Abstract
A facile, one-pot, and proficient method was developed for the production of various 2-arylaminobenzimidazoles. This methodology is based for the first time on a copper catalyst promoted domino C–N cross-coupling reaction for the generation of 2-arylaminobenzimidazoles. Mechanistic investigations revealed that the synthetic pathway involves a copper-based desulphurization/nucleophilic substitution and a subsequent domino intra and intermolecular C–N cross-coupling reactions. Some of the issues typically encountered during the synthesis of 2-arylaminobezimidazoles, including the use of expensive catalytic systems and the low reactivity of bromo precursors, were addressed using this newly developed copper-catalyzed method. The reaction procedure is simple, generally with excellent substrate tolerance, and provides good to high yields of the desired products.
1. Introduction
Heterocyclic compounds like benzimidazoles are very important molecules that can be found in a number of natural and synthetically prepared biologically active compounds such as 3-chloro-1-5-(2-methyl-1H-bezimidazol-2-yl)-4-(substituted) phenylazetidin-2-one (an antimicrobial compound [1]), 5, 6-dichloro-1-(β-d-ribofuranosyl)benzimidazoles(an antiviral agent [2]), pyrimidyl-thio-methyl-benzimidazole(an antiulcer molecule [3]), 1-ethyl-2-(4-phenyl)phenyl-1H-benzo[d]imidazole(an antiasthmatic compound [4]), and 1-butyl-2-(3,4-dihydro-4,4-dimethyl-2H-thiochromyl)phenyl-1H-benzo[d]imidazole (an anti-diabetic molecule [4]), shown in Figure 1. Furthermore, these compounds have been identified in N-methyl-d-aspartate (NMDA) antagonists [5], in analgesic [6], anti-inflammatory [7], anti-cancer [8] drugs, in factor Xa(FXa) inhibitors [9], in poly(ADP-ribose)polymerase (PARP) inhibitors [10] and in non-peptide thrombin inhibitordrugs [11].
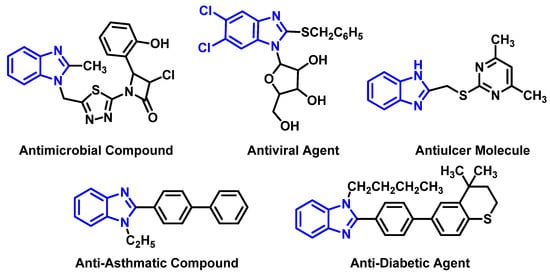
Figure 1.
Examples of some biologically active benzimidazoles.
Thus, enormous efforts have been focused on the preparation of benzimidazole structural frameworks. Classical methods may involve (i) the inter cyclocondensation of 2-aminophenylaniline precursors with either carboxylic acids or aldehydes followed by oxidation [12], (ii) the diazotization on 1-benzimidazolylidenehydrazine [13], (iii) the cyclocondensation of esters with diaminobenzene under microwave irradiation [14], (iv) the reductive cyclization of o-nitroaniline with aldehyde using sodium dithionite [15] and (v) a solid-phase route [16]. However, most of these protocols suffer from major shortcomings such as limited suitable substituents for diverse synthesis, strong alkaline conditions, troublesome management of the chemical process, and elevated temperature. Therefore, we reasoned that a catalytic approach involving C–N bond formation would overcome the abovementioned disadvantages.
In the past decade, efficient methods have been described for the synthesis of heterocyclic compounds by aryl halides with copper catalysts [17,18]. Buchwald and co-authors developed the preparation of indulines [19], 2-aryl-4-quinolones [20] and N-alkylbenzimidazoles [21]. The group of Ma described a cascade approach for the production of benzofurans [22], dihydrobenzimidazole-2-ones [23], benzimidazoles [24], isoquinolines [25], pyrrolo [1,2-a]quinoxaline [26], and indoles [27]. Intramolecular C–X bond formation has been established by Batey’s group to synthesize benzoxazoles [28], benzothiazoles [29] and aminobenzimidazoles [30]. In addition, C–N bond formation has been well explored using various transition metals such as copper [31,32], palladium [33], cobalt [34], zinc [35], ruthenium [36] for the production of various heterocycles, but, to the best of our knowledge, no report is available on the synthesis of substituted 2-aminophenylbenzimidazoles employing a copper catalyst through the reporting strategy.
Very recently, 2-phenylaminobenzimidazoles have been reported from thiourea using cobalt catalysis [37]; however, they have failed to develop from bromo precursors. Therefore, in continuation of our research towards the development of efficient methodologies for the synthesis of heterocycles [38,39], herein, we developed a methodology for the synthesis of 2-phenylamino benzimidazoles from molecules having a bromo moiety, under moderate reaction conditions using copper catalysis. Another advantage of our method is the use of copper, which is cheaper, more air-stable and more easily available than cobalt.
2. Results and Discussion
In this context, 2-phenylaminobenzimidazoles were prepared from thiourea through an approach consisting of consecutive desulphurization/nucleophilic substitution and C–N cross-coupling. Thiourea reacted with 2-bromoaniline at room temperature using copper salt as a catalyst to give N-(2-bromophenyl)-guanidine, which further underwent a domino intra and intermolecular C–N cross-coupling reaction with aryliodide to yield the desired product 2-phenylaminobenzimidazole 1a (Scheme 1). Optimization was achieved by taking thiourea and bromoaniline as model substrates and using various solvents, bases, ligands, and copper salts at different temperatures. We were pleased to observe that thiourea gave 2-bromoguanidine in quantitative conversion using NaOAc as a base and both copper (I) and copper (II) salts in the presence of DMSO/DMF at room temperature. The reaction was further continued at room temperature using K2CO3 (2 equiv) as a base, Cu salt (20 mol%), ligand L4 (20 mol%) for 18 h. Unfortunately, no target product was observed (Table 1, entry 1). Later, the reaction was performed at 50 °C, however, no product formed (Table 1, entry 2). In contrast, target product 1a was observed in 15% yield when the reaction was carried out at 80 °C (Table 1, entry 3). The yield of the product increased to 52% when the reaction was conducted at 100 °C (Table 1, entry 4). Very interestingly, the reaction provided the desired product 1a quantitatively at 120 °C (Table 1, entry 5).
