Interactions among Odorants, Phenolic Compounds, and Oral Components and Their Effects on Wine Aroma Volatility
Abstract
1. Introduction
2. Results
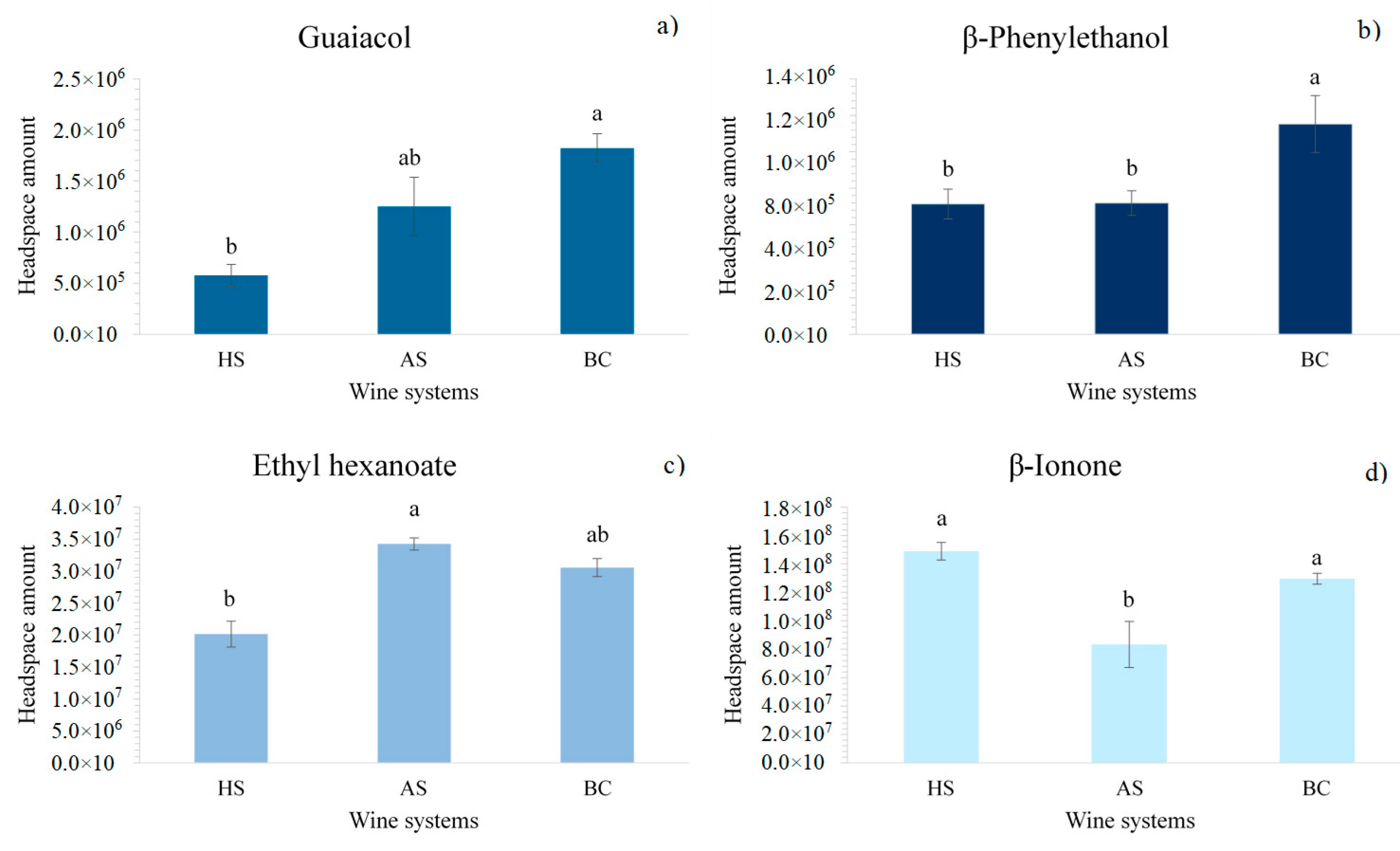
2.1. Effect of Oral Components on Wine Aroma Volatility
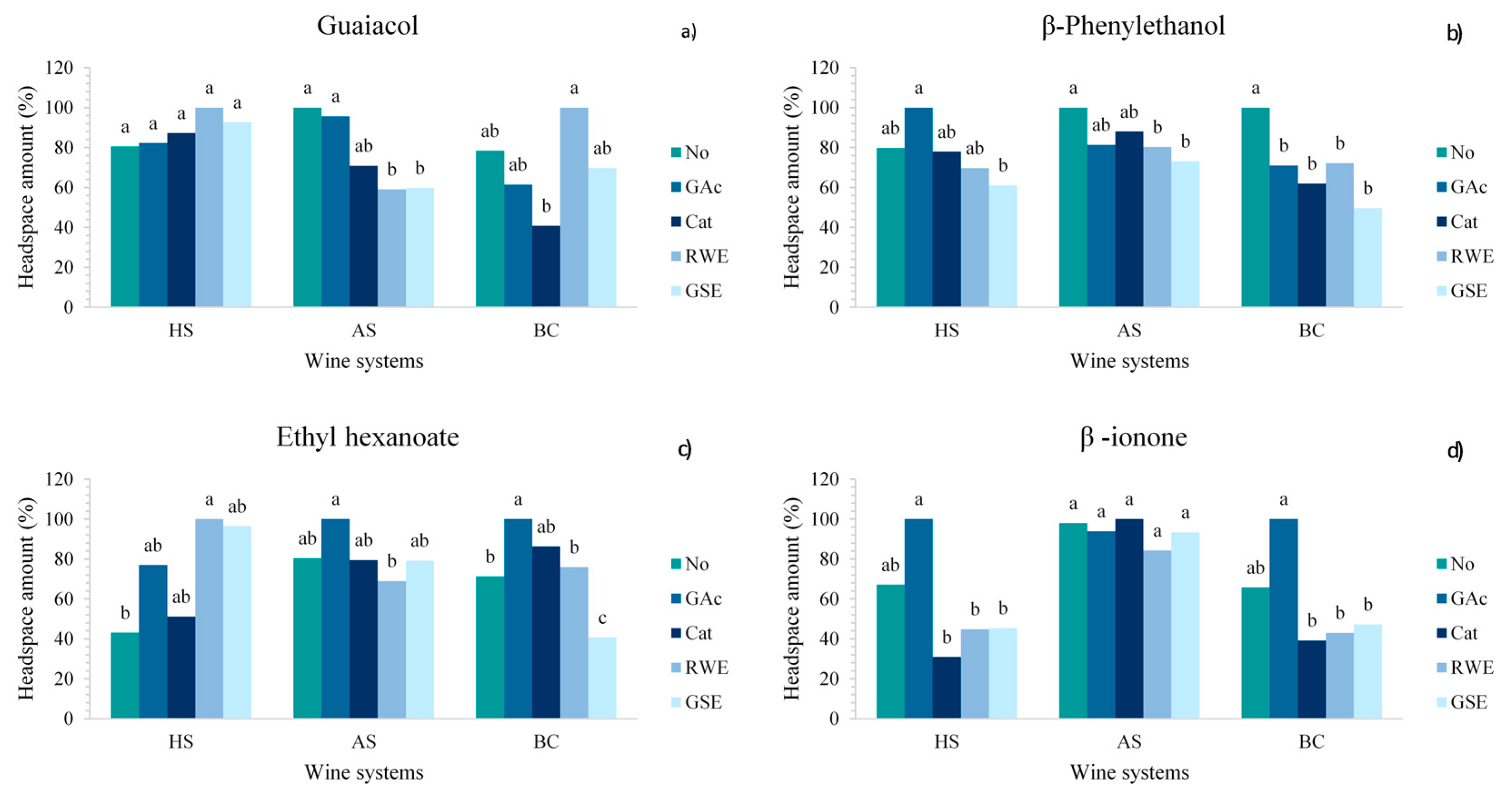
2.2. Effect of Phenolic Compounds on Aroma Volatility in Wines with Different Oral Components
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Synthetic Wines
3.3. Aroma Compounds
3.4. Oral Components (Human Saliva and Buccal Cells)
3.5. Artificial Saliva
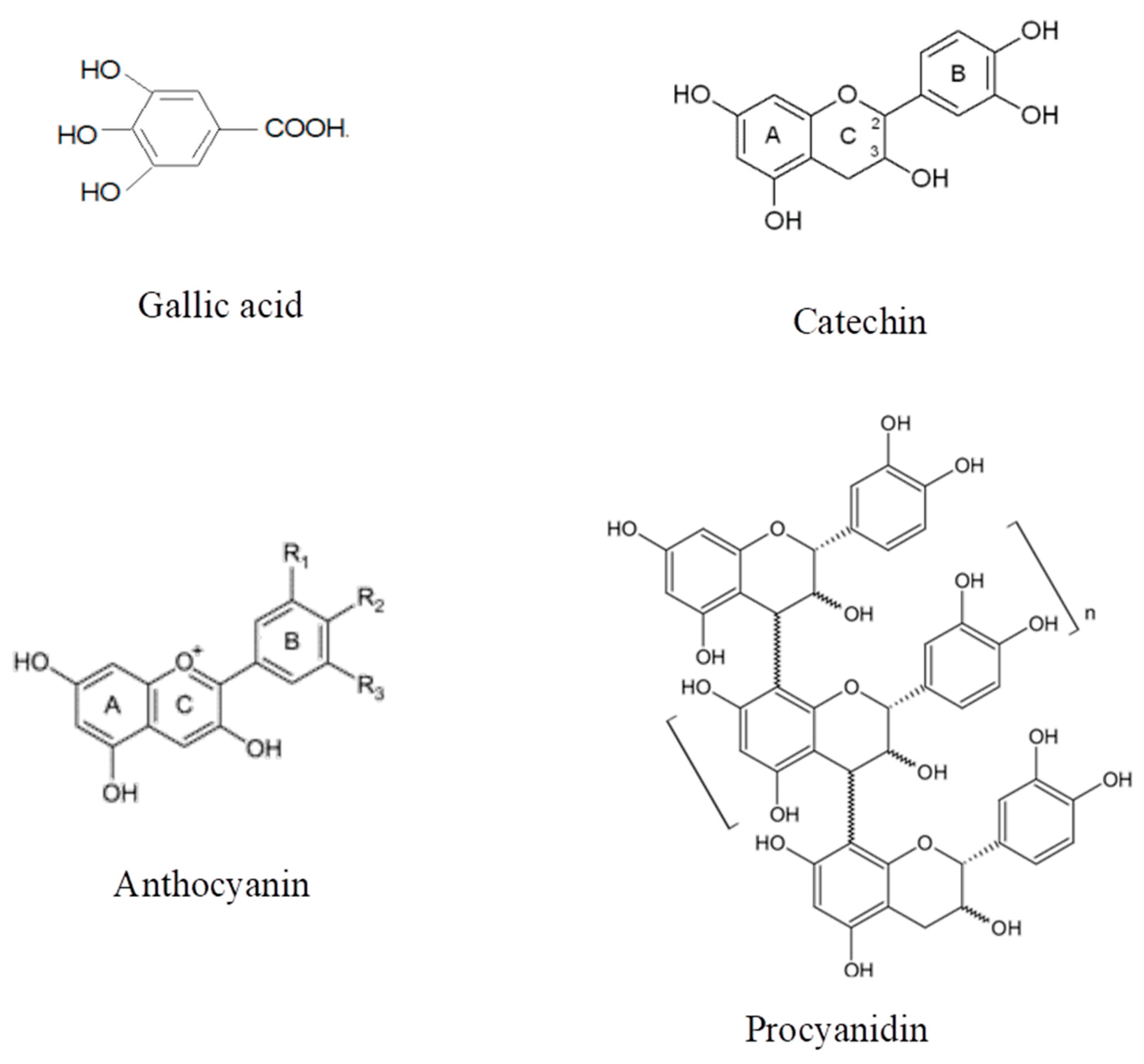
3.6. Phenolic Compounds
3.7. Headspace Solid Phase Microextraction (HS-SPME) Procedure
3.8. GC/MS Analyses
3.9. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Landy, P.; Druaux, C.; Voilley, A. Retention of aroma compounds by proteins in aqueous solution. Food Chem. 1995, 54, 387–392. [Google Scholar] [CrossRef]
- Pozo-Bayon, M.A.; Reineccius, G. Interactions Between Wine Matrix Macro-Components and Aroma Compounds. In Wine Chemistry and Biochemistry; Springer Science and Business Media LLC: Berlin, Germany, 2008; pp. 417–435. [Google Scholar]
- Dufour, C.; Bayonove, C.L. Influence of wine structurally different polysaccharides on the volatility of aroma substances in a model system. J. Agric. Food Chem. 1999, 47, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Bayonove, C.L. Interactions between wine polyphenols and aroma substances. An insight at the molecular level. J. Agric. Food Chem. 1999, 47, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Dufour, C.; Sauvaitre, I. Interactions between anthocyanins and aroma substances in a model system. Effect on the flavor of grape-derived beverages. J. Agric. Food Chem. 2000, 48, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.-M.; De Ropp, J.S.; Ebeler, S.E. Study of interactions between food phenolics and aromatic flavors using one- and two-dimensional (1)H NMR spectroscopy. J. Agric. Food Chem. 2000, 48, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, B.; Tempere, S.; Iturmendi, N.; Moine, V.; De Revel, G.; Teissedre, P.