Analysis of Total Thiols in the Urine of a Cystathionine β-Synthase-Deficient Mouse Model of Homocystinuria Using Hydrophilic Interaction Chromatography
Abstract
1. Introduction
2. Results and Discussion
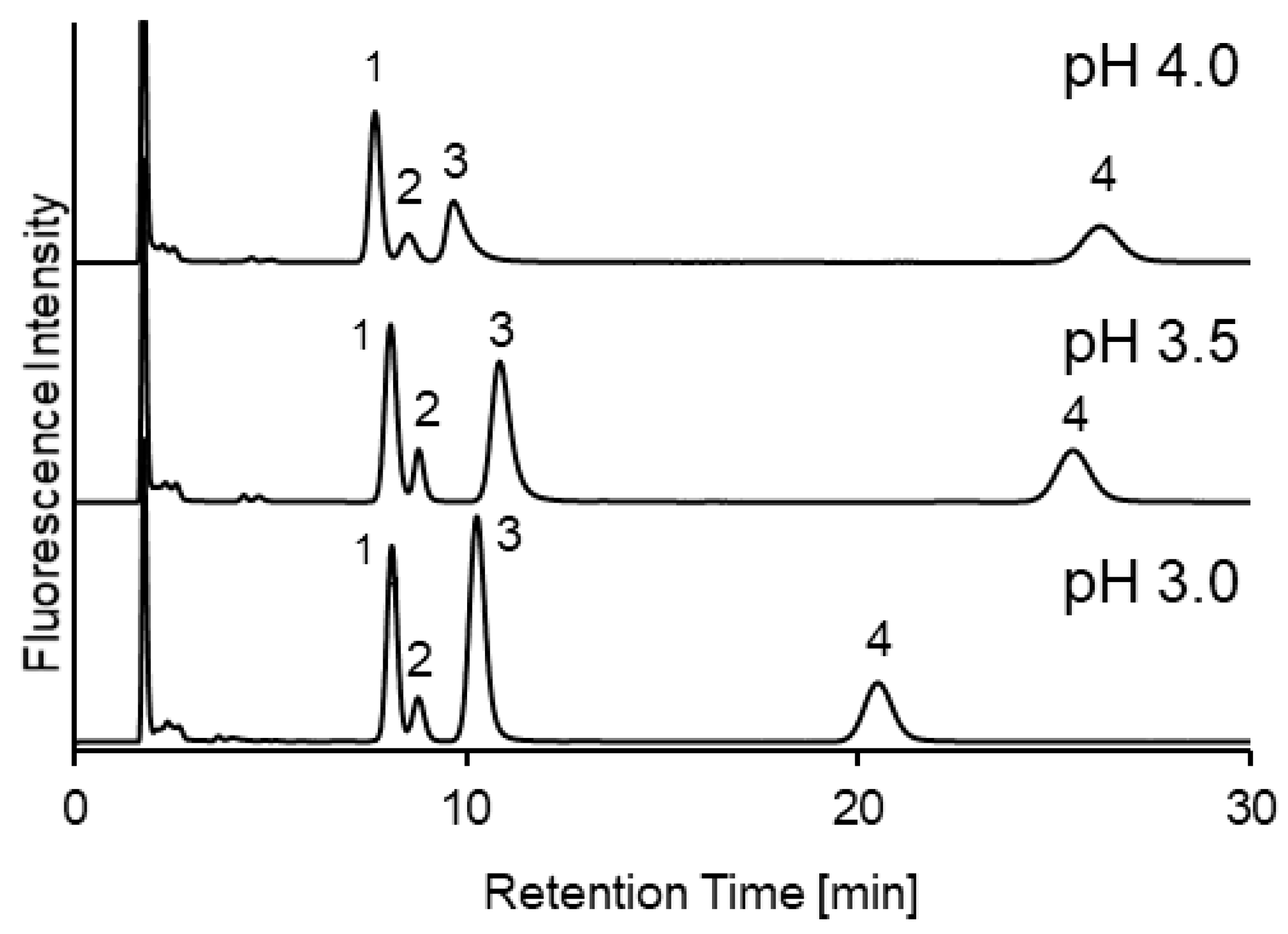
