Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application
Abstract
1. Introduction
2. Experimental Models of Hyperlipidemia Used to Study Hypolipidemic Effects of Polysaccharides
3. Dyslipidemia Models
4. β-d-Glucans
Hypolipidemic Effects of Carboxymethylated β-Glucan (CMG) and Statin (Atorvastatin) in Acute Lipemia in Mice
5. Mannans
Hypolipidemic Effects of Mannans
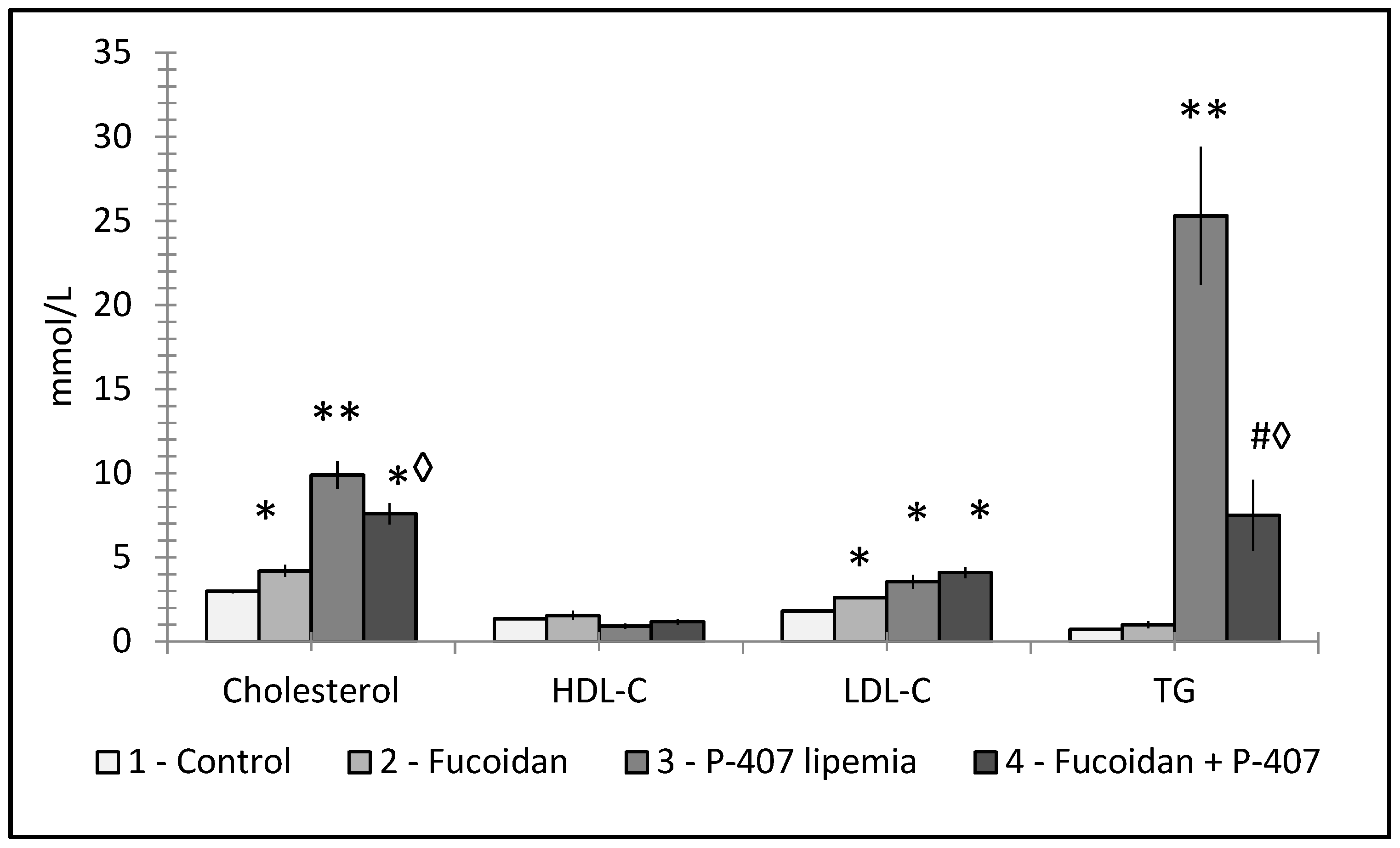
6. Fucoidans
7. Autophagy/Lipophagy Induction and Lipid Metabolism Changes Induced by Polysaccharides
8. Some Perspectives of Prevention and Treatment of Dyslipidemia by Polysaccharides in Medicine
9. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Barter, P.J.; Rye, K.-A. New Era of Lipid-Lowering Drugs. Pharmacol. Rev. 2016, 68, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Berent, T.; Berent, R.; Steiner, S.; Sinzinger, H. Statin-induced muscular side effects at rest and exercise–An anatomical mapping. Atheroscler. Suppl. 2019, 40, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Chaiyasothi, T.; Nathisuwan, S.; Dilokthornsakul, P.; Vathesatogkit, P.; Thakkinstian, A.; Reid, C.; Wongcharoen, W.; Chaiyakunapruk, N. Effects of Non-statin Lipid-Modifying Agents on Cardiovascular Morbidity and Mortality Among Statin-Treated Patients: A Systematic Review and Network Meta-Analysis. Front. Pharmacol. 2019, 10, 547. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-T.; Tsou, Y.-C.; Chen, Y.-T.; Lu, W.-J.; Hwang, P.-A. Effects of Low-Molecular-Weight Fucoidan and High Stability Fucoxanthin on Glucose Homeostasis, Lipid Metabolism, and Liver Function in a Mouse Model of Type II Diabetes. Mar. Drugs 2017, 15, 113. [Google Scholar] [CrossRef] [PubMed]
- Johnston, T.P.; Korolenko, T.; Sahebkar, A. P-407-induced Mouse Model of Dose-controlled Hyperlipidemia and Atherosclerosis. J. Cardiovasc. Pharmacol. 2017, 70, 339–352. [Google Scholar] [CrossRef]
- Loginova, V.M.; Tuzikov, F.V.; Tuzikova, N.A.; Korolenko, T. Comparative characteristics of lipemia models induced by injections of Triton WR-1339 and poloxamer 407 in mice. Bull. Exp. Boil. Med. 2013, 155, 284–287. [Google Scholar] [CrossRef]
- Chen, H.; Nie, Q.; Hu, J.; Huang, X.; Zhang, K.; Pan, S.; Nie, S. Hypoglycemic and Hypolipidemic Effects of Glucomannan Extracted from Konjac on Type 2 Diabetic Rats. J. Agric. Food Chem. 2019, 67, 5278–5288. [Google Scholar] [CrossRef]
- Korolenko, T.; Johnston, T.P.; Tuzikov, F.V.; Tuzikova, N.A.; Pupyshev, A.; Spiridonov, V.K.; Goncharova, N.V.; Maiborodin, I.V.; Zhukova, N.A. Early-stage atherosclerosis in poloxamer 407-induced hyperlipidemic mice: Pathological features and changes in the lipid composition of serum lipoprotein fractions and subfractions. Lipids Heal. Dis. 2016, 15, 16. [Google Scholar] [CrossRef]
- Korolenko, T.; Tuzikov, F.V.; Johnston, T.P.; Tuzikova, N.A.; Kisarova, Y.A.; Zhanaeva, S.Y.; Alexeenko, T.V.; Zhukova, N.A.; Brak, I.V.; Spiridonov, V.K.; et al. The influence of repeated administration of poloxamer 407 on serum lipoproteins and protease activity in mouse liver and heart. Can. J. Physiol. Pharmacol. 2012, 90, 1456–1468. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Effects of yeast-derived β-glucans on blood cholesterol and macrophage functionality. J. Immunotoxicol. 2009, 6, 30–35. [Google Scholar] [CrossRef]
- Du, B.; Meenu, M.; Liu, H.; Xu, B. A Concise Review on the Molecular Structure and Function Relationship of β-Glucan. Int. J. Mol. Sci. 2019, 20, 4032. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Xu, X.; Zeng, F. Correlation between antitumor activity, molecular weight, and conformation of lentinan. Carbohydr. Res. 2005, 340, 1515–1521. [Google Scholar] [CrossRef] [PubMed]