-L. Influence of phenolic compounds on the sensorial perception and volatility of red wine esters in model solution: An insight at the molecular level. Food Chem. 2013, 140, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; E Ebeler, S.; Heymann, H.; Boss, P.K.; Solomon, P.; Trengove, R. Interactions between Wine Volatile Compounds and Grape and Wine Matrix Components Influence Aroma Compound Headspace Partitioning. J. Agric. Food Chem. 2009, 57, 10313–10322. [Google Scholar] [CrossRef]
- Villamor, R.R.; Evans, M.A.; Mattinson, D.S.; Ross, C.F. Effects of ethanol, tannin and fructose on the headspace concentration and potential sensory significance of odorants in a model wine. Food Res. Int. 2013, 50, 38–45. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; González, C.M.; Andújar-Ortiz, I.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayon, M.A. Assessment of the effect of the non?volatile wine matrix on the volatility of typical wine aroma compounds by headspace solid phase microextraction/gas chromatography analysis. J. Sci. Food Agric. 2011, 91, 2484–2494. [Google Scholar] [CrossRef]
- Saenz-Navajas, M.-P.; Campo, E.; Cullereé, L.; Zurbano, P.F.; Valentin, D.; Ferreira, V. Effects of the Nonvolatile Matrix on the Aroma Perception of Wine. J. Agric. Food Chem. 2010, 58, 5574–5585. [Google Scholar] [CrossRef]
- Pineau, B.; Barbe, J.-C.; Van Leeuwen, K.; Dubourdieu, D. Which Impact for β-Damascenone on Red Wines Aroma? J. Agric. Food Chem. 2007, 55, 4103–4108. [Google Scholar] [CrossRef] [PubMed]
- Pozo-Bayon, M.A.; González, C.M.; Esteban-Fernández, A. Wine Preference and Wine Aroma Perception. In Wine Safety, Consumer Preference, and Human Health; Springer Science and Business Media LLC: Berlin, Germany, 2016; pp. 139–162. [Google Scholar]
