
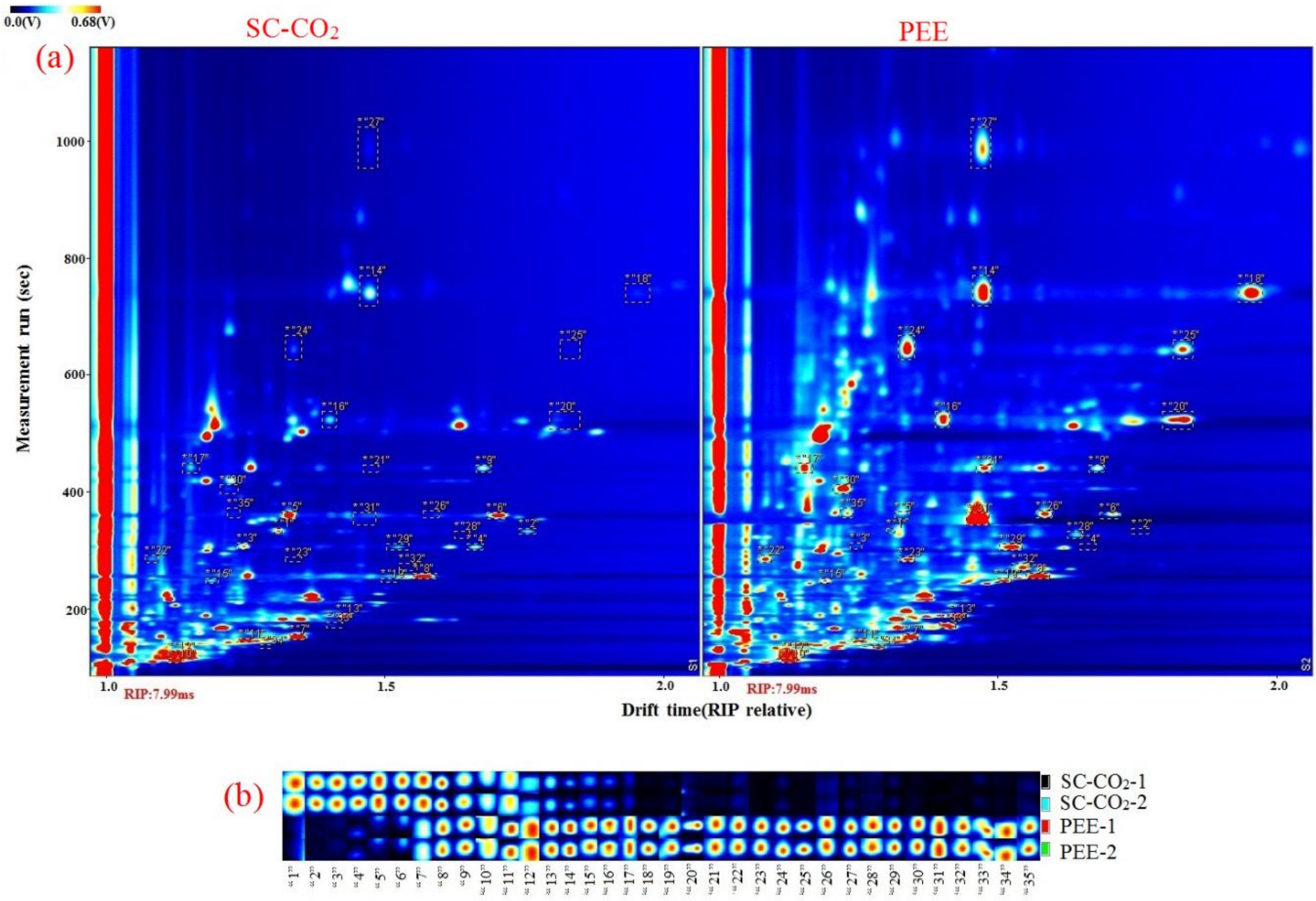
2.6. Volatile Organic Compounds
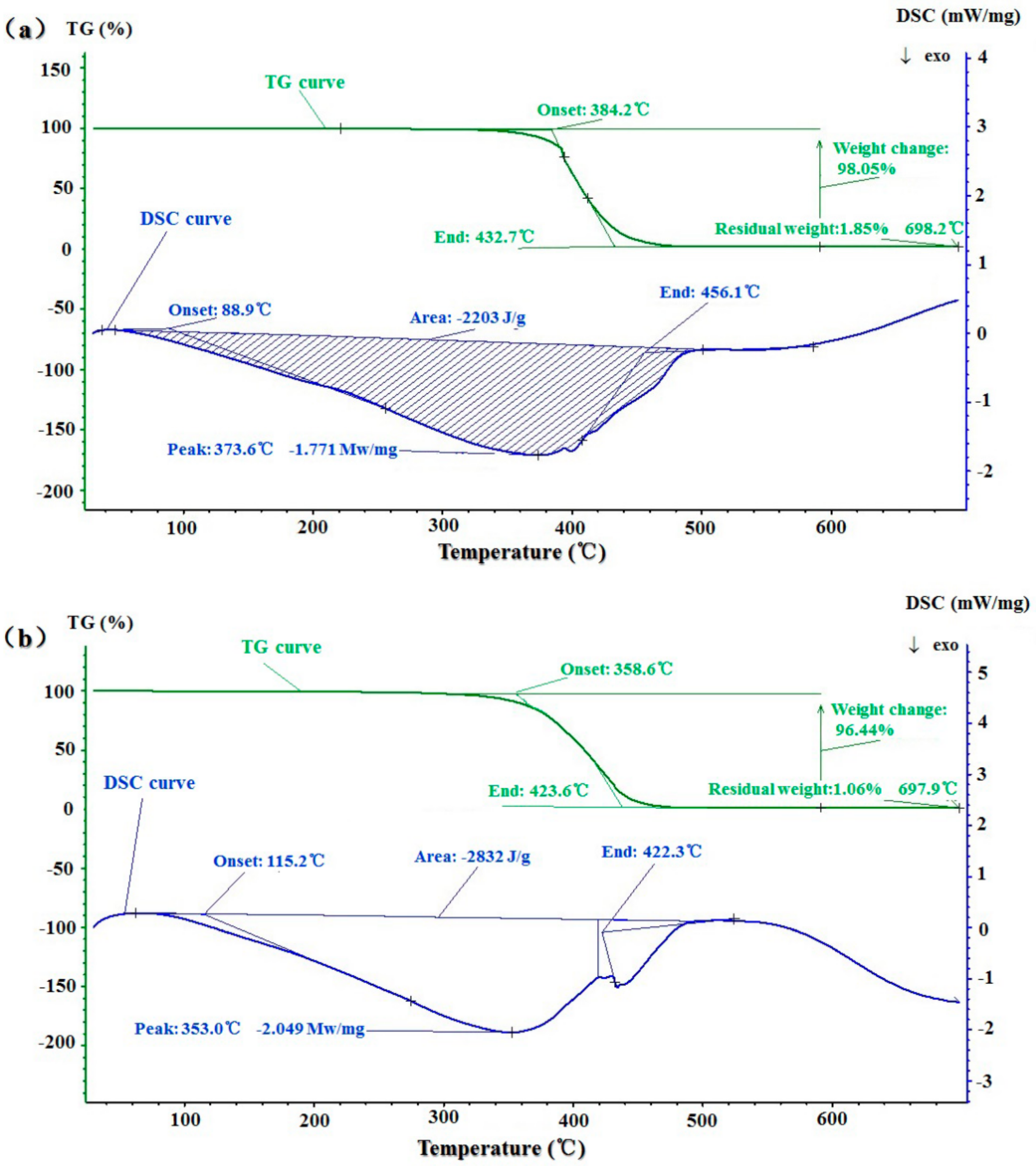
2.7. Thermal Stability and Thermal Behavior
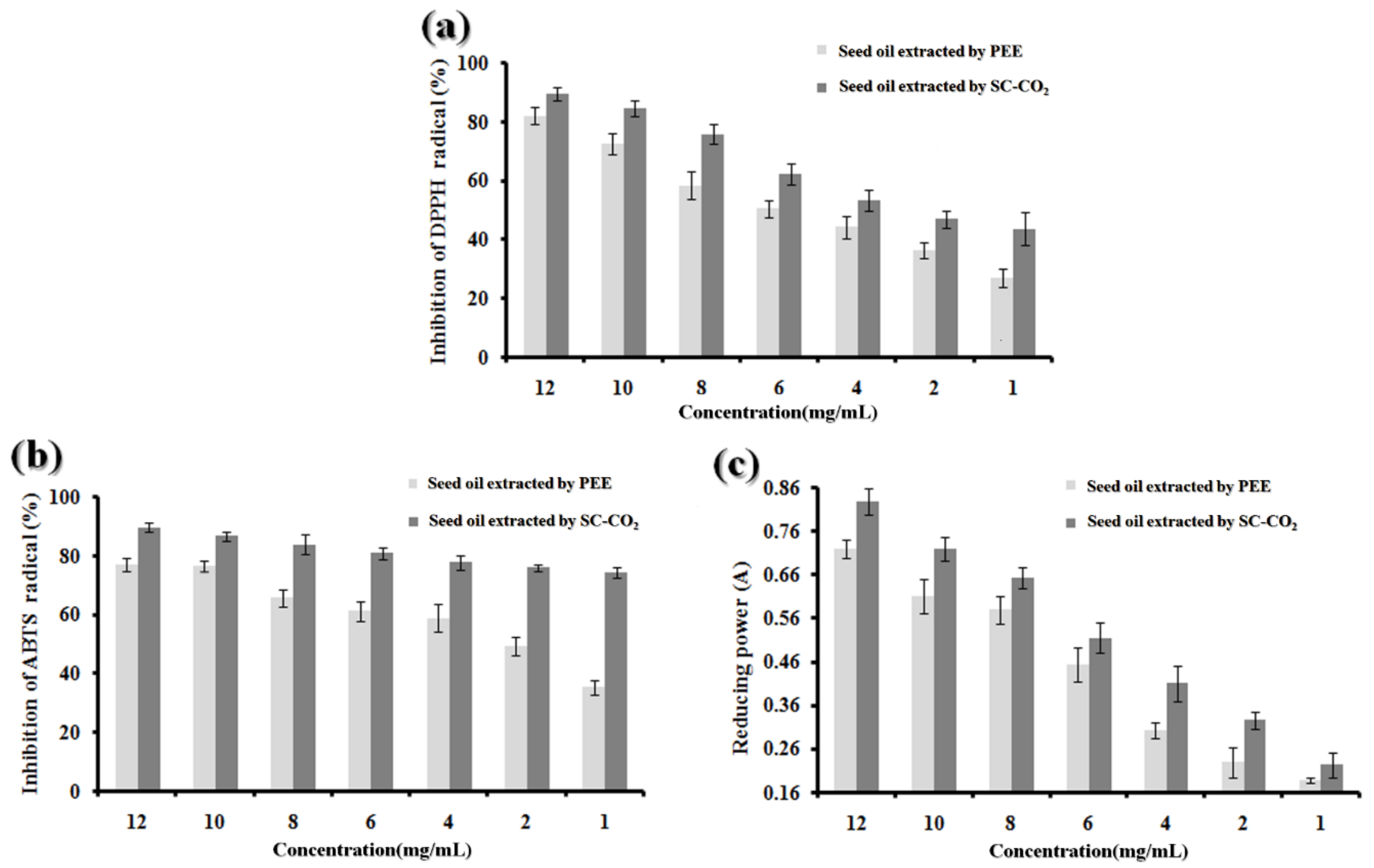
2.8. In Vitro Antioxidant Ability
2.8.1. DPPH Radical Scavenging Activity
2.8.2. ABTS Radical-Scavenging Activity
2.8.3. Reducing Power Assay
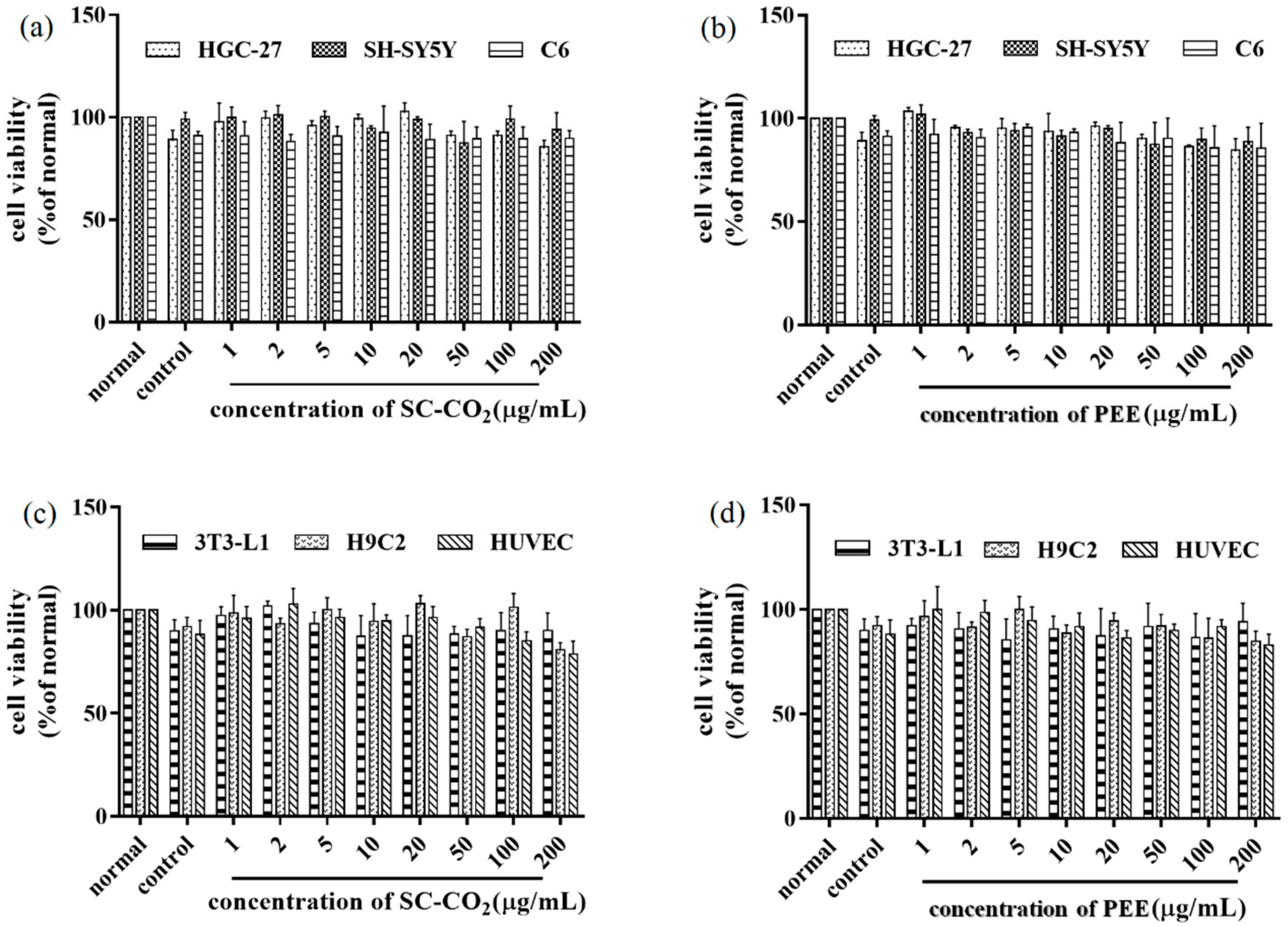
2.9. In Vitro Cytotoxicity
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Petroleum Ether Extraction
3.3. Supercritical Fluid Extraction with Carbon Dioxide
3.4. Determination of Extraction Yield
3.5. Experimental Design and Statistical Analysis
3.6. Chemical Characteristics
3.6.1. FT-IR Analysis
3.6.2. Determination of Fatty Acid Composition by GC–FID
3.6.3. Determination of Volatile Organic Compounds by GC–IMS
3.6.4. Thermal Stability and Thermal Behavior Analysis
3.7. Determination of In Vitro Antioxidant Activity
3.7.1. DPPH Radical Scavenging Activity Assay
3.7.2. ABTS Radical Scavenging Activity Assay
3.7.3. Reducing Power Assay
3.8. Determination of In Vitro Cell-Viability Assay
3.8.1. Preparation of BDSO Samples Obtained from SC-CO2 and PEE for Cellular Experiments
3.8.2. Cell Culture
3.8.3. Cellular Viability Assessment
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Guo, B.Z. Economic Flora of Qinhai, 2nd ed.; Qinhai People’s Press: Xi’ning, China, 1987; pp. 188–193. [Google Scholar]
- Ji, L.J. The analysis of free amino acid and ascorbic acid in Sea buckthorn, white-thorn, yellow-thorn. Acta Botanic. Sinica 1989, 6, 487–488. [Google Scholar]
- Gong, Z.Q.; Zhang, L.; Shi, W.Y.; Liu, P.L. The analysis of mechanisms of facial skin aging treated by three thorn cream. J. J. Qinghai Med. Coll. 1988, 2, 55–59. [Google Scholar]
- Wang, L.; Wu, M.; Liu, H.M.; Ma, Y.X.; Wang, X.D.; Qin, G.Y. Subcritical fluid extraction of Chinese quince seed: Optimization and product characterization. Molecules 2017, 22, 528. [Google Scholar] [CrossRef]
- Akubugwo, I.E.; Obasi, A.N.; Ginika, S.C. Nutritional potential of the leaves and seeds of black nightshade-Solanum Nigrum L. Var virginicum from afikponigeria. Pak. J. Nutr. 2007, 6, 323–326. [Google Scholar] [CrossRef]
- Piras, A.; Rosa, A.; Falconieri, D.; Porcedda, S.; Dessì, M.; Marongiu, B. Extraction of oil from wheat germ by supercritical CO2. Molecules 2009, 14, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Cifuentes, A.; Ibaῆez, E. Sub- and supercritical fluid extraction of functional ingredients from different natural sources: Plants, food-byproducts, algae and microalgae: A review. Food. Chem. 2006, 98, 136–148. [Google Scholar] [CrossRef]
- Wang, H.; Suo, Y.; Wang, X.; Li, Y.; You, J.; Zhao, X. Extraction of Nitraria tangutorum seed oil by supercritical carbon dioxide and determination of free fatty acids by HPLC/APCI/MS with fluorescence detection. Sep. Purif. Technol. 2007, 3, 371–377. [Google Scholar] [CrossRef]
