Impact of Isoorientin on Metabolic Activity and Lipid Accumulation in Differentiated Adipocytes
Abstract
:1. Introduction
2. Results
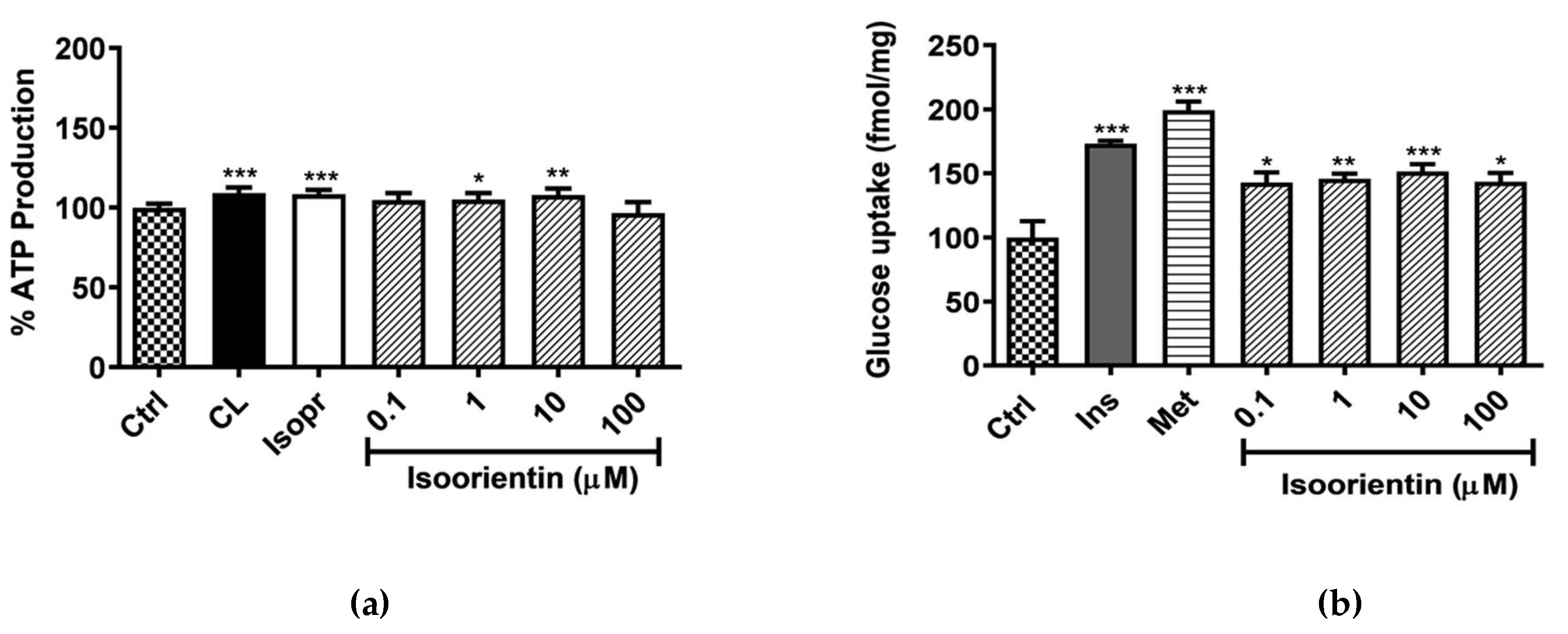
2.1. Isoorientin Enhanced ATP Production and Glucose Uptake without Inducing Any Cytotoxicity in Matured 3T3-L1 Adipocytes
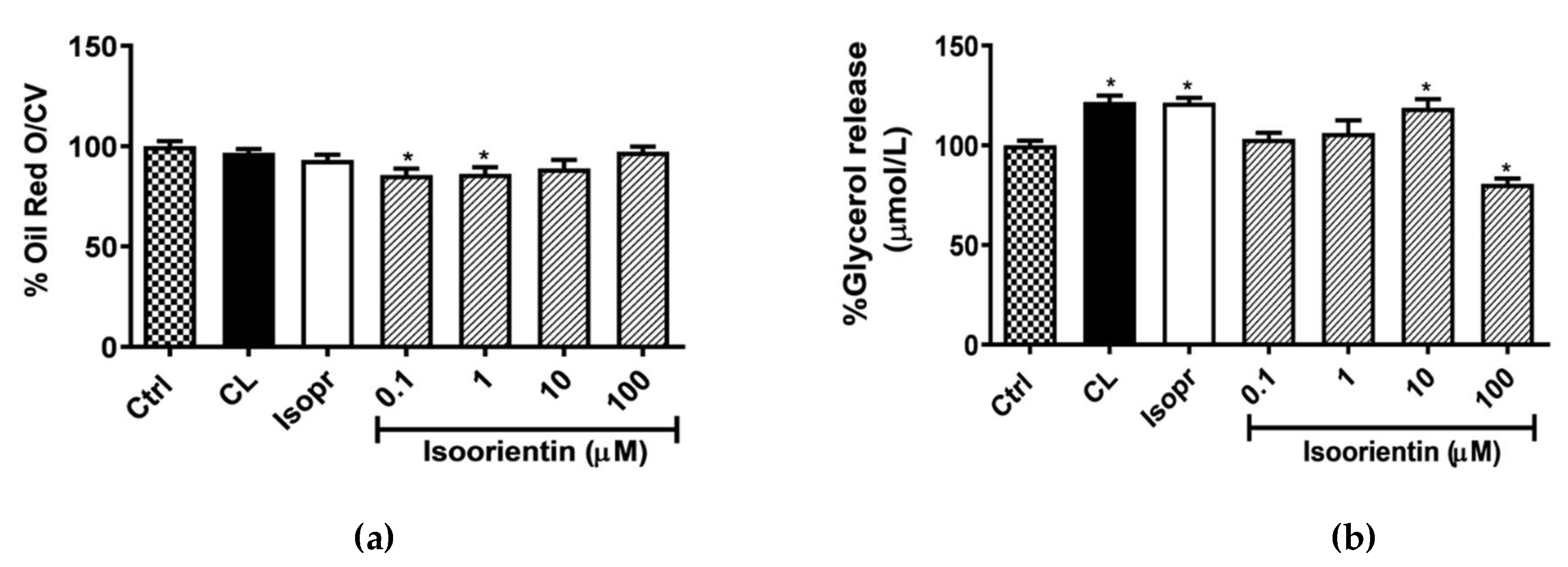
2.2. Isoorientin Reduced Intracellular Lipid Accumulation and Enhanced Lipolysis in Matured 3T3-L1 Adipocytes
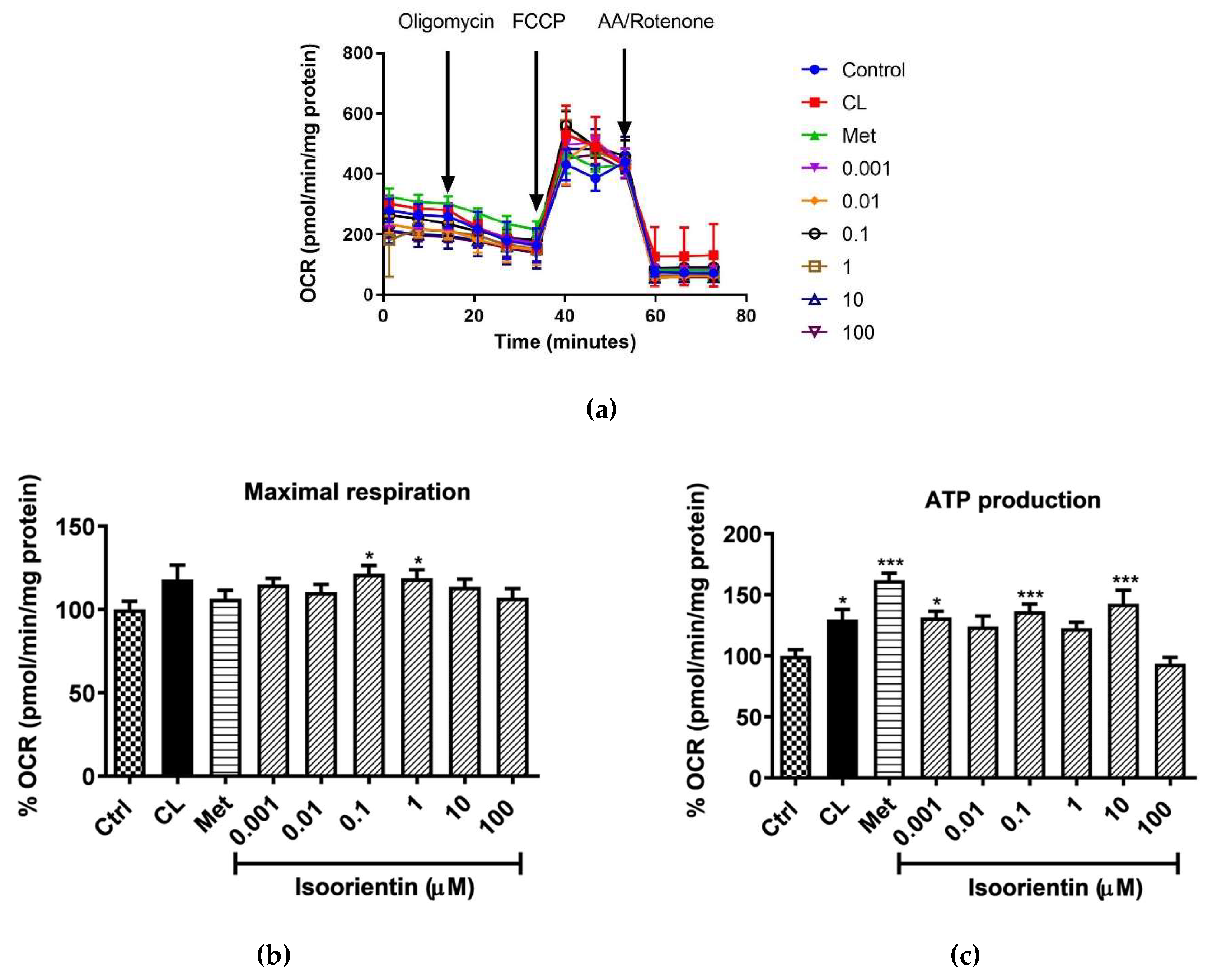
2.3. Isoorientin Improved Mitochondrial Respiration in Matured 3T3-L1 Adipocytes
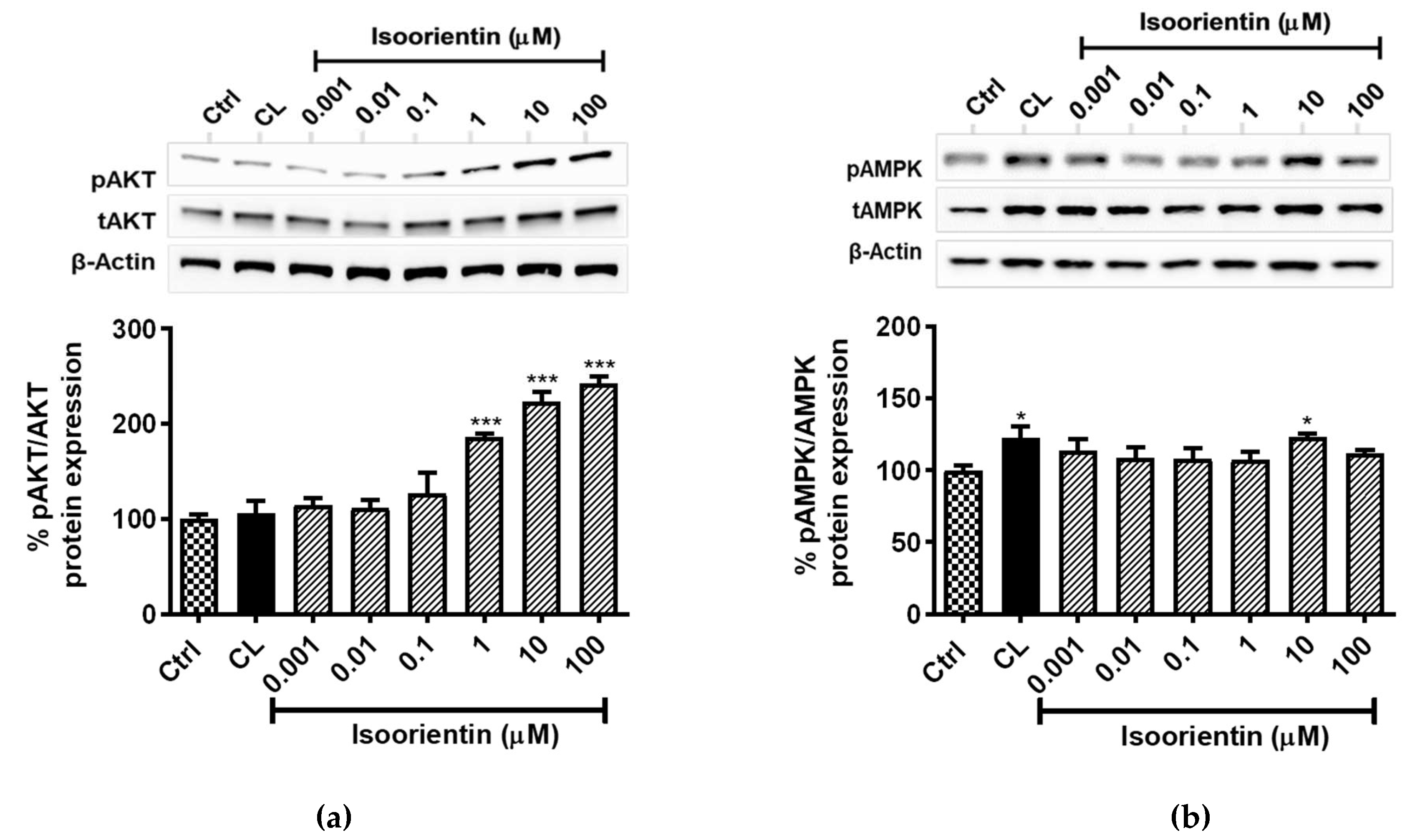
2.4. Isoorientin Increased AKT and AMPK Activation in Fully Differentiated 3T3-L1 Adipocytes
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Cell Culture and Differentiation of 3T3-L1 Adipocytes
3.2.2. Preparation of Compounds and Treatment
3.2.3. ATP Content and Glucose Uptake Determination
3.2.4. Intracellular Lipid Content and Glycerol Determination
3.2.5. Assessing Mitochondrial Bioenergetics
3.2.6. Western Blot Analysis
3.2.7. Statistical Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Patti, A.M.; Al-Rasadi, K.; Giglio, R.V.; Nikolic, D.; Mannina, C.; Castellino, G.; Chianetta, R.; Banach, M.; Cicero, A.F.G.; Lippi, G.; et al. Natural approaches in metabolic syndrome management. Arch. Med. Sci. AMS 2018, 14, 422–441. [Google Scholar] [CrossRef]
- Fu, C.; Jiang, Y.; Guo, J.; Su, Z. Natural Products with Anti-Obesity Effects and Different Mechanisms of Action. J. Agric. Food Chem. 2016, 64, 9571–9585. [Google Scholar] [CrossRef]
- Thounaojam, M.C.; Nammi, S.; Jadeja, R. Natural products for the treatment of obesity, metabolic syndrome, and type 2 diabetes. Evid.-Based Complement. Altern. Med. eCAM 2013, 2013, 871018. