Design, Molecular Modeling and Synthesis of Metal-Free Sensitizers of Thieno Pyridine Dyes as Light-Harvesting Materials with Efficiency Improvement Using Plasmonic Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
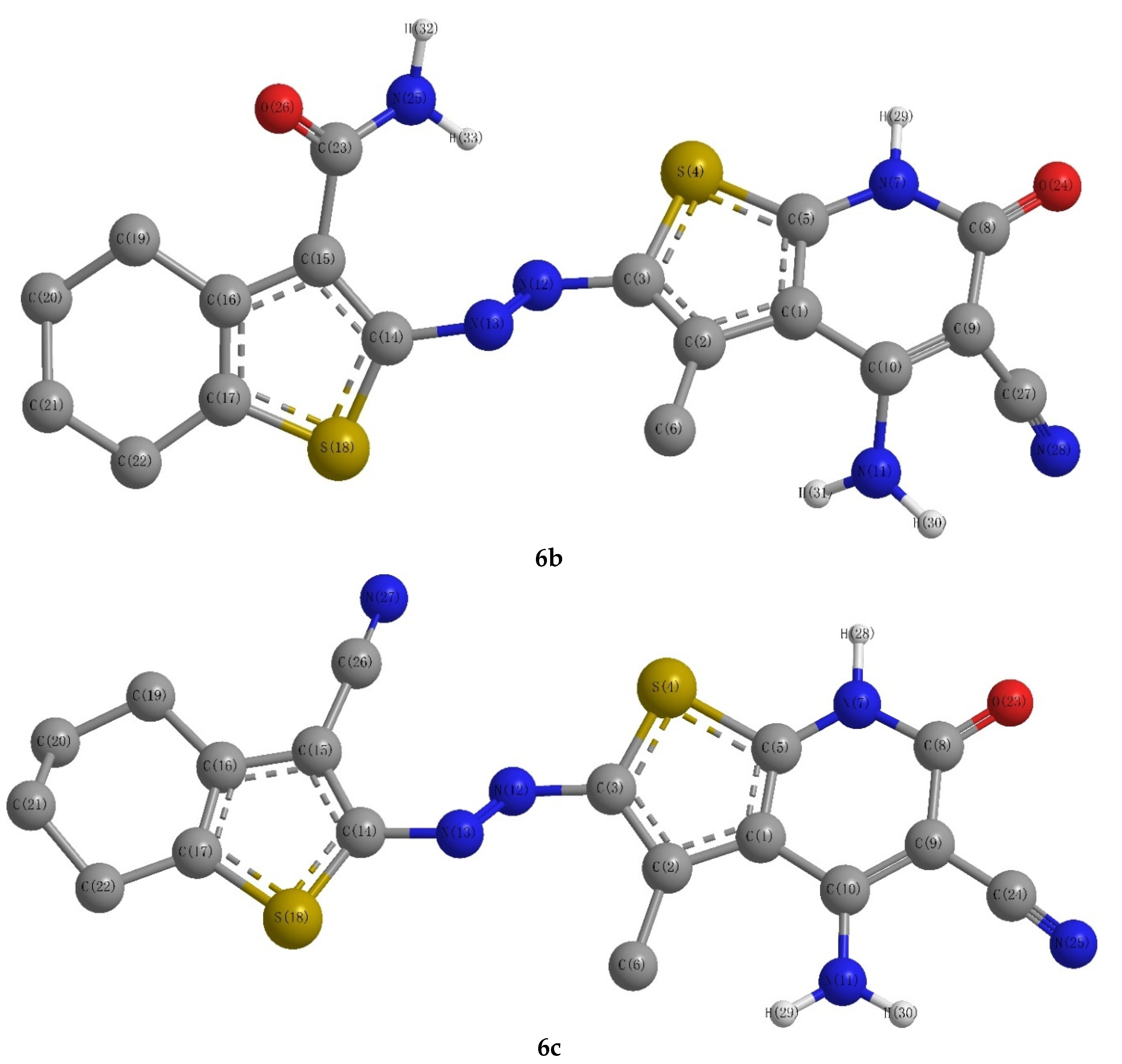
2.1. Chemistry
2.2. Metal Nanoparticles (MNPs) Characterization
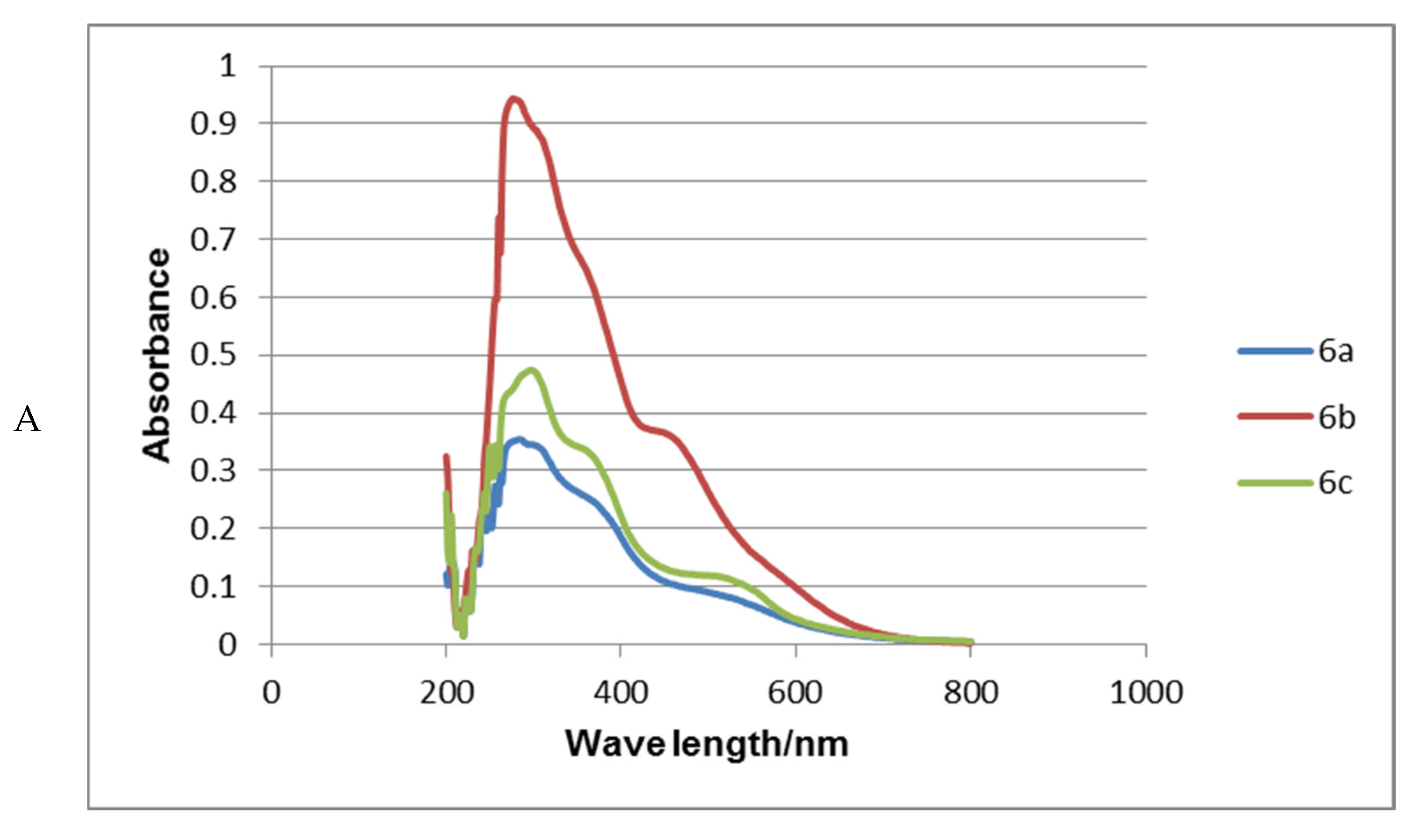
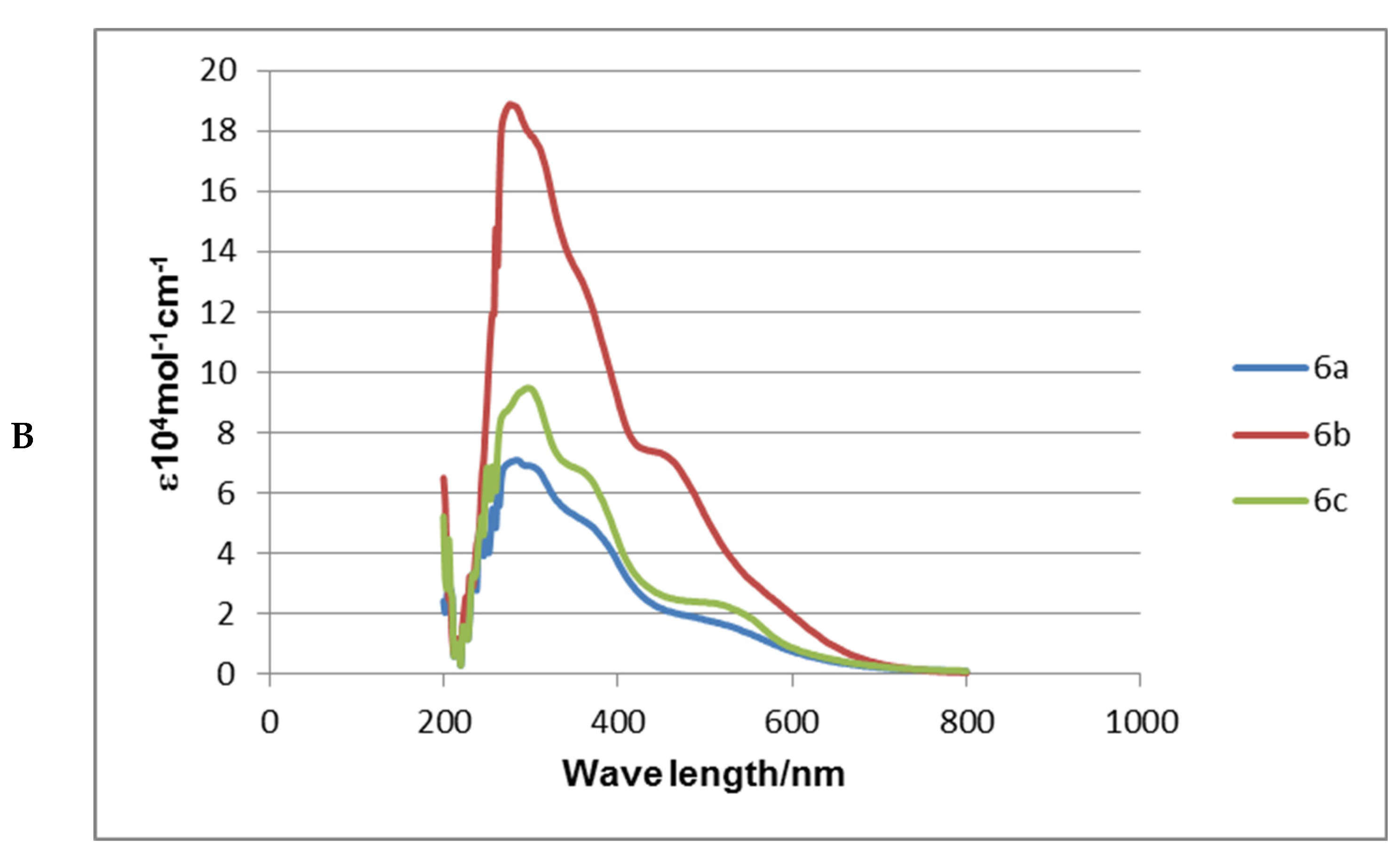
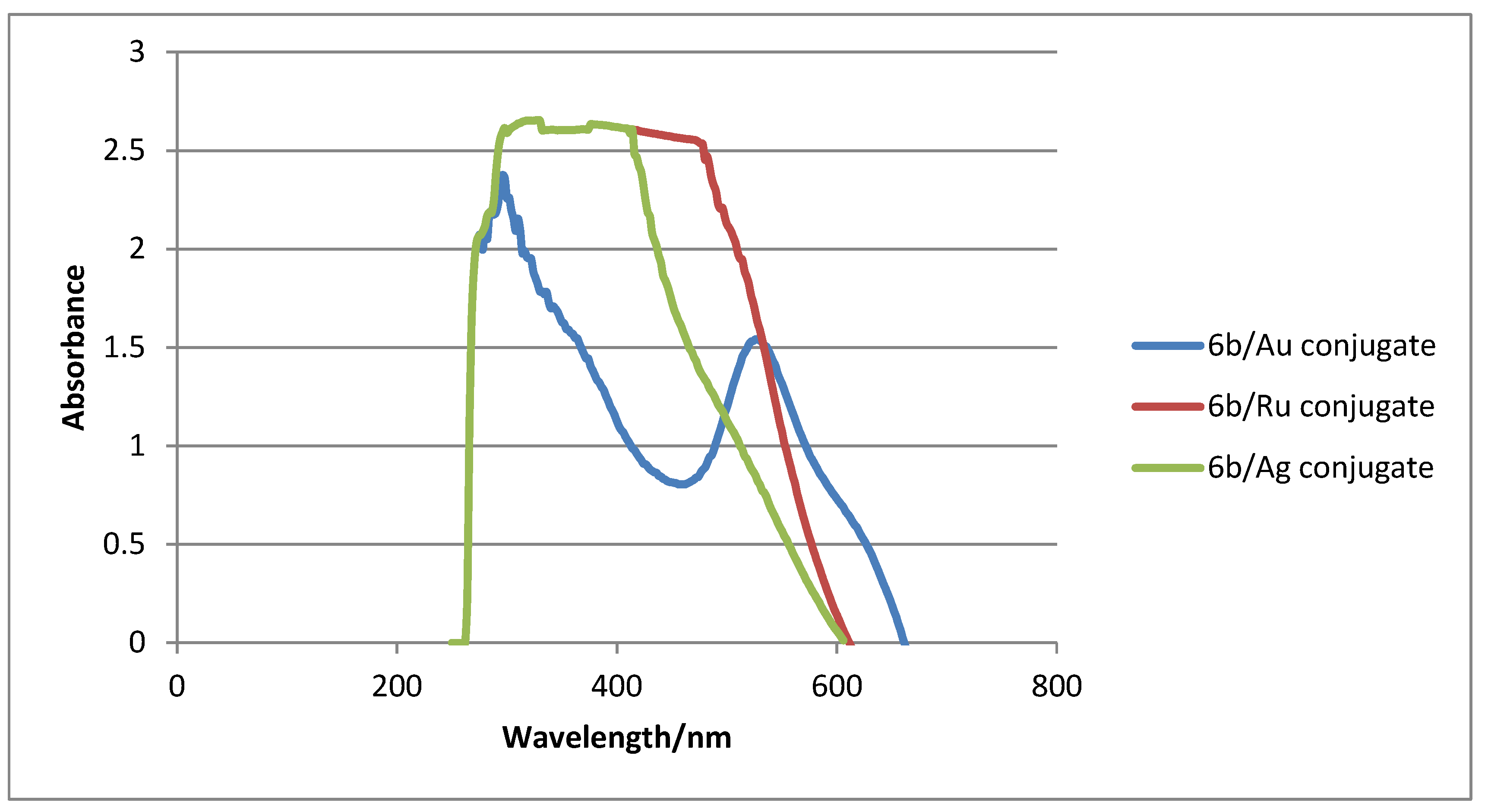
2.3. Absorption Spectra Analysis
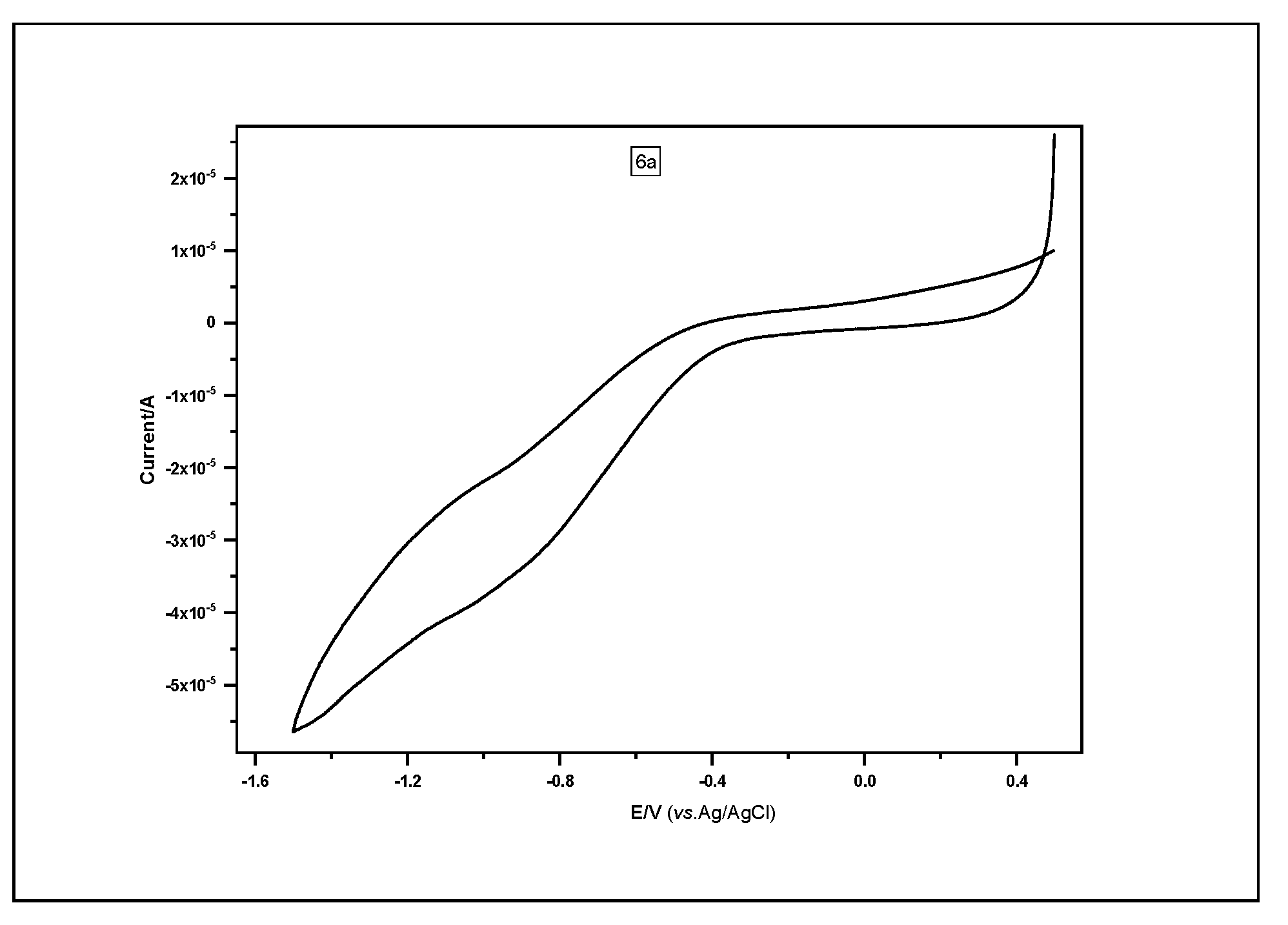
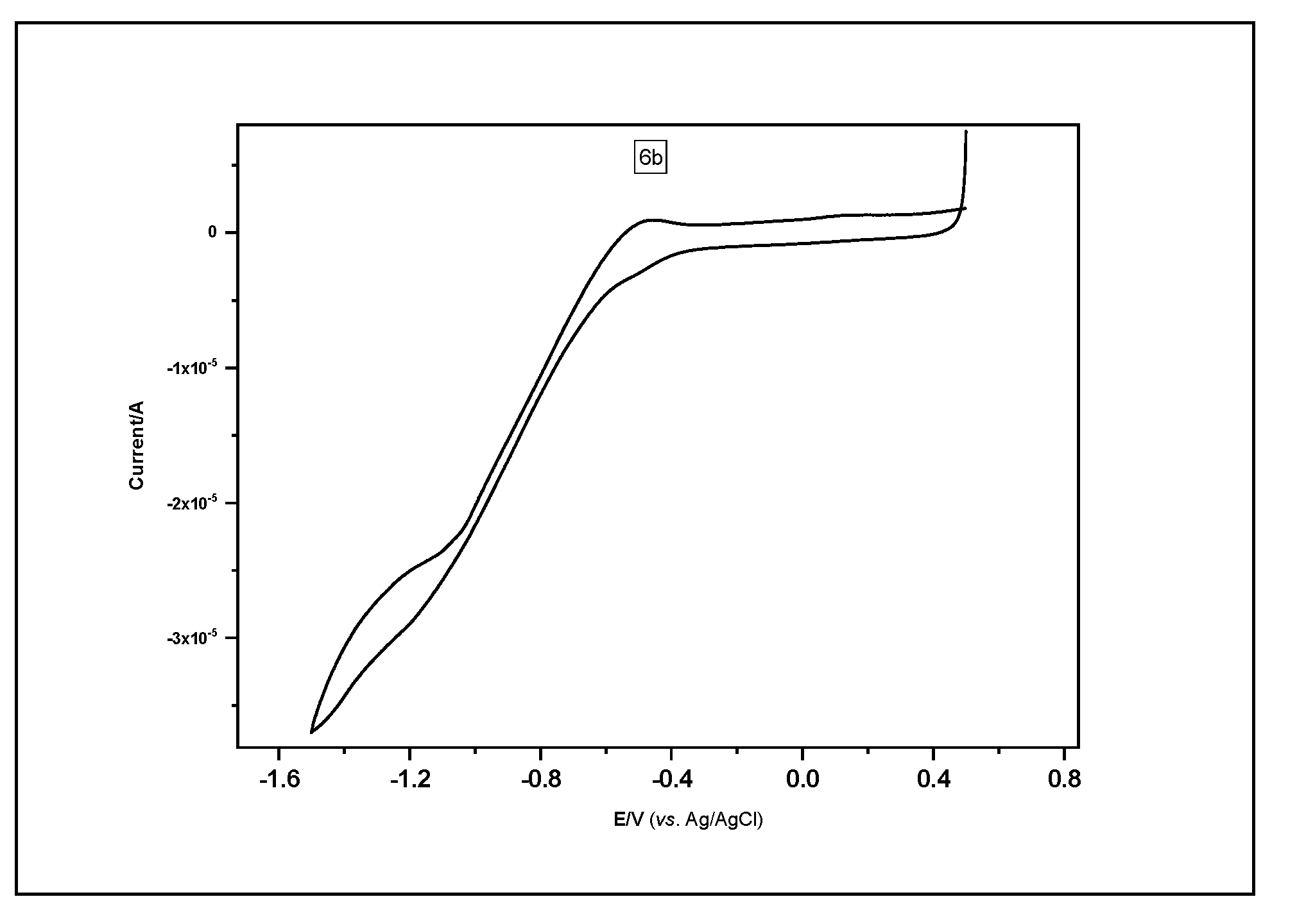
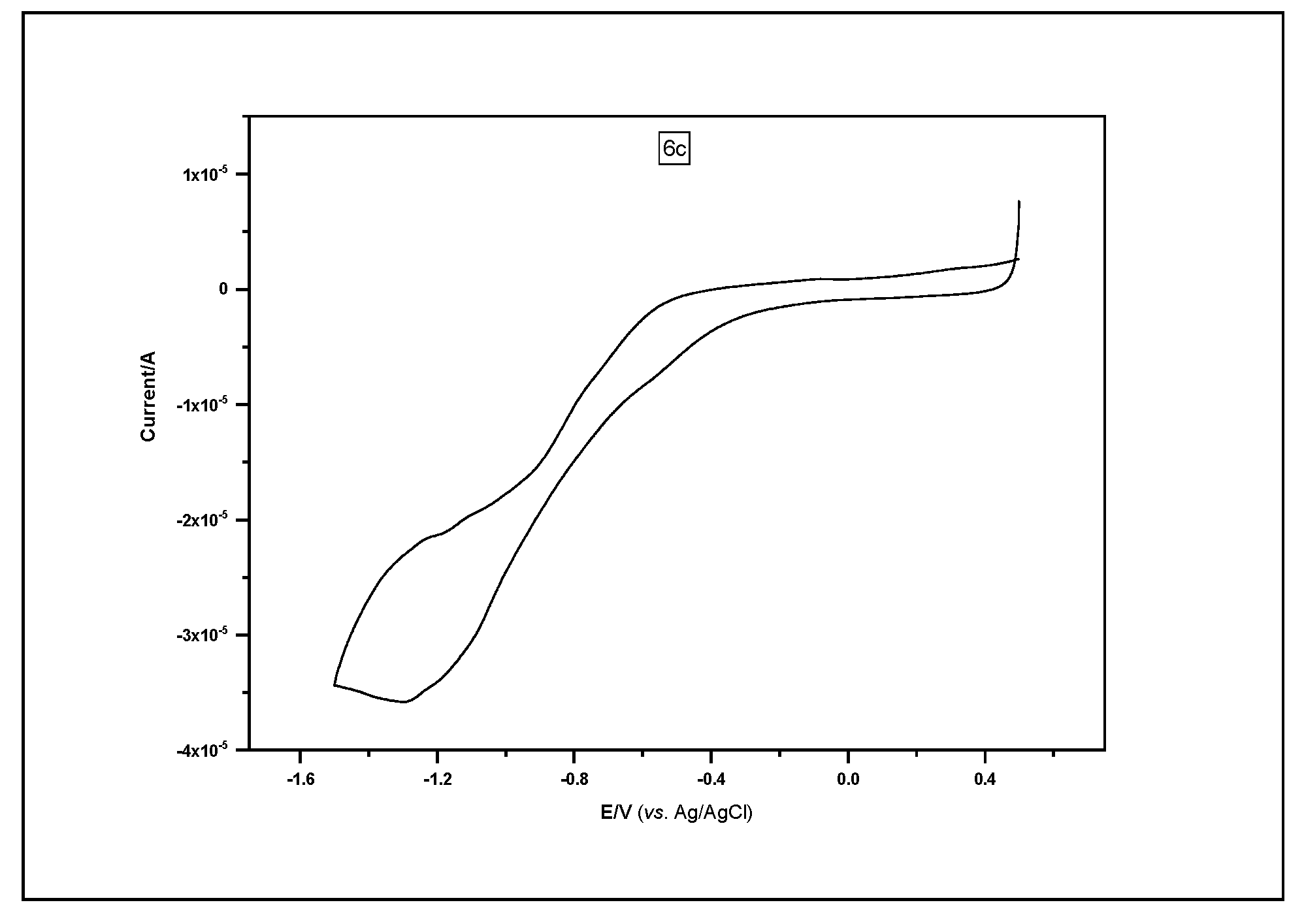
2.4. Electrochemical Characterization
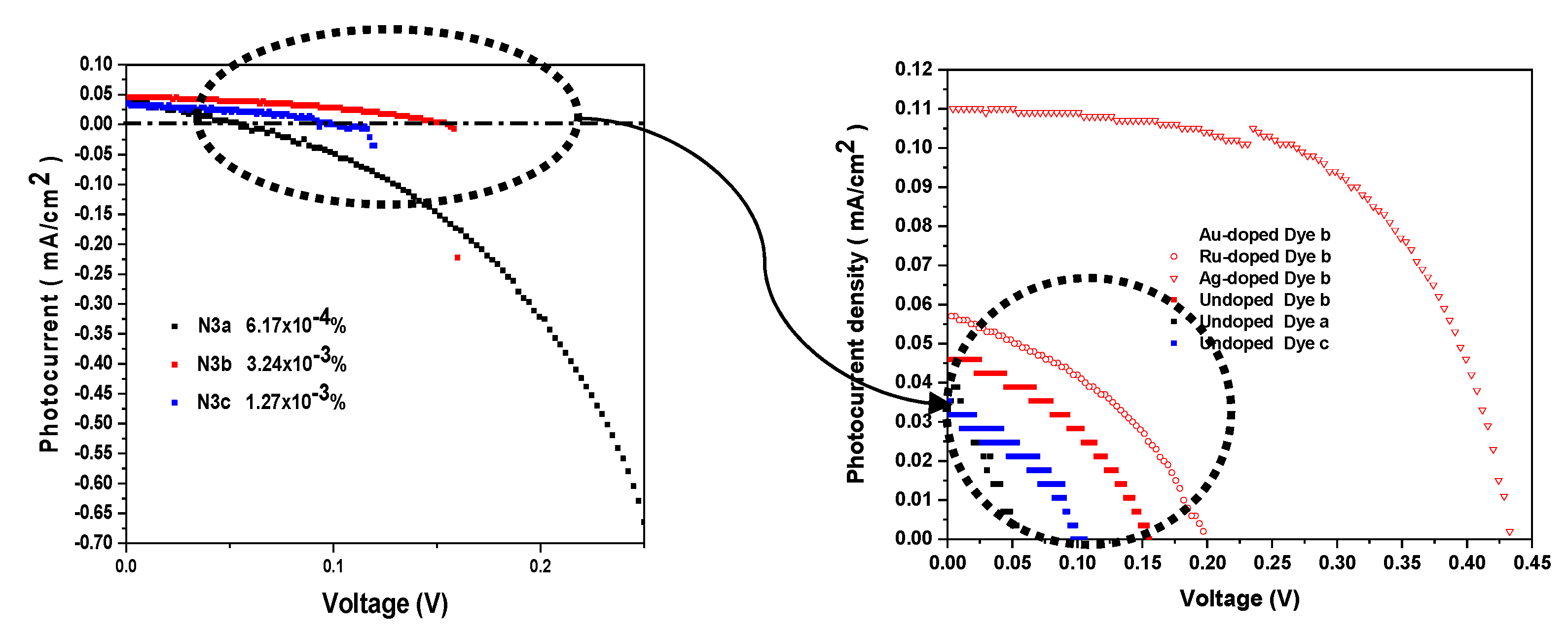
2.5. Photovoltaic Performance (PV) Study
2.6. Computational Study
2.6.1. Molecular Geometry
2.6.2. Frontier Molecular Orbitals
2.6.3. Mulliken Atomic Charges
2.6.4. Chemical Reactivity Descriptors
3. Materials and Methods
3.1. General
3.2. Preparation and Characterization of Metal Nanoparticles (MNPs) and their Dye Conjugates
3.3. Electrochemical Analysis
3.4. Fabrication of Dye-Sensitized Solar Cell (DSSCs) and Photovoltaic Characterizations
3.5. Molecular Modeling
3.6. Synthesis and Characterization
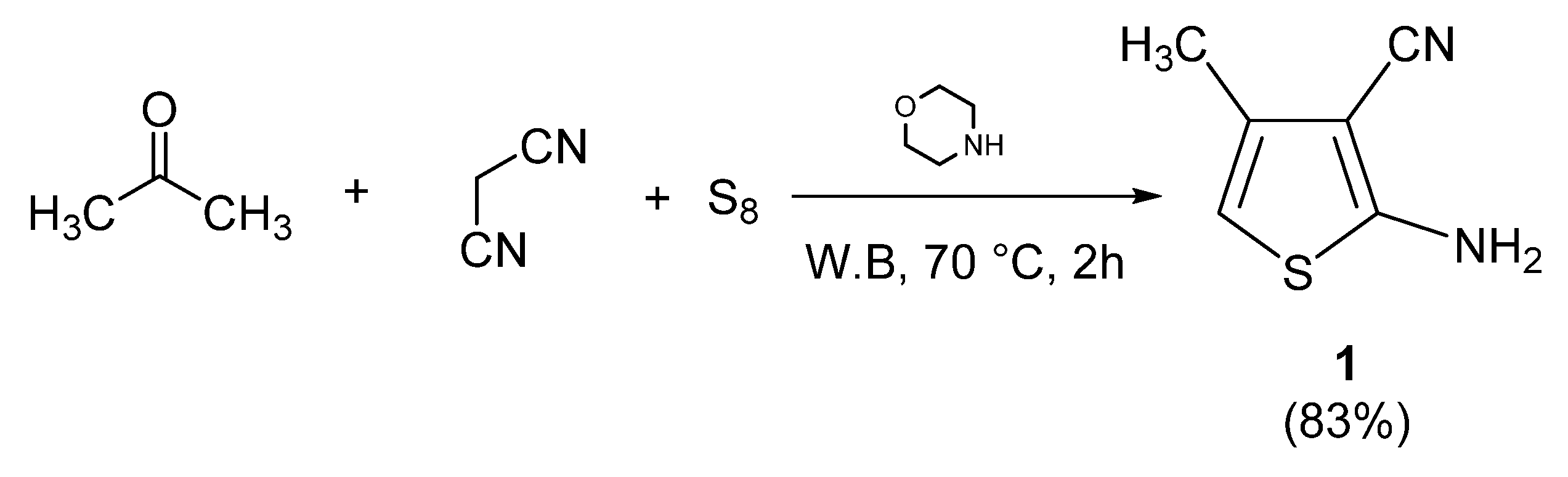
3.6.1. Synthesis of 2-amino-3-cyano-4-methylthiophene (1)
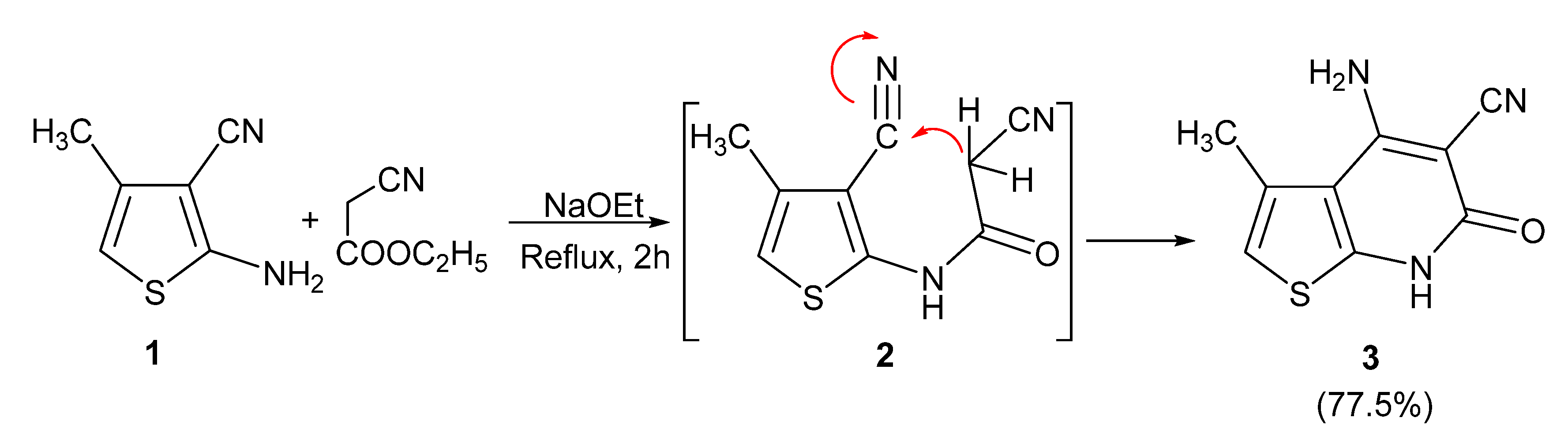
3.6.2. Synthesis of 4-amino-6,7-dihydro-3-methyl-6-oxothieno[2,3-b]pyridine-5-carbonitrile (3)
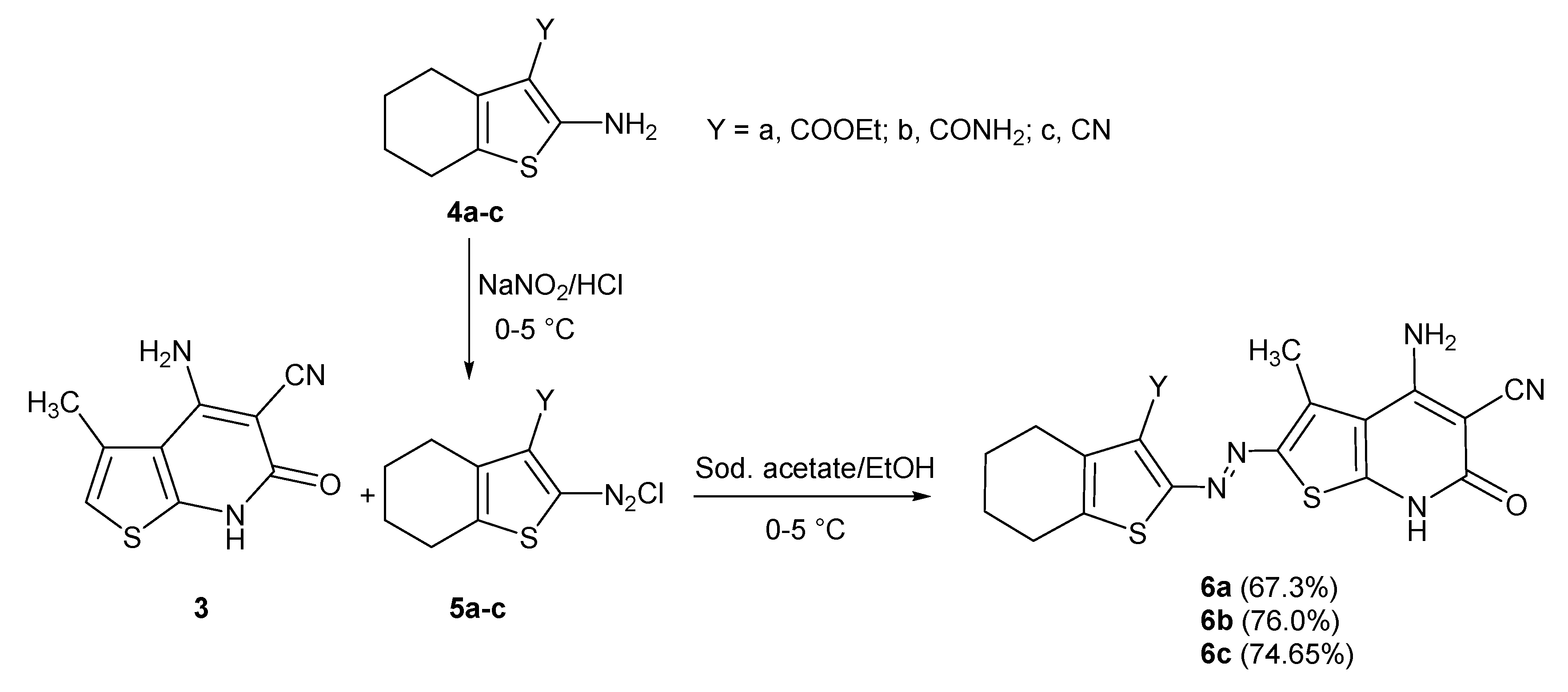
3.6.3. General Procedure for the Synthesis of 4,5,6,7-tetrahydrobenzo[b]thiophenes (4a–c)
3.6.4. General Procedure for Synthesis of thienopyridyl-azo-tetrahydrothiophene Derivatives (6a–c)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Zhou, N.; Prabakaran, K.; Lee, B.; Chang, S.H.; Harutyunyan, B.; Guo, P.; Butler, M.R.; Timalsina, A.; Bedzyk, M.J.; Ratner, M.A.; et al. Metal-Free Tetrathienoacene Sensitizers for High-Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2015, 137, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-P.; Li, C.-T.; Ho, K.-C. Use of organic materials in dye-sensitized solar cells. Mater. Today 2017, 20, 267–283. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-Free Organic Dyes for Dye-Sensitized Solar Cells: From Structure: Property Relationships to Design Rules. Angew. Chem. Int. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Batista, R.M.; Oliveira, E.; Nuñez, C.; Costa, S.P.; Lodeiro, C.; Raposo, M.M.M. Synthesis and evaluation of new thienyl and bithienyl-bis-indolylmethanes as colorimetric sensors for anions. J. Phys. Org. Chem. 2009, 22, 362–366. [Google Scholar] [CrossRef]
- Ahmad, S.; Guillén, E.; Kavan, L.; Grätzel, M.; Nazeeruddin, M.K. Metal free sensitizer and catalyst for dye sensitized solar cells. Energy Environ. Sci. 2013, 6, 3439–3466. [Google Scholar] [CrossRef]
- Eom, Y.K.; Hong, J.Y.; Kim, J.; Kim, H.K. Triphenylamine-based organic sensitizers with π-spacer structural engineering for dye-sensitized solar cells: Synthesis, theoretical calculations, molecular spectroscopy and structure-property-performance relationships. Dyes Pigm. 2017, 136, 496–504. [Google Scholar] [CrossRef]
- Obotowo, I.N.; Obot, I.B.; Ekpe, U.J. Organic sensitizers for dye-sensitized solar cell (DSSC): Properties from computation, progress and future perspectives. J. Mol. Struct. 2016, 1122, 80–87. [Google Scholar] [CrossRef]
- Wu, X.; Lu, G.Q.; Wang, L. Shell-in-shell TiO2 hollow spheres synthesized by one-pot hydrothermal method for dye-sensitized solar cell application. Energy Environ. Sci. 2011, 4, 3565–3572. [Google Scholar] [CrossRef]
- Kavan, L. Electrochemistry and dye-sensitized solar cells. Curr. Opin. Electrochem. 2017, 2, 88–96. [Google Scholar] [CrossRef]
- Ito, S.; Chen, P.; Comte, P.; Nazeeruddin, M.K.; Liska, P.; Péchy, P.; Grätzel, M. Fabrication of screen-printing pastes from TiO2 powders for dye-sensitised solar cells. Prog. Photovolt. Res. Appl. 2007, 15, 603–612. [Google Scholar] [CrossRef]
- Batista, R.M.F.; Costa, S.P.G.; Belsley, M.; Raposo, M.M.M. Synthesis and optical properties of novel, thermally stable phenanthrolines bearing an arylthienyl-imidazo conjugation pathway. Dyes Pigm. 2009, 80, 329–336. [Google Scholar] [CrossRef][Green Version]