- Větvička, V.C. [beta]-Glucans as Natural Biological Response Modifiers; Nova Science Publishers, Inc.: New York, NY, USA, 2013; 142p. [Google Scholar]
- Babineau, T.J.; Marcello, P.; Swails, W.; Kenler, A.; Bistrian, B.; Forse, R.A. Randomized Phase I/II Trial of a Macrophage-Specific Immunomodulator (PGG-Glucan) in High-Risk Surgical Patients. Ann. Surg. 1994, 220, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yamada, K.; Iwata, O.; Sugimoto, R.; Atsuji, K.; Ogawa, T.; Ishibashi-Ohgo, N.; Suzuki, K. β-Glucan in Foods and Its Physiological Functions. J. Nutr. Sci. Vitaminol. 2018, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Sima, P.; Richter, J.; Vetvicka, V. Glucans as New Anticancer Agents. Anticancer. Res. 2019, 39, 3373–3378. [Google Scholar] [CrossRef]
- Vetvicka, V.; Fernandez-Botran, R. B-glucan and parasites. Helminthologia 2018, 55, 177–184. [Google Scholar] [CrossRef]
- Brown, G.D.; Gordon, S. Fungal β-Glucans and Mammalian Immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef]
- Legentil, L.; Paris, F.; Ballet, C.; Trouvelot, S.; Daire, X.; Vetvicka, V.; Ferrières, V. Molecular Interactions of β-(1→3)-Glucans with Their Receptors. Molecules 2015, 20, 9745–9766. [Google Scholar] [CrossRef]
- Elcombe, S.E.; Naqvi, S.; Bosch, M.W.M.V.D.; MacKenzie, K.F.; Cianfanelli, F.; Brown, G.D.; Arthur, J.S.C. Dectin-1 Regulates IL-10 Production via a MSK1/2 and CREB Dependent Pathway and Promotes the Induction of Regulatory Macrophage Markers. PLoS ONE 2013, 8, e60086. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, J.Y.; Lee, H.K.; Kim, M.S.; Lee, S.R.; Kang, J.S.; Kim, H.M.; Lee, K.-A.; Hong, J.T.; Kim, Y.; et al. Dendritic Cell Activation by Glucan Isolated from Umbilicaria Esculenta. Immune Netw. 2010, 10, 188–197. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Allendorf, D.J.; Hansen, R.; Marroquin, J.; Ding, C.; Cramer, D.E.; Yan, J. Yeast β-Glucan Amplifies Phagocyte Killing of iC3b-Opsonized Tumor Cells via Complement Receptor 3-Syk-Phosphatidylinositol 3-Kinase Pathway. J. Immunol. 2006, 177, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ha, T.; Kelley, J.; Gao, X.; Qiu, Y.; Kao, R.L.; Browder, W.; Williams, D.L. Modulating Toll-like receptor mediated signaling by (1-->3)-beta-D-glucan rapidly induces cardioprotection. Cardiovasc. Res. 2004, 61, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.L.; Ha, T.; Li, C.; Laffan, J.; Kalbfleisch, J.; Browder, W. Inhibition of LPS-induced NFkappaB activation by a glucan ligand involves down-regulation of IKKbeta kinase activity and altered phosphorylation and degradation of IkappaBalpha. Shock 2000, 13, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Fadel, J.G.; Newman, R.K.; Newman, C.W.; Barnes, A.E. Hypocholesterolemic effects of beta-glucans in different barley diets fed to broiler chicks. Nutr. Rep. Int. 1987, 35, 1049–1058. [Google Scholar]
- Lim, M.-K.; Ku, S.-K.; Choi, J.-S.; Kim, J.-W. Effect of polycan, a β-glucan originating from Aureobasidium, on a high-fat diet-induced hyperlipemic hamster model. Exp. Ther. Med. 2015, 9, 1369–1378. [Google Scholar] [CrossRef]
- Food and Drug Administration. FDA Allows Food Containing Psyllium to Make Health Claim on Reducing Risk of Heart Disease; US Department of Health and Human Services: Rockville, MD, USA, 1998.
- Food and Drug Administration. Food Labeling: Health Claims; Oats and Coronary Heart Disease: Final Rule; Food and Drug Administration: Rockville, MD, USA, 1997.