- Esteban-Fernández, A.; Rocha-Alcubilla, N.; González, C.M.; Moreno-Arribas, M.V.; Pozo-Bayon, M.A. Intra-oral adsorption and release of aroma compounds following in-mouth wine exposure. Food Chem. 2016, 205, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Perez-Jiménez, M.; Chaya, C.; Pozo-Bayon, M.A. Individual differences and effect of phenolic compounds in the immediate and prolonged in-mouth aroma release and retronasal aroma intensity during wine tasting. Food Chem. 2019, 285, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-González, C.; Canon, F.; Feron, G.; Guichard, E.; Pozo-Bayon, M.A. Assessment Wine Aroma Persistence by Using an in Vivo PTR-ToF-MS Approach and Its Relationship with Salivary Parameters. Molecules 2019, 24, 1277. [Google Scholar] [CrossRef]
- Genovese, A.; Piombino, P.; Gambuti, A.; Moio, L. Simulation of retronasal aroma of white and red wine in a model mouth system. Investigating the influence of saliva on volatile compound concentrations. Food Chem. 2009, 114, 100–107. [Google Scholar] [CrossRef]
- Mitropoulou, A.; Hatzidimitriou, E.; Paraskevopoulou, A. Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides and artificial saliva. Food Res. Int. 2011, 44, 1561–1570. [Google Scholar] [CrossRef]
- González, C.M.; Feron, G.; Guichard, E.; Rodríguez-Bencomo, J.J.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Pozo-Bayón, M. Ángeles Understanding the Role of Saliva in Aroma Release from Wine by Using Static and Dynamic Headspace Conditions. J. Agric. Food Chem. 2014, 62, 8274–8288. [Google Scholar] [CrossRef]
- Friel, E.N.; Taylor, A.J. Effect of salivary components on volatile partitioning from solutions. J. Agric. Food Chem. 2001, 49, 3898–3905. [Google Scholar] [CrossRef]
- Pagès-Hélary, S.; Andriot, I.; Guichard, E.; Canon, F. Retention effect of human saliva on aroma release and respective contribution of salivary mucin and α-amylase. Food Res. Int. 2014, 64, 424–431. [Google Scholar] [CrossRef]
