Synthesis and Antibacterial Evaluation of N-phenylacetamide Derivatives Containing 4-Arylthiazole Moieties
Abstract
1. Introduction
2. Results and Discussion
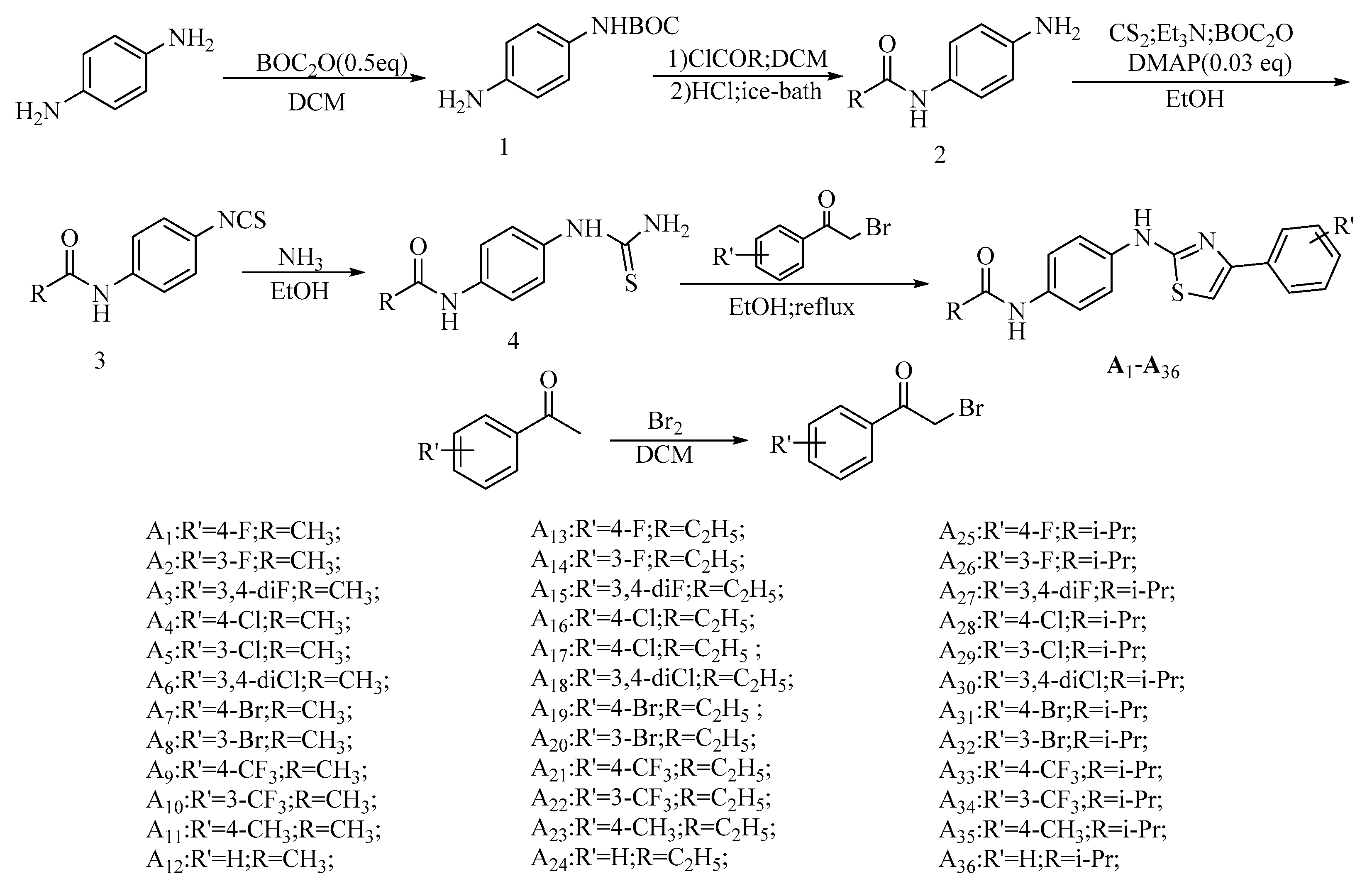
2.1. Chemistry
2.2. In Vitro Antibacterial Activity
2.3. Structure-Activity Relationship Analyses
2.4. Scanning Electron Microscopy Studies
2.5. Nematicidal Biological Activities
3. Experimental
3.1. Chemicals and Instruments
3.2. General Synthetic Procedure for the Target Compounds
3.2.1. Synthesis of Intermediate 1
3.2.2. Synthesis of Intermediates 2
3.2.3. Synthesis of Intermediates 3
3.2.4. Synthesis of the Intermediates 4
3.2.5. Synthesis of the α-Bromophenylethanone Intermediates
3.2.6. Synthesis of the Target Compounds A
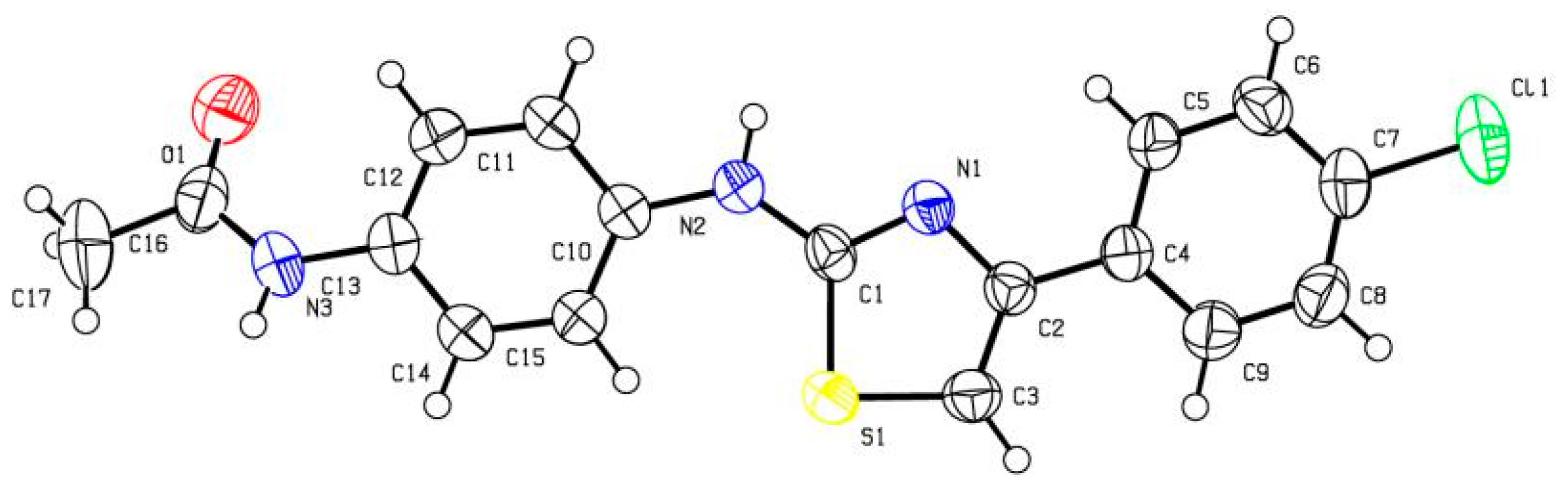
3.3. X-ray Diffraction Analysis
3.4. In Vitro Antibacterial Activity Bioassays
3.5. Scanning Electron Microscopy
3.6. Nematicidal Biological Activity In Vitro
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Spago, F.R.; Ishii Mauro, C.S.; Oliveira, A.G.; Beranger, J.P.O.; Cely, M.V.T.; Stanganelli, M.M.; Simionato, A.S.; San Martin, J.A.B.; Andrade, C.G.T.J.; Mello, J.C.P.; et al. Pseudomonas aeruginosa produces secondary metabolites that have biological activity against plant pathogenic Xanthomonas species. Crop. Prot. 2014, 62, 46–54. [Google Scholar] [CrossRef]
- Li, P.; Tian, P.Y.; Song, X.; Xue, W.; Jin, L.H.; Hu, D.Y.; Yang, S.; Song, B.A. Novel bisthioether derivatives containing a 1,3,4-oxadiazole moiety: Design, synthesis, antibacterial and nematocidal activities. Pest. Manag. Sci. 2018, 74, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.Y.; Zhou, L.; Zhou, J.; Wu, Z.; Xue, W.; Song, B.A.; Yang, S. Synthesis and antibacterial activity of pyridinium-tailored 2,5-substituted-1,3,4-oxadiazole thioether/sulfoxide/sulfone derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Iihama, T.; Miyazawa, M.; Miyahara, O.; Marumo, S.; Sano, S.; Hamamura, H.; Yokota, C.; Kawaguchi, M.; Takahashi, H.; Takkagi, M. Preparation of Thiazole Compounds as Pest Control Agents and Fungicides. JP Patent WO9940076, 12 August 1999. [Google Scholar]
