Antioxidative Activity of 1,3,5-Triazine Analogues Incorporating Aminobenzene Sulfonamide, Aminoalcohol/Phenol, Piperazine, Chalcone, or Stilbene Motifs
Abstract
1. Introduction
2. Results and Discussion
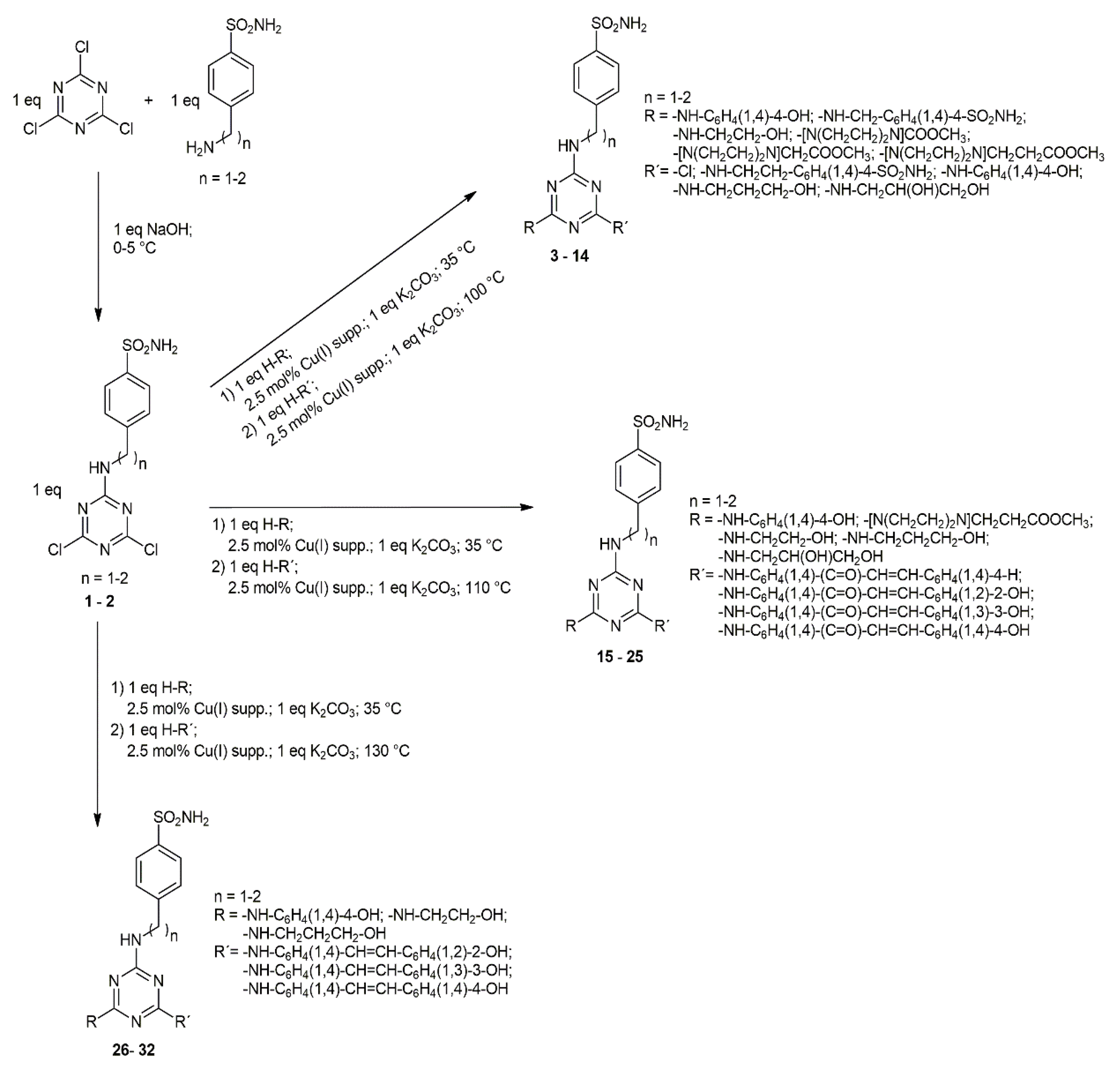
2.1. Chemistry
2.2. Antioxidant Evaluation
3. Conclusions
4. Experimental Section
4.1. General Information
4.2. General Synthetic Procedures
4.2.1. General Method for Synthesis of Trisubstituted Derivatives of 1,3,5-Triazine Containing Aminoalcohol/Phenol or Piperazine Structural Motifs (3–14)
4.2.2. General Method for Synthesis of Trisubstituted Derivatives of 1,3,5-Triazine Containing Chalcone Structural Motif (15–25)
4.2.3. General Method for Synthesis of Trisubstituted Derivatives of 1,3,5-Triazine Containing Stilbene Structural Motif (26–32)
4.2.4. Characterization of New Compounds
4.3. Determination of Antioxidant Activity by ABTS Method
Author Contributions
Funding
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef] [PubMed]
- Persson, T.; Popescu, B.O.; Cedazo-Minguez, A. Oxidative Stress in Alzheimer’s Disease: Why Did Antioxidant Therapy Fail? Oxid. Med. Cell. Longev. 2014, 2014, 1–11. [Google Scholar] [CrossRef]
- Gonzalez, P.; Pota, K.; Turan, L.S.; da Costa, V.C.P.; Akkaraju, G.; Green, K.N. Synthesis, Characterization, and Activity of a Triazine Bridged Antioxidant Small Molecule. ACS Chem. Neurosci. 2017, 8, 2414–2423. [Google Scholar] [CrossRef]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef]
- Sisein, E.A. Biochemistry of free radicals and antioxidants. Scholars Acad. J. Biosci. 2014, 2, 110–118. [Google Scholar]
- Kumar, S. The importance of antioxidant and their role in pharmaceutical science—A review. Asian. J. Med. Chem. Pharm. Sci. 2014, 1, 27–44. [Google Scholar]
- López-Alarcón, C.; Denicola, A. Evaluating the antioxidant capacity of natural products: A review on chemical and cellular-based assays. Anal. Chim. Acta 2013, 763, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Maulik, N.; McFadden, D.; Otani, H.; Thirunavukkarasu, M.; Parinandi, N.L. Antioxidants in Longevity and Medicine. Oxid. Med. Cell. Longev. 2013, 2013, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Toda, S. Polyphenol Content and Antioxidant Effects in Herb Teas. Chin. Med. 2011, 2, 29–31. [Google Scholar] [CrossRef]