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Jack, B.; Mkandla, Z.; Mutize, T.; Silvestri, S.; Orlando, P.; Tiano, L.; Louw, J.; Mazibuko-Mbeje, S.E. Inflammation and Oxidative Stress in an Obese State and the Protective Effects of Gallic Acid. Nutrients 2018, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Castro, A.J.; Zapata-Bustos, R.; Gomez-Espinoza, G.; Salazar-Olivo, L.A. Isoorientin reverts TNF-alpha-induced insulin resistance in adipocytes activating the insulin signaling pathway. Endocrinology 2012, 153, 5222–5230. [Google Scholar] [CrossRef]
- Poudel, B.; Nepali, S.; Xin, M.; Ki, H.H.; Kim, Y.H.; Kim, D.K.; Lee, Y.M. Flavonoids from Triticum aestivum inhibit adipogenesis in 3T3-L1 cells by upregulating the insig pathway. Mol. Med. Rep. 2015, 12, 3139–3145. [Google Scholar] [CrossRef] [Green Version]
- Koeppen, B.H.; Smit, J.B.; Roux, D.G. The flavone C-glycosides and flavonol O-glycosides of Aspalathus acuminatus (rooibos tea). Biochem. J. 1962, 83, 507–511. [Google Scholar] [CrossRef] [Green Version]
- Joubert, E.; de Beer, D.; Malherbe, C.J.; Muller, N.; Bonnet, S.L.; van der Westhuizen, J.H.; Ferreira, D. Occurrence and sensory perception of Z-2-(beta-d-glucopyranosyloxy)-3-phenylpropenoic acid in rooibos (Aspalathus linearis). Food Chem. 2013, 136, 1078–1085. [Google Scholar] [CrossRef]
- Sezik, E.; Aslan, M.; Yesilada, E.; Ito, S. Hypoglycaemic activity of Gentiana olivieri and isolation of the active constituent through bioassay-directed fractionation techniques. Life Sci. 2005, 76, 1223–1238. [Google Scholar] [CrossRef]
- Aragao, D.M.; Guarize, L.; Lanini, J.; da Costa, J.C.; Garcia, R.M.; Scio, E. Hypoglycemic effects of Cecropia pachystachya in normal and alloxan-induced diabetic rats. J. Ethnopharmacol. 2010, 128, 629–633. [Google Scholar] [CrossRef]
- Im, J.Y.; Ki, H.H.; Xin, M.; Kwon, S.U.; Kim, Y.H.; Kim, D.K.; Hong, S.P.; Jin, J.S.; Lee, Y.M. Anti-obesity effect of Triticum aestivum sprout extract in high-fat-diet-induced obese mice. Biosci. Biotechnol. Biochem. 2015, 79, 1133–1140. [Google Scholar] [CrossRef] [Green Version]