- Huang, D.L.; Liu, A.P.; Liu, W.D.; Liu, A.P.; Liu, W.D.; Liu, X.P.; Chen, X.Y.; Pei, H.; Sun, J.; Yin, D.L. Synthesis and biological evaluation of 1H-pyrazole-5-carboxamide derivatives as potential fungicidal and insecticidal agents. Synth. Commun. 2017, 47, 455–460. [Google Scholar] [CrossRef]
- Liao, G.P.; Zhou, X.; Xiao, W.; Xie, Y.; Jin, L.H. Synthesis and antimicrobial activity of novel 2-substituted phenoxy-N-(4-substituted phenyl-5-(1H-1,2,4-triazol-1-yl) thiazol-2-yl) acetamide derivatives. J. Heterocycl. Chem. 2016, 52, 1506–1513. [Google Scholar] [CrossRef]
- Bharti, S.K.; Nath, G.; Tilak, R.; Singh, S.K. Synthesis, antibacterial and antifungal activities of some novel Schiff bases containing 2,4-disubstituted thiazole ring. Eur. J. Med. Chem. 2010, 45, 651–660. [Google Scholar] [CrossRef]
- Wang, M.W.; Zhu, H.H.; Wang, P.Y.; Zeng, D.; Wu, Y.Y.; Liu, L.W.; Wu, Z.B.; Li, Z.; Yang, S. Synthesis of thiazolium-labeled 1,3,4-oxadiazole thioethers as prospective antimicrobials: In vitro and in vivo bioactivity and mechanism of action. J. Agric. Food Chem. 2019, 67, 12696–12708. [Google Scholar] [CrossRef]
- Wang, T.T.; Bing, G.F.; Zhang, X.; Qin, Z.; Yu, H.; Qin, X.; Dai, H.; Miao, W.; Wu, S.; Fang, J. Synthesis and herbicidal activities of 2-cyano-3-benzylaminoacrylates containing thiazole moiety. Bioorg. Med. Chem. Lett. 2010, 20, 3348–3351. [Google Scholar] [CrossRef]
- Guo, J.C.; Hao, Y.N.; Wang, X.F.; Liu, Y.; Ma, D.; Li, Y.; Pang, H.; Ni, J.; Wang, Q. Optimization, structure-activity relationship, and mode of action of nortopsentin analogues containing thiazole and oxazole moieties. J. Agric. Food Chem. 2019, 67, 10018–10031. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, B.; Fan, Z.J.; Hu, M.; Li, Q.; Hu, W.; Li, J.; Zhang, J. Discovery of novel isothiazole 1,2,3-thiadiazole and thiazole-based cinnamamides as fungicidal candidates. J. Agric. Food Chem. 2019, 67, 12357–12365. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.B.; Dai, H.; Fang, J.X. Synthesis and biological activities evaluation of new 4-(2,4-difluorophenyl)-N-aryl-5-(1H-1,2,4-triazol-1-yl)thiazol-2-amine. Communications 2011, 41, 3197–3206. [Google Scholar]