- Ployon, S.; Morzel, M.; Canon, F. The role of saliva in aroma release and perception. Food Chem. 2017, 226, 212–220. [Google Scholar] [CrossRef]
- Buettner, A. Influence of Human Salivary Enzymes on Odorant Concentration Changes Occurring in Vivo. 1. Esters and Thiols. J. Agric. Food Chem. 2002, 50, 3283–3289. [Google Scholar] [CrossRef] [PubMed]
- Buettner, A. Influence of Human Saliva on Odorant Concentrations. 2. Aldehydes, Alcohols, 3-Alkyl-2-methoxypyrazines, Methoxyphenols, and 3-Hydroxy-4,5-dimethyl-2(5H)-furanone. J. Agric. Food Chem. 2002, 50, 7105–7110. [Google Scholar] [CrossRef] [PubMed]
- Ijichi, C.; Wakabayashi, H.; Sugiyama, S.; Ihara, Y.; Nogi, Y.; Nagashima, A.; Ihara, S.; Niimura, Y.; Shimizu, Y.; Kondo, K.; et al. Metabolism of Odorant Molecules in Human Nasal/Oral Cavity Affects the Odorant Perception. Chem. Senses 2019, 44, 465–481. [Google Scholar] [CrossRef] [PubMed]
- González, C.M.; Brulé, M.; Feron, G.; Canon, F. Does interindividual variability of saliva affect the release and metabolization of aroma compounds ex vivo? The particular case of elderly suffering or not from hyposalivation. J. Texture Stud. 2018, 50, 36–44. [Google Scholar] [CrossRef] [PubMed]
- González, C.M.; Feron, G.; Brulé, M.; Canon, F. Understanding the release and metabolism of aroma compounds using micro-volume saliva samples by ex vivo approaches. Food Chem. 2017, 240, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, M.; Rocha-Alcubilla, N.; Pozo-Bayon, M.A. Effect of saliva esterase activity on ester solutions and possible consequences for thein-mouthester release during wine intake. J. Texture Stud. 2018, 50, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Canon, F.; Neiers, F.; Guichard, E. Saliva and Flavor Perception: Perspectives. J. Agric. Food Chem. 2018, 66, 7873–7879. [Google Scholar] [CrossRef]
- Isaac, G.; Koren, E.; Shalish, M.; Kanner, J.; Kohen, R. Saliva increases the availability of lipophilic polyphenols as antioxidants and enhances their retention in the oral cavity. Arch. Oral Boil. 2012, 57, 1327–1334. [Google Scholar]