- Liu, Z.G.; Mei, L.J.; Wang, Q.L.; Shao, Y.; Tao, Y.D. Optimization of subcritical fluid extraction of seed oil from Nitraria tangutorum using response surface methodology. LWT-Food Sci. Technol. 2014, 1, 168–174. [Google Scholar] [CrossRef]
- Wu, D.M.; Gao, T.T.; Yang, H.X.; Du, Y.Z.; Li, C.; Wei, L.X.; Zhou, T.Y.; Lu, J.M.; Bi, H.T. Simultaneous microwave/ultrasonic-assisted enzymatic extraction of antioxidant ingredients from Nitraria tangutorun Bobr. juice by-products. Ind. Crops Prod. 2015, 66, 229–238. [Google Scholar] [CrossRef]
- Bouras, K.; Kopsidas, K.; Bariotakis, M.; Kitsiou, P.; Kapodistria, K.; Agrogiannis, G. Effects of dietary supplementation with sea buckthorn (Hippophae rhamnoides L.) seed oil on an experimental model of hypertensive retinopathy in wistar rats. Biomed. Hub. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Han, L.; Meng, J.; Yang, Y.; Ye, Y.; Sou, Y.R. Antioxidant and Anti-fatigue Activities of Seed Oil from the Berries of Three Indigenous Plants in Tibetan Plateau. J. Food Nutr. Res. 2015, 3, 445–457. [Google Scholar] [CrossRef]
- Sbihi, H.M.; Nehdi, I.A.; Tan, C.P.; Al-Resayes, S.I. Bitter and sweet lupin (Lupinus albus L.) seeds and seed oils: A comparison study of their compositions and physicochemical properties. Ind. Crops Prod. 2013, 49, 573–579. [Google Scholar] [CrossRef]
- Devittori, C.; Gumy, D.; Kusy, A.; Colarow, L.; Bertoli, C.; Lambelet, P. Supercritical fluid extraction of oil from millet bran. J. Am. Oil Chem. Soc. 2000, 77, 573–579. [Google Scholar] [CrossRef]
- Naila, B.R.; Barba, F.J.; Danielle, B.; Isabelle, C. Supercritical CO2 extraction of oil, fatty acids and flavonolignans from milk thistle seeds: Evaluation of their antioxidant and cytotoxic activities in Caco-2 cells. Food Chem. Toxicol. 2015, 83, 275–282. [Google Scholar]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Šarkanj, B.; Cikoš, A.M.; Aladić, K.; Jokić, S. Chemical diversity of Codium bursa (Olivi) C. agardh headspace compounds, volatiles, fatty Acids and insight into its antifungal activity. Molecules 2019, 5, 842. [Google Scholar]
- Wang, C.; Duan, Z.; Fan, L.; Li, J. Supercritical CO2 fluid extraction of Elaeagnus mollis Diels seed oil and its antioxidant ability. Molecules 2019, 5, 911. [Google Scholar] [CrossRef] [PubMed]
- Nimet, G.; Silva, E.A.D.; Palú, F.; Dariva, C.; Freitas, L.d.S.; Neto, A.M.; Filho, L.C. Extraction of sunflower (Heliantus annuus L.) oil with supercritical CO2 and subcritical propane: Experimental and modeling. Chem. Eng. J. 2011, 168, 262–268. [Google Scholar] [CrossRef]
- Yang, F.; Xue, C.Y. Research advancement of nutritional characteristics and functions of common edible oils. Food Nutr. China 2013, 19, 63–66. [Google Scholar]
- Lopez-Perez, O.; Picon, A.; Nunez, M. Volatile compounds and odour characteristics of seven species of dehydrated edible seaweeds. Food Res. Int. 2016, 99, 1002–1010. [Google Scholar] [CrossRef]