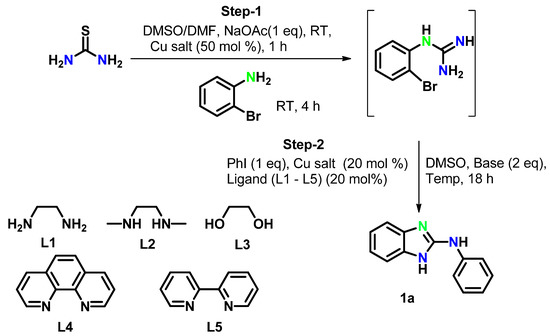
Scheme 1.
Synthetic route for the synthesis of N-phenyl-1H-benzo[d]imidazol-2-amine.

Table 1.
Optimization of the reaction for the synthesis of 2-aminophenyl benzoimidazole a.
Bases like K2CO3 and Cs2CO3 were found to be effective for the reaction (Table 1, entries 5 and 7), whereas KOH was less effective (Table 1, entry 6). All the tested copper salts, i.e., CuI, CuBr, CuCl, CuSO4·5H2O, Cu(OAc)2·H2O, exhibited similar catalytic activity (Table 1, entries 5 and 8–11). Among the tested ligands, L4 was efficient (Table 1, entry 5), while the other ligands L1–L3 and L5 provided the target product in lower yields (Table 1, entries 13–16). In contrast, the reaction didnot proceed in the absence of ligand (Table 1, entry 12).
By lowering the quantity of the copper source (10 mol%) or of the base (1.0 equiv), the reaction led to N-arylation, affording a lower amount of the target product (Table 1, entries 17–18). Control experiments also confirmed that the reaction could not yield the target product in the absence of a catalyst, and the intermediate 2-bromophenylguanidine was recovered intact (Table 1, entry 19). This control experiments demonstrated that the reaction requires a catalyst. Despite the similar catalytic activity of all the copper salts tested, considering the cost effectiveness of CuSO4·5H2O over the other examined catalysts, the authors chosen CuSO4·5H2O as a reasonable copper source for both step 1 and step 2 in Scheme 1. Finally, the total outcome of the above optimization studies is presented in Scheme 2.
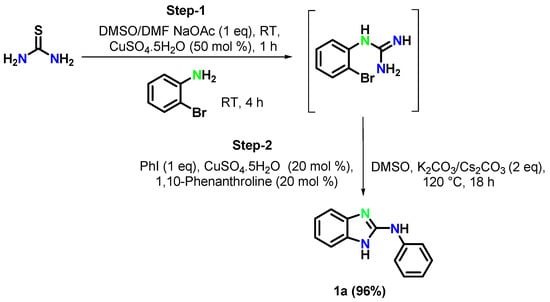
Scheme 2.
Optimized synthetic pathway for the synthesis of 2-phenylaminobenzimidazole from thiourea.
The compound 1a was further confirmed based on spectral data, for example, the appearance of characteristic peaks at 1542(m), 1478(s), 1413(s) cm−1, clearlyshowing Ar–C=C stretching, a peak at 3098(w) cm−1 showing aromatic =CH stretching, and peak at 3294(s) cm−1 indicating –N–H stretching. In the 1H NMR spectrum of compound 1a, adoublet at δ 7.78 ppm and amultiplet at δ 7.26–7.11 ppm were assigned to aromatic protons, and a broad singlet at δ 6.12 ppm (br s, 1H) to a –N–H proton. This spectral data allowed determining the structure of compound 1a as N-Phenyl-1H-benzo[d]imidazol-2-amine, which was further confirmed by its 13C NMR spectrum which exposed 11 signals between δ 139.2 and 110.8 ppm, indicates the existence of 11 different carbons in the compound. Further, the formation of compound 1a was supported by the molecular ion peak at 210.10[M + H]+ in its mass spectrum (EI).
Next, to validate the efficiency of the protocol, we investigated the substrate scope towards the production of 15 more 2-arylaminobenzimidazoles 1b–1p under optimized conditions, and the results are indicated in Figure 2. N-(2-bromophenyl)-guanidine was reacted with various derivatives of aryl iodide having 2-CH3, 3-CH3, 4-CH3, 4-OCH3, 4-COOCH3, 4-Cl, 2,4-DiCH3, 2,6-DiCH3 and naphthyl to obtain the final products 1b–1j in 62–97% yields. Similarly, the reaction of 2-bromoaniline holding substituents such as 2-Me, 3-Me, 4-Me, 4-CN readily provided the desired products 1k–1n in 56–96% yields. In addition, several substrates were tested to understand the reactivity of aryl halides. For example, thiourea reacted with 4-chloro-2-bromoaniline to provide guanidine which consecutively underwent a C–N cross-coupling reaction with 4-methyliodobenzene and 4-nitroiodobenzene to afford the target products 1o and 1p in 79% and 62% yields respectively. The above mentioned results clearly confirmed that the substrates having electron-donating and electron-withdrawing groups are compatible with this process, affording the substituted 2-aminobenzimidazoles in moderate to excellent yield. All the spectroscopic and analytical data of the synthesized compounds were in full agreement with the anticipated structures.
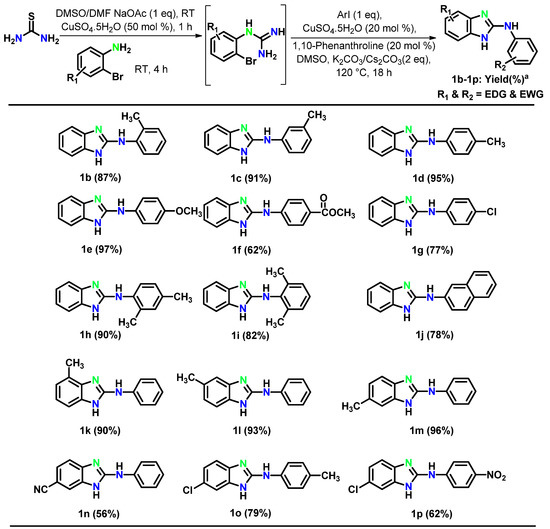
Figure 2.