- Payne, C.; Bowyer, P.K.; Herderich, M.J.; Bastian, S.E. Interaction of astringent grape seed procyanidins with oral epithelial cells. Food Chem. 2009, 115, 551–557. [Google Scholar] [CrossRef]
- Jung, D.-M.; Ebeler, S.E. Headspace Solid-Phase Microextraction Method for the Study of the Volatility of Selected Flavor Compounds. J. Agric. Food Chem. 2003, 51, 200–205. [Google Scholar] [CrossRef]
- Aybeke, E.N.; Ployon, S.; Brulé, M.; De Fonseca, B.; Bourillot, E.; Morzel, M.; Lesniewska, E.; Canon, F. Nanoscale Mapping of the Physical Surface Properties of Human Buccal Cells and Changes Induced by Saliva. Langmuir 2019, 35, 12647–12655. [Google Scholar] [CrossRef] [PubMed]
- Robert-Hazotte, A.; Schoumacker, R.; Semon, E.; Briand, L.; Guichard, E.; Le Quéré, J.-L.; Faure, P.; Heydel, J.-M. Ex vivo real-time monitoring of volatile metabolites resulting from nasal odorant metabolism. Sci. Rep. 2019, 9, 2492. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, N.; Da Silva, S.V.; Jakob, I.; Sicard, G.; Chevalier, J.; Ménétrier, F.; Berdeaux, O.; Artur, Y.; Heydel, J.-M.; Le Bon, A.-M. Odorant Metabolism Catalyzed by Olfactory Mucosal Enzymes Influences Peripheral Olfactory Responses in Rats. PLoS ONE 2013, 8, e59547. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, P.J.; McNair, H.M.; Zoecklein, B.W. Measurement of 3-alkyl-2-methoxypyrazine by headspace solid-phase microextraction in spiked model wines. Am. J. Enol. Viticult. 2002, 53, 285–288. [Google Scholar]
- Gibbins, H.; Proctor, G.B.; Yakubov, G.; Wilson, S.; Carpenter, G.; Yakubov, G. Concentration of salivary protective proteins within the bound oral mucosal pellicle. Oral Dis. 2013, 20, 707–713. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; González, C.M.; Jiménez-Girón, A.; Pérez-Jiménez, M.; Pozo-Bayon, M.A. Aroma release in the oral cavity after wine intake is influenced by wine matrix composition. Food Chem. 2017, 243, 125–133. [Google Scholar] [CrossRef]