- Genin, E.; Hugues, V.; Clermont, G.; Herbivo, C.; Castro, M.C.R.; Comel, A.; Raposo, M.M.M.; Blanchard-Desce, M. Fluorescence and two-photon absorption of push–pull aryl (bi) thiophenes: Structure–property relationships. Photochem. Photobiol. Sci. 2012, 11, 1756–1766. [Google Scholar] [CrossRef]
- Raposo, M.M.M.; Kirsch, G. Formylation, dicyanovinylation and tricyanovinylation of 5-alkoxy-and 5-amino-substituted 2, 2′-bithiophenes. Tetrahedron 2003, 59, 4891–4899. [Google Scholar] [CrossRef]
- Ishikawa, K.; Wen, C.-J.; Yamada, K.; Okubo, T. The Photocurrent of Dye-Sensitized Solar Cells Enhanced by the Surface Plasmon Resonance. J. Chem. Eng. Jpn. 2004, 37, 645–649. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Song, D.H.; Yoon, H.; Suh, J.S. Surface plasmon-enhanced dye-sensitized solar cells based on double-layered composite films consisting of TiO2/Ag and TiO2/Au nanoparticles. RSC Adv. 2015, 5, 27464–27469. [Google Scholar] [CrossRef]
- Neese, F. Prediction of molecular properties and molecular spectroscopy with density functional theory: From fundamental theory to exchange-coupling. Coord. Chem. Rev. 2009, 253, 526–563. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.A.; El-Shishtawy, R.M.; Raffah, B.M. New two rings Schiff base liquid crystals; ball mill synthesis, mesomorphic, Hammett and DFT studies. J. Mol. Liq. 2020, 299, 112161. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef]
- Zając, A.; Dymińska, L.; Lorenc, J.; Ptak, M.; Hanuza, J. Syntheses, spectroscopic properties and molecular structure of silver phytate complexes-IR, UV-VIS studies and DFT calculations. J. Mol. Struct. 2018, 1156, 483–491. [Google Scholar] [CrossRef]
- Ramesh, G.; Reddy, B.V. Spectroscopic investigation on structure (monomer and dimer), molecular characteristics and comparative study on vibrational analysis of picolinic and isonicotinic acids using experimental and theoretical (DFT & IVP) methods. J. Mol. Struct. 2018, 1160, 271–292. [Google Scholar]
- Orief, M.I.; Abdel-Rhman, M.H. Molecular modeling, spectroscopic and structural studies on newly synthesized ligand N-benzoyl-2-isonicotinoylhydrazine-1-carboxamide. J. Mol. Struct. 2018. [Google Scholar] [CrossRef]