Substrate scope evaluation; Reaction conditions: thiourea (1 mmol); DMSO (2 mL); NaOAc (1 equiv); CuSO4·5H2O (50 mol%), 1 h, room temperature; 2-bromo aniline/substituted 2-bromo aniline (2 mmol), room temperature, 4 h; Next, ArI (1 mmol); CuSO4·5H2O (20 mol%); 1,10-Phenanthroline (20 mol%); K2CO3 (2 mmol); 18 h; 120 °C; a Isolated yield.
The reaction of aryl halides was next examined under optimized reaction conditions. Iodobenzene afforded the target product 1a in 96% yield (Equation (1)). On the other hand, bromobenzene led to the target product 1a in 12% yield and to the product 2-aminobenzimidazole B in 81% yield (Equation (2)). However, no target product could be obtained when using chlorobenzene, which provided 2-aminobenzimidazole B in 97% yield (Equation (3)). In addition, we also examined the activity of different haloanilines. It was observed that 2-iodoaniline readily reacted with thiourea to give iodoguanidine C, which required a shorter reaction time than bromoguanidine to undergo a domino C–N cross-coupling reaction with iodobenzene, affording the desired product 1a in 96% yield (Equation (4)). However, 2-chloroaniline gave the intermediate chloroguanidine D in complete conversion, but no domin O C–N cross-coupled product was observed with iodobenzene under optimized reaction conditions (Equation (5)).
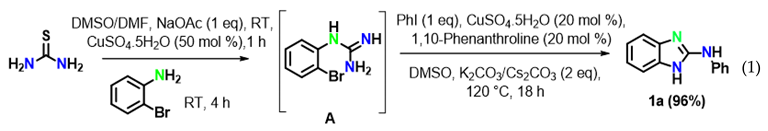
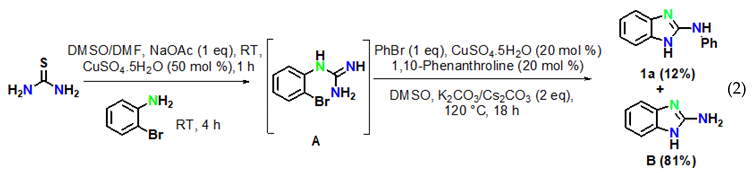
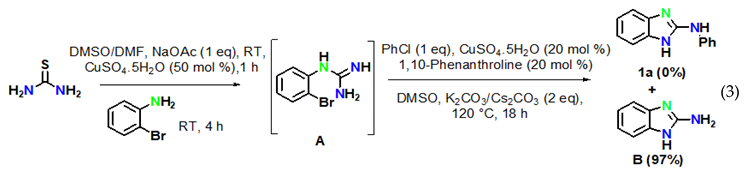
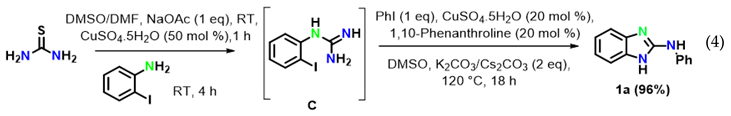
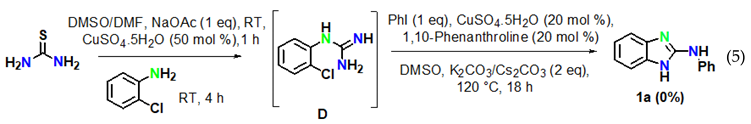





To reveal the mechanism, control experiments were conducted. The reaction was performed in the absence of aryl iodide and resulted inthe intramolecular C–N cyclized product 2-aminobenzimidazole B in 97% yield (Equation (6)) under optimized reaction conditions. Furthermore, 2-aminobenzimidazole readily reacted with iodobenzene under optimized conditions to afford the target product 1a in 96% yield (Equation (7)).
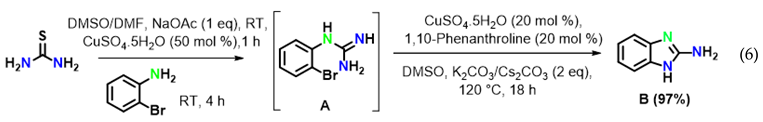
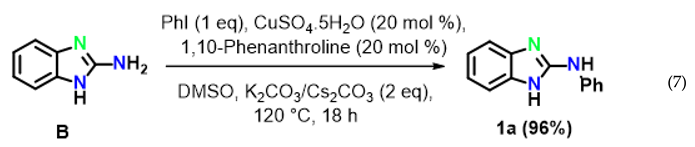


The above results clearly suggested that thiourea treated with 2-bromoaniline to provide N-(2-bromophenyl)-guanidine A gave first the intramolecular C–N cyclised product 2-amino benzimidazole B, which reacted with iodobenzene to providethe final product 1a by an intermolecular C–N cross-coupling reaction.
Moreover, the formation of the key intermediate B was confirmed based on its spectral data. In fact, in the IR spectrum of compound B, thepeaks at 3421(w) and 3396(w) cm−1 corresponds to N–H stretching, the peak at 3063(w) cm−1 represents –C=C–H stretching, the peaks at 1626(s), 1530(s), 1444(s) cm−1 correspond to Ar–C=C stretching; 1H-NMR analysis showed a broad signal at δ 5.62(2H) ppm, indicating amino protons and signals between δ7.58 and 7.09 ppm representing aromatic protons. Moreover, in the 13C NMR spectrum, seven signals between δ167.0 and 118.2 ppm also confirmed that compound B is 1H-benzo[d]imidazol-2-amine. Finally, the structure of B was confirmed by the appearanceof a peak at m/z 134.07 [M + H]+ in its mass spectrum (EI).
Based on these experimental investigations and the available literature [37,40,41,42,43,44,45,46,47,48,49,50], the mechanism for the formation of the final product 2-phenylaminobenzimidazole is very much similar to the mechanism reported for cobalt catalysis [37] (see supporting information Scheme S1).
