Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1—Azides, Porphyrins and Corroles
Abstract
1. Introduction
2. Porphyrins
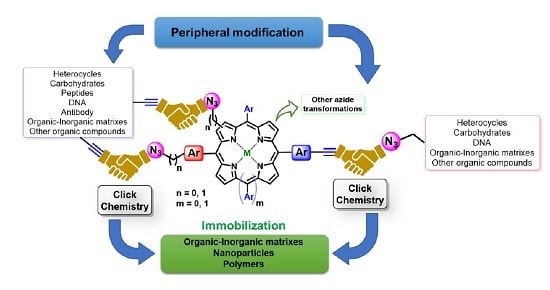
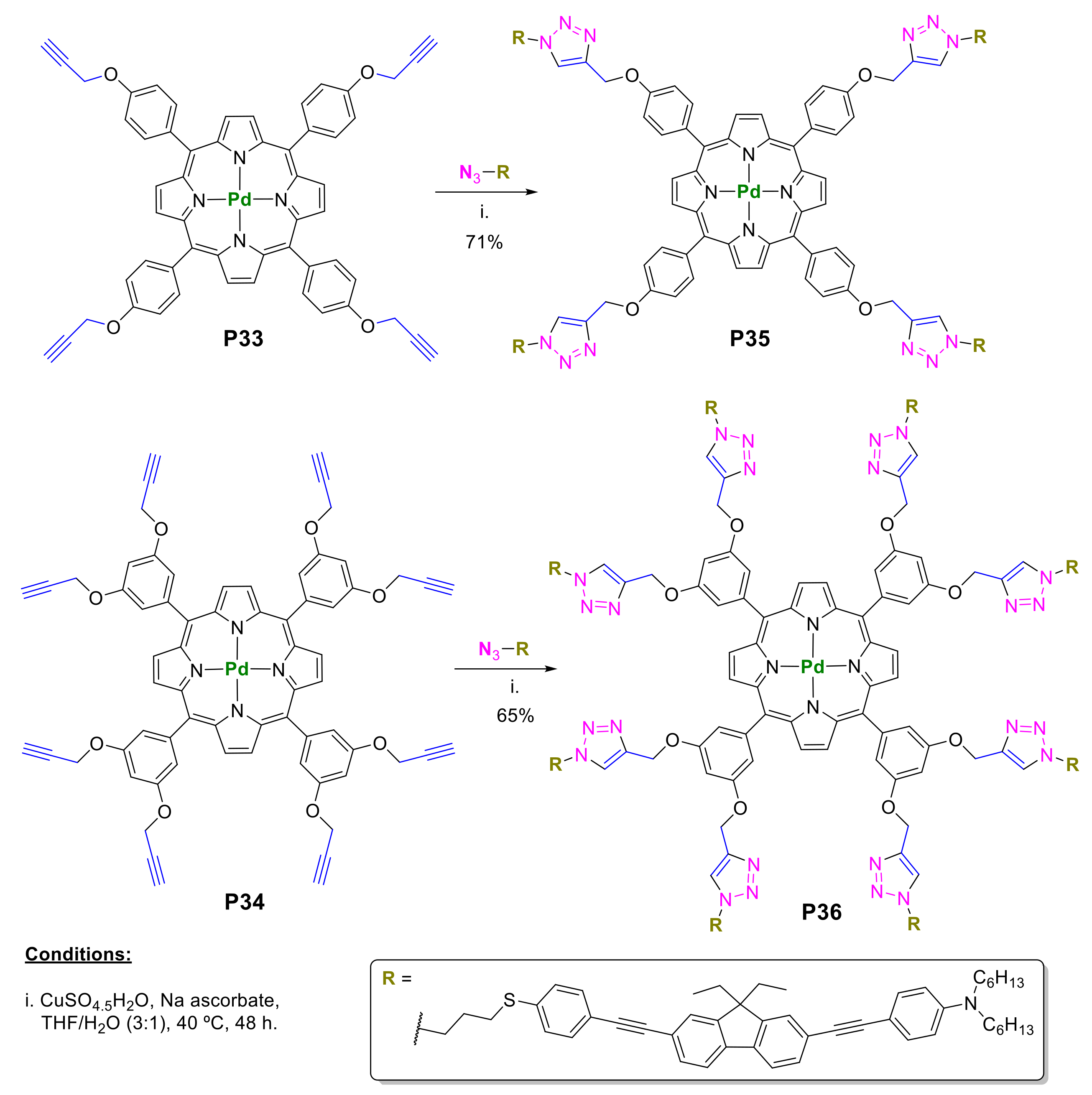
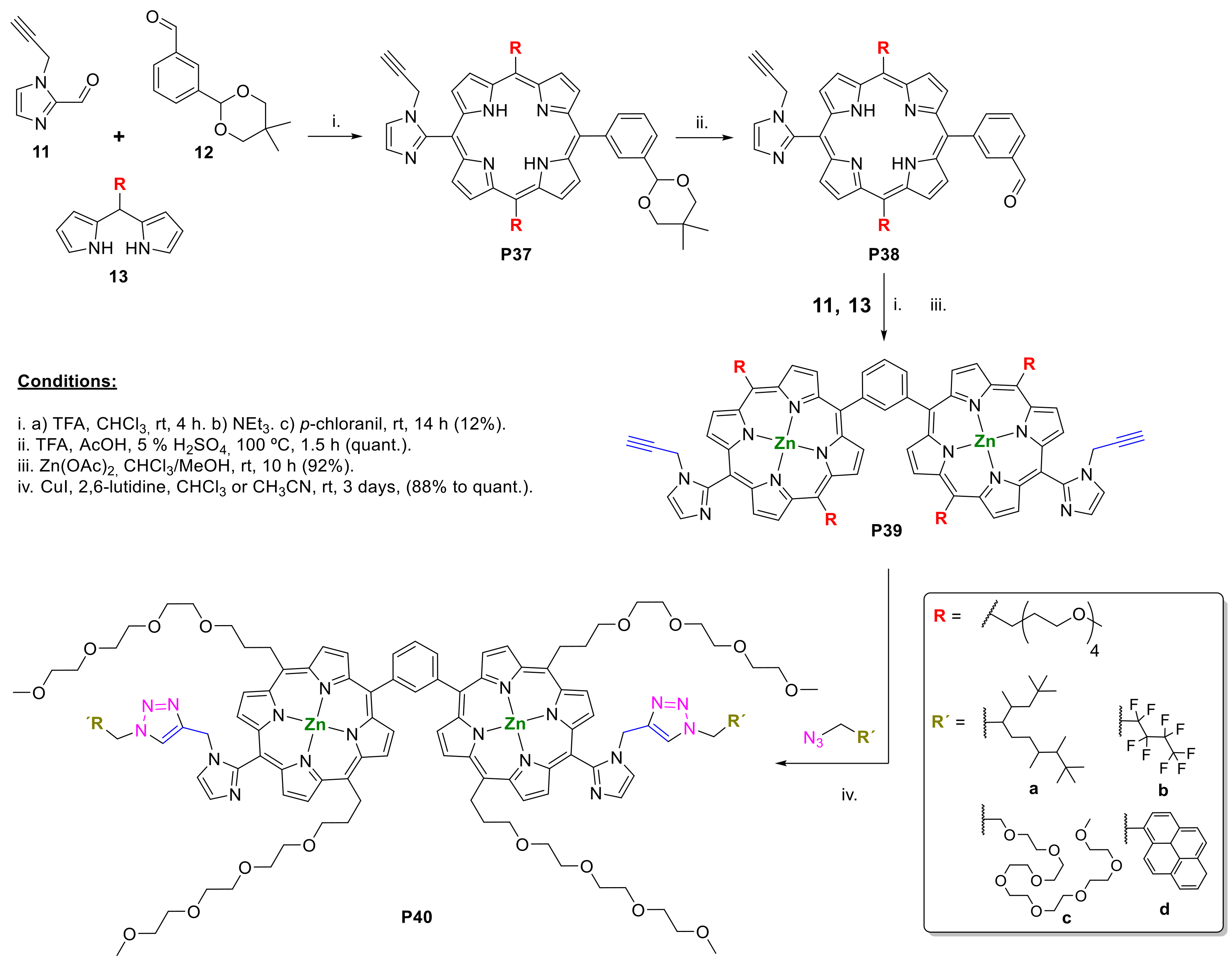