- Dludla, P.V.; Muller, C.J.; Louw, J.; Joubert, E.; Salie, R.; Opoku, A.R.; Johnson, R. The cardioprotective effect of an aqueous extract of fermented rooibos (Aspalathus linearis) on cultured cardiomyocytes derived from diabetic rats. Phytomed. Int. J. Phytother. Phytopharmacol. 2014, 21, 595–601. [Google Scholar] [CrossRef]
- Mazibuko, S.E.; Muller, C.J.; Joubert, E.; de Beer, D.; Johnson, R.; Opoku, A.R.; Louw, J. Amelioration of palmitate-induced insulin resistance in C(2)C(1)(2) muscle cells by rooibos (Aspalathus linearis). Phytomed. Int. J. Phytother. Phytopharmacol. 2013, 20, 813–819. [Google Scholar]
- Sanderson, M.; Mazibuko, S.E.; Joubert, E.; de Beer, D.; Johnson, R.; Pheiffer, C.; Louw, J.; Muller, C.J. Effects of fermented rooibos (Aspalathus linearis) on adipocyte differentiation. Phytomed. Int. J. Phytother. Phytopharmacol. 2014, 21, 109–117. [Google Scholar] [CrossRef]
- Kakkar, A.K.; Dahiya, N. Drug treatment of obesity: Current status and future prospects. Eur. J. Intern. Med. 2015, 26, 89–94. [Google Scholar] [CrossRef]
- Kupeli, E.; Aslan, M.; Gurbuz, I.; Yesilada, E. Evaluation of in vivo biological activity profile of isoorientin. Zeitschrift fur Naturforschung C 2004, 59, 787–790. [Google Scholar] [CrossRef]
- Lim, J.H.; Park, H.S.; Choi, J.K.; Lee, I.S.; Choi, H.J. Isoorientin induces Nrf2 pathway-driven antioxidant response through phosphatidylinositol 3-kinase signaling. Arch. Pharmacal Res. 2007, 30, 1590–1598. [Google Scholar] [CrossRef]
- Yuan, L.; Han, X.; Li, W.; Ren, D.; Yang, X. Isoorientin Prevents Hyperlipidemia and Liver Injury by Regulating Lipid Metabolism, Antioxidant Capability, and Inflammatory Cytokine Release in High-Fructose-Fed Mice. J. Agric. Food Chem. 2016, 64, 2682–2689. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, J.; Wu, W.; Liu, Q.; Liu, X. Effect of isoorientin on intracellular antioxidant defence mechanisms in hepatoma and liver cell lines. Biomed. Pharmacother. 2016, 81, 356–362. [Google Scholar] [CrossRef]