3. Experimental
3.1. Material and Methods
General information: 2-bromoaniline, iodobenzene, CuSO4∙5H2O (98%), CuI (98%), CuBr (98%), Cu2O (97%), CuCl (99%), Cu(OAc)2∙H2O (98%), sodium acetate, KOH, K2CO3, and Cs2CO3 were purchased from Sigma-Aldrich (St. Luis, MO, USA) and were used without further purification. The solvents were purchased and dried according to standard procedures prior to use. The reactions were monitored by using pre-coated Merck60 F254 (Merck, Darmstadt, Germany) TLC silica gel plates with 0.25 mm thickness. Column chromatography was performed for purification, utilizing silica gel (60–120 mesh, Merck, Darmsadt, Germany). A Cintex melting point apparatus (Cintex, Mumbai, India) was used to determine the melting points. Infrared (IR) spectra were recorded on a Perkin Elmer Spectrum one FT-IR spectrometer (Perkin Elmer, Waltham, Massachusetts, USA). A Varian 400 MHz spectrometer (Varian, Palo Alto, CA, USA) was utilized to record the 1H NMR and 13C-NMR spectra in CDCl3/DMSO-d6. Chemical shifts are presented in δ ppm, employing TMS as internal reference. A Jeol SX-102 spectrometer (JEOL Ltd., Akishima, Tokyo, Japan) was used to record the Mass spectra.
3.2. General Procedure for the Synthesis of 2-(N-arylamino)Benzimidazole
To a stirred solution of DMSO (2–3 mL), thiourea (1 mmol, 76 mg) was added slowly, followed by the addition of NaOAc (1 mmol, 82 mg) and a copper source (50 mol%) at room temperature. The whole reaction mixture was stirred for 1 h (until a black-colored solution was obtained) at room temperature. To this, 2-bromoaniline (2 mmol, 344 mg) was added. After completion of the reaction (monitored by TLC), the reaction mixture was transferred into tubes and the mixture was centrifuged for 10 min. A black solid was then recovered from the tubes, and to this a clear solution of iodobenzene (1 mmol, 204 mg), K2CO3 (2 mmol, 277 mg), Cu(SO)4.5H2O (20 mol%, 50 mg), and 1,10-phenanthroline (20 mol%, 36 mg) was added slowly in several minutes, and the reaction mixture was then allowed to stir for 18 h at 120 °C. Progress of the reaction was monitored by TLC, using ethyl acetate and hexane (1:4). After completion of the reaction, the reaction mixture was cooled to room temperature. Then, the solution was washed with ethyl acetate (7 mL) and water (3 mL) for 5 times. The organic layer was evaporated, and the crude reaction mixture was purified on asilica gel (60–120 mesh) column by chromatography to obtain the final product 2-(N-arylamino)benzimidazole, which was characterized by NMR (1H and 13C), IR, and mass spectral data.
N-Phenyl-1H-benzo[d]imidazol-2-amine (1a): White solid; yield 96%; mp 97–99 °C; 1H NMR (400 MHz, CDCl3) δ 7.78 (d,J = 6.8 Hz, 2H), 7.26–7.11 (m, 7H), 6.12 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 139.2, 137.2, 134.2, 132.6, 130.5, 129.9, 129.7, 127.3, 115.0, 114.7, 110.8; FT-IR (KBr) 3294, 3098, 1676, 1618, 1597, 1542, 1478, 1413, 1253, 1076 cm−1. m/z (ESI–MS) 210.10 [M + H]+.
N-O-Tolyl-1H-benzo[d]imidazol-2-amine (1b): White solid; yield 87%; mp 151–152 °C; 1H NMR (400 MHz, DMSO) δ 7.35 (d, J = 12 Hz, 1H), 7.25–7.19 (m, 2H), 7.00 (d,J = 7.4 Hz, 2H), 6.88–6.77 (m, 3H), 2.18 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 138.2, 137.5, 137.1, 135.7, 133.9, 132.2, 131.6, 130.9, 129.0, 125.3, 123.2, 118.2, 115.2, 20.7; FT-IR (KBr) 3314, 2924, 2859, 3109, 2115, 1619, 1509, 1330, 1250, 1112, 1088, 1025 cm−1. m/z (ESI–MS) 224.11 [M + H]+.
N-m-Tolyl-1H-benzo[d]imidazol-2-amine (1c): White solid; yield 91%; mp 148–149 °C; 1H NMR (400 MHz, CDCl3) δ 7.55 (s, 1H), 7.43–7.34 (m, 3H), 7.31–7.25 (m, 3H), 6.70 (br s, 1H), 2.36 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 149.0, 139.4, 137.4, 134.9, 134.7, 133.6, 133.1, 130.5, 124.0, 122.0, 115.5, 113.1, 110.4, 20.6; FT-IR (KBr) 3257, 3191, 2958, 1501, 1495, 1403, 1386, 1341, 1286, 1061 cm−1. m/z (ESI–MS) 224.11 [M + H]+.
N-p-Tolyl-1H-benzo[d]imidazol-2-amine (1d): White solid; yield 95%; mp 145–147 °C; 1H NMR (400 MHz, CDCl3) δ 7.45–7.32 (m, 6H), 7.12 (d,J = 8 Hz, 2H), 5.98 (br s, 1H), 2.29 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ 152.3, 142.2, 135.9, 133.0, 130.8, 129.8, 128.7, 128.5, 123.2, 120.6, 117.8, 20.0; FT-IR (KBr) 3256, 3121, 2963, 1643, 1586, 1514, 1367, 1234, 1136, 1094, 1017 cm−1. m/z (ESI–MS) 224.11 [M + H]+.
N-(4-Methoxyphenyl)-1H-benzo[d]imidazol-2-amine (1e): White solid; yield 97%; mp 153–154 °C; 1H NMR (400 MHz, CDCl3) δ 7.47–7.42 (m, 3H), 7.30–7.16 (m, 2H), 7.14–7.08 (m, 3H), 7.00 (br s, 1H), 3.87 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ 154.7, 153.1, 142.9, 133.7, 132.6, 129.7, 129.5, 129.3, 126.1, 121.3, 114.9, 55.02; FT-IR (KBr) 3345, 2958, 2857, 1567, 1535, 1506, 1321, 1271, 1235, 1182, 1123, 1074, 1033 cm−1. m/z (ESI–MS) 240.11 [M + H]+.