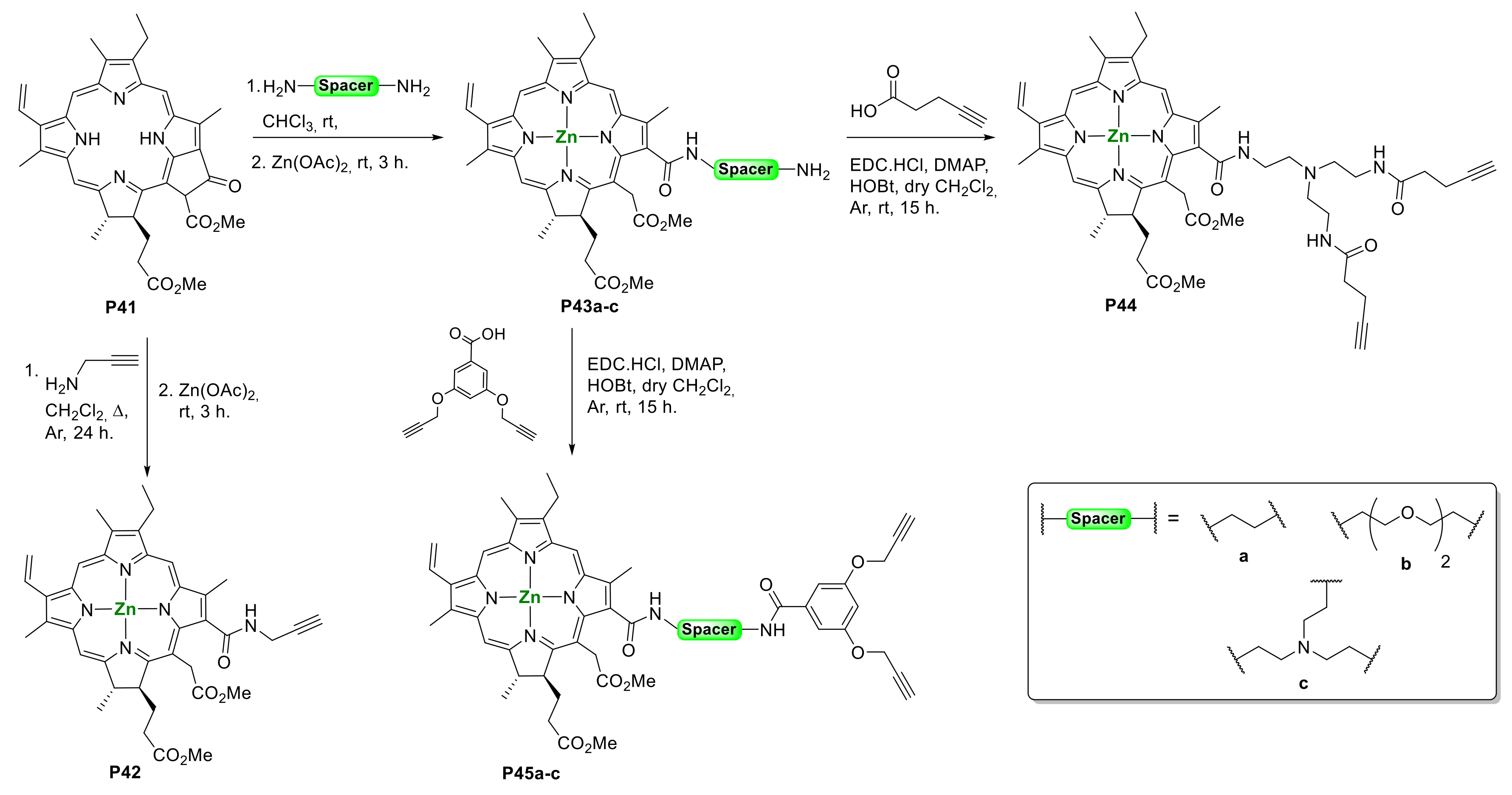
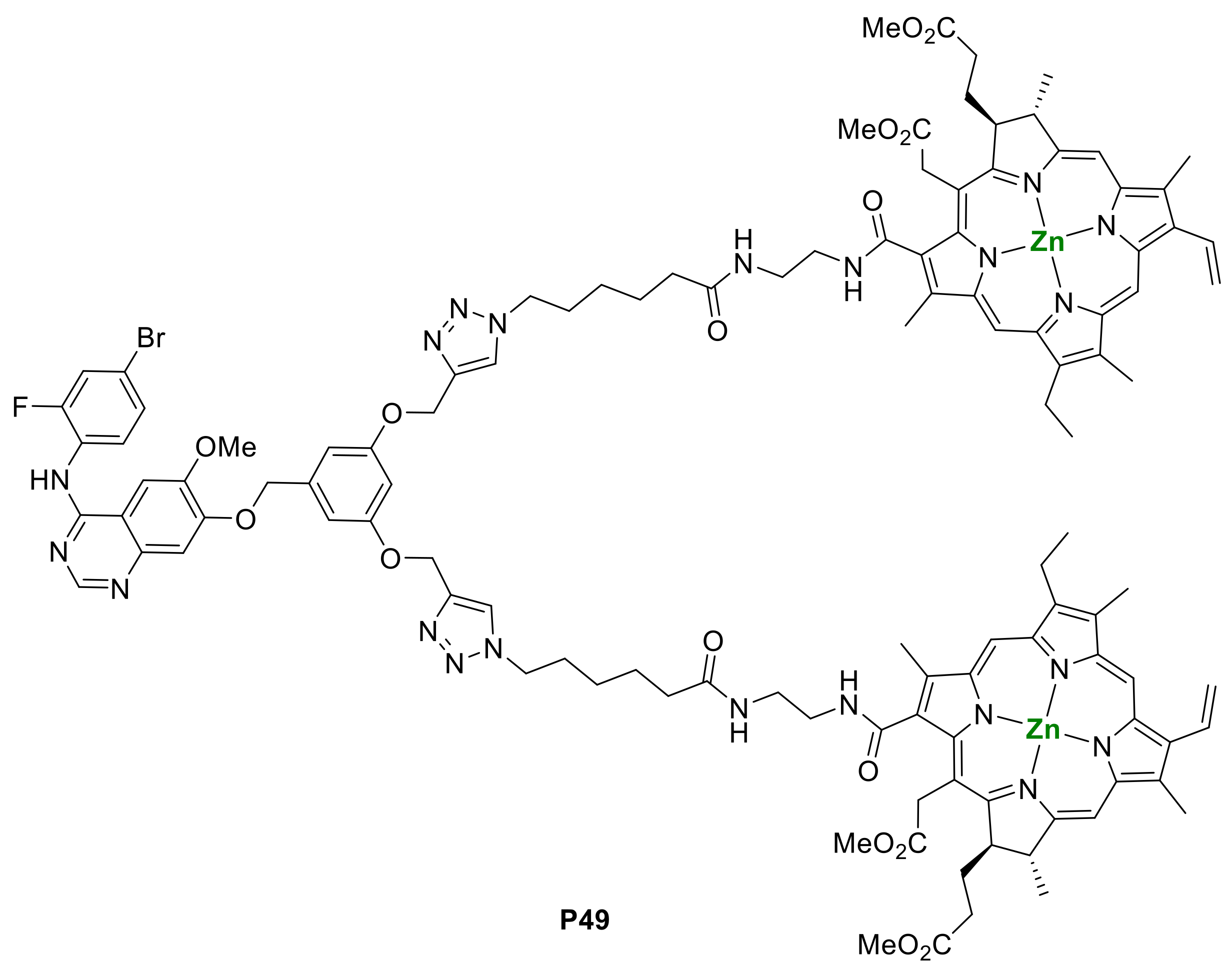
2.1. Porphyrins