- Anilkumar, K.; Reddy, G.V.; Azad, R.; Yarla, N.S.; Dharmapuri, G.; Srivastava, A.; Kamal, M.A.; Pallu, R. Evaluation of Anti-Inflammatory Properties of Isoorientin Isolated from Tubers of Pueraria tuberosa. Oxid. Med. Cell. Longev. 2017, 2017, 5498054. [Google Scholar] [CrossRef] [Green Version]
- Mazibuko, S.E.; Joubert, E.; Johnson, R.; Louw, J.; Opoku, A.R.; Muller, C.J. Aspalathin improves glucose and lipid metabolism in 3T3-L1 adipocytes exposed to palmitate. Mol. Nutr. Food Res. 2015, 59, 2199–2208. [Google Scholar] [CrossRef]
- Dludla, P.V.; Jack, B.; Viraragavan, A.; Pheiffer, C.; Johnson, R.; Louw, J.; Muller, C.J. A dose-dependent effect of dimethyl sulfoxide on lipid content, cell viability and oxidative stress in 3T3-L1 adipocytes. Toxicol. Rep. 2018, 5, 1014–1020. [Google Scholar] [CrossRef]
- Asano, H.; Kanamori, Y.; Higurashi, S.; Nara, T.; Kato, K.; Matsui, T.; Funaba, M. Induction of beige-like adipocytes in 3T3-L1 cells. J. Vet. Med. Sci. 2014, 76, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Mazibuko, S. In Vitro and In Vivo Effect of Aspalathu linearis and Its Major Polyphenols on Carbohydrate and Lipid Metabolism in Insulin Resistant Models; University of Zululand: Richards Bay, South Africa, 2014. [Google Scholar]
- Johnson, R.; Dludla, P.; Joubert, E.; February, F.; Mazibuko, S.; Ghoor, S.; Muller, C.; Louw, J. Aspalathin, a dihydrochalcone C-glucoside, protects H9c2 cardiomyocytes against high glucose induced shifts in substrate preference and apoptosis. Mol. Nutr. Food Res. 2016, 60, 922–934. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Johnson, R.; Joubert, E.; Louw, J.; Ziqubu, K.; Tiano, L.; Silvestri, S.; Orlando, P.; Opoku, A.R.; et al. Aspalathin, a natural product with the potential to reverse hepatic insulin resistance by improving energy metabolism and mitochondrial respiration. PLoS ONE 2019, 14, e0216172. [Google Scholar] [CrossRef] [Green Version]