- Bartley, J.P.; Foley, P. Supercritical fluid extraction of Australian-grown ginger (Zingiber officinale). J. Sci. Food Agric. 1994, 66, 365–371. [Google Scholar] [CrossRef]
- Wei, M.C.; Xiao, J.; Yang, Y.C. Extraction of α-humulene-enriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 2016, 210, 172–181. [Google Scholar] [CrossRef] [PubMed]
- González-Mas, M.C.; Rambla, J.L.; López-Gresa, M.P.; Blázquez, M.A.; Granell, A. Volatile Compounds in Citrus essential oils: A Comprehensive Review. Front. Plant Sci. 2019, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Gerhardt, N.; Schwolow, S.; Rohn, S.; Pérez-Cacho, P.R.; Galán-Soldevilla, H.; Arce, L.; Weller, P. Quality assessment of olive oils based on temperature-ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM. Food Chem. 2019, 278, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Karpas, Z. Applications of ion mobility spectrometry (IMS) in the field of foodomics. Food Res. Int. 2013, 1, 1146–1151. [Google Scholar] [CrossRef]
- Chen, T.; Qi, X.; Chen, M.; Chen, B. Gas Chromatography-Ion Mobility Spectrometry Detection of Odor Fingerprint as Markers of Rapeseed Oil Refined Grade. J. Anal. Methods Chem. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Armenta, S.; Alcala, M.; Blanco, M. A review of recent, unconventional applications of ion mobility spectrometry (IMS). Anal. Chim. Acta 2011, 2, 114–123. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Torbati, M.; Azadmard-Damirchi, S.; Savage, G.P. Vegetable oil blending: A review of physicochemical, nutritional and health effects. Trends Food Sci. Technol. 2016, 57, 52–58. [Google Scholar]
- Bhattacharjee, P.; Singhal, R.S.; Tiwari, S.R. Supercritical carbon dioxide extraction of cotton seed oil. J. Food Eng. 2007, 79, 892–898. [Google Scholar] [CrossRef]
- Shi, L.N.; Ren, F.; Zhao, X.D.; Du, Y.G.; Han, F. Supercritical carbon dioxide extraction of Microula sikkimensis seed oil. J. Am. Oil Chem. Soc. 2010, 87, 1221–1226. [Google Scholar]
- Belbaki, A.; Louaer, W.; Meniai, A.H. Supercritical CO2 extraction of oil from Crushed Algerian olives. J. Supercrit. Fluid 2017, 130, 165–171. [Google Scholar] [CrossRef]
- Shen, Y.B.; Zheng, L.Y.; Jin, J.; Li, X.J.; Fu, J.N.; Wang, M.Z.; Guan, Y.F.; Song, X. Phytochemical and Biological Characteristics of Mexican Chia Seed Oil. Molecules 2018, 12, 3219. [Google Scholar] [CrossRef] [PubMed]
- Timilsena, Y.P.; Vongsvivut, J.; Adhikari, R.; Adhikari, B. Physicochemical and thermal characteristics of Australian chia seed oil. Food Chem. 2017, 228, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Hamdan, D.; El-Readi, M.Z.; Nibret, E.; Sporer, F.; Farrag, N.; El-Shazly, A.; Wink, M. Chemical composition of the essential oils of two Citrus species and their biological activities. Pharmazie 2010, 65, 141–147. [Google Scholar] [PubMed]