- Sima, P.; Vannucci, L.; Větvička, V. β-glucans and cholesterol (Review). Int. J. Mol. Med. 2018, 41, 1799–1808. [Google Scholar] [CrossRef]
- Slavin, J. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef]
- Lia, A.; Hallmans, G.; Sandberg, A.S.; Sundberg, B.; Åman, P.; Andersson, H. Oat beta-glucan increases bile acid excretion and a fiber-rich barley fraction increases cholesterol excretion in ileostomy subjects. Am. J. Clin. Nutr. 1995, 62, 1245–1251. [Google Scholar] [CrossRef]
- Zhu, X.; Sun, X.; Wang, M.; Zhang, C.; Cao, Y.; Mo, G.; Liang, J.; Zhu, S. Quantitative assessment of the effects of beta-glucan consumption on serum lipid profile and glucose level in hypercholesterolemic subjects. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 714–723. [Google Scholar] [CrossRef]
- Newman, A.R.K.; Lewis, S.E.; Newman, C.W.; Boik, R.J.; Ramage, R.T. Hypocholesterolemic effects of beta-glucans in different barley diets fed to broilers chicks. Nutr. Rep. Int. 1989, 39, 749–760. [Google Scholar]
- Talati, R.; Baker, W.L.; Pabilonia, M.S.; White, C.M.; Coleman, C.I. The Effects of Barley-Derived Soluble Fiber on Serum Lipids. Ann. Fam. Med. 2009, 7, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J. Effect of beta-glucan level in oat fiber extracts on blood lipids in men and women. J. Am. Coll. Nutr. 1997, 16, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, K.; Dinesen, B.; Hoie, L.H.; Morgenstern, E.; Gruenwald, J. Effects of soy and other natural products on LDL:HDL ratio and other lipid parameters: A literature review. Adv. Ther. 2003, 20, 50–78. [Google Scholar] [CrossRef] [PubMed]
- Keogh, G.F.; Cooper, G.J.; Mulvey, T.B.; McArdle, B.H.; Coles, G.D.; Monro, J.A.; Poppitt, S. Randomized controlled crossover study of the effect of a highly β-glucan–enriched barley on cardiovascular disease risk factors in mildly hypercholesterolemic men. Am. J. Clin. Nutr. 2003, 78, 711–718. [Google Scholar] [CrossRef]
- Ho, H.V.T.; Sievenpiper, J.L.; Zurbau, A.; Mejia, S.B.; Jovanovski, E.; Au-Yeung, F.; Jenkins, A.L.; Vuksan, V. The effect of oatβ-glucan on LDL-cholesterol, non-HDL-cholesterol and apoB for CVD risk reduction: A systematic review and meta-analysis of randomised-controlled trials. Br. J. Nutr. 2016, 116, 1369–1382. [Google Scholar] [CrossRef]
- Thandapilly, S.J.; Ndou, S.P.; Wang, Y.; Nyachoti, C.M.; Ames, N. Barley β-glucan increases fecal bile acid excretion and short chain fatty acid levels in mildly hypercholesterolemic individuals. Food Funct. 2018, 9, 3092–3096. [Google Scholar] [CrossRef]
- Wang, Y.; Harding, S.; Thandapilly, S.J.; Tosh, S.M.; Jones, P.J.H.; Ames, N. Barley β-glucan reduces blood cholesterol levels via interrupting bile acid metabolism. Br. J. Nutr. 2017, 118, 822–829. [Google Scholar] [CrossRef]
- Velikonja, A.; Lipoglavšek, L.; Zorec, M.; Orel, R.; Avguštin, G. Alterations in gut microbiota composition and metabolic parameters after dietary intervention with barley beta glucans in patients with high risk for metabolic syndrome development. Anaerobe 2019, 55, 67–77. [Google Scholar] [CrossRef]
- Mikkelsen, M.S.; Jensen, M.G.; Nielsen, T.S. Barley beta-glucans varying in molecular mass and oligomer structure affect cecal fermentation and microbial composition but not blood lipid profiles in hypercholesterolemic rats. Food Funct. 2017, 8, 4723–4732. [Google Scholar] [CrossRef]