Bearing Alkyne Groups in CuAAC Reactions
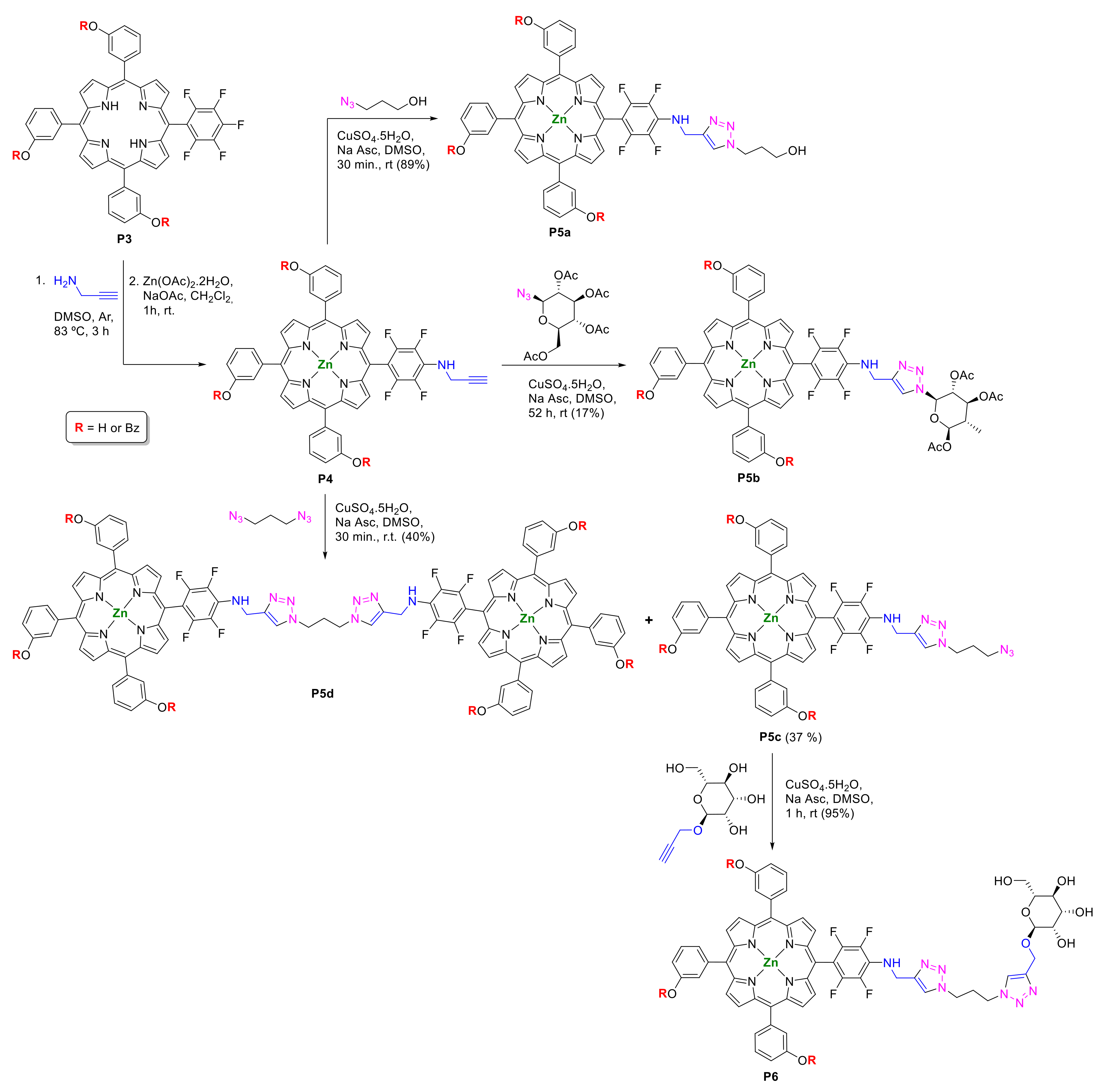