- Esfahani, A.; Ghoreishi, Z.; Nikanfar, A.; Sanaat, Z.; Ghorbanihaghjo, A. Influence of Chemotherapy on the Lipid Peroxidation and Antioxidant Status in Patients with Acute Myeloid Leukemia. Acta Med. Iran. 2012, 50, 454–458. [Google Scholar]
- Singh, K.; Bhori, M.; Kasu, Y.A.; Bhat, G.; Marar, T. Antioxidants as precision weapons in war against cancer chemotherapy induced toxicity—Exploring the armoury of obscurity. Saudi Pharm. J. 2018, 26, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Conklin, K.A. Chemotherapy-Associated Oxidative Stress: Impact on Chemotherapeutic Effectiveness. Integr. Cancer Ther. 2016, 3, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, H. Systemic chemotherapy for cancer: From weapon to treatment. Lancet Oncol. 2008, 9, 304. [Google Scholar] [CrossRef]
- Perumal, S.S.; Shanthi, P.; Sachdanandam, P. Combined efficacy of tamoxifen and coenzyme Q10 on the status of lipid peroxidation and antioxidants in DMBA induced breast cancer. Mol. Cell. Biochem. 2005, 273, 151–160. [Google Scholar] [CrossRef]
- Perumal, S.S.; Shanthi, P.; Sachdanandam, P. Augmented efficacy of tamoxifen in rat breast tumorigenesis when gavaged along with riboflavin, niacin, and CoQ10: Effects on lipid peroxidation and antioxidants in mitochondria. Chem. Biol. Interact. 2005, 152, 49–58. [Google Scholar] [CrossRef]
- Simone, C.B., 2nd; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part I. Altern. Ther. Health Med. 2007, 13, 22–28. [Google Scholar]
- Simone, C.B., 2nd; Simone, N.L.; Simone, V.; Simone, C.B. Antioxidants and other nutrients do not interfere with chemotherapy or radiation therapy and can increase kill and increase survival, part II. Altern. Ther. Health Med. 2007, 13, 40–47. [Google Scholar]
- Huthmacher, K.; Most, D. Cyanuric Acid and Cyanuric Chloride. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2000; pp. 1–21. ISBN 3527306730. [Google Scholar]
- European Chemicals Agency. Substance Infocard: 2,4,6-trichloro-1,3,5-triazine. Available online: Echa.europa.eu/substance-information/-/substanceinfo/100.003.287 (accessed on 10 April 2020).
