Towards Building Blocks for Supramolecular Architectures Based on Azacryptates
Abstract
1. Introduction
2. Results and Discussion
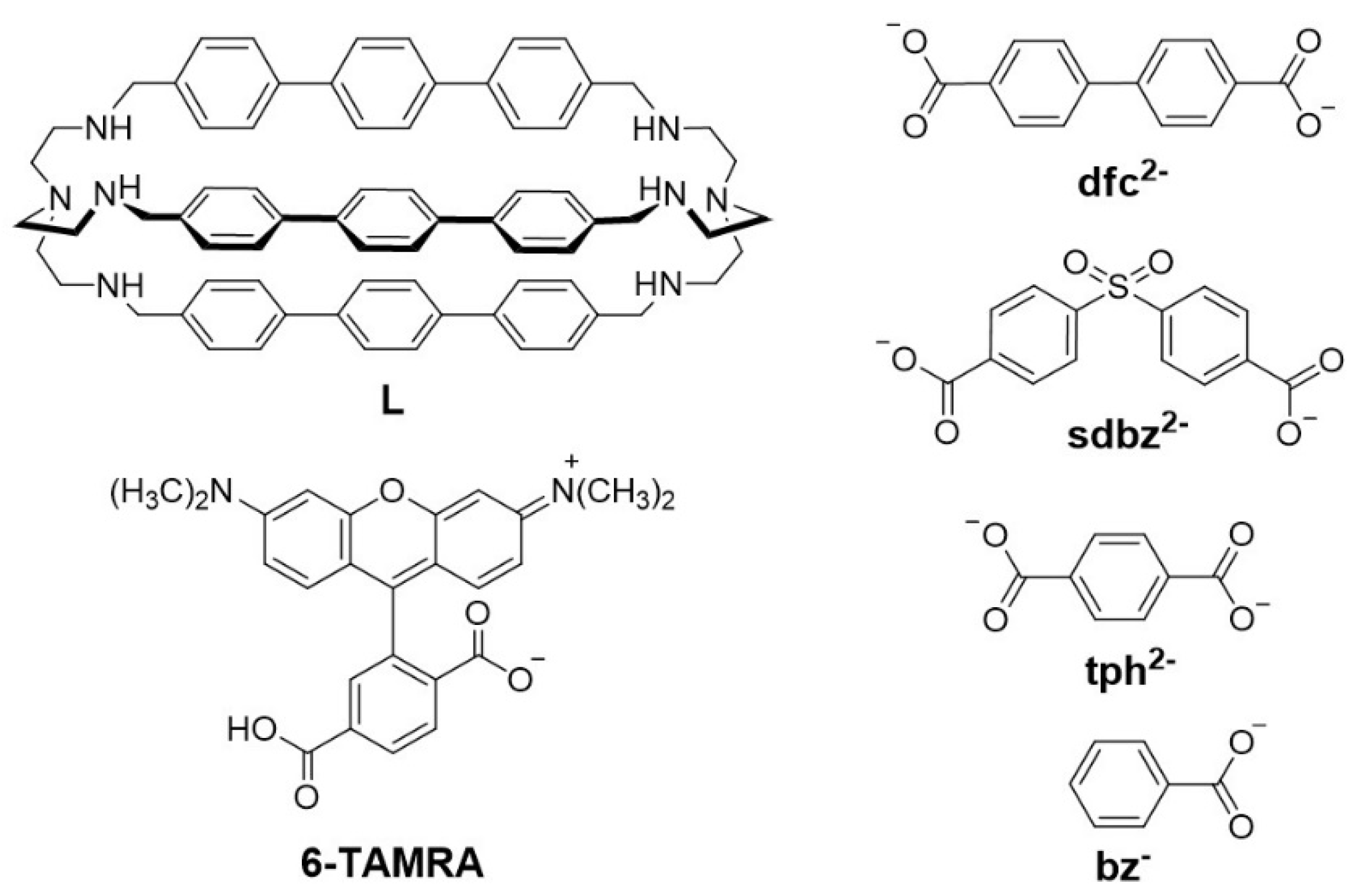
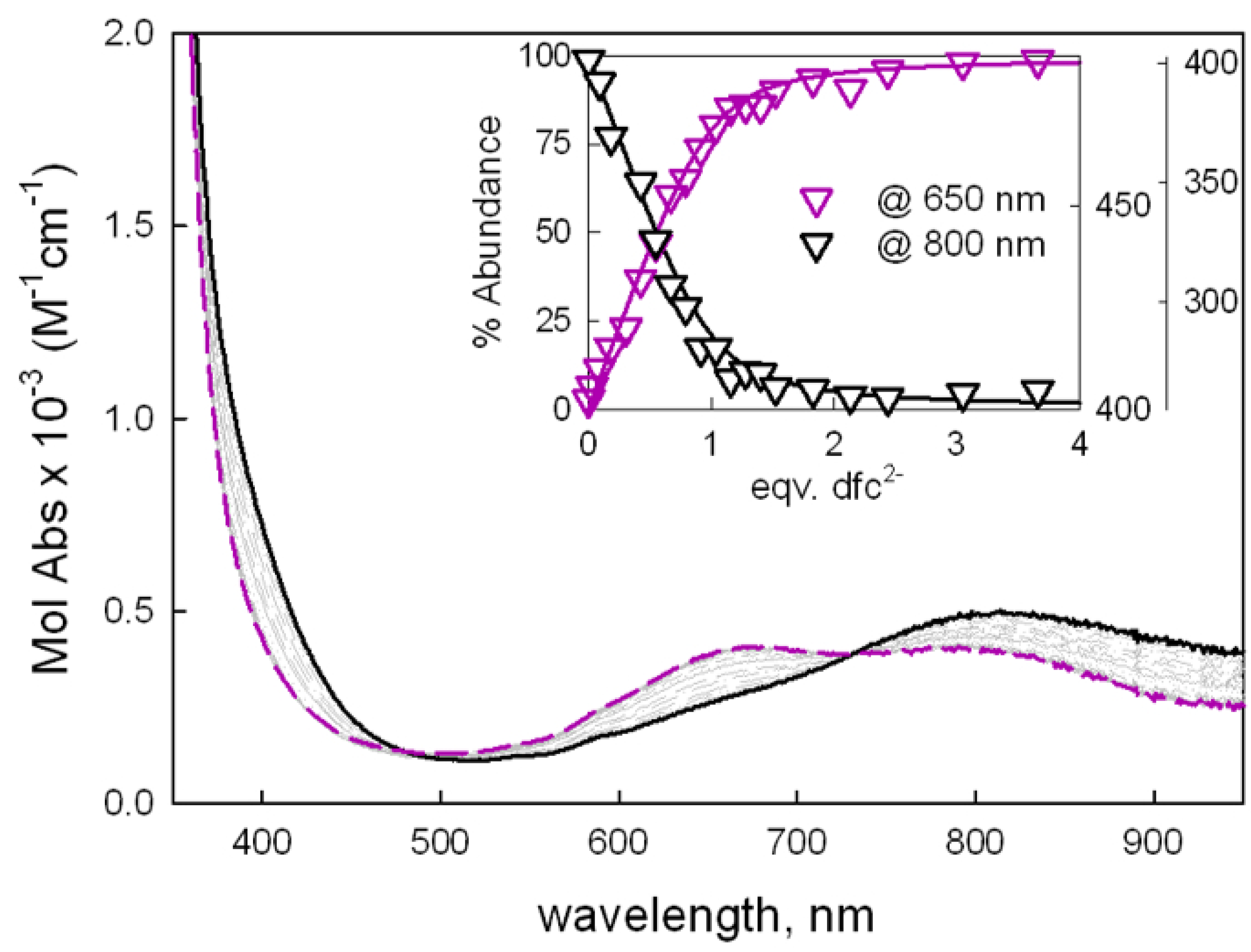
2.1. UV-Vis Investigations with Aromatic Dicarboxylates of Different Size/Length
2.2. Anion Binding Studies Using the ID Approach
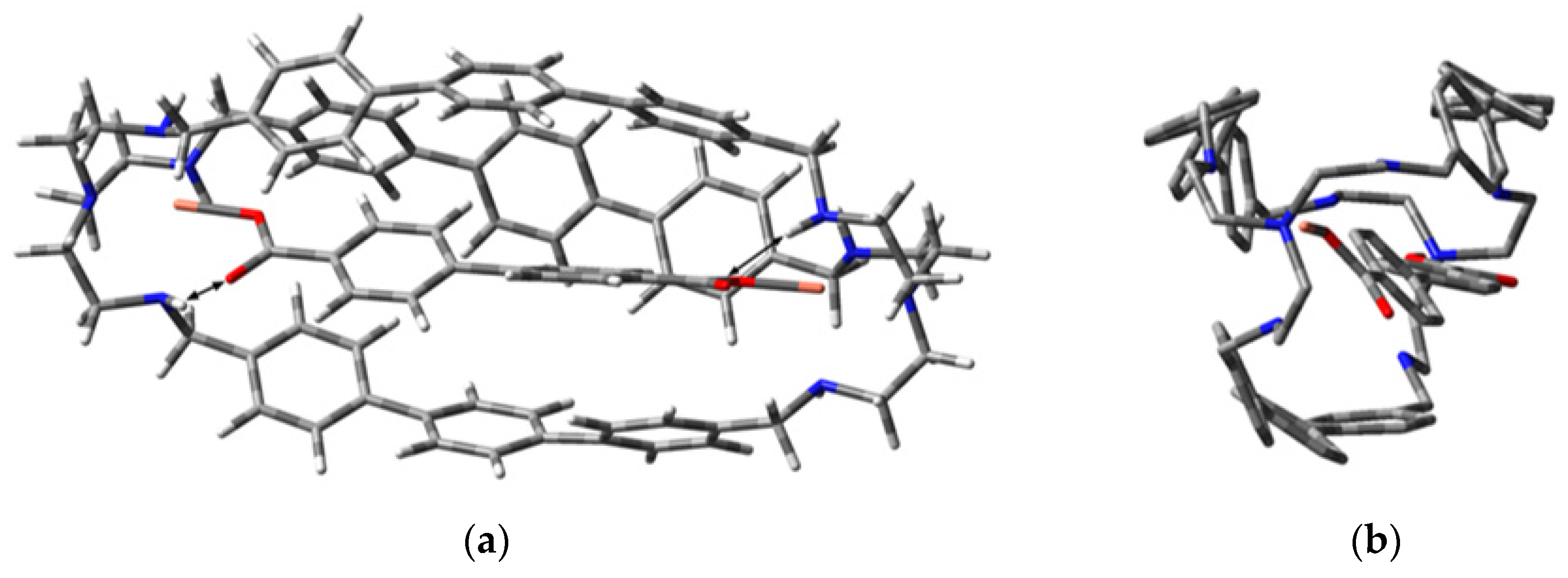
2.3. Computational Investigations on the Inclusion Complexes
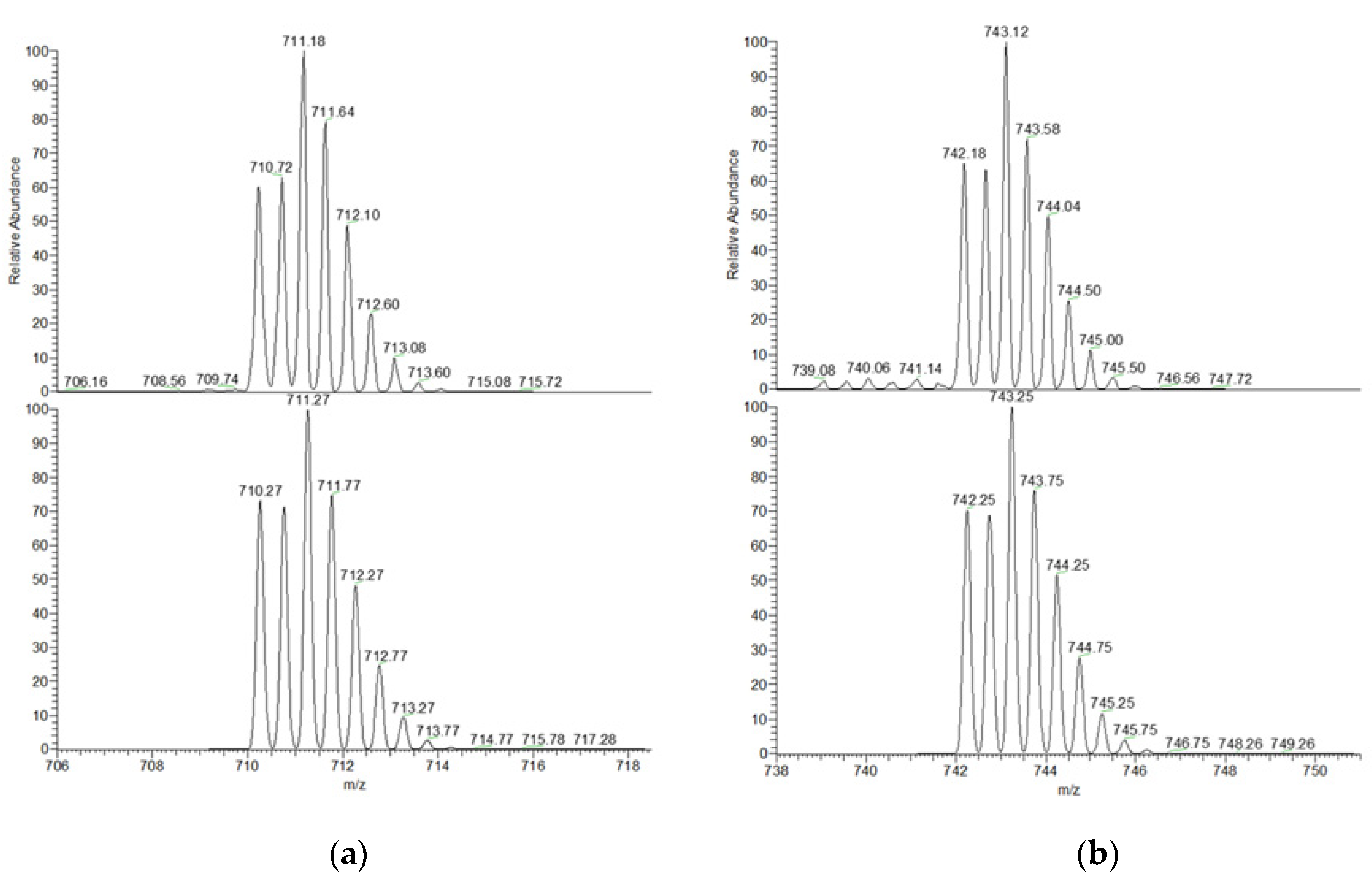
2.4. ESI-MS Characterization of the Inclusion Complexes
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bissell, R.A.; Córdova, E.; Kaifer, A.E.; Stoddart, J.F. A chemically and electrochemically switchable molecular shuttle. Nature 1994, 369, 133–137. [Google Scholar] [CrossRef]
- Balzani, V.; Gómez-López, M.; Stoddart, J.F. Molecular Machines. Acc. Chem. Res. 1998, 31, 405–414. [Google Scholar] [CrossRef]
- Silvi, S.; Venturi, M.; Credi, A. Light operated molecular machines. Chem. Commun. 2011, 47, 2483–2489. [Google Scholar] [CrossRef]
- Liu, Y.; Flood, A.H.; Bonvallet, P.A.; Vignon, S.A.; Northrop, B.H.; Tseng, H.-R.; Jeppesen, J.O.; Huang, T.J.; Brough, B.; Baller, M.; et al. Linear Artificial Molecular Muscles. J. Am. Chem. Soc. 2005, 127, 9745–9759. [Google Scholar] [CrossRef] [PubMed]
- Erbas-Cakmak, S.; Leigh, D.A.; McTernan, C.T.; Nussbaumer, A.L. Artificial Molecular Machines. Chem. Rev. 2015, 115, 10081–10206. [Google Scholar] [CrossRef] [PubMed]
- Balzani, V.; Credi, A.; Venturi, M. Molecular Devices and Machines: Concepts and Perspectives for the Nano World, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008; ISBN 978-3-527-62168-2. [Google Scholar]