- El Asbahani, A.; Miladi, K.; Badri, W.; Sala, M.; Addi, E.H.A.; Casabianca, H.; El Mousadik, A.; Hartmann, D.; Jilale, A.; Renaud, F.N.R.; et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015, 483, 220–243. [Google Scholar] [CrossRef]
- Jiang, M.H.; Yang, L.; Zhu, L.; Piao, J.H.; Jiang, J.G. Comparative GC/MS analysis of essential oils extracted by 3 methods from the bud of Citrus aurantium L. var. amara Engl. J. Food Sci. 2011, 76, 1219–1225. [Google Scholar] [CrossRef]
- Temelli, F. Perspectives on supercritical fluid processing of fats and oils. J. Supercrit. Fluid 2009, 47, 583–590. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Mitrev, S.; Stafilov, T.; Markova, N.; Leitner, E.; Lankmayr, E.; Siegmund, B. Characterisation of traditional Macedonian edible oils by their fatty acid composition and their volatile compounds. Food Res. Int. 2015, 77, 506–514. [Google Scholar] [CrossRef]
- Tian, L.L.; Zeng, Y.Y.; Zheng, X.Q.; Chui, Y.H.; Liu, T. Detection of Peanut oil adulteration mixed with Rapeseed Oil using Gas Chromatography and Gas Chromatography–Ion Mobility Spectrometry. Food Anal. Method 2019, 9, 2282–2292. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Y.Y.; Wang, Y.; Liao, H.Z.; Han, Z.T. Comparison analysis of volatile components in two kinds of rattan pepper oil produced from different processing. Sci. Tech. Food Ind. 2019, 40, 215–220. [Google Scholar]
- Hao, S.X.; Wei, Y.; Li, L.H.; Yang, X.Q.; Cen, J.W.; Huang, H.; Lin, W.L.; Yuan, X.M. The effects of different extraction methods on composition and storage stability of sturgeon oil. Food Chem. 2015, 173, 274–282. [Google Scholar] [CrossRef]
- Yu, W.L.; Han, N.; Wu, K.J.; Sang, Y.X.; Wang, X.H. Analysis of characteristic aroma components of flaxseed oils obtained by different processing techniques by HS-SPME-GC-MS-O. J. Food Sci. 2019, 18, 266–272. [Google Scholar]
- Xi, W.P.; Zheng, H.W.; Zhang, Q.Y.; Li, W.H. Profiling taste and aroma compound metabolism during apricot fruit development and ripening. Int. J. Mol. Sci. 2016, 7, 998. [Google Scholar] [CrossRef] [PubMed]
- Pripis-Nicolau, L.; Bertrand, A.; Maujean, A. Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. J. Agric. Food Chem. 2000, 48, 3761–3766. [Google Scholar] [CrossRef] [PubMed]
- Javed, H.U.; Wang, D.; Wu, G.F.; Kaleeme, Q.M.; Duan, C.Q.; Shi, Y. Post-storage changes of volatile compounds in air- and sun-dried raisins with different packaging materials using HS-SPME with GC/MS. Food Res. Int. 2019, 119, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Wang, R.R.; Guo, J.J.; Ge, K.; Li, G.X.; Fu, F.H.; Ding, S.H.; Yang, S. Changes in the volatile components of Candied Kumquats in different processing methodologies with Headspace-Gas Chromatography-Ion Mobility Spectrometry. Molecules 2019, 17, 3053. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Araújo, L.; Enguix, L.; Verdú, A.; García-García, E.; Carbonell-Barrachina, A.A. Investigation of aromatic compounds in toasted almonds used for the manufacture of turrón. Eur. Food Res. Technol. 2008, 227, 243–254. [Google Scholar] [CrossRef]