- Karri, S.; Sharma, S.; Hatware, K.; Patil, K. Natural anti-obesity agents and their therapeutic role in management of obesity: A future trend perspective. Biomed. Pharmacother. 2019, 110, 224–238. [Google Scholar] [CrossRef]
- Valli, V.; Heilmann, K.; Danesi, F.; Bordoni, A.; Gerhauser, C. Modulation of Adipocyte Differentiation and Proadipogenic Gene Expression by Sulforaphane, Genistein, and Docosahexaenoic Acid as a First Step to Counteract Obesity. Oxid. Med. Cell. Longev. 2018, 2018, 1617202. [Google Scholar] [CrossRef]
- Ruiz-Ojeda, F.J.; Ruperez, A.I.; Gomez-Llorente, C.; Gil, A.; Aguilera, C.M. Cell Models and Their Application for Studying Adipogenic Differentiation in Relation to Obesity: A Review. Int. J. Mol. Sci. 2016, 17, 1040. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.Y.; Lee, M.H.; Hsu, C.C.; Wei, C.L.; Tsai, Y.C. Methyl cinnamate inhibits adipocyte differentiation via activation of the CaMKK2-AMPK pathway in 3T3-L1 preadipocytes. J. Agric. Food Chem. 2012, 60, 955–963. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Liu, J. Akt activation: A potential strategy to ameliorate insulin resistance. Diabetes Res. Clin. Pract. 2019, 156, 107092. [Google Scholar] [CrossRef]
- Huang, B.; Yuan, H.D.; Kim, D.Y.; Quan, H.Y.; Chung, S.H. Cinnamaldehyde prevents adipocyte differentiation and adipogenesis via regulation of peroxisome proliferator-activated receptor-gamma (PPARgamma) and AMP-activated protein kinase (AMPK) pathways. J. Agric. Food Chem. 2011, 59, 3666–3673. [Google Scholar] [CrossRef]