- Shafei, A.Z.; Nagaty, H.F.; Rifaat, M.A.; Salem, S. Piperazine as Anthelmintic. Lancet 1955, 266, 827–828. [Google Scholar] [CrossRef]
- Wood, W.B.; Austrian, R. Studies on the Antibacterial Action of the Sulfonamide Drugs. J. Exp. Med. 1942, 75, 383–394. [Google Scholar] [CrossRef][Green Version]
- Shah, D.R.; Modh, R.P.; Chikhalia, K.H. Privileged s -triazines: Structure and pharmacological applications. Fut. Med. Chem. 2014, 6, 463–477. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Paul, K. Triazine as a promising scaffold for its versatile biological behavior. Eur. J. Med. Chem. 2015, 102, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Cascioferro, S.; Parrino, B.; Spanò, V.; Carbone, A.; Montalbano, A.; Barraja, P.; Diana, P.; Cirrincione, G. 1,3,5-Triazines: A promising scaffold for anticancer drugs development. Eur. J. Med. Chem. 2017, 142, 523–549. [Google Scholar] [CrossRef] [PubMed]
- Marín-Ocampo, L.; Veloza, L.A.; Abonia, R.; Sepúlveda-Arias, J.C. Anti-inflammatory activity of triazine derivatives: A systematic review. Eur. J. Med. Chem. 2019, 162, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.N.; Srivastava, P.; Sharma, P.; Tripathi, M.K.; Seth, A.; Tripathi, A.; Rai, S.N.; Singh, S.P.; Shrivastava, S.K. Biphenyl–3-oxo-1,2,4-triazine linked piperazine derivatives as potential cholinesterase inhibitors with anti-oxidant property to improve the learning and memory. Bioorg. Chem. 2019, 85, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Iraji, A.; Firuzi, O.; Khoshneviszadeh, M.; Nadri, H.; Edraki, N.; Miri, R. Synthesis and structure-activity relationship study of multi-target triazine derivatives as innovative candidates for treatment of Alzheimer’s disease. Bioorg. Chem. 2018, 77, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Akocak, S.; Boga, M.; Lolak, N.; Tuneg, M.; Sanku, R.K.K. Design, synthesis and biological evaluation of 1,3-diaryltriazene-substituted sulfonamides as antioxidant, acetylcholinesterase and butyrylcholinesterase inhibitors. J. Turk. Chem. Soc. Sect. A Chem. 2019, 6, 63–70. [Google Scholar] [CrossRef]
- Narsinghani, T.; Sharma, M.C.; Bhargav, S. Synthesis, docking studies and antioxidant activity of some chalcone and aurone derivatives. Med. Chem. Res. 2013, 22, 4059–4068. [Google Scholar] [CrossRef]
- Phenolic Antioxidant Capacity: A Review of the State of the Art. In Phenolic Compounds: Biological Activity; IntechOpen: London, UK, 2017; pp. 59–74. ISBN 978-953-51-2960-8.