Methyl 4-(1H-benzo[d]imidazol-2-ylamino)benzoate (1f): White solid; yield 62%; mp 207–209 °C; 1H NMR (300 MHz, d6-DMSO, ppm) δ 7.64–7.51 (m, 3H), 6.83–6.61 (m, 5H), 5.83 (br s, 1H), 3.83 (s, 3H);13C NMR (75 MHz, d6-DMSO) d = 166.3, 152.7, 145.7, 144.2, 141.3, 132.3, 131.2, 128.1, 119.8, 119.1, 106.6, 106.3, 45.3; FT-IR (KBr) 3234, 3157, 2853, 1658, 1599, 1516, 1428, 1411, 1242, 1197, 1121, 1087, 1065, 1022 cm−1. m/z (ESI-MS) 268.10 [M + H]+.
N-(4-Chlorophenyl)-1H-benzo[d]imidazol-2-amine (1g): White solid; yield 77%; mp 158–159 °C; 1H NMR (400 MHz, CDCl3) δ 7.77–7.50 (m, 4H), 7.26 (d, J = 7.6 Hz, 2H), 7.16 (d, J= 8.8 Hz, 2H), 4.66 (br s, 1H); 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ 151.1, 141.7, 136.9, 132.3, 128.3, 128.1, 127.7, 127.0, 124.7, 120.0, 118.1; FT-IR (KBr) 3076, 2958, 2150, 1637, 1504, 1421, 1374, 1330, 1256, 1207, 1030 cm−1. m/z (ESI–MS) 245.05 [M + H]+.
N-(2,4-Dimethylphenyl)-1H-benzo[d]imidazol-2-amine (1h): White solid; yield 90%; mp 143–144 °C;1H NMR (400 MHz, CDCl3) δ 7.65 (s, 1H), 7.38–7.33 (m, 3H), 7.27–7.09 (m, 3H), 6.74 (br s, 1H), 2.28 (s, 3H), 2.27 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 141.3, 138.3, 137.2, 136.9, 132.9, 132.4, 130.6, 127.2, 117.7, 115.1, 111.2, 21.4, 20.4; FT-IR (KBr) 3278, 3201, 2922, 2858, 1607, 1581, 1453, 1410, 1389, 1268, 1155, 1018 cm−1. m/z (ESI–MS) 238.13 [M + H]+.
N-(2,6-Dimethylphenyl)-1H-benzo[d]imidazol-2-amine (1i): White solid; yield 82%; mp 148–149 °C; 1H NMR (400 MHz, CDCl3) δ 7.22–7.17 (m, 2H), 7.13 (d,J = 8.4 Hz, 2H), 7.06–6.97 (m, 3H), 6.35 (br s, 1H), 2.43 (s, 6H), 2.18 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 142.8, 135.2, 134.4, 134.3, 130.5, 130.1, 129.5, 128.7, 118.4, 115.5, 111.2, 24.5, 20.7; FT-IR (KBr) 3094, 2921, 2867, 2222, 1574, 1486, 1456, 1374, 1241, 1208, 1027 cm−1. m/z (ESI–MS) 238.13 [M + H]+.
N-(Naphthalen-3-yl)-1H-benzo[d]imidazol-2-amine (1j): White solid; yield 78%; mp 168–169 °C; 1H NMR (400 MHz, CDCl3) δ 8.02–7.63 (m, 5H), 7.51–7.47 (m, 3H), 7.35 (d, J = 8 Hz, 1H), 7.26 (d, J = 5.2 Hz, 2H), 6.60 (br s, 1H); 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ 154.6, 142.6, 134.2, 133.8, 133.6, 131.2, 129.4, 129.1, 128.8, 128.3, 127.8, 127.2, 125.8, 125.4, 122.4, 121.1, 120.8. m/z (ESI–MS) 260.11 [M + H]+.
4-Methyl-N-phenyl-1H-benzo[d]imidazol-2-amine (1k): White solid; yield 90%; mp 151–153 °C;1H NMR (400 MHz, CDCl3) δ 7.47-7.25 (m, 4H), 7.07 (d, J = 7.6 Hz, 2H), 6.94 (d, J = 8 Hz, 1H), 6.74 (br s, 1H), 6.54 (s, 1H), 2.35 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 146.7, 139.5, 135.5, 134.4, 132.8, 129.7, 128.7, 126.4, 124.5, 117.9, 110.0, 21.8; FT-IR (KBr) 3290, 3144, 2984, 2917, 2859, 1604, 1584, 1498, 1449, 1413, 1391, 1289, 1270, 1213, 1153, 1057, 934 cm−1. m/z (ESI–MS) 224.11 [M + H]+.
5-Methyl-N-phenyl-1H-benzo[d]imidazol-2-amine (1l): White solid; yield 93%; mp 151–153 °C; 1H NMR (400 MHz, CDCl3) δ 7.76–7.49 (m, 4H), 7.25–6.85 (m, 4H), 2.37 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ 151.6, 141.5, 135.7, 134.8, 132.2, 128.3, 128.0, 127.8, 119.9, 118.0, 114.3, 19.1; FT-IR (KBr) 3306, 2920, 2847, 1603, 1515, 1444, 1302, 1236, 1302, 1236, 1191, 1115, 1095, 1020 cm−1. m/z (ESI–MS) 224.11 [M + H]+.
6-Methyl-N-phenyl-1H-benzo[d]imidazol-2-amine (1m): White solid; yield 96%; mp 146–147 °C; 1H NMR (400 MHz, CDCl3) δ 7.40 (s, 1H), 7.30–7.19 (m, 5H), 7.04–7.01 (m, 2H), 6.81 (br s, 1H), 2.32 (s, 3H); 13C NMR (100 MHz, CDCl3) δ 138.0, 137.4, 135.3, 134.1, 132.7, 129.6, 127.0, 126.7, 115.3, 110.0, 20.3; FT-IR (KBr) 3285, 3056, 2854, 1603, 1574, 1534, 1497, 1456, 1321, 1234, 1121, 1085 cm−1. m/z (ESI–MS) 224.11 [M + H]+.
2-(Phenylamino)-3H-benzo[d]imidazole-5-carbonitrile (1n): White solid: yield 56%; mp 169–170 °C; 1H NMR (400 MHz, CDCl3) δ 7.53 (s, 1H), 7.29–7.22 (m, 3H), 7.16 (d, J = 6.8 Hz, 2H), 6.97 (d, J = 7.2 Hz, 2H), 6.84 (br s, 1H); 13C NMR (100 MHz, CDCl3) δ 139.2, 137.3, 134.2, 132.6, 130.5, 130.0, 129.7, 127.3, 115.0, 114.8, 111.0, 110.9; FT-IR (KBr) 3253, 3198, 1654, 1588, 1488, 1407, 1317, 1287, 1164, 1019, 927 cm−1. m/z (ESI–MS) 235.09 [M + H]+.