- Talens, P.; Escriche, I.; Martínez-Navarrete, N.; Chiralt, A. Influence of osmotic dehydration and freezing on the volatile profile of kiwi fruit. Food Res. Int. 2003, 36, 635–642. [Google Scholar] [CrossRef]
- Risch, S.J.; Ho, C.T. Flavor chemistry. In Industrial and Academic Research; American Chemical Society: Washington, DC, USA, 2000. [Google Scholar]
- Hossein, M.; Seyed, M.S.; Fatemeh, E.; Ali, A. Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind. Crops Prod. 2019, 131, 106–116. [Google Scholar]
- Pu, W.; Pang, S.; Jia, H. Using DSC/TG/DTA techniques to re-evaluate the effect of clays on crude oil oxidation kinetics. J. Petrol. Sci. Eng. 2015, 134, 123–130. [Google Scholar] [CrossRef]
- Jiao, J.; Li, Z.G.; Gai, Q.Y.; Li, X.J.; Wei, F.Y.; Fu, Y.J.; Ma, W. Microwave-assisted aqueous enzymatic extraction of oil from pumpkin seeds and evaluation of its physicochemical properties, fatty acid compositions and antioxidant activities. Food Chem. 2014, 147, 17–24. [Google Scholar] [CrossRef]
- Eller, F.J.; Cermak, S.C.; Taylor, S.L. Supercritical carbon dioxide extraction of cuphea seed oil. Ind. Crops. Prod. 2011, 33, 554–557. [Google Scholar] [CrossRef]
- Liu, S.C.; Yang, F.; Zhang, C.H.; Ji, H.W.; Hong, P.Z.; Deng, C.J. Optimization of process parameters for supercritical carbon dioxide extraction of Passiflora seed oil by response surface methodology. J. Supercrit. Fluid 2009, 48, 9–14. [Google Scholar] [CrossRef]
- Ruttarattanamongkol, K.; Petrasch, A. Oxidative susceptibility and thermal properties of Moringa oliefera seed oil obtained by pilotscale subcritical and supercritical carbon dioxide extraction. J. Food Process. Eng. 2016, 39, 226–236. [Google Scholar] [CrossRef]
- Aleksandar, S.C.; Agnieszka, T. Antioxidant capacity of rapeseed extracts obtained by conventional and ultrasound-assisted extraction. J. Am. Oil Chem. Soc. 2014, 91, 2011–2019. [Google Scholar]
- Zeng, L.B.; Zhang, Z.R.; Luo, Z.H.; Zhu, J.X. Antioxidant activity and chemical constituents of essential oil and extracts of Rhizoma homalomenae. Food Chem. 2011, 125, 456–463. [Google Scholar] [CrossRef]
- Chen, X.; Li, L.; Liu, X.; Luo, R.; Liao, G.; Li, L.; Liu, J.P.; Cheng, J.Q.; Lu, Y.R.; Chen, Y.N. Oleic acid protects saturated fatty acid mediated lipotoxicity in hepatocytes and rat of non-alcoholic steatohepatitis. Life Sci. 2018, 203, 291–304. [Google Scholar] [CrossRef]
- Jian, T.Y.; Wu, Y.X.; Ding, X.Q.; Lv, H.; Ma, L.; Zuo, Y.Y.; Ren, B.R.; Zhao, L.; Tong, B.; Chen, J.; et al. A novel sesquiterpene glycoside from Loquat leaf alleviates oleic acid-induced steatosis and oxidative stress in HepG2 cells. Biomed. Pharmacother. 2017, 97, 1125–1130. [Google Scholar] [CrossRef]