- Li, D.; Paxton, W.F.; Baughman, R.H.; Huang, T.J.; Stoddart, J.F.; Weiss, P.S. Molecular, Supramolecular, and Macromolecular Motors and Artificial Muscles. MRS Bull. 2009, 34, 671–681. [Google Scholar] [CrossRef]
- Kay, E.R.; Leigh, D.A. Rise of the Molecular Machines. Angew. Chem. Int. Ed. 2015, 54, 10080–10088. [Google Scholar] [CrossRef]
- Lewis, J.E.M.; Galli, M.; Goldup, S.M. Properties and emerging applications of mechanically interlocked ligands. Chem. Commun. 2017, 53, 298–312. [Google Scholar] [CrossRef]
- Kay, E.R.; Leigh, D.A.; Zerbetto, F. Synthetic Molecular Motors and Mechanical Machines. Angew. Chem. Int. Ed. 2007, 46, 72–191. [Google Scholar] [CrossRef]
- Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Development of Pseudorotaxanes and Rotaxanes: From Synthesis to Stimuli-Responsive Motions to Applications. Chem. Rev. 2015, 115, 7398–7501. [Google Scholar] [CrossRef]
- Clever, G.H.; Shionoya, M. A pH Switchable Pseudorotaxane Based on a Metal Cage and a Bis-anionic Thread. Chem. Eur. J. 2010, 16, 11792–11796. [Google Scholar] [CrossRef]
- Lehn, J.-M. Supramolecular Chemistry: Concepts and Perspectives; Wiley-VCH: Weinheim, Germany, 1995; ISBN 978-3-527-29311-7. [Google Scholar]
- Mateus, P.; Bernier, N.; Delgado, R. Recognition of anions by polyammonium macrocyclic and cryptand receptors: Influence of the dimensionality on the binding behavior. Coord. Chem. Rev. 2010, 254, 1726–1747. [Google Scholar] [CrossRef]
- Fabbrizzi, L. Cryptands and Cryptates; World Scientific Publishing Europe Ltd.: London, UK, 2018; ISBN 1-78634-369-X. [Google Scholar]