6-Chloro-N-p-tolyl-1H-benzo[d]imidazol-2-amine (1o): White solid: yield 79%; mp 151–152 °C; 1H NMR (400 MHz, CDCl3) δ 7.77 (s, 1H), 7.50 (t, J = 9.2 Hz, 2H), 7.26 (d, J = 7.6 Hz, 2H), 7.16 (d, J = 8.8 Hz, 2H), 4.66 (br s, 1H), 2.40 (s, 3H); 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ 151.1, 141.7, 136.9, 132.3, 128.3, 128.1, 127.7, 127.0, 124.7, 120.0, 118.1, 19.2; FT-IR (KBr) 3076, 2958, 2150, 1637, 1504, 1421, 1374, 1330, 1256, 1207, 1030 cm−1. m/z (ESI–MS) 259.12 [M + H]+.
6-Chloro-N-(4-nitrophenyl)-1H-benzo[d]imidazol-2-amine(1p): White solid: yield 62%; mp 178–180 °C; 1H NMR (400 MHz, CDCl3) δ 7.74 (d, J = 2.0 Hz, 1H), 7.47–7.37 (m, 3H), 7.10 (d, J = 9.2 Hz, 1H), 6.94 (br s, 1H), 6.81 (dd, J = 7.2, 2.4 Hz, 2H); 13C NMR (100 MHz, CDCl3 + DMSO-d6) δ 153.9, 152.1, 135.8, 131.9, 131.2, 130.1, 129.9, 127.9, 121.8, 119.2, 112.7; FT-IR (KBr) 3275, 3085, 2834, 1607, 1579, 1486, 1302, 1233, 1179, 1085, 1036 cm−1. m/z (ESI–MS) 290.04 [M + H]+.
1H-benzo[d]imidazol-2-amine(B): White solid; mp 129–130 °C; 1H NMR (400 MHz, CDCl3) δ 7.58–7.56 (td, J = 8.0, 0.4 Hz, 1H), 7.53–7.50 (dd, J = 8.0, 0.8 Hz, 1H), 7.31–7.27 (m, 1H), 7.13–7.09 (d, J = 7.6, 1.2 Hz, 1H), 5.62 (br s, 2H); 13C NMR (100 MHz, CDCl3) δ 167.0, 151.9, 131.0, 125.5, 121.4, 120.6, 118.2; FT-IR (KBr) 3421, 3396, 3063, 1626, 1530, 1444, 1309, 1105 cm−1. m/z (ESI–MS) 134.07 [M + H]+.
4. Conclusions
In conclusion, we have developed a methodology for the synthesis of substituted 2-aminophenyl benzimidazoles from thiourea through a multistep reaction. This methodology involves consecutive a desulphurization/nucleophilic substitution and domino intra and inter molecular C–N cross-coupling reaction. Control experiments were performed to understand themechanism. Many researchers have reported reactions for the generationof benzimidazoles; however, the simplicity, environmental acceptability, and cost effectiveness of copper makes this method more practical. Although the single yields look moderate, considering that the reaction consists of multiple processes, the yields are in fact good to high.
Supplementary Materials
The following are available online. Scheme S1. Proposed mechanism.
Author Contributions
S.N.M.B., R.T. and H.B.B. designed the project; S.F.A., R.K.G. and S.N. helped in the preparation of the manuscript; R.T., R.K.G. and H.B.B. performed the experimental section, while S.F.A., M.E.A. and H.B.B. contributed to the characterization; along with H.B.B. who provided scientific guidance and helped to draft the manuscript. O.A. and M.R.H.S. helped procure the funding for the successful completion of the project. All authors have read and agreed to the published version of the manuscript.
Funding
Research group project No. RG-1440-068.
Acknowledgments
The authors are grateful to the Deanship of Scientific Research at King Saud University for funding this work through the research group project No. RG-1440-068.
Conflicts of Interest
The authors declare there are no conflicts to declare.
References
- Ansari, K.; Lal, C. Synthesis and evaluation of some new benzimidazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 2294–2299. [Google Scholar] [CrossRef]
- Devivar, R.V.; Kawashima, E.; Revankar, G.R.; Breitenbach, J.M.; Kreske, E.D.; Drach, J.C.; Townsend, L.B. BenzimidazoleRibonucleosides: Design, Synthesis, and Antiviral Activity of Certain 2-(Alkylthio)-and 2-(Benzylthio)-5, 6-dichloro-1-(. beta.-D-ribofuranosyl) benzimidazoles. J. Med. Chem. 1994, 37, 2942–2949. [Google Scholar] [CrossRef]
- Katiyar, A.; Rai, J.; Gangwar, S.; Mohanty, A.K.; Mishra, A.P. Biological Activities of Substituted Benzimidazole Derivatives. J. Drug Discov. Dev. 2018, 2, 2–10. [Google Scholar]
- Kumar, B.; Rao, P. Synthesis and structural studies on transition metal complexes derived from 1-(2-thienyl)-1-ethanole-1H-benzimidazole. Asian J. Chem. 2006, 18, 3060–3064. [Google Scholar]
- Baudy, R.B.; Fletcher, H., III; Yardley, J.P.; Zaleska, M.M.; Bramlett, D.R.; Tasse, R.P.; Kowal, D.M.; Katz, A.H.; Moyer, J.A.; Abou-Gharbia, M. Design, synthesis, SAR, and biological evaluation of highly potent benzimidazole-spaced phosphono-α-amino acid competitive NMDA antagonists of the AP-6 type. J. Med. Chem. 2001, 44, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Carcanague, D.; Shue, Y.-K.; Wuonola, M.A.; Uria-Nickelsen, M.; Joubran, C.; Abedi, J.K.; Jones, J.; Kühler, T.C. Novel Structures Derived from 2-[[(2-Pyridyl) methyl] thio]-1 H-benzimidazole as Anti-Helicobacter p ylori Agents, Part 2. J. Med. Chem. 2002, 45, 4300–4309. [Google Scholar] [CrossRef]
- Gaba, M.; Singh, S.; Mohan, C. Benzimidazole: An emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 2014, 76, 494–505. [Google Scholar] [CrossRef]
- Alaqeel, S.I. Synthetic approaches to benzimidazoles from o-phenylenediamine: A literature review. J. Saudi Chem. Soc. 2017, 21, 229–237. [Google Scholar] [CrossRef]