- Khalifa, M.; Abdel-Latif, E.; Gobouri, A. Disperse Dyes Based on 5-Arylazo-thiazol-2-ylcarbamoyl-thiophenes: Synthesis, Antimicrobial Activity and Their Application on Polyester. J. Heterocycl. Chem. 2015, 52, 674–680. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Al-Amoudi, M.S.; Gobouri, A.A.; Merazga, A.; Fadda, A.A. Synthesis of Novel Arylazothiazolyl-thiophene Dyes for Solar Cell and Nonlinear Optical Materials. Acta Chim. Slov. 2015, 63, 121–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Al-Baradi, A.M.; Al-Shehri, W.A.; Badawi, A.; Almalki, A.S.A.; Merazga, A. A study of the nanostructure and efficiency of solid-state dye-sensitized solar cells based on a conducting polymer. Heliyon 2019, 5, e01472. [Google Scholar] [CrossRef]
- Almalki, A.S.A.; Alhadhrami, A.; Obaid, R.J.; Alsharif, M.A.; Adam, A.M.A.; Grabchev, I.; Refat, M.S. Preparation of some compounds and study their thermal stability for use in dye sensitized solar cells. J. Mol. Liq. 2018, 261, 565–582. [Google Scholar] [CrossRef]
- Gewald, K. Zur Reaktion von α-Oxo-mercaptanen mit Nitrilen. Angew. Chem. 1961, 73, 114. [Google Scholar] [CrossRef]
- Pak, F.; Ekinci, D.; Tümer, F.; Demir, Ü. A Mechanistic and Characteristic Investigation of Electrooxidation of 2-Amino-3-cyano-4-methylthiophene. Macromol. Chem. Phys. 2007, 208, 2367–2374. [Google Scholar] [CrossRef]
- Gewald, K.; Schäfer, H.; Sattler, K. Synthesen von 4-Amino-thieno[2,3-b]pyridinen. Monatsh. Chem. 1979, 110, 1189–1196. [Google Scholar] [CrossRef]
- Baron, H.; Remfry, F.G.P.; Thorpe, J.F. The formation and reactions of imino-compounds. Part I. Condensation of ethyl cyanoacetate with its sodium derivative. J. Chem. Soc. Trans. 1904, 85, 1726–1761. [Google Scholar] [CrossRef]
- Csukonyi, K.; Lázár, J.; Bernáth, G.; Hermecz, I.; Mészáros, Z. Saturated heterocycles, 75. Preparation of tetracyclic thiophene derivatives with bridgehead nitrogen. Synthesis of polymethylenethieno [2, 3—d] dihydropyrrolo-, tetrahydropyrido-and tetrahydroazepino [1, 2—a] pyrimidin-4-ones and-4-thiones. Monatsh. Chem. 1986, 117, 1295–1303. [Google Scholar] [CrossRef]