Sample Availability:B. dasystachya oil obtained by the SC-CO2 method and PEE method are available from the authors. |
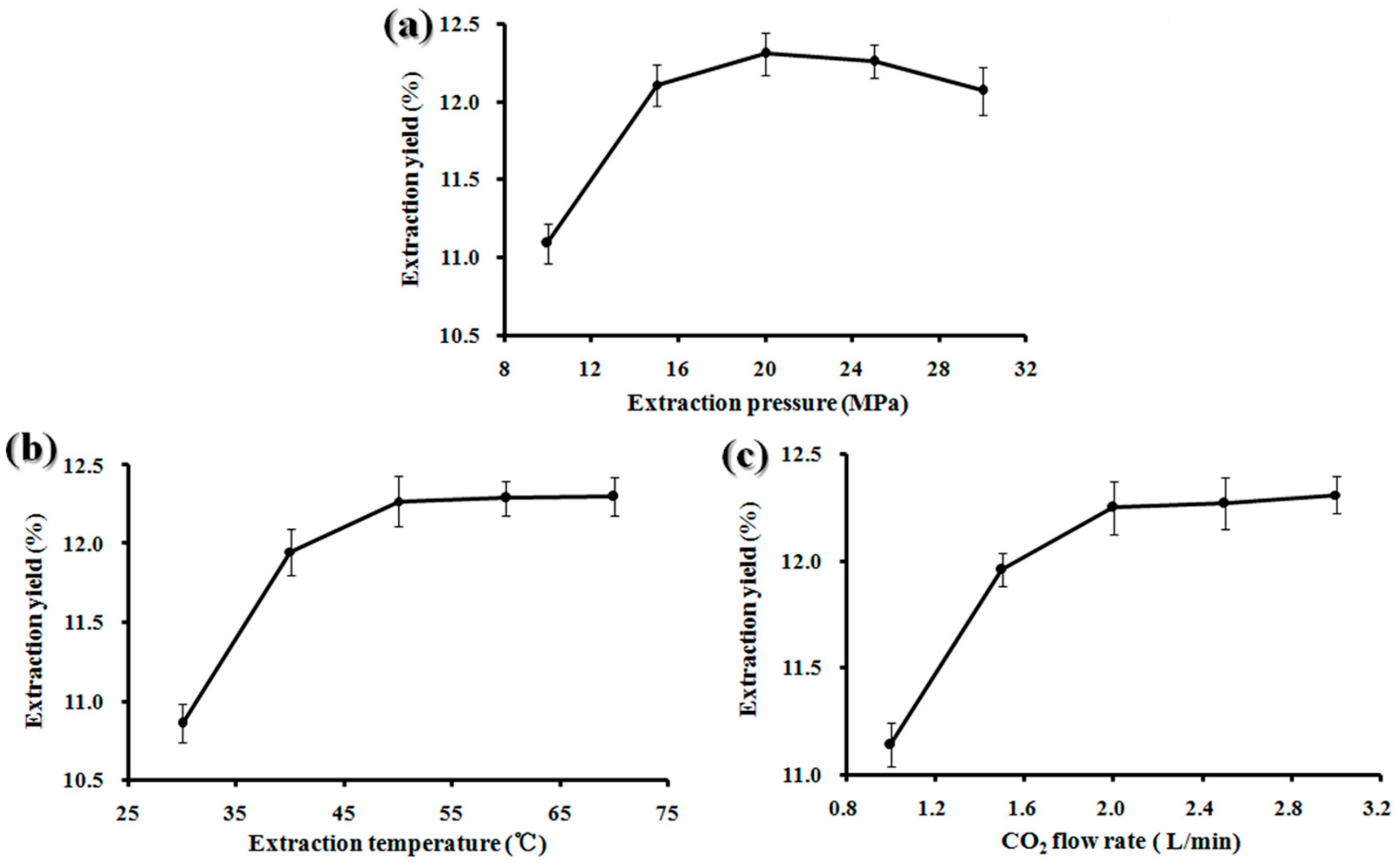
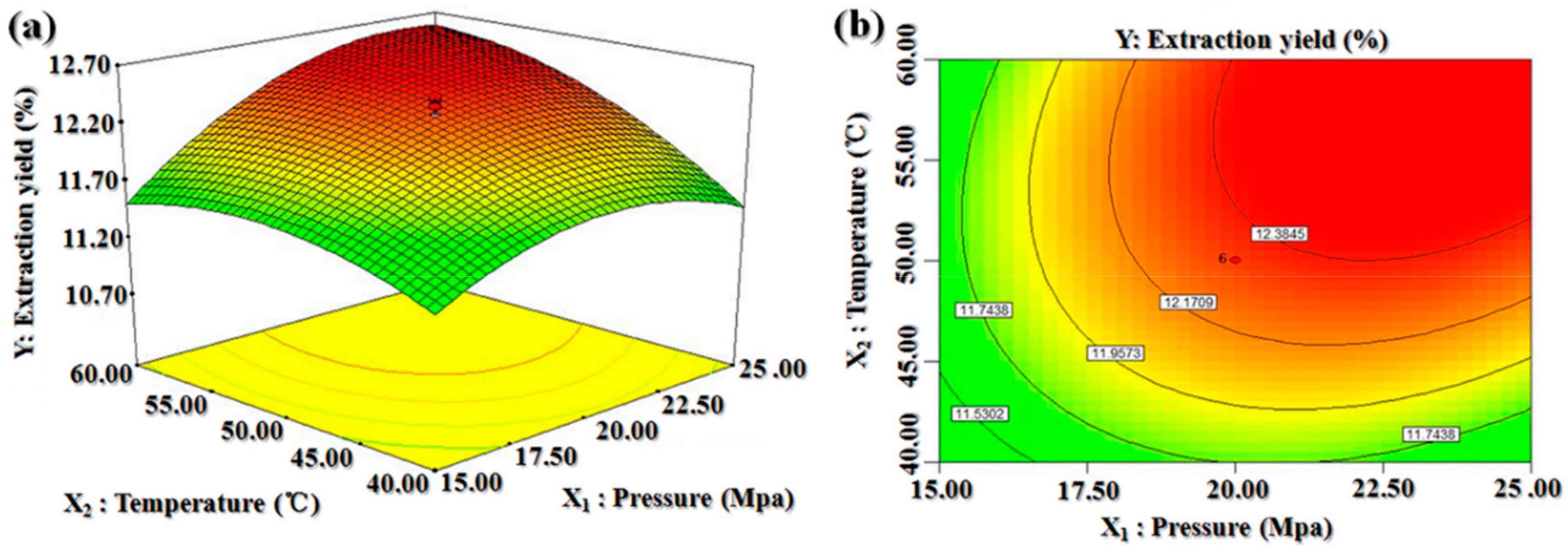
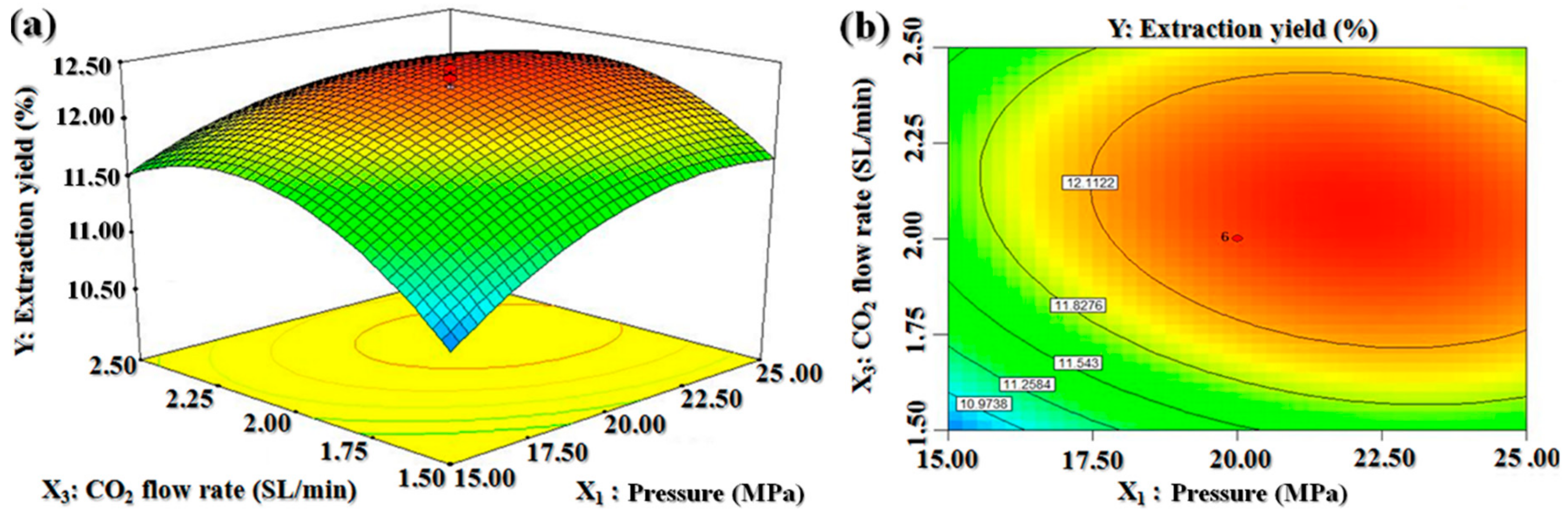
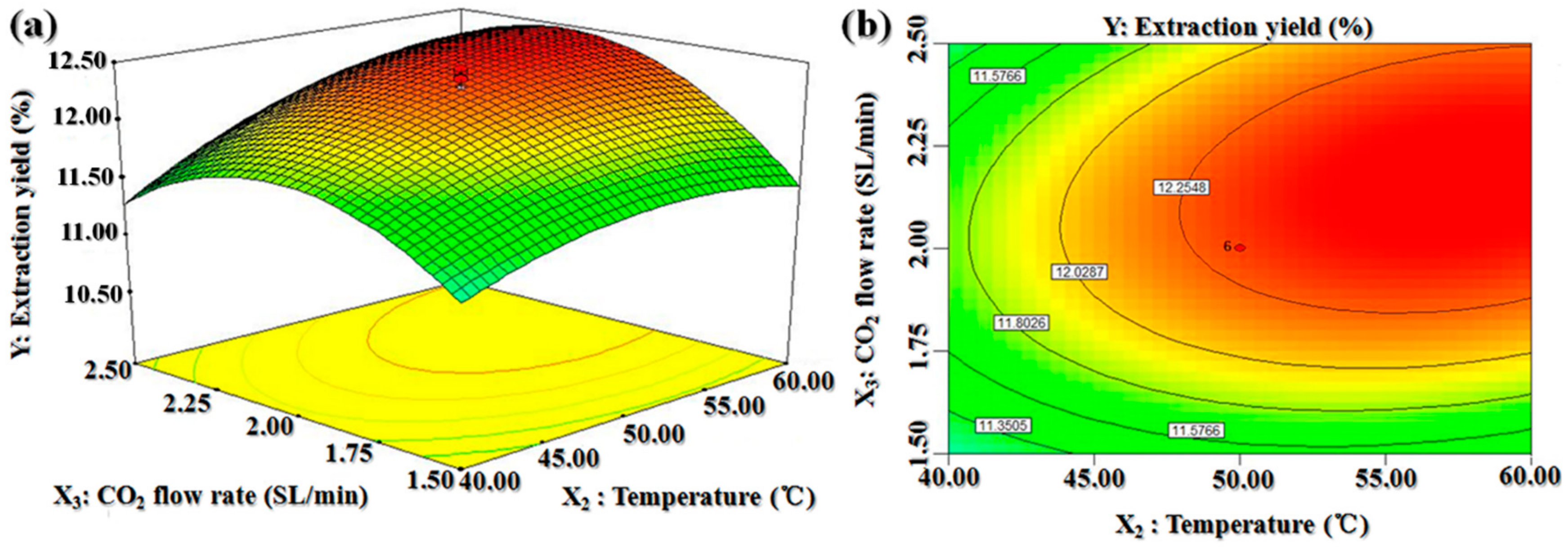
| Run | Pressure (X1) (MPa) | Temperature (X2) (°C) | CO2 Flow Rate (X3) (SL/min) | BDSO Yield (%) | |
|---|---|---|---|---|---|
| Measured Value | Predicted Value | ||||
| 1 | −1 | −1 | −1 | 10.39 | 11.23 |
| 2 | 1 | 1 | 1 | 12.44 | 12.35 |
| 3 | 1.682 | 0 | 0 | 11.89 | 11.77 |
| 4 | 0 | 0 | 0 | 12.42 | 12.35 |
| 5 | 1 | 1 | −1 | 11.56 | 11.69 |
| 6 | 1 | −1 | 1 | 10.72 | 11.08 |
| 7 | −1 | 1 | −1 | 10.66 | 10.36 |
| 8 | 0 | 0 | 0 | 12.36 | 12.35 |
| 9 | −1 | −1 | 1 | 11.07 | 10.34 |
| 10 | 0 | 0 | 1.682 | 10.95 | 11.77 |
| 11 | 0 | 0 | 0 | 12.44 | 12.35 |
| 12 | 0 | 1.682 | 0 | 12.02 | 12.07 |
| 13 | −1.682 | 0 | 0 | 10.74 | 10.78 |
| 14 | 0 | 0 | 0 | 12.35 | 12.35 |
| 15 | 0 | 0 | 0 | 12.25 | 12.35 |
| 16 | 0 | 0 | 0 | 12.29 | 12.35 |
| 17 | 0 | 0 | −1.682 | 10.41 | 10.38 |
| 18 | 0 | −1.682 | 0 | 11.18 | 10.96 |
| 19 | 1 | −1 | −1 | 10.98 | 10.90 |
| 20 | −1 | 1 | 1 | 11.39 | 11.76 |
| Standard Deviation | Coefficient of Variation (CV, %) | Adjusted R2 | Adeq Precision | Predicted R2 | R2 |
|---|---|---|---|---|---|
| 0.16 | 1.38 | 0.9560 | 17.878 | 0.8679 | 0.9768 |
| Source | Sum of Squares (SS) | Degree of Freedom (df) | Mean Square (MS) | F-Value | p-Value (Prob > F) |
|---|---|---|---|---|---|
| Model | 10.72 | 9 | 1.19 | 46.84 | <0.0001 ** |
| X1 | 1.31 | 1 | 1.31 | 51.39 | 0.0001 ** |
| X2 | 1.42 | 1 | 1.42 | 55.83 | <0.0001 ** |
| X3 | 0.77 | 1 | 0.77 | 30.20 | 0.0003 ** |
| X1X2 | 0.41 | 1 | 0.41 | 16.11 | 0.0025 ** |
| X1X3 | 0.21 | 1 | 0.21 | 8.18 | 0.0169 * |
| X2X3 | 0.28 | 1 | 0.28 | 10.92 | 0.00080 ** |
| X1X1 | 1.74 | 1 | 1.74 | 68.31 | <0.0001 ** |
| X2X2 | 0.87 | 1 | 0.87 | 34.41 | 0.0002 ** |
| X3X3 | 4.71 | 1 | 4.71 | 185.24 | <0.0001 ** |
| Residual | 0.25 | 10 | 0.025 | - | - |
| Lack of Fit | 0.16 | 5 | 0.033 | 1.85 | 0.2587 |
| Pure Error | 0.089 | 5 | 0.018 | - | - |
| Cor Total | 10.97 | 19 | - | - | - |
| Pressure (X1) (MPa) | Temperature (X2) (°C) | CO2 Flow Rate (X3) (SL/min) | B. dasystachya Seed Oil Yields (g/100 g) | |
|---|---|---|---|---|
| Actual Value | Predicted Value | |||
| 25 | 59.03 | 2.25 | 12.54 ± 0.56 | 12.553 |
| SFA | C11 | C13 | C14 | C15 | C16 | C18 | C20 | ∑SPA |
| PEE a | 0.11 ± 0.02 | 0.24 ± 0.04 | 3.65 ± 0.63 | - | 12.19 ± 1.12 | 2.03 ± 0.44 | 0.90 ± 0.16 | 19.12 |
| SC-CO2 b | 0.15 ± 0.01 | 0.18 ± 0.03 | 3.69 ± 0.77 | 0.47 ± 0.06 | 5.98 ± 1.33 | 3.16 ± 0.57 | 0.74 ± 0.09 | 14.37 |
| UFA | C16:1 | C18:1 | C18:2 | C18:3 | C22:1 | ∑UFA | ∑PUFA | SFA/UFA |