- Mazibuko-Mbeje, S.E.; Dludla, P.V.; Roux, C.; Johnson, R.; Ghoor, S.; Joubert, E.; Louw, J.; Opoku, A.R.; Muller, C.J. Aspalathin-Enriched Green Rooibos Extract Reduces Hepatic Insulin Resistance by Modulating PI3K/AKT and AMPK Pathways. Int. J. Mol. Sci. 2019, 20, 633. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, H.M. AMPK and Exercise: Glucose Uptake and Insulin Sensitivity. Diabetes Metab. J. 2013, 37, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Zheng, M.; Cai, D.; Xie, J.; Jin, Z.; Liu, H.; Liu, J. Zeaxanthin promotes mitochondrial biogenesis and adipocyte browning via AMPKalpha1 activation. Food Funct. 2019, 10, 2221–2233. [Google Scholar] [CrossRef]
- Luan, G.; Wang, Y.; Wang, Z.; Zhou, W.; Hu, N.; Li, G.; Wang, H. Flavonoid Glycosides from Fenugreek Seeds Regulate Glycolipid Metabolism by Improving Mitochondrial Function in 3T3-L1 Adipocytes in Vitro. J. Agric. Food Chem. 2018, 66, 3169–3178. [Google Scholar] [CrossRef]
- Xiao, J.; Hogger, P. Stability of dietary polyphenols under the cell culture conditions: Avoiding erroneous conclusions. J. Agric. Food Chem. 2015, 63, 1547–1557. [Google Scholar] [CrossRef]
- Malik, A.; Jamil, U.; Butt, T.T.; Waquar, S.; Gan, S.H.; Shafique, H.; Jafar, T.H. In silico and in vitro studies of lupeol and iso-orientin as potential antidiabetic agents in a rat model. Drug Des. Dev. Ther. 2019, 13, 1501–1513. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.K.; Qin, X.Y.; Wang, G.K.; Xie, G.Y.; Li, X.S.; Sun, C.Y.; Liu, B.L.; Qin, M.J. Antihyperglycemic, antihyperlipidemic and antioxidant effects of standard ethanol extract of Bombax ceiba leaves in high-fat-diet- and streptozotocin-induced Type 2 diabetic rats. Chin. J. Nat. Med. 2017, 15, 168–177. [Google Scholar] [CrossRef]
- Goss, M.J.; Nunes, M.L.O.; Machado, I.D.; Merlin, L.; Macedo, N.B.; Silva, A.M.O.; Bresolin, T.M.B.; Santin, J.R. Peel flour of Passiflora edulis Var. Flavicarpa supplementation prevents the insulin resistance and hepatic steatosis induced by low-fructose-diet in young rats. Biomed. Pharmacother. 2018, 102, 848–854. [Google Scholar] [CrossRef]