- Perrissin, M.; Favre, M.; Luu-Duc, C.; Bakri-Logeais, F.; Huguet, F.; Narcisse, G. Thieno (2.3-d)-4-pyrimidones: Synthesis, structure and pharmacological properties. Eur. J. Med. Chem. 1984, 19, 420–424. [Google Scholar]
- Offermann, W.; Eger, K.; Roth, H.J. Synthesen bicyclischer 1, 2, 6-Thiadiazine. Arch. Pharm. 1981, 314, 168–175. [Google Scholar] [CrossRef]
- Amr, A.E.-G.E.; Sherif, M.H.; Assy, M.G.; Al-Omar, M.A.; Ragab, I. Antiarrhythmic, serotonin antagonist and antianxiety activities of novel substituted thiophene derivatives synthesized from 2-amino-4, 5, 6, 7-tetrahydro-N-phenylbenzo [b] thiophene-3-carboxamide. Eur. J. Med. Chem. 2010, 45, 5935–5942. [Google Scholar] [CrossRef]
- Maruoka, H.; Yamagata, K.; Yamazaki, M. Studies on Heterocyclic Enaminonitriles, XV. A Convenient One-pot Synthesis of Ethyl 4-Amino-2, 3-dihydrothieno-[2, 3-b] pyridine-5-carboxylates. Liebigs Ann. Chem. 1993, 1993, 1269–1271. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Elkhawass, E.A.; Pardede, A.; Ninomiya, M.; Tanaka, K.; Koketsu, M. A facile synthesis of formazan dyes conjugated with plasmonic nanoparticles as photosensitizers in photodynamic therapy against leukemia cell line. Monatsh. Chem. 2018, 149, 2195–2206. [Google Scholar] [CrossRef]
- Chauhan, A.; Zubair, S.; Tufail, S.; Sherwani, A.; Sajid, M.; Raman, S.C.; Azam, A.; Owais, M. Fungus-mediated biological synthesis of gold nanoparticles: Potential in detection of liver cancer. Int. J. Nanomedicine 2011, 6, 2305–2319. [Google Scholar]
- Kusada, K.; Kobayashi, H.; Yamamoto, T.; Matsumura, S.; Sumi, N.; Sato, K.; Nagaoka, K.; Kubota, Y.; Kitagawa, H. Discovery of Face-Centered-Cubic Ruthenium Nanoparticles: Facile Size-Controlled Synthesis Using the Chemical Reduction Method. J. Am. Chem. Soc. 2013, 135, 5493–5496. [Google Scholar] [CrossRef]
- Asiri, A.M.; Khan, S.A. Synthesis, characterization and optical properties of mono- and bis-chalcone. Mater. Lett. 2011, 65, 1749–1752. [Google Scholar] [CrossRef]