- Tong, L.-T.; Zhong, K.; Liu, L.; Zhou, X.; Qiu, J.; Zhou, S. Effects of dietary hull-less barley β-glucan on the cholesterol metabolism of hypercholesterolemic hamsters. Food Chem. 2015, 169, 344–349. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Quint, J.; Rieder, A.; Balance, S.; Dreiss, C.A.; Cross, K.L.; Gray, R.; Bajka, B.; Butterworth, P.J.; Ellis, P.R.; et al. The impact of oat structure and β-glucan on in vitro lipid digestion. J. Funct. Foods 2017, 38, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Uusitupa, M.I.; Ruuskanen, E.; Mäkinen, E.; Laitinen, J.; Toskala, E.; Kervinen, K.; Kesaniemi, Y.A. A controlled study on the effect of beta-glucan-rich oat bran on serum lipids in hypercholesterolemic subjects: Relation to apolipoprotein E phenotype. J. Am. Coll. Nutr. 1992, 11, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.G.; Endrighi, M.; Lisenko, K.G.; De Oliveira, M.R.D.; Damasceno, M.R.; Claudino, J.A.; Gutierres, P.G.; Peconick, A.P.; Saad, F.M.D.O.B.; Zangeronimo, M. Oat beta-glucan as a dietary supplement for dogs. PLoS ONE 2018, 13, e0201133. [Google Scholar] [CrossRef] [PubMed]
- Bub, A.; Malpuech-Brugère, C.; Orfila, C.; Amat, J.; Arianna, A.; Blot, A.; Di Nunzio, M.; Holmes, M.; Kertész, Z.; Marshall, L.; et al. A Dietary Intervention of Bioactive Enriched Foods Aimed at Adults at Risk of Metabolic Syndrome: Protocol and Results from PATHWAY-27 Pilot Study. Nutrients 2019, 11, 1814. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Sun, Y.; Zou, S.; Li, M.; Xu, X. Orally Administered Baker’s Yeast β-Glucan Promotes Glucose and Lipid Homeostasis in the Livers of Obesity and Diabetes Model Mice. J. Agric. Food Chem. 2017, 65, 9665–9674. [Google Scholar] [CrossRef]
- Vetvicka, V.; Vetvickova, J. Physiological effects of different types of β-glucan. Biomed. Pap. 2007, 151, 225–231. [Google Scholar] [CrossRef]
- Nicolosi, R.; Bell, S.J.; Bistrian, B.R.; Greenberg, I.; Forse, R.A.; Blackburn, G.L. Plasma lipid changes after supplementation with β-glucan fiber from yeast. Am. J. Clin. Nutr. 1999, 70, 208–212. [Google Scholar] [CrossRef]
- Závorková, M.; Větvička, V.; Richter, J.; Kral, V.; Liehnova, I.; Rajnohova, D.L. Effects of Glucan and Vitamin D Supplementation on Obesity and Lipid Metabolism in Diabetic Retinopathy. Open Biochem. J. 2018, 12, 36–45. [Google Scholar] [CrossRef]
- Kubo, K.; Nanba, H. The effect of maitake mushrooms on liver and serum lipids. Altern. Ther. Heal. Med. 1996, 2, 62–66. [Google Scholar]
- Aoe, S.; Yamanaka, C.; Koketsu, K.; Nishioka, M.; Onaka, N.; Nishida, N.; Takahashi, M. Effects of Paramylon Extracted from Euglena gracilis EOD-1 on Parameters Related to Metabolic Syndrome in Diet-Induced Obese Mice. Nutrients 2019, 11, 1674. [Google Scholar] [CrossRef]
- Korolenko, T.; Cherkanova, M.S.; Tuzikov, F.V.; Johnston, T.P.; Tuzikova, N.A.; Loginova, V.M.; Kaledin, V.I. Influence of atorvastatin on fractional and subfractional composition of serum lipoproteins and MMP activity in mice with Triton WR 1339-induced lipaemia. J. Pharm. Pharmacol. 2011, 63, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Kogan, G.; Pajtinka, M.; Babincova, M.; Miadoková, E.; Rauko, P.; Slamenova, D.; Korolenko, T. Yeast cell wall polysaccharides as antioxidants and antimutagens: Can they fight cancer? Neoplasma 2008, 55, 387–393. [Google Scholar] [PubMed]