- Treml, J.; Leláková, V.; Šmejkal, K.; Paulíčková, T.; Labuda, Š.; Granica, S.; Havlík, J.; Jankovská, D.; Padrtová, T.; Hošek, J. Antioxidant Activity of Selected Stilbenoid Derivatives in a Cellular Model System. Biomolecules 2019, 9, 468. [Google Scholar] [CrossRef]
- Taslimi, P.; Köksal, E.; Gören, A.C.; Bursal, E.; Aras, A.; Kılıç, Ö.; Alwasel, S.; Gülçin, İ. Anti-Alzheimer, antidiabetic and antioxidant potential of Satureja cuneifolia and analysis of its phenolic contents by LC-MS/MS. Arab. J. Chem. 2020, 13, 4528–4537. [Google Scholar] [CrossRef]
- Murlimanju, B.V. Neuroprotective effects of resveratrol in Alzheimer rsquo s disease. Front. Biosci. 2020, 12, 139–149. [Google Scholar] [CrossRef]
- Wu, P.-Q.; Li, B.; Yu, Y.-F.; Su, P.-J.; Liu, X.; Zhang, Z.-P.; Zhi, D.-J.; Qi, F.-M.; Fei, D.-Q.; Zhang, Z.-X. Isolation, characterization, and possible anti-Alzheimer’s disease activities of bisabolane-type sesquiterpenoid derivatives and phenolics from the rhizomes of Curcuma longa. Chem. Biodivers. 2020, 17. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-N.; Zhu, D.; Wang, G.-H.; Lin, T.; Sun, C.-L.; Ding, R.; Tian, W.-J.; Chen, H.-F. Phenolic glycosides and flavonoids with antioxidant and anticancer activities from Desmodium caudatum. Nat. Prod. Res. 2020, 34. [Google Scholar] [CrossRef] [PubMed]
- Sharman, M.J.; Verdile, G.; Kirubakaran, S.; Münch, G. Inflammation in Alzheimer’s Disease, and Prevention with Antioxidants and Phenolic Compounds—What Are the Most Promising Candidates? In Neurodegeneration and Alzheimer’s Disease; John Wiley: Chichester, UK, 2019; pp. 233–266. ISBN 9781119356752. [Google Scholar]
- Pashirova, T.N.; Burilova, E.A.; Tagasheva, R.G.; Zueva, I.V.; Gibadullina, E.M.; Nizameev, I.R.; Sudakov, I.A.; Vyshtakalyuk, A.B.; Voloshina, A.D.; Kadirov, M.K.; et al. Delivery nanosystems based on sterically hindered phenol derivatives containing a quaternary ammonium moiety: Synthesis, cholinesterase inhibition and antioxidant activity. Chem. Biol. Interact. 2019, 310, 108753. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yu, Z.; Xia, J.; Zhang, X.; Liu, K.; Sik, A.; Jin, M. Anti-Parkinson’s disease activity of phenolic acids from Eucommia ulmoides Oliver leaf extracts and their autophagy activation mechanism. Food Funct. 2020, 11, 1425–1440. [Google Scholar] [CrossRef]
- Garcia-Moreno, J.C.; Porta de la Riva, M.; Martínez-Lara, E.; Siles, E.; Cañuelo, A. Tyrosol, a simple phenol from EVOO, targets multiple pathogenic mechanisms of neurodegeneration in a C. elegans model of Parkinson’s disease. Neurobiol. Aging 2019, 82, 60–68. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Menezes, R.; Foito, A.; da Silva, M.H.; Braga, A.; Dekker, W.; Sevillano, D.M.; Rosado-Ramos, R.; Jardim, C.; Oliveira, J.; et al. Identification and Microbial Production of the Raspberry Phenol Salidroside that Is Active against Huntington’s Disease. Plant Physiol. 2019, 179, 969–985. [Google Scholar] [CrossRef]