- Kong, Z.; Liu, D.; He, J.; Wang, X. Electrode buffer layers producing high performance nonvolatile organic write-once-read-many-times memory devices. RSC Adv. 2017, 7, 13171–13176. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, S.; Zhang, M.; Cai, N.; Wang, Y.; Wang, P. An Extremely High Molar Extinction Coefficient Ruthenium Sensitizer in Dye-Sensitized Solar Cells: The Effects of π-Conjugation Extension. J. Phys. Chem. C 2009, 113, 14559–14566. [Google Scholar] [CrossRef]
- Bonomo, M.; Carella, A.; Borbone, F.; Rosato, L.; Dini, D.; Gontrani, L. New pyran-based molecules as both n-and p-type sensitizers in semi-transparent Dye Sensitized Solar Cells. Dyes Pigm. 2020, 175, 108140. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView, 5th ed.; Semichem Inc.: Shawnee, KS, USA, 2009. [Google Scholar]
- Karelson, M.; Lobanov, V.S.; Katritzky, A.R. Quantum-chemical descriptors in QSAR/QSPR studies. Chem. Rev. 1996, 96, 1027–1044. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Pérez, P.; Sáez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Fukui, K.; Yonezawa, T.; Shingu, H. A molecular orbital theory of reactivity in aromatic hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Chen, H.; Liu, M.-G. Synthesis, characterization and crystal structure of heterocyclic tetrahydropyrido [4′, 3′: 4, 5] thieno [2, 3-d] pyrimidinone derivatives via sequential aza-Wittig/base catalyzed cyclization. J. Mol. Struct. 2019, 1180, 31–40. [Google Scholar] [CrossRef]
- Khojasteh, V.; Kakanejadifard, A.; Zabardasti, A.; Azarbani, F. Spectral, structural, solvatochromism, biological and computational investigation of some new azo–azomethines containing N-alkylpyridinium salts. J. Mol. Struct. 2019, 1175, 261–268. [Google Scholar] [CrossRef]
- Datta, A. Role of metal ions (M= Li+, Na+, and K+) and pore sizes (Crown-4, Crown-5, and Crown-6) on linear and nonlinear optical properties: New materials for optical birefringence. J. Phys. Chem. C 2009, 113, 3339–3344. [Google Scholar] [CrossRef]
- Xavier, S.; Periandy, S.; Ramalingam, S. NBO, conformational, NLO, HOMO–LUMO, NMR and electronic spectral study on 1-phenyl-1-propanol by quantum computational methods. Spectrochim. Acta A 2015, 137, 306–320. [Google Scholar] [CrossRef]
- Arjunan, V.; Balamourougane, P.; Kalaivani, M.; Raj, A.; Mohan, S. Experimental and theoretical quantum chemical investigations of 8-hydroxy-5-nitroquinoline. Spectrochim. Acta A 2012, 96, 506–516. [Google Scholar] [CrossRef]