- Korolenko, T.; Tuzikov, F.V.; Cherkanova, M.S.; Johnston, T.P.; Tuzikova, N.A.; Loginova, V.M.; Filjushina, E.E.; Kaledin, V.I. Influence of atorvastatin and carboxymethylated glucan on the serum lipoprotein profile and MMP activity of mice with lipemia induced by poloxamer. Can. J. Physiol. Pharmacol. 2012, 90, 141–153. [Google Scholar] [CrossRef]
- Kagimura, F.Y.; Da Cunha, M.A.A.; Theis, T.V.; Malfatti, C.R.M.; Dekker, R.F.; Barbosa, A.M.; Teixeira, S.D.; Salome, K. Carboxymethylation of (1 --> 6)-beta-glucan (lasiodiplodan): Preparation, characterization and antioxidant evaluation. Carbohydr. Polym. 2015, 127, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Drozdowski, L.A.; Reimer, R.A.; Temelli, F.; Bell, R.C.; Vasanthan, T.; Thomson, A.B. Beta-glucan extracts inhibit the in vitro intestinal uptake of long-chain fatty acids and cholesterol and down-regulate genes involved in lipogenesis and lipid transport in rats. J. Nutr. Biochem. 2009, 21, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.R.S.; Filho, E.X.F. An overview of mannan structure and mannan-degrading enzyme systems. Appl. Microbiol. Biotechnol. 2008, 79, 165–178. [Google Scholar] [CrossRef]
- Križková, L.; Ďuračková, Z.; Šandula, J.; Sasinková, V.; Krajčovič, J. Antioxidative and antimutagenic activity of yeast cell wall mannans in vitro. Mutat. Res. Toxicol. Environ. Mutagen. 2001, 497, 213–222. [Google Scholar] [CrossRef]
- Thiel, S.; Gadjeva, M. Humoral pattern recognition molecules: Mannan-binding lectin and ficolins. Adv. Exp. Med. Biol. 2009, 653, 58–73. [Google Scholar] [CrossRef]
- Ruseva, M.; Kolev, M.; Dagnæs-Hansen, F.; Hansen, S.B.; Takahashi, K.; Ezekowitz, A.; Thiel, S.; Jensenius, J.C.; Gadjeva, M. Mannan-binding lectin deficiency modulates the humoral immune response dependent on the genetic environment. Immunology 2009, 127, 279–288. [Google Scholar] [CrossRef]
- Dong, L.; Wu, J.; Chen, K.; Xie, J.; Wang, Y.; Li, D.; Liu, Y.; Yin, A.; Zhao, Y.; Han, Y.; et al. Mannan-Binding Lectin Attenuates Inflammatory Arthritis Through the Suppression of Osteoclastogenesis. Front. Immunol. 2019, 10, 1239. [Google Scholar] [CrossRef]
- Worthley, D.L.; Bardy, P.G.; Gordon, D.L.; Mullighan, C.G. Mannose-binding lectin and maladies of the bowel and liver. World J. Gastroenterol. 2006, 12, 6420–6428. [Google Scholar] [CrossRef]
- Watanabe, Y.; Bowden, T.A.; Wilson, I.A.; Crispin, M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 1480–1497. [Google Scholar] [CrossRef] [PubMed]
- Eisen, D. Mannose-Binding Lectin Deficiency and Respiratory Tract Infection. J. Innate Immun. 2010, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Heitzeneder, S.; Seidel, M.G.; Förster-Waldl, E.; Heitger, A. Mannan-binding lectin deficiency—Good news, bad news, doesn’t matter? Clin. Immunol. 2012, 143, 22–38. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vannucci, L.; Sima, P.; Richter, J. Beta Glucan: Supplement or Drug? From Laboratory to Clinical Trials. Molecules 2019, 24, 1251. [Google Scholar] [CrossRef]
- Korolenko, T.; Bgatova, N.; Vetvicka, V. Glucan and Mannan-Two Peas in a Pod. Int. J. Mol. Sci. 2019, 20, 3189. [Google Scholar] [CrossRef]
- Korolenko, T.; Svechnikova, I.G.; Filjushina, E.E.; Kaledin, V.I.; Vakulin, G.M.; Usynin, I.F.; Tsyrendordjiev, D.D. Macrophage stimulation and antitumor effect of Ukrain. Drugs Exp. Clin. Res. 1998, 24, 253–260. [Google Scholar]
- Safina, A.F.; Korolenko, T.A.; Mynkina, G.I.; Dushkin, M.I.; Krasnoselskaya, G.A. Liver and serum lysosomal enzymes activity during zymosan-induced inflammation in mice. Agents Actions Suppl. 1992, 38 Pt 3, 370–375. [Google Scholar]