- Yang, H.J.; Lim, J.H.; Park, K.J.; Kang, S.; Kim, D.S.; Park, S. Methyl jasmolate treated buckwheat sprout powder enhances glucose metabolism by potentiating hepatic insulin signaling in estrogen-deficient rats. Nutrition 2016, 32, 129–137. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available through commercial sources. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziqubu, K.; Muller, C.J.F.; Dludla, P.V.; Mthembu, S.X.H.; Obonye, N.; Louw, J.; Kappo, A.P.; Silvestri, S.; Orlando, P.; Tiano, L.; et al. Impact of Isoorientin on Metabolic Activity and Lipid Accumulation in Differentiated Adipocytes. Molecules 2020, 25, 1773. https://doi.org/10.3390/molecules25081773
Ziqubu K, Muller CJF, Dludla PV, Mthembu SXH, Obonye N, Louw J, Kappo AP, Silvestri S, Orlando P, Tiano L, et al. Impact of Isoorientin on Metabolic Activity and Lipid Accumulation in Differentiated Adipocytes. Molecules. 2020; 25(8):1773. https://doi.org/10.3390/molecules25081773
Chicago/Turabian StyleZiqubu, Khanyisani, Christo J. F. Muller, Phiwayinkosi V. Dludla, Sinenhlanhla X. H. Mthembu, Nnini Obonye, Johan Louw, Abidemi P. Kappo, Sonia Silvestri, Patrick Orlando, Luca Tiano, and et al. 2020. "Impact of Isoorientin on Metabolic Activity and Lipid Accumulation in Differentiated Adipocytes" Molecules 25, no. 8: 1773. https://doi.org/10.3390/molecules25081773
APA StyleZiqubu, K., Muller, C. J. F., Dludla, P. V., Mthembu, S. X. H., Obonye, N., Louw, J., Kappo, A. P., Silvestri, S., Orlando, P., Tiano, L., & Mazibuko-Mbeje, S. E. (2020). Impact of Isoorientin on Metabolic Activity and Lipid Accumulation in Differentiated Adipocytes. Molecules, 25(8), 1773. https://doi.org/10.3390/molecules25081773