| PEE a | 0.63 ± 0.14 | 23.08 ± 1.58 | 21.41 ± 1.09 | 32.28 ± 2.02 | 3.47 ± 0.25 | 80.87 | 53.69 | 0.2364 |
| SC-CO2 b | 1.77 ± 0.32 | 20.34 ± 1.30 | 23.16 ± 1.97 | 34.74 ± 1.91 | 5.61 ± 0.83 | 85.62 | 57.90 | 0.1678 |
| Marker Number | Volatile Organic Compounds | Chemical Abstracts Service (CAS#) | Retention Index | Retention Time (s) | Drift Time (ms) | Formula | Comment | Peak Intensity | Peak Intensity |
|---|---|---|---|---|---|---|---|---|---|
| PEE a | SC-CO2 b | ||||||||
| Esters | |||||||||
| 1 | Isoamyl acetate | C123922 | 877.3 | 333.077 | 1.3124 | C7H14O2 | Monomer | - | 271.3 ± 15.03 |
| 2 | Isoamyl acetate | C123922 | 876.7 | 332.413 | 1.7576 | C7H14O2 | Dimer | - | 166.7 ± 7.02 |
| 3 | Ethyl isovalerate | C108645 | 854.2 | 308.514 | 1.2494 | C7H14O2 | Monomer | 31.33 ± 1.54 | 394.0 ± 10.53 |
| 4 | Ethyl isovalerate | C108645 | 852.2 | 306.523 | 1.664 | C7H14O2 | Dimer | 66.32 ± 1.87 | 248.43 ± 8.02 |
| 7 | Ethyl acetate | C141786 | 611.5 | 153.041 | 1.3413 | C4H8O2 | Monomer | 989.0 ± 4.58 | 2176.01 ± 46.36 |
| 27 | Butyl hexanoate | C626824 | 1176.6 | 985.551 | 1.4731 | C10H20O2 | Monomer | 412.3 ± 2.08 | 37.50 ± 1.43 |
| 32 | Ethyl lactate | C97643 | 815 | 271.866 | 1.5439 | C5H10O3 | Monomer | 551.50 ± 4.58 | - |
| Aldehydes | |||||||||
| 5 | Heptanal | C111717 | 901.2 | 361.114 | 1.3312 | C7H14O | Monomer | 304.33 ± 1.53 | 862.23 ± 28.09 |
| 6 | Heptanal | C111717 | 900.1 | 359.762 | 1.7035 | C7H14O | Dimer | 443.56 ± 1.57 | 1070.68 ± 18.24 |
| 8 | Hexanal | C66251 | 796.4 | 256.461 | 1.5682 | C6H12O | Monomer | 1877.63 ± 11.06 | 2000.56 ± 15.54 |
| 9 | t-2-heptenal | C18829555 | 958.8 | 440.986 | 1.6788 | C7H12O | Monomer | 304.76 ± 1.15 | 252.51 ± 13.65 |
| 13 | Pentanal | C110623 | 694.3 | 189.878 | 1.431 | C5H10O | Monomer | 1172.30 ± 1.52 | 665.25 ± 22.34 |
| 14 | n-Nonanal | C124196 | 1100.6 | 740.792 | 1.4731 | C9H18O | Monomer | 756.21 ± 5.50 | 302.11 ± 4.36 |
| 16 | Octanal | C124130 | 1007.7 | 525.295 | 1.4019 | C8H16O | Monomer | 598.06 ± 15.71 | 174.48 ± 2.09 |
| 17 | Benzaldehyde | C100527 | 958.3 | 440.108 | 1.1555 | C7H6O | Monomer | 519.57 ± 17.06 | 103.02 ± 5.67 |
| 18 | n-Nonanal | C124196 | 1101.3 | 742.815 | 1.9518 | C9H18O | Dimer | 839.77 ± 7.02 | 27.09 ± 0.58 |
| 20 | Octanal | C124130 | 1006.3 | 522.661 | 1.8334 | C8H16O | Dimer | 729.35 ± 5.86 | - |
| 21 | Benzaldehyde | C100527 | 958.8 | 440.986 | 1.4756 | C7H6O | Dimer | 574.41 ± 8.32 | - |
| 22 | Furfural | C98011 | 831.5 | 286.572 | 1.0841 | C5H4O2 | Monomer | 623.59 ± 7.04 | 69.28 ± 6.65 |
| 23 | Furfural | C98011 | 830.3 | 285.522 | 1.3376 | C5H4O2 | Dimer | 871.76 ± 13.22 | - |
| 24 | t-2-octenal | C2548870 | 1063.9 | 645.72 | 1.337 | C8H14O | Monomer | 752.24 ± 9.61 | 71.03 ± 5.29 |
| 25 | t-2-octenal | C2548870 | 1063 | 643.698 | 1.8288 | C8H14O | Dimer | 524.25 ± 1.27 | - |
| 33 | 3-methylbutanal | C590863 | 660.8 | 173.425 | 1.4074 | C5H10O | Monomer | 956.55 ± 3.21 | 78.45 ± 4.06 |