- Wei, Q.Y.; Wang, X.M.; Cheng, J.H.; Zeng, G.; Sun, D.W. Synthesis and antimicrobial activities of novel sorbic and benzoic acid amide derivatives. Food Chem. 2018, 268, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.J.; Rao, X.P.; Shang, S.B.; Song, Z.; Shen, M.; Liu, H. Synthesis, structure analysis and antibacterial activity of N-[5-dehydroabietyl-[1,3,4]thiadiazol-2-yl]-aromatic amide derivatives. J. Saudi Chem. Soc. 2017, 21, S258–S263. [Google Scholar] [CrossRef]
- Wang, Z.J.; Gao, Y.; Hou, Y.L.; Zhang, C.; Yu, S.J.; Bian, Q.; Li, Z.M.; Zhao, W.G. Design, synthesis, and fungicidal evaluation of a series of novel 5- methyl-1H-1,2,3-trizole-4-carboxyl amide and ester analogues. Eur. J. Med. Chem. 2016, 86, 87–94. [Google Scholar] [CrossRef]
- Chen, J.X.; Yi, C.F.; Wang, S.B.; Wu, S.; Li, S.; Hu, D.; Song, B. Novel amide derivatives containing 1,3,4-thiadiazole moiety: Design, synthesis, nematocidal and antibacterial activities. Bioorg. Med. Chem. Lett. 2019, 29, 1203–1210. [Google Scholar] [CrossRef]
- Che, Z.; Zhang, S.; Shao, Y.; Fan, L.; Xu, H.; Yu, X.; Zhi, X.; Yao, X.; Zhang, R. Synthesis and quantitative structure-activity relationship (QSAR) study of novel N-arylsulfonyl-3-acylindole arylcarbonyl hydrazone derivatives as nematicidal agents. J. Agric. Food Chem. 2013, 61, 5696–5705. [Google Scholar] [CrossRef]
- Chankeswara, S.V.; Chakraborti, A.K. Catalyst-free chemoselective N-tert-butyloxycarbonylation of amines in water. Org. Lett. 2006, 8, 3259–3262. [Google Scholar] [CrossRef]
- Basel, Y.; Hassner, A. Di-tert-butyl dicarbonate and 4-(dimethylamino)pyridine revisited. Their reactions with amines and alcohols. J. Org. Chem. 2000, 65, 6368–6380. [Google Scholar] [CrossRef]