- Cueva, C.; Sánchez-Patán, F.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martín-Álvarez, P.J.; Bartolomé, B.; Moreno-Arribas, M.V. In vitrofermentation of grape seed flavan-3-ol fractions by human faecal microbiota: Changes in microbial groups and phenolic metabolites. FEMS Microbiol. Ecol. 2012, 83, 792–805. [Google Scholar] [CrossRef]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson M, G.R.; Quintanilla-López, J.E.; Lebrón-Aguilar, R.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. In Vitro Fermentation of a Red Wine Extract by Human Gut Microbiota: Changes in Microbial Groups and Formation of Phenolic Metabolites. J. Agric. Food Chem. 2012, 60, 2136–2147. [Google Scholar]
- Hara, Y.; Honda, M. The Inhibition of ?-Amylase by Tea Polyphenols. Agric. Boil. Chem. 1990, 54, 1939–1945. [Google Scholar] [CrossRef]
- Kandra, L.; Gyémánt, G.; Zajácz, Á.; Batta, G. Inhibitory effects of tannin on human salivary α-amylase. Biochem. Biophys. Res. Commun. 2004, 319, 1265–1271. [Google Scholar] [CrossRef]
- Wang, D.; Zou, L.; Jin, Q.; Hou, J.; Ge, G.-B.; Yang, L. Human carboxylesterases: A comprehensive review. Acta Pharm. Sin. B 2018, 8, 699–712. [Google Scholar] [CrossRef]
- Weng, Z.-M.; Ge, G.-B.; Dou, T.-Y.; Wang, P.; Liu, P.-K.; Tian, X.-H.; Qiao, N.; Yü, Y.; Zou, L.-W.; Zhou, Q.; et al. Characterization and structure-activity relationship studies of flavonoids as inhibitors against human carboxylesterase 2. Bioorganic Chem. 2018, 77, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Criado, C.; Chaya, C.; Fernández-Ruíz, V.; Alvarez, M.D.; Herranz, B.; Pozo-Bayon, M.A. Effect of saliva composition and flow on inter-individual differences in the temporal perception of retronasal aroma during wine tasting. Food Res. Int. 2019, 126, 108677. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Smith, A.; Grist, N.; Barnett, P.; Smart, J.D. An in vitro mucosal model predictive of bioadhesive agents in the oral cavity. J. Control. Release 1999, 61, 175–183. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