- Arnaiz, D.O.; Griedel, B.; Sakata, S.; Dallas, J.L.; Whitlow, M.; Trinh, L.; Post, J.; Liang, A.; Morrissey, M.M.; Shaw, K.J. Design, synthesis, and in vitro biological activity of benzimidazole based factor Xa inhibitors. Bioorgan. Med. Chem. Lett. 2000, 10, 963–966. [Google Scholar] [CrossRef]
- White, A.W.; Almassy, R.; Calvert, A.H.; Curtin, N.J.; Griffin, R.J.; Hostomsky, Z.; Maegley, K.; Newell, D.R.; Srinivasan, S.; Golding, B.T. Resistance-modifying agents. 9. Synthesis and biological properties of benzimidazole inhibitors of the DNA repair enzyme poly (ADP-ribose) polymerase. J. Med. Chem. 2000, 43, 4084–4097. [Google Scholar] [CrossRef]
- Hauel, N.H.; Nar, H.; Priepke, H.; Ries, U.; Stassen, J.-M.; Wienen, W. Structure-based design of novel potent nonpeptide thrombin inhibitors. J. Med. Chem. 2002, 45, 1757–1766. [Google Scholar] [CrossRef] [PubMed]
- Benincori, T.; Sannicolo, F. New benzimidazole synthesis. J. Heterocycl. Chem. 1988, 25, 1029–1033. [Google Scholar] [CrossRef]
- Zornik, D.; Meudtner, R.M.; El Malah, T.; Thiele, C.M.; Hecht, S. Designing Structural Motifs for Clickamers: Exploiting the 1, 2, 3-Triazole Moiety to Generate Conformationally Restricted Molecular Architectures. Chem. A Eur. J. 2011, 17, 1473–1484. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-J.; Myung, D.; Lee, I.Y.C.; Jung, M.H. Microwave-assisted synthesis of benzimidazoles, benzoxazoles, and benzothiazoles from resin-bound esters. J. Comb. Chem. 2008, 10, 501–503. [Google Scholar] [CrossRef]
- Yang, D.; Fokas, D.; Li, J.; Yu, L.; Baldino, C.M. A versatile method for the synthesis of benzimidazoles from o-nitroanilines and aldehydes in one step via a reductive cyclization. Synthesis 2005, 2005, 47–56. [Google Scholar] [CrossRef]
- Wu, Z.; Rea, P.; Wickham, G. ‘One-pot’nitro reduction–cyclisation solid phase route to benzimidazoles. Tetrahedron Lett. 2000, 41, 9871–9874. [Google Scholar] [CrossRef]
- Ma, D.; Cai, Q. Copper/amino acid catalyzed cross-couplings of aryl and vinyl halides with nucleophiles. Acc. Chem. Res. 2008, 41, 1450–1460. [Google Scholar] [CrossRef]
- Chemler, S.R.; Fuller, P.H. Heterocycle synthesis by copper facilitated addition of heteroatoms to alkenes, alkynes and arenes. Chem. Soc. Rev. 2007, 36, 1153–1160. [Google Scholar] [CrossRef]
- Minatti, A.; Buchwald, S.L. Synthesis of indolines via a domino Cu-catalyzed amidation/cyclization reaction. Org. Lett. 2008, 10, 2721–2724. [Google Scholar] [CrossRef]
- Jones, C.P.; Anderson, K.W.; Buchwald, S.L. Sequential Cu-catalyzed amidation-base-mediated camps cyclization: A two-step synthesis of 2-aryl-4-quinolones from o-halophenones. J. Organ. Chem. 2007, 72, 7968–7973. [Google Scholar] [CrossRef]
- Zheng, N.; Buchwald, S.L. Copper-catalyzed regiospecific synthesis of N-alkylbenzimidazoles. Org. Lett. 2007, 9, 4749–4751. [Google Scholar] [CrossRef]
- Lu, B.; Wang, B.; Zhang, Y.; Ma, D. CuI-catalyzed domino process to 2, 3-disubstituted benzofurans from 1-bromo-2-iodobenzenes and β-keto esters. J. Organ. Chem. 2007, 72, 5337–5341. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Yuan, Q.; Ma, D. Cascade coupling/cyclization process to N-substituted 1, 3-dihydrobenzimidazol-2-ones. Org. Lett. 2007, 9, 4291–4294. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Yuan, Q.; Ma, D. Synthesis of 1, 2-disubstituted benzimidazoles by a Cu-catalyzed cascade aryl amination/condensation process. Angew. Chem. Int. Ed. 2007, 46, 2598–2601. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Lu, B.; Jiang, Y.; Zhang, Y.; Ma, D. Assembly of isoquinolines via CuI-catalyzed coupling of β-Keto esters and 2-halobenzylamines. Org. Lett. 2008, 10, 2761–2763. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Ma, D. A one-pot coupling/hydrolysis/condensation process to pyrrolo [1–a] quinoxaline. J. Organ. Chem. 2008, 73, 5159–5162. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Sun, Z.; Ma, D. Elaboration of 2-(Trifluoromethyl) indoles via a cascade coupling/condensation/deacylation Process. Org. Lett. 2008, 10, 625–628. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, X.; Ma, D. Facile access to polysubstituted indoles via a cascade Cu-catalyzed arylation− condensation process. J. Organ. Chem. 2007, 72, 9329–9334. [Google Scholar] [CrossRef]
- Evindar, G.; Batey, R.A. Parallel synthesis of a library of benzoxazoles and benzothiazoles using ligand-accelerated copper-catalyzed cyclizations of ortho-halobenzanilides. J. Organ. Chem. 2006, 71, 1802–1808. [Google Scholar] [CrossRef]
- Evindar, G.; Batey, R.A. Copper-and palladium-catalyzed intramolecular aryl guanidinylation: An efficient method for the synthesis of 2-aminobenzimidazoles. Org. Lett. 2003, 5, 133–136. [Google Scholar] [CrossRef]