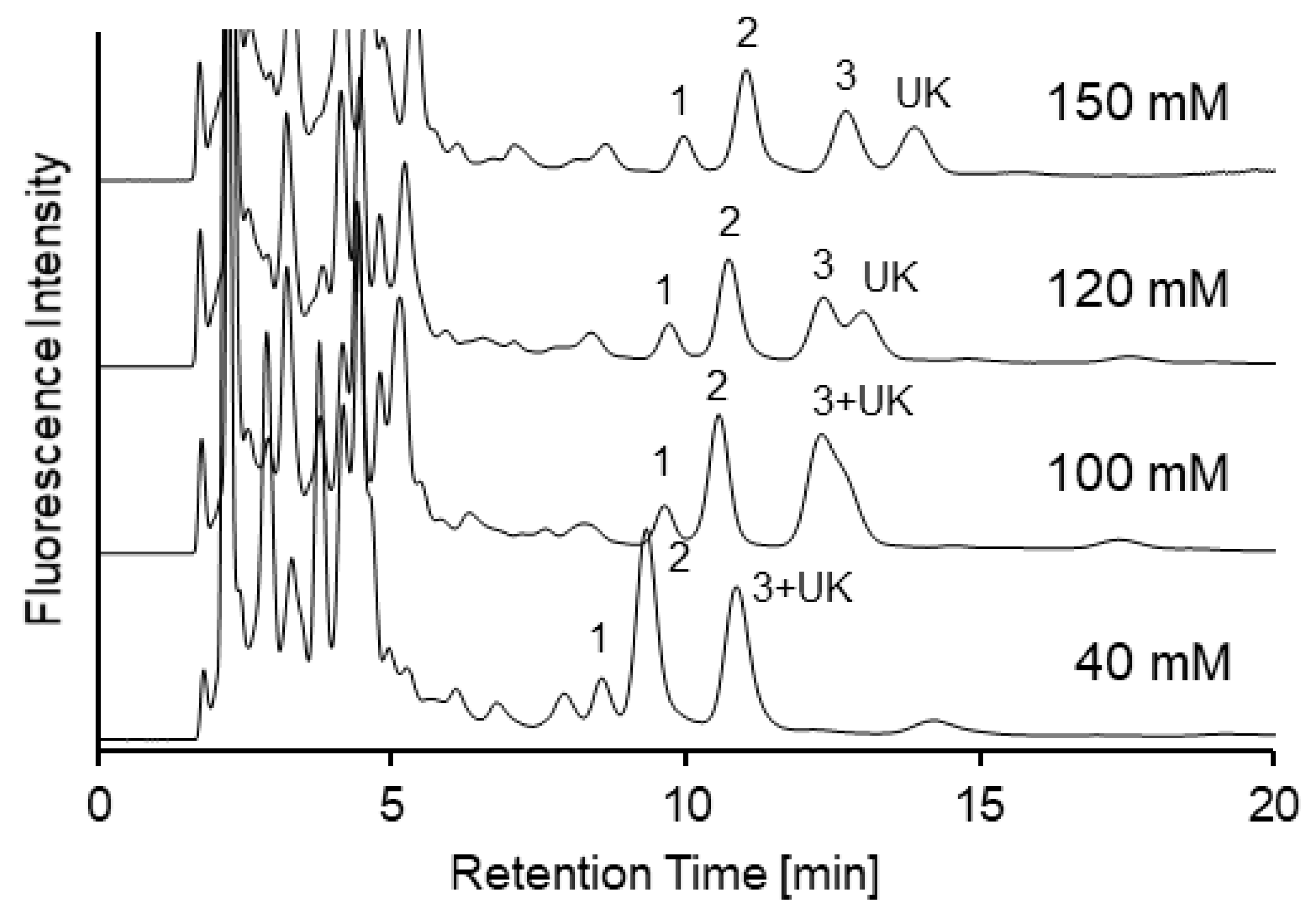
2.1. Optimization of Separation Conditions Using an Amide-Type Column for SBD-Thiols in Urine
2.2. Method Validation
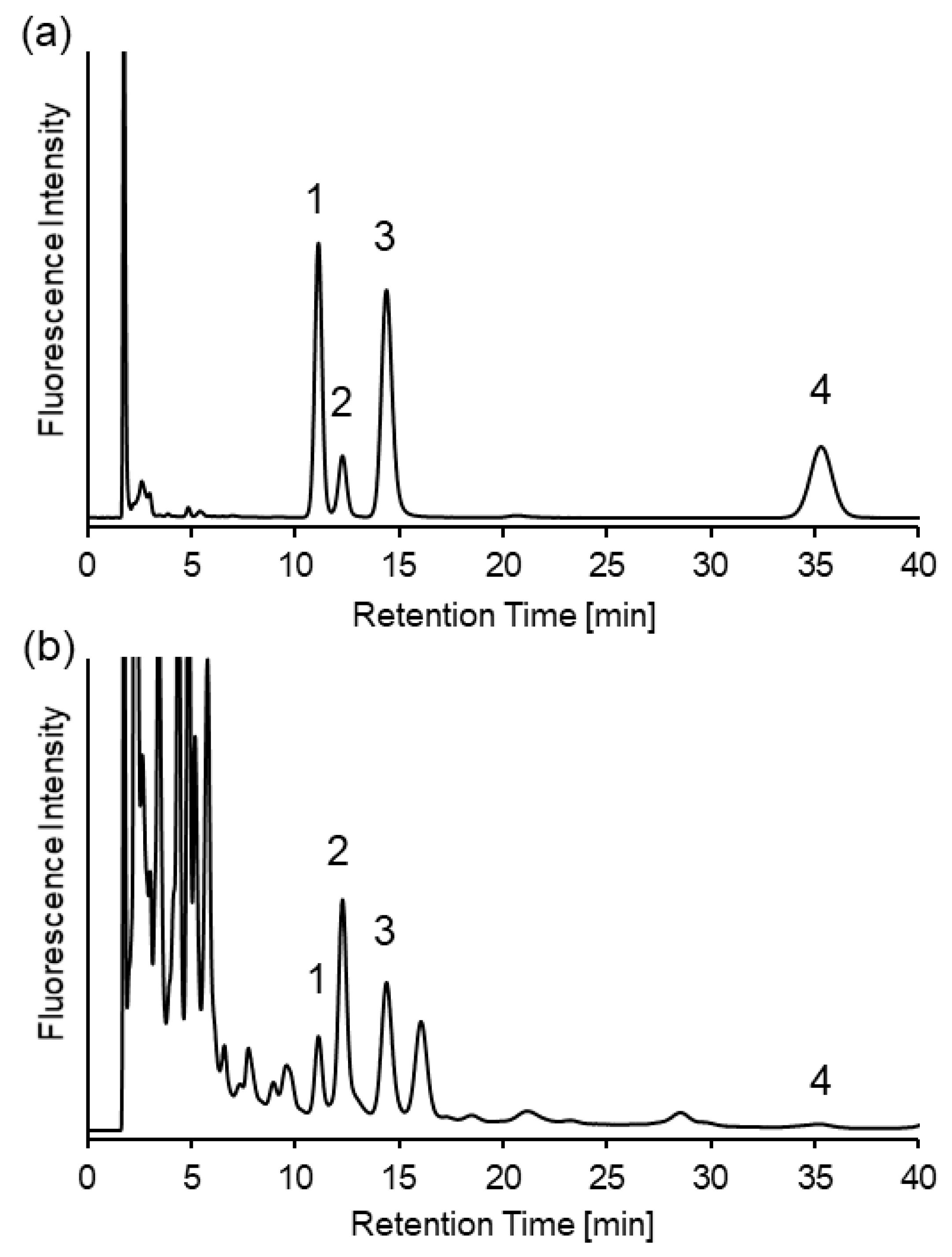
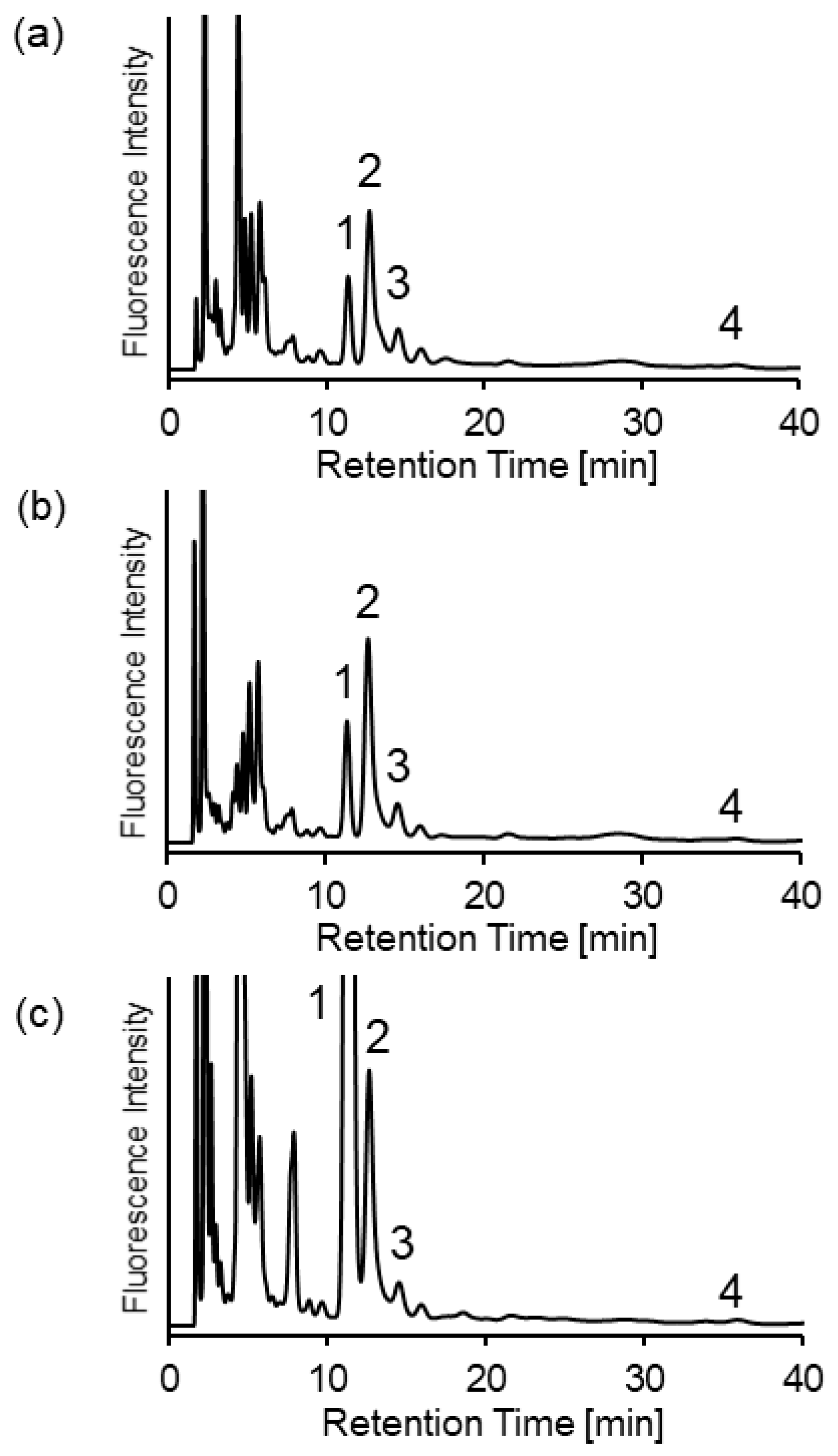