- Korolenko, T.A.; Kisarova, Y.A.; Filjushina, E.E.; Dergunova, M.A.; Machova, E. Macrophage stimulation and β-D-glucans as biological response modifiers: The role in experimental tumor development. In Handbook of Macrophages: Life Cycle, Functions and Diseases; Takahashi, R., Kai, H., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 249–275. [Google Scholar]
- Korolenko, T.; Johnston, T.; Machová, E.; Bgatova, N.; Lykov, A.P.; Goncharova, N.; Nescakova, Z.; Shintyapina, A.; Maiborodin, I.; Karmatskikh, O. Hypolipidemic effect of mannans from C. albicans serotypes A and B in acute hyperlipidemia in mice. Int. J. Boil. Macromol. 2018, 107, 2385–2394. [Google Scholar] [CrossRef]
- Torrecillas, S.; Makol, A.; Caballero, M.J.; Montero, D.; Robaina, L.; Real, F.; Sweetman, J.; Tort, L.; Izquierdo, M. Immune stimulation and improved infection resistance in European sea bass (Dicentrarchus labrax) fed mannan oligosaccharides. Fish Shellfish. Immunol. 2007, 23, 969–981. [Google Scholar] [CrossRef]
- Fitton, J.; Stringer, D.N.; Karpiniec, S.S. Therapies from Fucoidan: An Update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsova, T.A.; Ivanushko, L.A.; Persiyanova, E.V.; Ermakova, S.P.; Besednova, N.N. Markers of Systemic Inflammation in Experimental Dyslipidemia Induced by P-407: Modulation with Fucoidan from Brown Alga Fucus evanescens. Bull. Exp. Boil. Med. 2019, 166, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Berteau, O. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.R.R.; Arumugam, R.; Anantharaman, P.; Rengasamy, K.R. Pharmaceutical potential of a fucoidan-like sulphated polysaccharide isolated from Halodule pinifolia. Int. J. Boil. Macromol. 2013, 62, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, A.A.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide isolated from the Okhotsk Sea Fucus evanescens brown alga. Bull. Exp. Boil. Med. 2007, 143, 730–732. [Google Scholar] [CrossRef]
- Luthuli, S.; Wu, S.; Cheng, Y.; Zheng, X.; Wu, M.; Tong, H. Therapeutic Effects of Fucoidan: A Review on Recent Studies. Mar. Drugs 2019, 17, 487. [Google Scholar] [CrossRef]
- Fitton, J. Therapies from Fucoidan; Multifunctional Marine Polymers. Mar. Drugs 2011, 9, 1731–1760. [Google Scholar] [CrossRef]
- Chollet, L.; Saboural, P.; Chauvierre, C.; Villemin, J.-N.; Letourneur, D.; Chaubet, F. Fucoidans in Nanomedicine. Mar. Drugs 2016, 14, 145. [Google Scholar] [CrossRef]
- Van Weelden, G.; Bobiński, M.; Okła, K.; Van Weelden, W.J.; Romano, A.; Pijnenborg, J. Fucoidan Structure and Activity in Relation to Anti-Cancer Mechanisms. Mar. Drugs 2019, 17, 32. [Google Scholar] [CrossRef]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important Determinants for Fucoidan Bioactivity: A Critical Review of Structure-Function Relations and Extraction Methods for Fucose-Containing Sulfated Polysaccharides from Brown Seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Patil, N.P.; Le, V.; Sligar, A.D.; Mei, L.; Chavarria, D.; Yang, E.Y.; Baker, A.B. Algal Polysaccharides as Therapeutic Agents for Atherosclerosis. Front. Cardiovasc. Med. 2018, 5, 153. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.-O.; Yu, Q. Fucoidan delays apoptosis and induces pro-inflammatory cytokine production in human neutrophils. Int. J. Boil. Macromol. 