- Lee, L.; Leroux, Y.R.; Hapiot, P.; Downard, A.J. Amine-terminated monolayers on carbon: Preparation, characterization, and coupling Reactions. Langmuir 2015, 31, 5071–5077. [Google Scholar] [CrossRef]
- Henrik, M.; Jon, S.H.; Michael, P. A new efficient synthesis of isothiocyanates from amines using di-tert-butyl dicarbonate. Tetrahedron Lett. 2008, 49, 3117–3119. [Google Scholar]
- Stephensen, H.; Zaragoza, F. Resin-bound isothiocyanates and their synthetic equivalents as intermediates for the solid-phase synthesis of substituted thiophenes. J. Org. Chem. 1997, 62, 6096–6097. [Google Scholar] [CrossRef]
- Seelam, M.; Shaikh, B.V.; Tamminana, R.; Kammela, P. An efficient methodology for the synthesis of thioureas from amine mediated by a cobalt source. Tetrahedron Lett. 2016, 57, 5297–5300. [Google Scholar] [CrossRef]
- Baek, S.H.; Kim, N.J.; Kim, S.H.; Park, K.-H. Inhibitory effect of 4-aryl 2-substituted aniline-thiazole analogs on growth of human prostate cancer LNCap cell. Bull. Korean Chem. Soc. 2012, 33, 111–114. [Google Scholar] [CrossRef]
- Jeong, K.; Lee, J.H.; Park, S.M.; Choi, J.H.; Jeong, D.Y.; Choi, D.H.; Nam, Y.; Park, J.H.; Lee, K.N.; Kim, S.M.; et al. Synthesis and in-vitro evaluation of 2-amino-4-arylthiazole as inhibitor of 3D polymerase against foot-and-mouth disease (FMD). Eur. J. Med. Chem. 2015, 387–397. [Google Scholar] [CrossRef]
- Su, S.H.; Zhou, X.; Liao, G.P.; Qi, P.Y.; Jin, L.H. Synthesis and antibacterial evaluation of new sulfone derivatives containing 2-aroxymethyl-1,3,4-oxadiazole/thiadiazole moiety. Molecules 2017, 22, 64. [Google Scholar] [CrossRef]
- Zhou, J.; Tao, Q.Q.; Wang, P.Y.; Shao, W.B.; Wu, Z.B.; Li, Z.; Yang, S. Antimicrobial evaluation and action mechanism of pyridinium-decorated 1,4-pentadien-3-one derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 1742–1746. [Google Scholar] [CrossRef]
- Montasser, S.A.; Abd El-Wahab, A.E.; Abd-Elgawad, M.M.M.; Abd-El-Khair, H.; Faika, F.H.K.; Hammam, M.M.A. Effects of some fungi and bacteria as bio-control agents against citrus nematode Tylenchulus semipenetrans Cobb. J. Appl. Sci. Res. 2012, 8, 5436–5444. [Google Scholar]