- Boiocchi, M.; Bonizzoni, M.; Fabbrizzi, L.; Piovani, G.; Taglietti, A. A Dimetallic Cage with a Long Ellipsoidal Cavity for the Fluorescent Detection of Dicarboxylate Anions in Water. Angew. Chem. Int. Ed. 2004, 43, 3847–3852. [Google Scholar] [CrossRef]
- Amendola, V.; Miljkovic, A.; Legnani, L.; Toma, L.; Dondi, D.; Lazzaroni, S. Self-Assembly of Pseudorotaxane Structures from a Dicopper(II) Molecular Cage and Dicarboxylate Axles. Inorg. Chem. 2018, 57, 3540–3547. [Google Scholar] [CrossRef] [PubMed]
- Wiskur, S.L.; Ait-Haddou, H.; Lavigne, J.J.; Anslyn, E.V. Teaching Old Indicators New Tricks. Acc. Chem. Res. 2001, 34, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Bounos, G.; Ghosh, S.; Lee, A.K.; Plunkett, K.N.; DuBay, K.H.; Bolinger, J.C.; Zhang, R.; Friesner, R.A.; Nuckolls, C.; Reichman, D.R.; et al. Controlling Chain Conformation in Conjugated Polymers Using Defect Inclusion Strategies. J. Am. Chem. Soc. 2011, 133, 10155–10160. [Google Scholar] [CrossRef] [PubMed]
- Jazwinski, J.; Lehn, J.M.; Lilienbaum, D.; Ziessel, R.; Guilhem, J.; Pascard, C. Polyaza Macrobicyclic Cryptands: Synthesis, Crystal Structures of a Cyclophane Type Macrobicyclic Cryptand and of Its Dinuclear Copper(I) Cryptate, and Anion Binding Features. J. Chem. Soc. Chem. Commun. 1987, 1691–1694. [Google Scholar] [CrossRef]
- Duggan, M.; Ray, N.; Hathaway, B.; Tomlinson, G.; Brint, P.; Pelin, K. Crystal structure and electronic properties of ammine[tris(2-aminoethyl)amine]copper(II) diperchlorate and potassium penta-amminecopper(II) tris(hexafluorophosphate). J. Chem. Soc. Dalton Trans. 1980, 8, 1342–1348. [Google Scholar] [CrossRef]