2015, 73, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Vetvicka, V.; Vetvickova, J. Fucoidans Stimulate Immune Reaction and Suppress Cancer Growth. Anticancer. Res. 2017, 37, 6041–6046. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Citkowska, A.; Szekalska, M.; Winnicka, K. Possibilities of Fucoidan Utilization in the Development of Pharmaceutical Dosage Forms. Mar. Drugs 2019, 17, 458. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Huang, L.; Liu, X.; Liu, N.; Zhang, Q.; Liu, S. Antihyperlipidemic activity of high sulfate content derivative of polysaccharide extracted from Ulva pertusa (Chlorophyta). Carbohydr. Polym. 2012, 87, 1637–1640. [Google Scholar] [CrossRef]
- Park, M.-K.; Jung, U.; Roh, C. Fucoidan from Marine Brown Algae Inhibits Lipid Accumulation. Mar. Drugs 2011, 9, 1359–1367. [Google Scholar] [CrossRef]
- Huang, L.; Wen, K.; Gao, X.; Liu, Y. Hypolipidemic effect of fucoidan from Laminaria japonica in hyperlipidemic rats. Pharm. Boil. 2010, 48, 422–426. [Google Scholar] [CrossRef]
- Yokota, T.; Nomura, K.; Nagashima, M.; Kamimura, N. Fucoidan alleviates high-fat diet-induced dyslipidemia and atherosclerosis in ApoEshl mice deficient in apolipoprotein E expression. J. Nutr. Biochem. 2016, 32, 46–54. [Google Scholar] [CrossRef]
- Kim, M.-J.; Chang, U.-J.; Lee, J.-S. Inhibitory Effects of Fucoidan in 3T3-L1 Adipocyte Differentiation. Mar. Biotechnol. 2008, 11, 557–562. [Google Scholar] [CrossRef]
- Kim, M.-J.; Jeon, J.; Lee, J.-S. Fucoidan Prevents High-Fat Diet-Induced Obesity in Animals by Suppression of Fat Accumulation. Phytotherapy Res. 2013, 28, 137–143. [Google Scholar] [CrossRef]
- Park, J.; Yeom, M.; Hahm, D.-H. Fucoidan improves serum lipid levels and atherosclerosis through hepatic SREBP-2-mediated regulation. J. Pharmacol. Sci. 2016, 131, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, J.; Chang, Y.; Xu, J.; Wang, Y.; Long, T.; Xue, C. Fucoidan from the sea cucumber Acaudina molpadioides exhibits anti-adipogenic activity by modulating the Wnt/β-catenin pathway and down-regulating the SREBP-1c expression. Food Funct. 2014, 5, 1547–1555. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, L.; Chen, Q.; Zhang, P.; Jia, L.; Li, H.; Chen, C. Fucoidan alleviates dyslipidemia and modulates gut microbiota in high-fat diet-induced mice. J. Funct. Foods 2018, 48, 220–227. [Google Scholar] [CrossRef]
- Wang, H.; Sun, W.; Huang, S.; Xiang, W. Experimental study of weight-losing and hypolipidemic effects of Thallus laminariae polysaccharides and its mechanisms in rat. Chin. J. Mod. Appl. Pharm. 2008, 25. [Google Scholar]
- Ren, D.; Wang, Q.; Yang, Y.; Hu, Y.; Song, Y.; He, Y.; Liu, S.; Wu, L. Hypolipidemic effects of fucoidan fractions from Saccharina sculpera (Laminariales, Phaeophyceae). Int. J. Boil. Macromol. 2019, 140, 188–195. [Google Scholar] [CrossRef]
- Corona, D.M.H.; Martínez-Abundis, E.; González-Ortiz, M. Effect of Fucoidan Administration on Insulin Secretion and Insulin Resistance in Overweight or Obese Adults. J. Med. Food 2014, 17, 830–832. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, G.; Wang, Y.; Yin, J.; Wang, J.; Xia, B.; Li, T.; Yang, X.; Hou, P.; Hu, S.; et al. Fucoidan A2 from the Brown Seaweed Ascophyllum nodosum Lowers Lipid by Improving Reverse Cholesterol Transport in C57BL/6J Mice Fed a High-Fat Diet. J. Agric. Food Chem. 2019, 67, 5782–5791. [Google Scholar] [CrossRef]
- Kounakis, K.; Chaniotakis, M.; Markaki, M.; Tavernarakis, N. Emerging Roles of Lipophagy in Health and Disease. Front. Cell Dev. Boil. 2019, 7, 185. [Google Scholar] [CrossRef]