- Amorim, R.; de Meneses, M.D.F.; Borges, J.C.; da Silva Pinheiro, L.C.; Caldas, L.A.; Cirne-Santos, C.C.; de Mello, M.V.P.; de Souza, A.M.T.; Castro, H.C.; de Palmer Paixao, I.C.N. Thieno [2, 3-b] pyridine derivatives: A new class of antiviral drugs against Mayaro virus. Arch. Virol. 2017, 162, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Tayfuroğlu, Ö.; Kılıçarslan, F.A.; Atmaca, G.Y.; Erdoğmuş, A. Synthesis, characterization of new phthalocyanines and investigation of photophysical, photochemical properties and theoretical studies. J. Porphyr. Phthalocyanines 2018, 22, 250–265. [Google Scholar] [CrossRef]
- Khalifa, M.; Gobouri, A.; Kabli, F.; Altalhi, T.; Almalki, A.; Mohamed, M. Synthesis, Antibacterial, and Anti HepG2 Cell Line Human Hepatocyte Carcinoma Activity of Some New Potentially Benzimidazole-5-(Aryldiazenyl)Thiazole Derivatives. Molecules 2018, 23, 3285. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liew, K.Y.; Li, J. Size-controlled synthesis of Ru nanoparticles by ethylene glycol reduction. Mater. Lett. 2008, 62, 1018–1021. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G. Gaussian 09, Revision, A.1; Gaussian: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Pair-distribution function and its coupling-constant average for the spin-polarized electron gas. Phys. Rev. B 1992, 46, 12947. [Google Scholar] [CrossRef]
- Fondjo, E.S.; Döpp, D.; Henkel, G. Reactions of some anellated 2-aminothiophenes with electron poor acetylenes. Tetrahedron 2006, 62, 7121–7131. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds(6a–c) are available from the authors. |


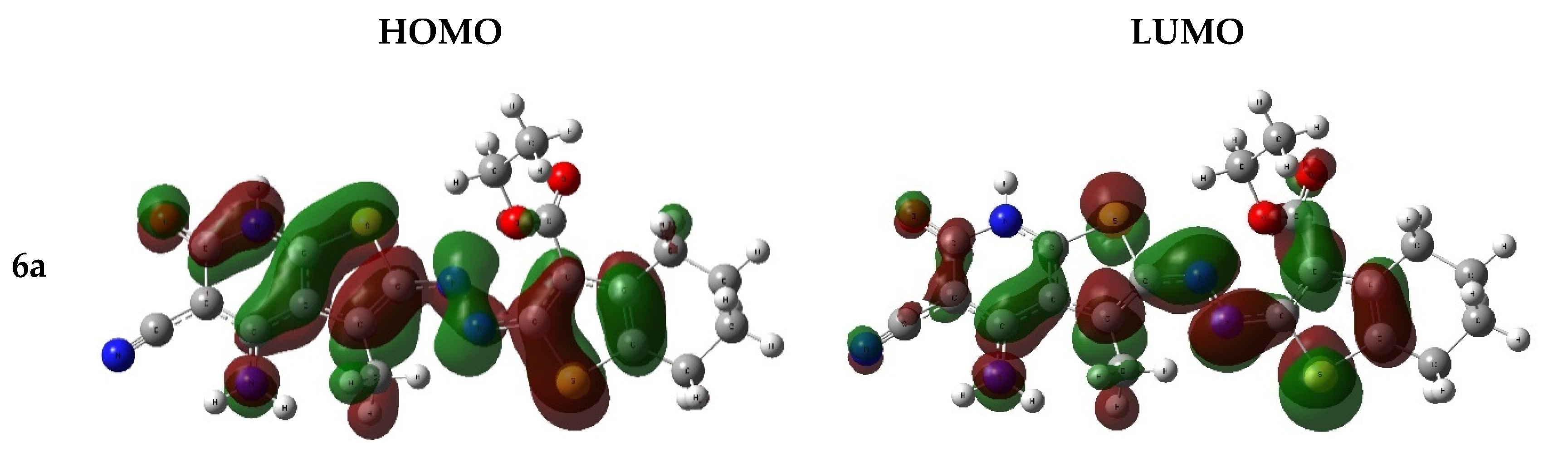
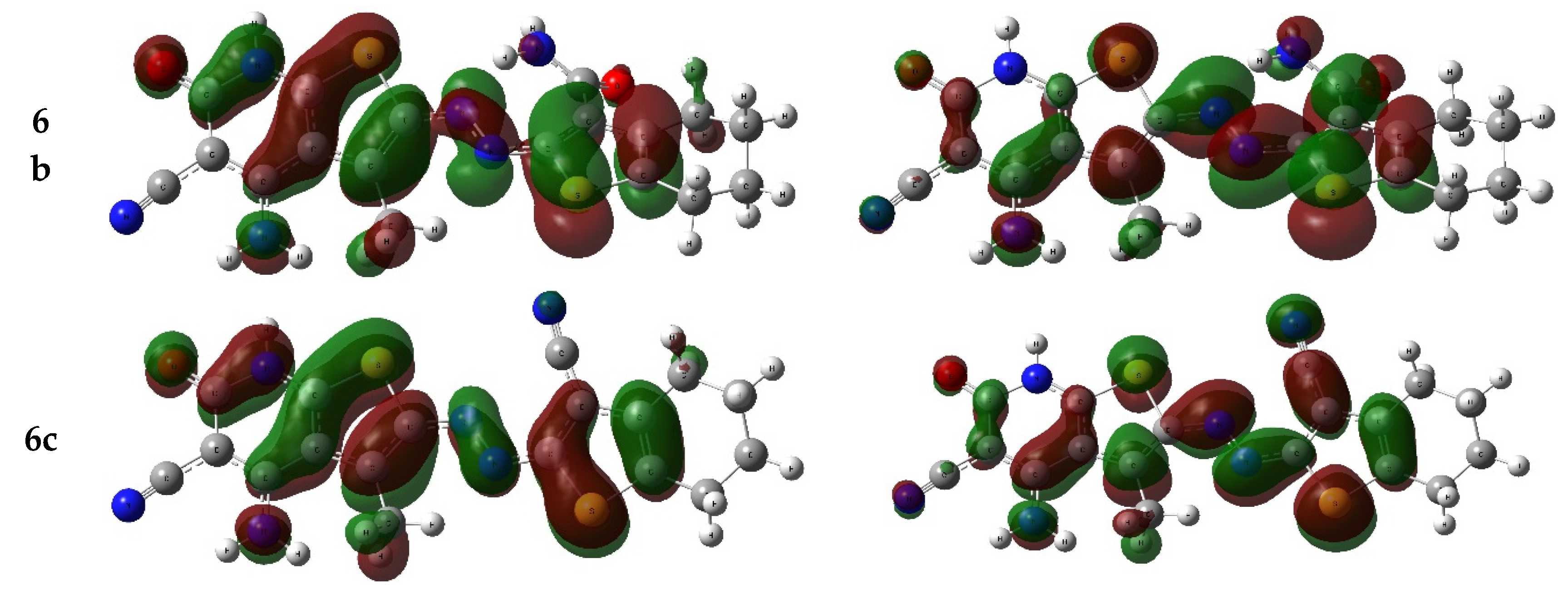
| Dye | E HOMO (eV) | E LUMO (eV) | Eg (eV) |
|---|---|---|---|
| 6a | −3.30 | −3.55 | 0.25 |
| 6b | −3.40 | −3.20 | 0.20 |
| 6c | −3.13 | −3.73 | 0.60 |
| Dye/MNPs | E HOMO (eV) | E LUMO (eV) | Eg (eV) |
|---|---|---|---|
| 6b/AuNPs | −3.10 | −3.43 | 0.33 |
| 6b/AgNPs | −3.31 | −3.40 | 0.09 |
| 6b/RuNPs | −2.91 | −3.43 | 0.52 |