| 34 | 2-methyl-propanal | C78842 | 559.5 | 135.569 | 1.2894 | C4H8O | Monomer | 783.14 ± 3.98 | - |
| Ketones | |||||||||
| 12 | Acetone | C67641 | 505.7 | 121.009 | 1.1222 | C3H6O | Monomer | 2407.02 ± 35.78 | 1640.79 ± 24.31 |
| 15 | 2-Hexanone | C591786 | 787 | 249.108 | 1.1906 | C6H12O | Monomer | 397.71 ± 5.86 | 157.04 ± 8.08 |
| 19 | 2-Hexanone | C591786 | 787 | 249.108 | 1.5089 | C6H12O | Dimer | 781.09 ± 4.53 | 49.17 ± 1.92 |
| 31 | Cyclohexanone | C108941 | 894.7 | 353.164 | 1.4612 | C6H10O | Monomer | 3594.16 ± 4.56 | - |
| Alcohols | |||||||||
| 10 | Ethanol | C64175 | 467.6 | 112.478 | 1.1279 | C2H6O | Monomer | 2822.06 ± 35.77 | 2406.98 ± 42.50 |
| 11 | 1-Propanol | C71238 | 592 | 146.039 | 1.2509 | C3H8O | Monomer | 1483.37 ± 14.03 | 946.48 ± 15.14 |
| 26 | 2-Butoxyethanol | C111762 | 902.6 | 362.824 | 1.5871 | C6H14O2 | Monomer | 638.25 ± 1.19 | - |
| 28 | 1-Hexanol | C111273 | 872.1 | 327.373 | 1.6389 | C6H14O | Monomer | 174.51 ± 2.64 | - |
| 29 | 2-Hexenol | C2305217 | 852.5 | 306.88 | 1.5264 | C6H12O | Monomer | 1471.66 ± 5.89 | 135.05 ± 3.82 |
| Terpenes | |||||||||
| 30 | α-Pinene | C80568 | 935.2 | 405.857 | 1.2239 | C10H16 | Monomer | 1102.94 ± 13.57 | 27.61 ± 2.07 |
| Organic acids | |||||||||
| 35 | Pentanoic acid | C109524 | 903.3 | 363.627 | 1.2299 | C5H10O2 | Monomer | 403.03 ± 4.09 | 29.58 ± 2.16 |
| Independent Variables | Level | ||||
|---|---|---|---|---|---|
| −1.682 | −1 | 0 | 1 | 1.682 | |
| Pressure (X1, MPa) | 11.59 | 15 | 20 | 25 | 28.41 |
| Temperature (X2, ℃) | 33.18 | 40 | 50 | 60 | 66.32 |
| CO2 flow rate (X3, SL/min) | 1.16 | 1.5 | 2.0 | 2.5 | 2.84 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Han, Q.; Yang, Y.; Wang, H.; Wang, S.; Li, G. Characterization and Biological Activities of Seed Oil Extracted from Berberis dasystachya Maxim. by the Supercritical Carbon Dioxide Extraction Method. Molecules 2020, 25, 1836. https://doi.org/10.3390/molecules25081836
Han L, Han Q, Yang Y, Wang H, Wang S, Li G. Characterization and Biological Activities of Seed Oil Extracted from Berberis dasystachya Maxim. by the Supercritical Carbon Dioxide Extraction Method. Molecules. 2020; 25(8):1836. https://doi.org/10.3390/molecules25081836
Chicago/Turabian StyleHan, Lijuan, Qingqing Han, Yongjing Yang, Honglun Wang, ShuLin Wang, and Gang Li. 2020. "Characterization and Biological Activities of Seed Oil Extracted from Berberis dasystachya Maxim. by the Supercritical Carbon Dioxide Extraction Method" Molecules 25, no. 8: 1836. https://doi.org/10.3390/molecules25081836
APA StyleHan, L., Han, Q., Yang, Y., Wang, H., Wang, S., & Li, G. (2020). Characterization and Biological Activities of Seed Oil Extracted from Berberis dasystachya Maxim. by the Supercritical Carbon Dioxide Extraction Method. Molecules, 25(8), 1836. https://doi.org/10.3390/molecules25081836