- Essa, M.M.; Moghadas, M.; Ba-Omar, T.; Walid Qoronfleh, M.; Guillemin, G.J.; Manivasagam, T.; Justin-Thenmozhi, A.; Ray, B.; Bhat, A.; Chidambaram, S.B.; et al. Protective Effects of Antioxidants in Huntington’s Disease: An Extensive Review. Neurotox. Res. 2019, 35, 739–774. [Google Scholar] [CrossRef]
- Antonenko, T.A.; Shpakovsky, D.B.; Berseneva, D.A.; Gracheva, Y.A.; Dubova, L.G.; Shevtsov, P.N.; Redkozubova, O.M.; Shevtsova, E.F.; Tafeenko, V.A.; Aslanov, L.A.; et al. Cytotoxic activity of organotin carboxylates based on synthetic phenolic antioxidants and polycyclic bile acids. J. Organomet. Chem. 2020, 909, 121089. [Google Scholar] [CrossRef]
- Teixeira-Guedes, C.I.; Oppolzer, D.; Barros, A.I.; Pereira-Wilson, C. Phenolic rich extracts from cowpea sprouts decrease cell proliferation and enhance 5-fluorouracil effect in human colorectal cancer cell lines. J. Funct. Foods 2019, 60, 103452. [Google Scholar] [CrossRef]
- Han, M.; Li, G.; Liu, X.; Li, A.; Mao, P.; Liu, P.; Li, H. Phenolic Profile, Antioxidant Activity and Anti-proliferative Activity of Crabapple Fruits. Hortic. Plant J. 2019, 5, 155–163. [Google Scholar] [CrossRef]
- Golonko, A.; Pienkowski, T.; Swislocka, R.; Lazny, R.; Roszko, M.; Lewandowski, W. Another look at phenolic compounds in cancer therapy the effect of polyphenols on ubiquitin-proteasome system. Eur. J. Med. Chem. 2019, 167, 291–311. [Google Scholar] [CrossRef] [PubMed]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, J.Y.; Montero, J.L.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: Synthesis and inhibition of cytosolic/tumor-associated carbonic anhydrase isozymes I, II, and IX with sulfonamides incorporating 1,2,4-triazine moieties. Bioorg. Med. Chem. Lett. 2004, 14, 5427–5433. [Google Scholar] [CrossRef] [PubMed]
- Havránková, E.; Csöllei, J.; Pazdera, P. New Approach for the One-Pot Synthesis of 1,3,5-Triazine Derivatives: Application of Cu(I) Supported on a Weakly Acidic Cation-Exchanger Resin in a Comparative Study. Molecules 2019, 24, 3586. [Google Scholar] [CrossRef] [PubMed]
- Havránková, E.; Csöllei, J.; Vullo, D.; Garaj, V.; Pazdera, P.; Supuran, C.T. Novel sulfonamide incorporating piperazine, aminoalcohol and 1,3,5-triazine structural motifs with carbonic anhydrase I, II and IX inhibitory action. Bioorg. Chem. 2018, 77, 25–37. [Google Scholar] [CrossRef]
- Mut-Salud, N.; Álvarez, P.J.; Garrido, J.M.; Carrasco, E.; Aránega, A.; Rodríguez-Serrano, F. Antioxidant Intake and Antitumor Therapy: Toward Nutritional Recommendations for Optimal Results. Oxid. Med. Cell. Longev. 2016, 2016, 1–19. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
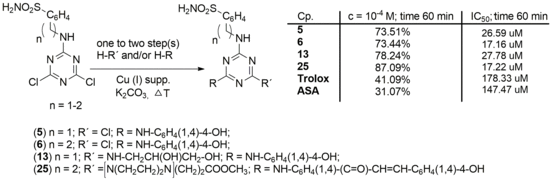