| Dye | JSC (mA.cm−2) | VOC (V) | FF | η (%) |
|---|---|---|---|---|
| 6a | 4.309 × 10−2 | 0.054 | 0.27 | 6.17 × 10−4 |
| 6b | 4.313 × 10−2 | 0.141 | 0.53 | 3.24 × 10−3 |
| 6c | 3.243 × 10−2 | 0.102 | 0.38 | 1.27 × 10−3 |
| 6b/Au-NPs | 4.065 × 10−2 | 0.182 | 0.38 | 2.79 × 10−3 |
| 6b/Ru-NPs | 5.561 × 10−2 | 0.197 | 0.39 | 4.16 × 10−3 |
| 6b/Ag-NPs | 1.136 × 10−1 | 0.436 | 0.57 | 2.82 × 10−2 |
| 6a | 6b | 6c | |
|---|---|---|---|
| EHOMO | −7.23 | −7.22 | −7.23 |
| ELUMO | −5.29 | −5.32 | −5.44 |
| ΔE | 1.94 | 1.90 | 1.79 |
| χ | 6.26 | 6.27 | 6.34 |
| η | 0.97 | 0.95 | 0.89 |
| δ | 1.03 | 1.05 | 1.12 |
| ω | 20.17 | 20.71 | 22.49 |
| I | 7.23 | 7.22 | 7.23 |
| A | 5.29 | 5.32 | 5.44 |
| μtotal | 10.09 | 8.32 | 11.54 |
| μx | 9.69 | −7.82 | 10.43 |
| μy | −1.80 | −2.53 | −4.93 |
| μz | 2.16 | −1.28 | −0.26 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalifa, M.E.; Almalki, A.S.A.; Merazga, A.; Mersal, G.A.M. Design, Molecular Modeling and Synthesis of Metal-Free Sensitizers of Thieno Pyridine Dyes as Light-Harvesting Materials with Efficiency Improvement Using Plasmonic Nanoparticles. Molecules 2020, 25, 1813. https://doi.org/10.3390/molecules25081813
Khalifa ME, Almalki ASA, Merazga A, Mersal GAM. Design, Molecular Modeling and Synthesis of Metal-Free Sensitizers of Thieno Pyridine Dyes as Light-Harvesting Materials with Efficiency Improvement Using Plasmonic Nanoparticles. Molecules. 2020; 25(8):1813. https://doi.org/10.3390/molecules25081813
Chicago/Turabian StyleKhalifa, Mohamed E., Abdulraheem S. A. Almalki, Amar Merazga, and Gaber A. M. Mersal. 2020. "Design, Molecular Modeling and Synthesis of Metal-Free Sensitizers of Thieno Pyridine Dyes as Light-Harvesting Materials with Efficiency Improvement Using Plasmonic Nanoparticles" Molecules 25, no. 8: 1813. https://doi.org/10.3390/molecules25081813
APA StyleKhalifa, M. E., Almalki, A. S. A., Merazga, A., & Mersal, G. A. M. (2020). Design, Molecular Modeling and Synthesis of Metal-Free Sensitizers of Thieno Pyridine Dyes as Light-Harvesting Materials with Efficiency Improvement Using Plasmonic Nanoparticles. Molecules, 25(8), 1813. https://doi.org/10.3390/molecules25081813