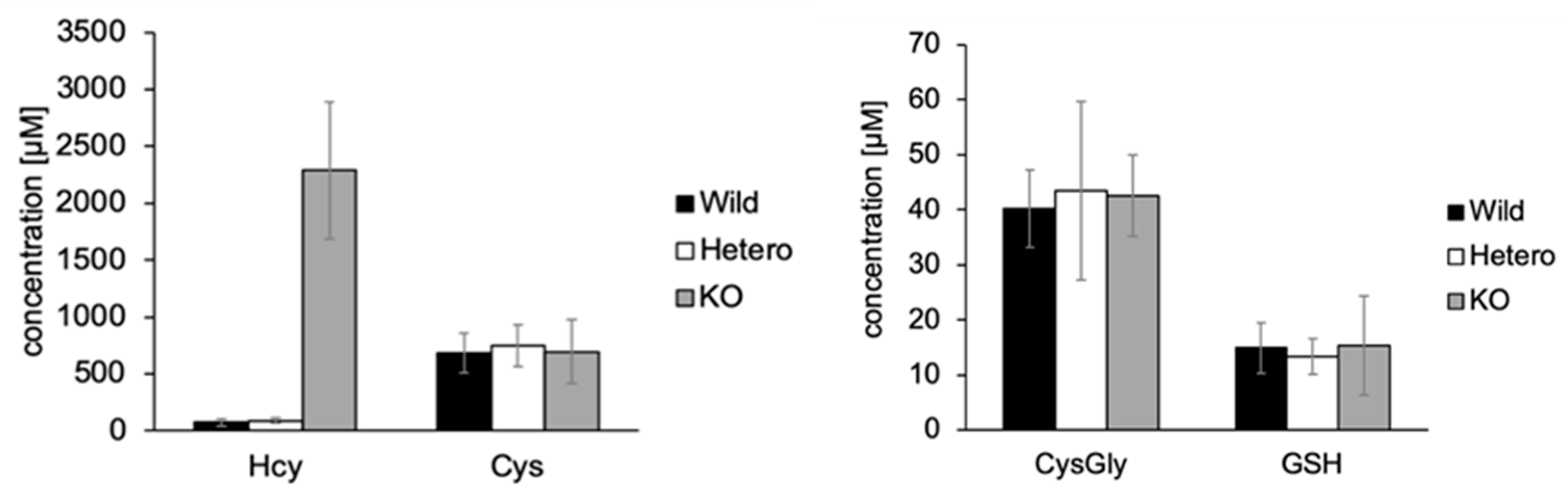
2.3. Analysis of Biothiols in Urine Samples of Mice with Cystathionine β-Synthase Deficiency
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Biological Samples
3.3. Sample Pretreatment
3.4. HPLC Conditions
3.5. Analytical Validation
Author Contributions
Funding
Conflicts of Interest
References
- Morris, A.A.; Kožich, V.; Santra, S.; Andria, G.; Ben-Omran, T.I.; Chakrapani, A.B.; Crushell, E.; Henderson, M.J.; Hochuli, M.; Huemer, M.; et al. Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J. Inherit. Metab. Dis. 2017, 40, 49–74. [Google Scholar] [PubMed]
- Sacharow, S.J.; Picker, J.D.; Levy, H.L. Homocystinuria Caused by Cystathionine Beta-Synthase Deficiency; GeneReviews: Seattle, WA, USA, 2004.