| Compound | n | R1 | R2 | 1 × 10−2 M; (%) 2 | 1 × 10−4 M; (%) 2 | EC50 (μM) 2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 min | 5 min | 30 min | 60 min | 0 min | 5 min | 30 min | 60 min | 60 min | ||||
| 3 | 1 | NH-CH2CH2OH | Cl | 45.07 | 56.11 | 64.33 | 72.56 | 31.56 | 39.88 | 45.67 | 58.34 | 136.56 |
| 4 | 2 | NH-CH2CH2OH | Cl | 49.34 | 61.78 | 76.45 | 80.89 | 32.59 | 43.27 | 46.78 | 52.47 | 128.37 |
| 51 | 1 | NH-C6H4(1,4)-4-OH | Cl | 92.05 | 92.00 | 91.88 | 91.88 | 31.52 | 38.51 | 44.71 | 73.51 | 26.59 |
| 61 | 2 | NH-C6H4(1,4)-4-OH | Cl | 91.38 | 91.26 | 91.21 | 91.21 | 45.38 | 49.84 | 63.65 | 73.44 | 17.16 |
| 71 | 1 | NH-CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 35.58 | 47.98 | 58.35 | 66.69 | <30 | <30 | <30 | 35.57 | 887.86 |
| 81 | 2 | NH-CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 51.53 | 74.64 | 79.54 | 90.36 | 31.46 | 35.24 | 40.99 | 41.83 | 900.00 |
| 91 | 1 | [N(CH2CH2)2N]COOCH3 | NH-CH2CH(OH)CH2OH | 90.70 | 92.84 | 92.95 | 92.90 | <30 | 31.01 | 39.92 | 52.99 | 61.29 |
| 101 | 1 | [N(CH2CH2)2N]CH2COOCH3 | NH-CH2CH(OH)CH2OH | 63.98 | 82.13 | 87.15 | 91.77 | <30 | 35.07 | 34.73 | 50.06 | 75.47 |
| 111 | 1 | [N(CH2CH2)2N]CH2COOCH3 | NH-CH2CH2-C6H4(1,4)-4-SO2NH2 | 79.20 | 86.47 | 88.33 | 88.39 | <30 | 34.28 | 42.90 | 64.38 | 43.84 |
| 121 | 1 | [N(CH2CH2)2N]CH2CH2COOCH3 | NH-C6H4(1,4)-4-OH | 89.63 | 89.69 | 89.69 | 89.69 | 38.28 | 39.41 | 41.89 | 60.66 | 51.47 |
| 131 | 1 | NH-C6H4(1,4)-4-OH | NH-CH2CH(OH)CH2OH | 89.40 | 89.29 | 89.18 | 89.12 | 33.55 | 36.31 | 48.71 | 78.24 | 27.78 |
| 141 | 1 | NH-CH2-C6H4(1,4)-4-SO2NH2 | NH-CH2CH2CH2OH | 43.81 | 62.80 | 75.54 | 82.19 | <30 | <30 | 30.67 | 49.16 | 138.21 |
| 15 | 1 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-H | 52.03 | 68.04 | 75.88 | 81.57 | <30 | <30 | <30 | 42.62 | 229.01 |
| 16 | 2 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-H | 52.60 | 70.01 | 85.29 | 89.12 | <30 | <30 | 31.63 | 46.34 | 158.12 |
| 17 | 1 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-3-OH | 89.57 | 89.57 | 89.63 | 89.63 | 24.70 | 31.91 | 34.62 | 50.79 | 66.39 |
| 18 | 1 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 90.70 | 90.36 | 89.97 | 89.74 | 34.28 | 35.41 | 37.32 | 57.56 | 64.43 |
| 19 | 2 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 91.43 | 91.32 | 91.04 | 90.76 | <30 | <30 | <30 | 40.03 | 170.44 |
| 20 | 1 | NH-CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 90.59 | 90.19 | 89.80 | 89.52 | <30 | <30 | 30.90 | 49.39 | 127.74 |
| 21 | 2 | NH-CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 90.08 | 89.40 | 88.84 | 89.07 | <30 | <30 | <30 | 47.07 | 153.70 |
| 22 | 2 | NH-CH2CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-3-OH | 89.12 | 89.01 | 89.01 | 89.12 | 25.49 | 34.79 | 47.81 | 65.28 | 20.16 |
| 23 | 2 | NH-CH2CH(OH)CH2OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 91.15 | 91.04 | 90.76 | 90.53 | 36.25 | 38.85 | 40.59 | 58.97 | 74.49 |