- Schulze, R.J.; Sathyanarayan, A.; Mashek, D.G. Breaking fat: The regulation and mechanisms of lipophagy. Biochim. Biophys. Acta (BBA)-Mol. Cell Boil. Lipids 2017, 1862, 1178–1187. [Google Scholar] [CrossRef]
- Li, X.; Gong, H.; Yang, S.; Yang, L.; Fan, Y.; Zhou, Y. Pectic Bee Pollen Polysaccharide from Rosa rugosa Alleviates Diet-Induced Hepatic Steatosis and Insulin Resistance via Induction of AMPK/mTOR-Mediated Autophagy. Molecules 2017, 22, 699. [Google Scholar] [CrossRef]
- Liu, C.; Liao, J.-Z.; Li, P. Traditional Chinese herbal extracts inducing autophagy as a novel approach in therapy of nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 1964–1973. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Wang, Q.; Fu, J.; Ren, R. Polysaccharides derived from natural sources regulate triglyceride and cholesterol metabolism: A review of the mechanisms. Food Funct. 2019, 10, 2330–2339. [Google Scholar] [CrossRef]
- Cingolani, F.; Czaja, M.J. Regulation and Functions of Autophagic Lipolysis. Trends Endocrinol. Metab. 2016, 27, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yao, Z.; Chen, Y.; Qian, L.; Jiang, S.; Zhou, J.; Shao, J.; Chen, A.; Zhang, F.; Zheng, S. Lipophagy and liver disease: New perspectives to better understanding and therapy. Biomed. Pharmacother. 2018, 97, 339–348. [Google Scholar] [CrossRef]
- Xiao, J.; Liong, E.C.; Ching, Y.P.; Chang, R.C.-C.; Fung, M.L.; Xu, A.M.; So, K.-F.; Tipoe, G.L. Lycium barbarum polysaccharides protect rat liver from non-alcoholic steatohepatitis-induced injury. Nutr. Diabetes 2013, 3, e81. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Zhang, Y.; Ren, J. Autophagic Regulation of Lipid Homeostasis in Cardiometabolic Syndrome. Front. Cardiovasc. Med. 2018, 5, 38. [Google Scholar] [CrossRef]
- Dai, Z.; Feng, S.; Liu, A.B.; Wang, H.; Zeng, X.; Yang, C.S. Protective effects of α-galacto-oligosaccharides against a high-fat/western-style diet-induced metabolic abnormalities in mice. Food Funct. 2019, 10, 3660–3670. [Google Scholar] [CrossRef]
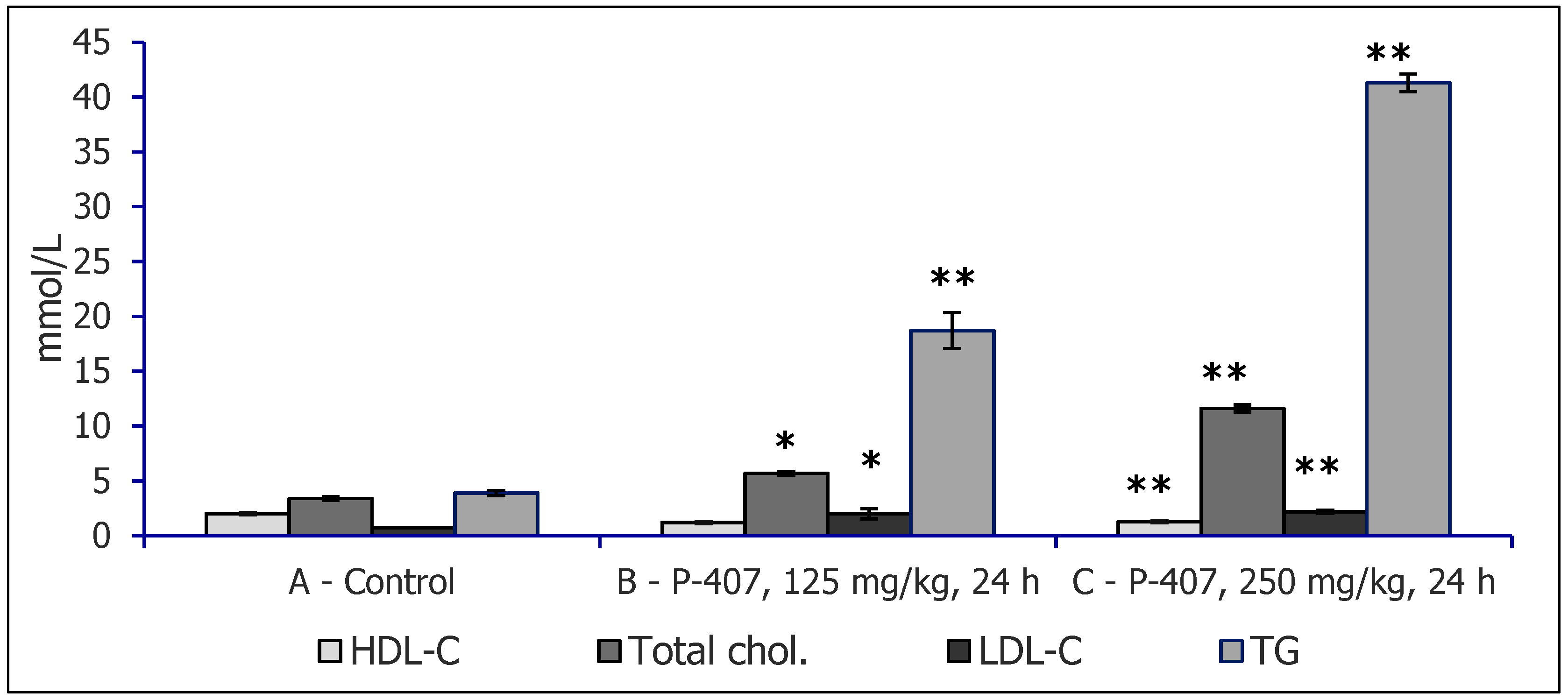
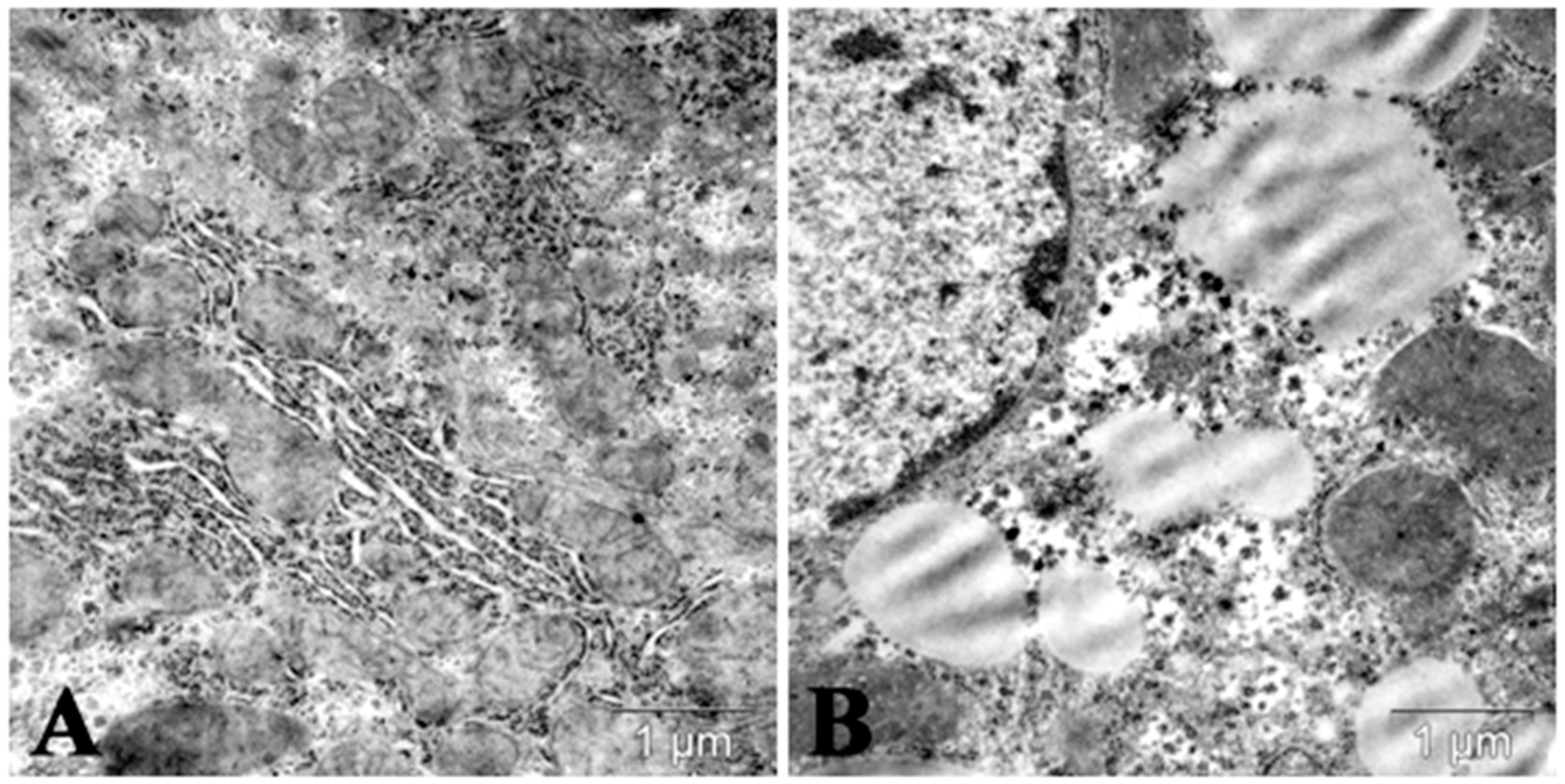
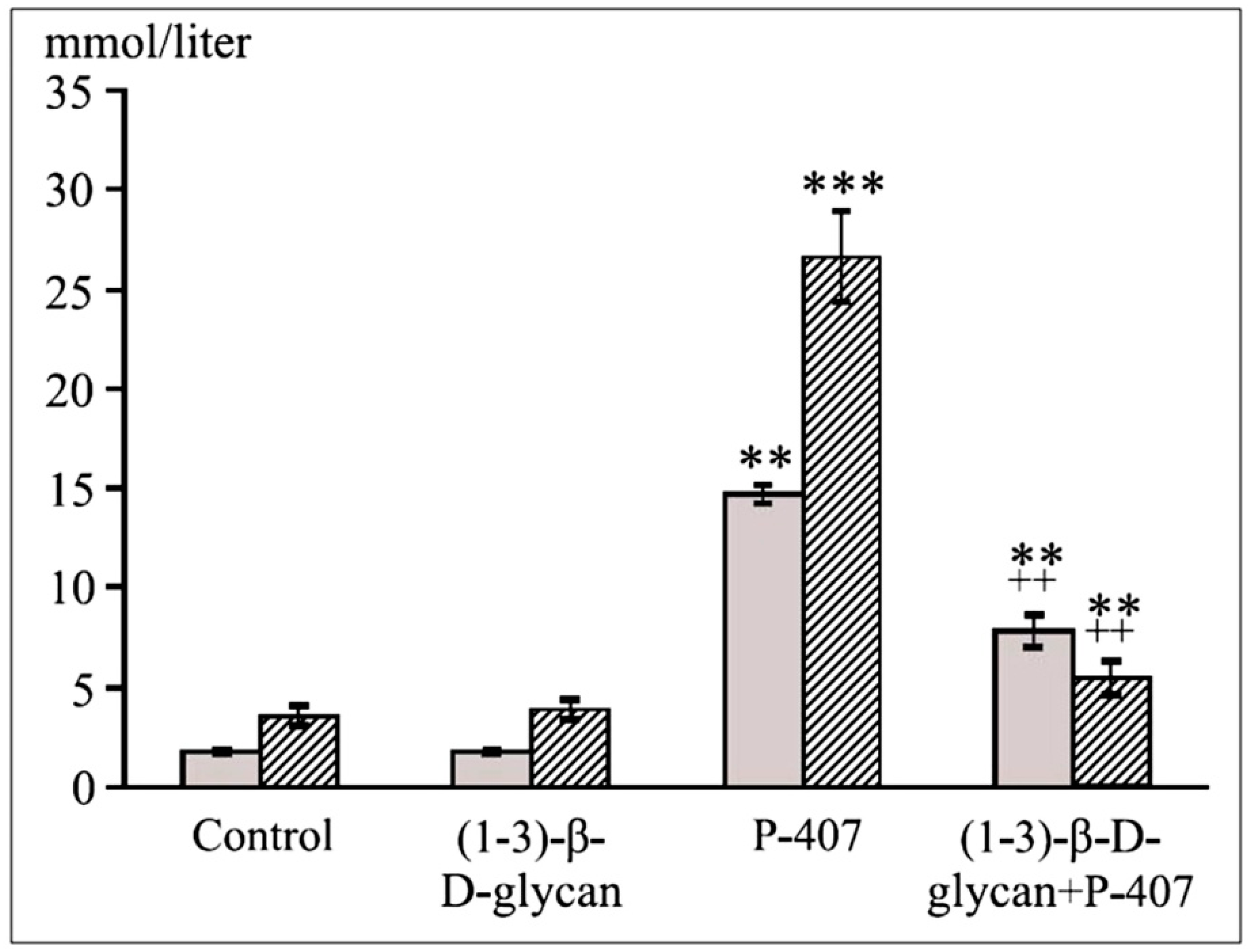
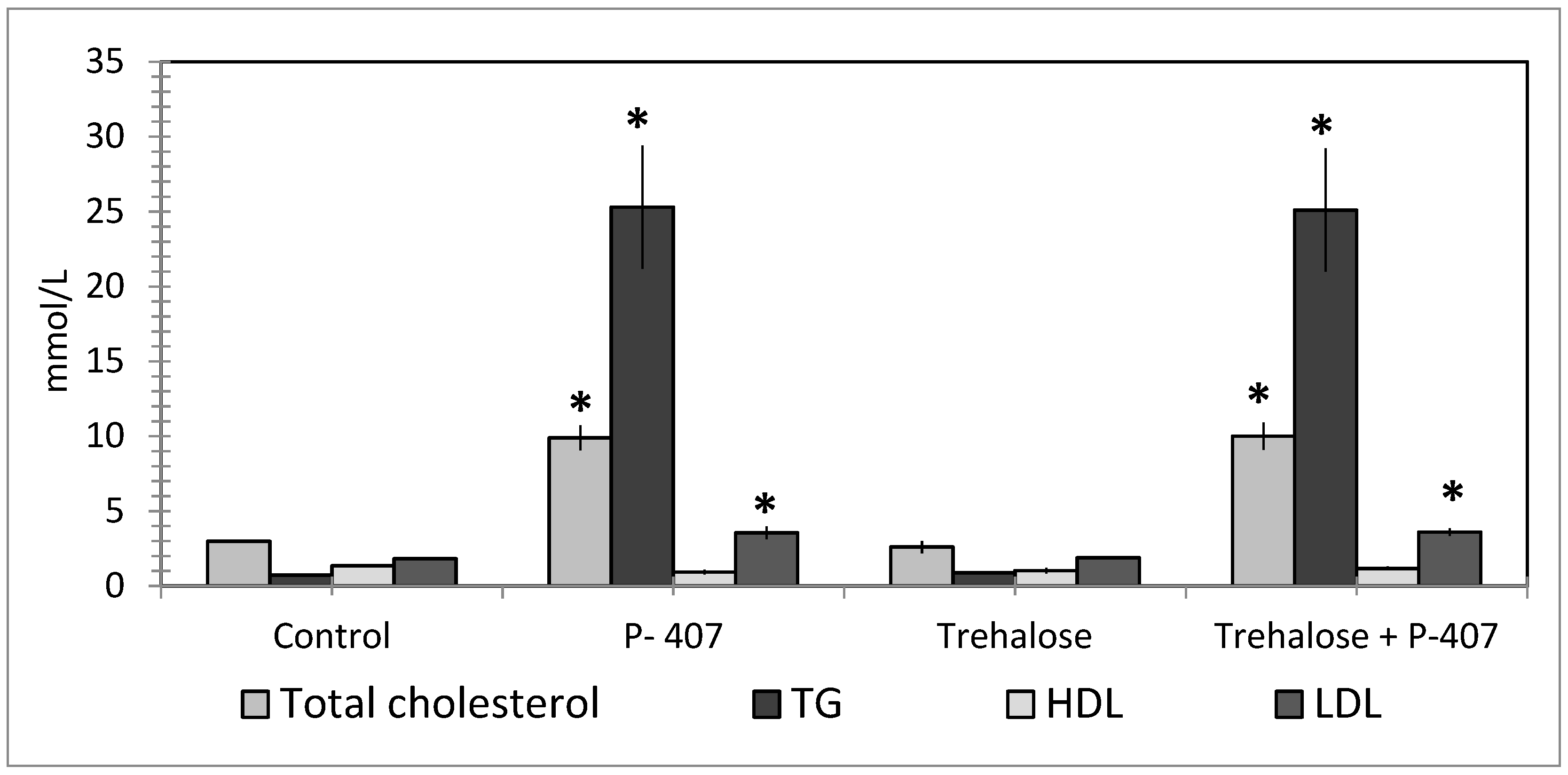


| Parameter | Control | Triton WR 1339 | Poloxamer 40 |
|---|---|---|---|
| Cholesterol | 108.4 ± 11.6 | 189 ± 11.6 * | 232.2 ± 2.3 *,# |
| TG | 115.1 ± 8.8 | 1610.7 ± 155.8 * | 2283.3 ± 88.5 *,# |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korolenko, T.A.; Bgatova, N.P.; Ovsyukova, M.V.; Shintyapina, A.; Vetvicka, V. Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application. Molecules 2020, 25, 1819. https://doi.org/10.3390/molecules25081819
Korolenko TA, Bgatova NP, Ovsyukova MV, Shintyapina A, Vetvicka V. Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application. Molecules. 2020; 25(8):1819. https://doi.org/10.3390/molecules25081819
Chicago/Turabian StyleKorolenko, Tatiana A., Nataliya P. Bgatova, Marina V. Ovsyukova, Alexandra Shintyapina, and Vaclav Vetvicka. 2020. "Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application" Molecules 25, no. 8: 1819. https://doi.org/10.3390/molecules25081819
APA StyleKorolenko, T. A., Bgatova, N. P., Ovsyukova, M. V., Shintyapina, A., & Vetvicka, V. (2020). Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application. Molecules, 25(8), 1819. https://doi.org/10.3390/molecules25081819