- Mortimer, P.P.; Parry, J.V. Non-invasive virological diagnosis: Are saliva and urine specimens adequate substitutes for blood? Rev. Med. Virol. 1991, 1, 73–78. [Google Scholar] [CrossRef]
- Kuśmierek, K.; Chwatko, G.; Głowacki, R.; Bald, E. Determination of endogenous thiols and thiol drugs in urine by HPLC with ultraviolet detection. J. Chromatogr. B. 2009, 877, 3300–3308. [Google Scholar] [CrossRef] [PubMed]
- Isokawa, M.; Kanamori, T.; Funatsu, T.; Tsunoda, M. Analytical methods involving separation techniques for determination of low-molecular-weight biothiols in human plasma and blood. J. Chromatogr. B. 2014, 964, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Isokawa, M.; Shimosawa, T.; Funatsu, T.; Tsunoda, M. Determination and characterization of total thiols in mouse serum samples using hydrophilic interaction liquid chromatography with fluorescence detection and mass spectrometry. J. Chromatogr. 2016, 1019, 59–65. [Google Scholar] [CrossRef]
- Isokawa, M.; Funatsu, T.; Tsunoda, M. Fast and simultaneous analysis of biothiols by high-performance liquid chromatography with fluorescence detection under hydrophilic interaction chromatography conditions. Analyst 2013, 138, 3802–3808. [Google Scholar] [CrossRef]
- Isokawa, M.; Kanamori, T.; Funatsu, T.; Tsunoda, M. Recent advances in hydrophilic interaction chromatography for quantitative analysis of endogenous and pharmaceutical compounds in plasma samples. Bioanalysis 2014, 6, 2421–2439. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Hayashi, Y.; Matsunaga, H.; Okada, F.; Kinoshita, M.; Suzuki, S. Analysis of 2-Aminobenzoic Acid-Labeled Monosaccharides and Glycoprotein-Derived Oligosaccharides by Online Cleanup Liquid Chromatography in the Reversed-Phase and Hydrophilic Interaction Liquid Chromatography Modes. Chromatography 2019, 40, 65–70. [Google Scholar] [CrossRef]
- Isokawa, M.; Kobayashi, K.; Miyoshi, Y.; Mita, M.; Funatsu, T.; Hamase, K.; Tsunoda, M. Quantification of Biological Thiols in the Plasma of a Homocystinuria Model with Cystathionine β-Synthase Deficiency Utilizing Hydrophilic Interaction Liquid Chromatography and Fluorescence Detection. Chromatography 2016, 37, 147–151. [Google Scholar] [CrossRef]
- Greco, G.; Letzel, T. Main interactions and influences of the chromatographic parameters in HILIC separations. J. Chromatogr. Sci. 2013, 51, 684–693. [Google Scholar] [CrossRef]
- Frick, B.; Schröcksnadel, K.; Neurauter, G.; Wirleitner, B.; Artner-Dworzak, E.; Fuchs, D. Rapid measurement of total plasma homocysteine by HPLC. Clin. Chim. Acta 2003, 331, 19–23. [Google Scholar] [CrossRef]
- Ichinose, S.; Nakamura, M.; Maeda, M.; Ikeda, R.; Wada, M.; Nakazato, M.; Ohba, Y.; Takamura, N.; Maeda, T.; Aoyagi, K.; et al. A validated HPLC-fluorescence method with a semi-micro column for routine determination of homocysteine, cysteine and cysteamine, and the relation between the thiol derivatives in normal human plasma. Biomed. Chromatogr. 2009, 23, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Ferin, R.; Pavão, M.L.; Baptista, J. Methodology for a rapid and simultaneous determination of total cysteine, homocysteine, cysteinylglycine and glutathione in plasma by isocratic RP-HPLC. J. Chromatogr. 2012, 911, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Isokawa, M.; Funatsu, T.; Tsunoda, M. Efficient Separation and Sensitive Detection of Biothiols by Hydrophilic Interaction Liquid Chromatography with Fluorescence Detection after Derivatization with 4-Aminosulfonyl-7-fluoro-2,1,3-benzoxadiazole. Chromatography 2014, 35, 169–172. [Google Scholar] [CrossRef]