| 24 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-2-OH | 89.40 | 87.32 | 88.56 | 90.19 | 37.72 | 39.47 | 42.28 | 69.34 | 45.78 |
| 25 | 2 | [N(CH2CH2)2N]CH2CH2COOCH3 | -NH-C6H4(1,4)-(C=O)-CH=CH-C6H4(1,4)-4-OH | 90.47 | 91.83 | 91.77 | 92.11 | 40.03 | 46.96 | 55.25 | 87.09 | 17.22 |
| 26 | 1 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-3-OH | 55.92 | 65.05 | 74.47 | 82.92 | <30 | <30 | <30 | 39.63 | 344.87 |
| 27 | 2 | NH-CH2CH2CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-3-OH | 83.37 | 90.19 | 91.66 | 91.71 | <30 | <30 | <30 | 43.19 | 264.71 |
| 28 | 1 | NH-CH2CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-2-OH | 80.60 | 84.05 | 88.03 | 89.45 | <30 | <30 | <30 | <30 | 305.67 |
| 29 | 2 | NH-CH2CH2OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-4-OH | 79.20 | 85.91 | 88.33 | 88.39 | <30 | <30 | <30 | 30.73 | 282.36 |
| 30 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-2-OH | 71.03 | 75.09 | 81.96 | 87.83 | <30 | <30 | <30 | 44.14 | 199.34 |
| 31 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-3-OH | 90.08 | 89.91 | 89.80 | 89.57 | 28.53 | 31.97 | 34.28 | 48.26 | 79.34 |
| 32 | 2 | NH-C6H4(1,4)-4-OH | -NH-C6H4(1,4)-CH=CH-C6H4(1,4)-4-OH | 91.04 | 90.98 | 90.81 | 90.64 | <30 | <30 | 32.31 | <30 | 289.51 |
| trolox | - | - | - | 89.29 | 89.23 | 88.18 | 89.23 | 34.96 | 35.41 | 37.61 | 41.49 | 178.33 |
| Ascorbic acid | - | - | - | 88.33 | 88.45 | 88.33 | 88.45 | 29.09 | 28.81 | 29.83 | 31.07 | 147.47 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Havránková, E.; Čalkovská, N.; Padrtová, T.; Csöllei, J.; Opatřilová, R.; Pazdera, P. Antioxidative Activity of 1,3,5-Triazine Analogues Incorporating Aminobenzene Sulfonamide, Aminoalcohol/Phenol, Piperazine, Chalcone, or Stilbene Motifs. Molecules 2020, 25, 1787. https://doi.org/10.3390/molecules25081787
Havránková E, Čalkovská N, Padrtová T, Csöllei J, Opatřilová R, Pazdera P. Antioxidative Activity of 1,3,5-Triazine Analogues Incorporating Aminobenzene Sulfonamide, Aminoalcohol/Phenol, Piperazine, Chalcone, or Stilbene Motifs. Molecules. 2020; 25(8):1787. https://doi.org/10.3390/molecules25081787
Chicago/Turabian StyleHavránková, Eva, Nikola Čalkovská, Tereza Padrtová, Jozef Csöllei, Radka Opatřilová, and Pavel Pazdera. 2020. "Antioxidative Activity of 1,3,5-Triazine Analogues Incorporating Aminobenzene Sulfonamide, Aminoalcohol/Phenol, Piperazine, Chalcone, or Stilbene Motifs" Molecules 25, no. 8: 1787. https://doi.org/10.3390/molecules25081787
APA StyleHavránková, E., Čalkovská, N., Padrtová, T., Csöllei, J., Opatřilová, R., & Pazdera, P. (2020). Antioxidative Activity of 1,3,5-Triazine Analogues Incorporating Aminobenzene Sulfonamide, Aminoalcohol/Phenol, Piperazine, Chalcone, or Stilbene Motifs. Molecules, 25(8), 1787. https://doi.org/10.3390/molecules25081787