- Mateus, P.; Delgado, R.; Brandão, P.; Félix, V. Recognition of Oxalate by a Copper(II) Polyaza Macrobicyclic Complex. Chem. A Eur. J. 2011, 17, 7020–7031. [Google Scholar] [CrossRef]
- Thaler, F.; Hubbard, C.D.; Heinemann, F.W.; van Eldik, R.; Schindler, S.; Fábián, I.; Dittler-Klingemann, A.M.; Hahn, F.E.; Orvig, C. Structural, Spectroscopic, Thermodynamic and Kinetic Properties of Copper(II) Complexes with Tripodal Tetraamines. Inorg. Chem. 1998, 37, 4022–4029. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Billing, D.E. The electronic properties and stereochemistry of mono-nuclear complexes of the copper(II) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Dudley, R.J.; Hathaway, B.J.; Hodgson, P.G.; Power, P.C.; Loose, D.J. Single-crystal electronic and electron spin resonance spectra of four trigonal bipyramidal copper( II ) complexes. J. Chem. Soc. Dalton Trans. 1974, 10, 1005–1009. [Google Scholar] [CrossRef]
- Alibrandi, G.; Amendola, V.; Bergamaschi, G.; Fabbrizzi, L.; Licchelli, M. Bistren cryptands and cryptates: Versatile receptors for anion inclusion and recognition in water. Org. Biomol. Chem. 2015, 13, 3510–3524. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Buttafava, A.; Fabbrizzi, L.; Monzani, E. Recognition and Sensing of Nucleoside Monophosphates by a Dicopper(II) Cryptate. J. Am. Chem. Soc. 2010, 132, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Bergamaschi, G.; Fabbrizzi, L.; Licchelli, M.; Mangano, C. The interaction of Mozobil TM with carboxylates. Org. Biomol. Chem. 2016, 14, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Mateus, P.; Delgado, R.; André, V.; Duarte, M.T. Dicarboxylate Recognition Properties of a Dinuclear Copper(II) Cryptate. Inorg. Chem. 2015, 54, 229–240. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Boiocchi, M.; Bonizzoni, M.; Ciarrocchi, C.; Fabbrizzi, L.; Invernici, M.; Licchelli, M. Anion Recognition in Water, Including Sulfate, by a Bicyclam Bimetallic Receptor: A Process Governed by the Enthalpy/Entropy Compensatory Relationship. Chem. A Eur. J. 2018, 24, 5659–5666. [Google Scholar] [CrossRef]
- Marcotte, N.; Taglietti, A. Transition-metal-based Chemosensing Ensembles: ATP Sensing in Physiological Conditions. Supramol. Chem. 2003, 15, 617–625. [Google Scholar] [CrossRef]
- McCleskey, S.C.; Metzger, A.; Simmons, C.S.; Anslyn, E.V. Competitive indicator methods for the analysis of citrate using colorimetric assays. Tetrahedron 2002, 58, 621–628. [Google Scholar] [CrossRef]
- Merli, D.; La Cognata, S.; Balduzzi, F.; Miljkovic, A.; Toma, L.; Amendola, V. A smart supramolecular device for the detection of t,t-muconic acid in urine. New J. Chem. 2019, 42, 15460–15465. [Google Scholar] [CrossRef]
- Amendola, V.; Bergamaschi, G.; Boiocchi, M.; Fabbrizzi, L.; Poggi, A.; Zema, M. Halide ion inclusion into a dicopper(II) bistren cryptate containing ‘active’ 2,5-dimethylfuran spacers: The origin of the bright yellow colour. Inorg. Chim. Acta 2008, 361, 4038–4046. [Google Scholar] [CrossRef]