- Cheng, W.L.; Yang, J.Y.; Nie, Q.Y.; Huang, D.; Yu, C.; Zheng, L.; Cai, M.; Thomashow, L.S.; Weller, D.M.; Yu, Z.; et al. Volatile organic compounds from paenibacillus polymyxa KM2501-1 control meloidogyne incognita by multiple strategies. Sci. Rep. 2017, 7, 16213. [Google Scholar] [CrossRef]
- Chen, J.X.; Chen, Y.Z.; Gan, X.H.; Song, B.J.; Hu, D.Y.; Song, B.A. Synthesis, nematicidal evaluation, and 3D-QSAR analysis of novel 1,3,4-oxadiazole-cinnamic acid hybrids. J. Agric. Food Chem. 2018, 66, 9616–9623. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds A1–A36 are available from the authors. |

| Compound | EC50 (µM) c | ||
|---|---|---|---|
| Xoo | Xac | Xoc | |
| A1 | 156.7±7.4 | 152.0±7.8 | 250.7±8.0 |
| A3 | 260.9±9.0 | 561.6±6.8 | 361.4±9.3 |
| A4 | 179.2±6.2 | 281.2±9.4 | 194.9±8.0 |
| A6 | 144.7±3.2 | 347.3±5.6 | 248.5±12.1 |
| A11 | 512.1±13.4 | 812.3±11.0 | 280.6±16.4 |
| A13 | 541.4±15.3 | 660.4±10.1 | 392.8±6.3 |
| A25 | 918.8±19.0 | 1186±11.9 | 716.5±19.0 |
| Bismerthiazolb | 230.5±7.5 | 162.7±3.9 | 254.9±8.2 |
| Thiodiazole copperb | 545.2±13.7 | 476.5±19.9 | 607.5±3.78 |
| Compound | R’/R | 500 μg/mL | 100 μg/mL | ||
|---|---|---|---|---|---|
| 24 h | 72 h | 24 h | 72 h | ||
| A1 | 4-F/CH3 | 26.6 | 26.9 | 9.3 | 9.9 |
| A2 | 3-F/CH3 | 11.9 | 13.1 | 0 | 5.4 |
| A3 | 3,4-diF/CH3 | 20.2 | 23.7 | 6.4 | 9.0 |
| A4 | 4-Cl/CH3 | 14.7 | 21.8 | 5.1 | 5.1 |
| A5 | 3-Cl/CH3 | 8.7 | 17.3 | 0 | 0 |
| A6 | 3,4-diCl/CH3 | 11.9 | 20.5 | 4.8 | 7.1 |
| A7 | 4-Br/CH3 | 14.1 | 21.2 | 5.8 | 8 |
| A8 | 3-Br/CH3 | 11.9 | 14.4 | 7.1 | 8.3 |
| A9 | 4- CF3/CH3 | 23.7 | 29.2 | 14.1 | 17.3 |
| A10 | 3-CF3/CH3 | 11.5 | 17.6 | 8 | 8.7 |
| A11 | 4-CH3/CH3 | 14.7 | 21.8 | 5.1 | 5.1 |
| A12 | H/CH3 | 21.2 | 23.4 | 3.5 | 5.4 |
| A13 | 4-F/CH2CH3 | 6.1 | 11.5 | 0.6 | 2.6 |
| A14 | 3-F/CH2CH3 | 6.7 | 9.9 | 1.0 | 3.2 |
| A15 | 3,4-diF/CH2CH3 | 15.1 | 14.1 | 2.6 | 2.9 |