- Jakubowski, H. Quantification of urinary S- and N-homocysteinylated protein and homocysteine-thiolactone in mice. Anal. Biochem. 2016, 508, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Kožich, V.; Ditrói, T.; Sokolová, J.; Křížková, M.; Krijt, J.; Ješina, P.; Nagy, P. Metabolism of sulfur compounds in homocystinurias. Br. J. Pharmacol. 2019, 176, 594–606. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Isokawa, M.; Funatsu, T.; Tsunoda, M. Optimization of tris(2-carboxyethyl) phosphine reduction conditions for fast analysis of total biothiols in mouse serum samples. Heliyon 2019, 5. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Thiol compounds and other chemicals are available from the authors. |

| SBD-Thiols | LOD [nM] | LOQ [nM] | Linearity [nM] |
|---|---|---|---|
| (S/N = 3) | (S/N = 10) | R2 > 0.999 | |
| Hcy | 3.5 | 12 | 20–2000 |
| Cys | 20 | 67 | 120–12000 |
| CysGly | 3.9 | 13 | 30–3000 |
| GSH | 16 | 53 | 60–6000 |
| Thiols | Added [µM] | Measured (Mean ± SD) [µM] | RSD [%] | Recovery [%] |
|---|---|---|---|---|
| Hcy | 0 | 13.3 ± 0.2 | 1.4 | - |
| 10 | 23.7 ± 0.5 | 2.1 | 104 | |
| 20 | 34.7 ± 1.3 | 3.8 | 107 | |
| 40 | 54.0 ± 1.3 | 2.4 | 102 | |
| Cys | 0 | 202 ± 6 | 3.1 | - |
| 125 | 323 ± 5 | 1.7 | 97 | |
| 250 | 426 ± 15 | 3.3 | 104 | |
| 500 | 684 ± 19 | 2.8 | 96 | |
| CysGly | 0 | 50.9 ± 0.5 | 1.1 | - |
| 30 | 80.3 ± 2.1 | 2.6 | 98 | |
| 60 | 116 ± 4 | 3.1 | 108 | |
| 120 | 163 ± 3 | 1.8 | 93 | |
| GSH | 0 | 7.76 ± 0.17 | 2.2 | - |
| 6 | 13.5 ± 0.4 | 3.0 | 96 | |
| 12 | 22.0 ± 0.6 | 2.6 | 119 | |
| 24 | 34.2 ± 0.8 | 2.4 | 110 |
| Thiols | Added [µM] | Measured (Mean ± SD) [µM] | RSD [%] | Recovery [%] |
|---|---|---|---|---|
| Hcy | 0 | 30.8 ± 1.5 | 4.9 | - |
| 10 | 40.5 ± 1.2 | 2.9 | 97 | |
| 20 | 50.4 ± 2.5 | 5.0 | 98 | |
| 40 | 72.1 ± 2.5 | 3.4 | 103 | |
| Cys | 0 | 368 ± 12 | 3.2 | - |
| 125 | 483 ± 16 | 3.3 | 91 | |
| 250 | 597 ± 33 | 5.5 | 91 | |
| 500 | 853 ± 35 | 4.1 | 97 | |
| CysGly | 0 | 116 ± 7 | 5.7 | - |
| 30 | 143 ± 7 | 4.6 | 92 | |
| 60 | 171 ± 13 | 7.3 | 91 | |
| 120 | 233 ± 14 | 6.0 | 97 | |
| GSH | 0 | 12.2 ± 0.5 | 3.8 | - |
| 6 | 17.4 ± 0.4 | 2.1 | 87 | |
| 12 | 24.2 ± 1.2 | 4.9 | 100 | |
| 24 | 38.6 ± 1.1 | 2.7 | 110 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-F.; Hamase, K.; Tsunoda, M. Analysis of Total Thiols in the Urine of a Cystathionine β-Synthase-Deficient Mouse Model of Homocystinuria Using Hydrophilic Interaction Chromatography. Molecules 2020, 25, 1735. https://doi.org/10.3390/molecules25071735
Chang C-F, Hamase K, Tsunoda M. Analysis of Total Thiols in the Urine of a Cystathionine β-Synthase-Deficient Mouse Model of Homocystinuria Using Hydrophilic Interaction Chromatography. Molecules. 2020; 25(7):1735. https://doi.org/10.3390/molecules25071735
Chicago/Turabian StyleChang, Chun-Fang, Kenji Hamase, and Makoto Tsunoda. 2020. "Analysis of Total Thiols in the Urine of a Cystathionine β-Synthase-Deficient Mouse Model of Homocystinuria Using Hydrophilic Interaction Chromatography" Molecules 25, no. 7: 1735. https://doi.org/10.3390/molecules25071735
APA StyleChang, C.-F., Hamase, K., & Tsunoda, M. (2020). Analysis of Total Thiols in the Urine of a Cystathionine β-Synthase-Deficient Mouse Model of Homocystinuria Using Hydrophilic Interaction Chromatography. Molecules, 25(7), 1735. https://doi.org/10.3390/molecules25071735