| Compound | Chemical Structure | CAS Number | MW (a) (g mol−1) | BP (b) (°C) | Log P(c) | OT (d) (µg/L) | Descriptor (e) |
|---|---|---|---|---|---|---|---|
| Guaiacol |  | 90-05-1 | 124 | 211 | 1.34 | 9.5–10 | spice, clove |
| β-phenyl ethanol | 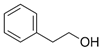 | 60-12-8 | 122 | 224 | 1.57 | 14,000–100,000 | honey, spice, rose |
| Ethyl hexanoate | 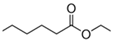 | 123-66-0 | 144 | 167 | 2.83 | 5–14 | apple, peel, fruit |
| β-ionone | 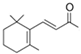 | 8013-90-9 | 192 | 262 | 4.42 | 0.09 | raspberry, violet, flower |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Jiménez, M.; Esteban-Fernández, A.; Muñoz-González, C.; Pozo-Bayón, M.A. Interactions among Odorants, Phenolic Compounds, and Oral Components and Their Effects on Wine Aroma Volatility. Molecules 2020, 25, 1701. https://doi.org/10.3390/molecules25071701
Perez-Jiménez M, Esteban-Fernández A, Muñoz-González C, Pozo-Bayón MA. Interactions among Odorants, Phenolic Compounds, and Oral Components and Their Effects on Wine Aroma Volatility. Molecules. 2020; 25(7):1701. https://doi.org/10.3390/molecules25071701
Chicago/Turabian StylePerez-Jiménez, María, Adelaida Esteban-Fernández, Carolina Muñoz-González, and María Angeles Pozo-Bayón. 2020. "Interactions among Odorants, Phenolic Compounds, and Oral Components and Their Effects on Wine Aroma Volatility" Molecules 25, no. 7: 1701. https://doi.org/10.3390/molecules25071701
APA StylePerez-Jiménez, M., Esteban-Fernández, A., Muñoz-González, C., & Pozo-Bayón, M. A. (2020). Interactions among Odorants, Phenolic Compounds, and Oral Components and Their Effects on Wine Aroma Volatility. Molecules, 25(7), 1701. https://doi.org/10.3390/molecules25071701