- Deng, X.; McAllister, H.; Mani, N.S. CuI-catalyzed amination of arylhalides with guanidines or amidines: A facile synthesis of 1-H-2-substituted benzimidazoles. J. Organ. Chem. 2009, 74, 5742–5745. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ye, M.; Zong, C.; Hu, F.; Feng, L.; Wang, X.; Wang, Y.; Chen, C. Copper-catalyzed intramolecular C− N bond formation: A straightforward synthesis of benzimidazole derivatives in water. J. Organ. Chem. 2011, 76, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Brain, C.T.; Steer, J.T. An improved procedure for the synthesis of benzimidazoles, using palladium-catalyzed aryl-amination chemistry. J. Organ. Chem. 2003, 68, 6814–6816. [Google Scholar] [CrossRef]
- Saha, P.; Ali, M.A.; Ghosh, P.; Punniyamurthy, T. Cobalt-catalyzed intramolecular C–N and C–O cross-coupling reactions: Synthesis of benzimidazolesand benzoxazoles. Organ. Biomol. Chem. 2010, 8, 5692–5699. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Payra, S.; Saha, A.; Sereda, G. ZnOnanoparticles: A green efficient catalyst for the room temperature synthesis of biologically active 2-aryl-1, 3-benzothiazole and 1, 3-benzoxazole derivatives. Tetrahedron Lett. 2014, 55, 5515–5520. [Google Scholar] [CrossRef]
- Ramachandran, R.; Prakash, G.; Selvamurugan, S.; Viswanathamurthi, P.; Malecki, J.G.; Ramkumar, V. Efficient and versatile catalysis of N-alkylation of heterocyclic amines with alcohols and one-pot synthesis of 2-aryl substituted benzazoles with newly designed ruthenium (II) complexes of PNS thiosemicarbazones. Dalton Trans. 2014, 43, 7889–7902. [Google Scholar] [CrossRef] [PubMed]
- Kondraganti, L.; Manabolu, S.b.; Dittakavi, R. Synthesis of Benzimidazoles via Domino Intra and Intermolecular C-N Cross-Coupling Reaction. Chem. Sel. 2018, 3, 11744–11748. [Google Scholar] [CrossRef]
- Boddapati, S.N.M.; Kola, A.E.; Kesana, S.B.; Bollikolla, H.B. Temperature dependent regioselective synthesis of aryl tetrazole amines using copper source. J. Organomet. Chem. 2018, 866, 177–183. [Google Scholar] [CrossRef]
- Boddapati, S.N.M.; Kurmarayuni, C.M.; Mutchu, B.R.; Tamminana, R.; Bollikolla, H.B. Copper-catalyzed synthesis of 2-aminophenyl benzothiazoles: A novel approach. Organ. Biomol. Chem. 2018, 16, 8267–8272. [Google Scholar] [CrossRef]
- Ramana, T.; Punniyamurthy, T. Preparation of 2-Azido-1-Substituted-1 H-Benzo [d] imidazoles Using a Copper-Promoted Three-Component Reaction and Their Further Conversion into 2-Amino and 2-Triazolyl Derivatives. Chem. A Eur. J. 2012, 18, 13279–13283. [Google Scholar] [CrossRef]
- Guin, S.; Rout, S.K.; Gogoi, A.; Nandi, S.; Ghara, K.K.; Patel, B.K. Desulfurization strategy in the construction of azoles possessing additional nitrogen, oxygen or sulfur using a copper (I) catalyst. Adv. Synth. Catal. 2012, 354, 2757–2770. [Google Scholar] [CrossRef]
- Yella, R.; Khatun, N.; Rout, S.K.; Patel, B.K. Tandem regioselective synthesis of tetrazoles and related heterocycles using iodine. Organ. Biomol. Chem. 2011, 9, 3235–3245. [Google Scholar] [CrossRef] [PubMed]
- Bowmaker, G.A.; Hanna, J.V.; Pakawatchai, C.; Skelton, B.W.; Thanyasirikul, Y.; White, A.H. For the reduction of copper (II) salts to copper (I) species using thiourea, see: Crystal structures and vibrational spectroscopy of copper (I) thiourea complexes. Inorg. Chem. 2009, 48, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kuang, C.; Yang, Q. Copper-Catalyzed Synthesis of 4-Aryl-1H-1, 2, 3-triazoles from 1, 1-Dibromoalkenes and Sodium Azide. Eur. J. Org. Chem. 2012, 2012, 424–428. [Google Scholar] [CrossRef]
- Chiba, S.; Zhang, L.; Ang, G.Y.; Hui, B.W.-Q. Generation of iminyl copper species from α-azido carbonyl compounds and their catalytic C–C bond cleavage under an oxygen atmosphere. Org. Lett. 2010, 12, 2052–2055. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.Y.-H.; Teo, Y.-C. Efficient cobalt-catalyzed C–N cross-coupling reaction between benzamide and aryl iodide in water. Organ. Biomol. Chem. 2014, 12, 7478–7481. [Google Scholar] [CrossRef]
- Ma, D.; Lu, X.; Shi, L.; Zhang, H.; Jiang, Y.; Liu, X. Domino Condensation/S-Arylation/Heterocyclization Reactions: Copper-Catalyzed Three-Component Synthesis of 2-N-Substituted Benzothiazoles. Angew. Chem. Int. Ed. 2011, 50, 1118–1121. [Google Scholar] [CrossRef]
- Cahiez, G.; Moyeux, A. Cobalt-catalyzed cross-coupling reactions. Chem. Rev. 2010, 110, 1435–1462. [Google Scholar] [CrossRef]
- Boddapati, S.N.M.; Polam, N.; Mutchu, B.R.; Bollikolla, H.B. The synthesis of arylcyanamides: A copper catalyzed consecutive desulfurization and C-N cross coupling strategy. N. J. Chem. 2018, 42, 918–922. [Google Scholar] [CrossRef]
- Boddapati, S.N.M.; Saketi, J.M.R.; Mutchu, B.R.; Bollikolla, H.B.; Adil, S.F.; Khan, M. Copper promoted desulfurization and C-N cross coupling reactions: Simple approach to the synthesis of substituted 2-aminobenzoxazoles and 2,5-disubstituted tetrazole amines. Arab. J. Chem. 2020, 13, 4477–4494. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).