| A16 | 4-Cl/CH2CH3 | 7.4 | 14.4 | 3.8 | 6.7 |
| A17 | 3-Cl/CH2CH3 | 7.4 | 10.0 | 1.0 | 2.3 |
| A18 | 3,4-diCl/CH2CH3 | 21.5 | 23.1 | 3.8 | 4.8 |
| A19 | 4-Br/CH2CH3 | 5.1 | 2.9 | 0 | 0 |
| A20 | 3-Br/CH2CH3 | 15.4 | 16.7 | 1.3 | 1.9 |
| A21 | 4- CF3/CH2CH3 | 17.3 | 18.3 | 4.2 | 5.3 |
| A22 | 3- CF3/CH2CH3 | ‘13.1 | 15.4 | 3.8 | 7.1 |
| A23 | 4-CH3/CH2CH3 | 100 | 100 | 51.3 | 53.2 |
| A24 | H/CH2CH3 | 29.5 | 30.1 | 7.7 | 9.3 |
| A25 | 4-F/CH(CH3)2 | 6.1 | 11.5 | 0.6 | 2.6 |
| A26 | 3-F/CH(CH3)2 | 6.7 | 10.0 | 1.0 | 5.2 |
| A27 | 3,4-diF/CH(CH3)2 | 15.4 | 21.2 | 5.4 | 7.7 |
| A28 | 4-Cl/CH(CH3)2 | 19.9 | 20.8 | 5.8 | 6.1 |
| A29 | 3-Cl/CH(CH3)2 | 7.4 | 15.5 | 0 | 0 |
| A30 | 4-CF3/CH(CH3)2 | 22.1 | 26 | 10.3 | 12.8 |
| A31 | 3-CF3/CH(CH3)2 | 17.6 | 17.6 | 0 | 0 |
| A32 | 3,4-diCl/CH(CH3)2 | 17.0 | 17.3 | 0 | 0 |
| A33 | 4-Br/CH(CH3)2 | 16.0 | 24.7 | 7.4 | 8.3 |
| A34 | 3-Br/CH(CH3)2 | 29.8 | 30.4 | 9.9 | 10.9 |
| A35 | 4-CH3/CH(CH3)2 | 19.6 | 20.8 | 8 | 9.3 |
| A36 | H/CH(CH3)2 | 18.6 | 19.9 | 8.7 | 9.3 |
| Avermectinb | 100 | 100 | 71.8 | 79.3 | |
| CKc | 0 | 0 | 0 | 0 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, H.; Zhou, X.; Wang, L.; Jin, L. Synthesis and Antibacterial Evaluation of N-phenylacetamide Derivatives Containing 4-Arylthiazole Moieties. Molecules 2020, 25, 1772. https://doi.org/10.3390/molecules25081772
Lu H, Zhou X, Wang L, Jin L. Synthesis and Antibacterial Evaluation of N-phenylacetamide Derivatives Containing 4-Arylthiazole Moieties. Molecules. 2020; 25(8):1772. https://doi.org/10.3390/molecules25081772
Chicago/Turabian StyleLu, Hui, Xia Zhou, Lei Wang, and Linhong Jin. 2020. "Synthesis and Antibacterial Evaluation of N-phenylacetamide Derivatives Containing 4-Arylthiazole Moieties" Molecules 25, no. 8: 1772. https://doi.org/10.3390/molecules25081772
APA StyleLu, H., Zhou, X., Wang, L., & Jin, L. (2020). Synthesis and Antibacterial Evaluation of N-phenylacetamide Derivatives Containing 4-Arylthiazole Moieties. Molecules, 25(8), 1772. https://doi.org/10.3390/molecules25081772