- Yang, L.-Z.; Li, Y.; Zhuang, X.-M.; Jiang, L.; Chen, J.-M.; Luck, R.L.; Lu, T.-B. Mechanistic Studies of C-C Bond Cleavage of Nitriles by Dinuclear Metal Cryptates. Chem. Eur. J. 2009, 15, 12399–12407. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, J.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Borch, R.F.; Bernstein, M.D.; Durst, H.D. Cyanohydridoborate anion as a selective reducing agent. J. Am. Chem. Soc. 1971, 93, 2897–2904. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |



| Anion | LogK11UV-Vis | λfin UV-Vis (nm) | LogK11ID | % I/Imax |
|---|---|---|---|---|
| dfc2− | 5.46(3) | 670, ~780 | 5.46(4) | 75% |
| sdbz2− | 5.08(1) | 686, ~780 | 5.01(1) | 70% |
| tph2− | 4.43(4) | 690(sh.), 800 | n.a. | 46% |
| bz− | n.a. | n.a. | n.a. | 36% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miljkovic, A.; La Cognata, S.; Bergamaschi, G.; Freccero, M.; Poggi, A.; Amendola, V. Towards Building Blocks for Supramolecular Architectures Based on Azacryptates. Molecules 2020, 25, 1733. https://doi.org/10.3390/molecules25071733
Miljkovic A, La Cognata S, Bergamaschi G, Freccero M, Poggi A, Amendola V. Towards Building Blocks for Supramolecular Architectures Based on Azacryptates. Molecules. 2020; 25(7):1733. https://doi.org/10.3390/molecules25071733
Chicago/Turabian StyleMiljkovic, Ana, Sonia La Cognata, Greta Bergamaschi, Mauro Freccero, Antonio Poggi, and Valeria Amendola. 2020. "Towards Building Blocks for Supramolecular Architectures Based on Azacryptates" Molecules 25, no. 7: 1733. https://doi.org/10.3390/molecules25071733
APA StyleMiljkovic, A., La Cognata, S., Bergamaschi, G., Freccero, M., Poggi, A., & Amendola, V. (2020). Towards Building Blocks for Supramolecular Architectures Based on Azacryptates. Molecules, 25(7), 1733. https://doi.org/10.3390/molecules25071733