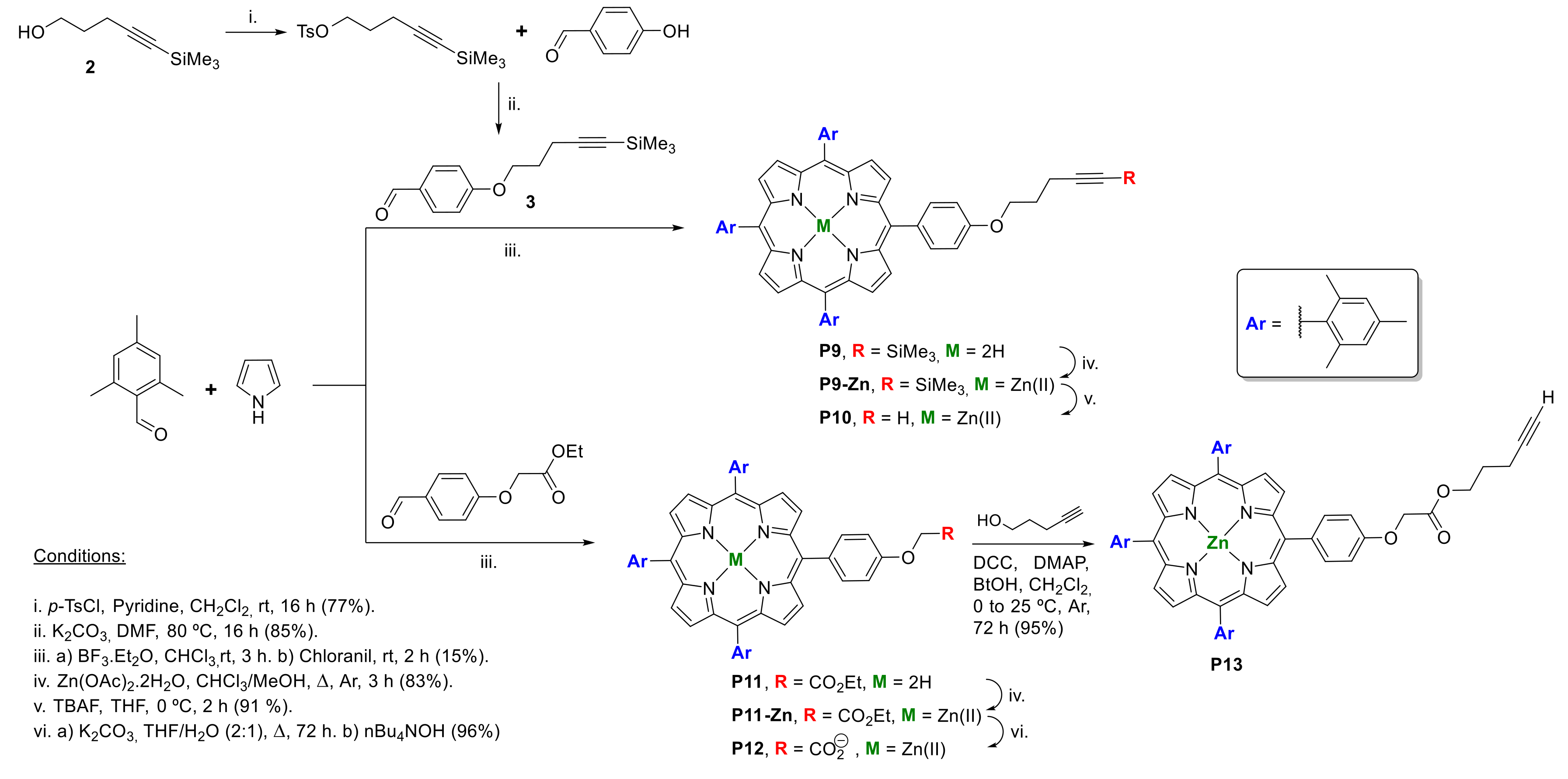
2.1.1. Modification at Peripheral Substituents
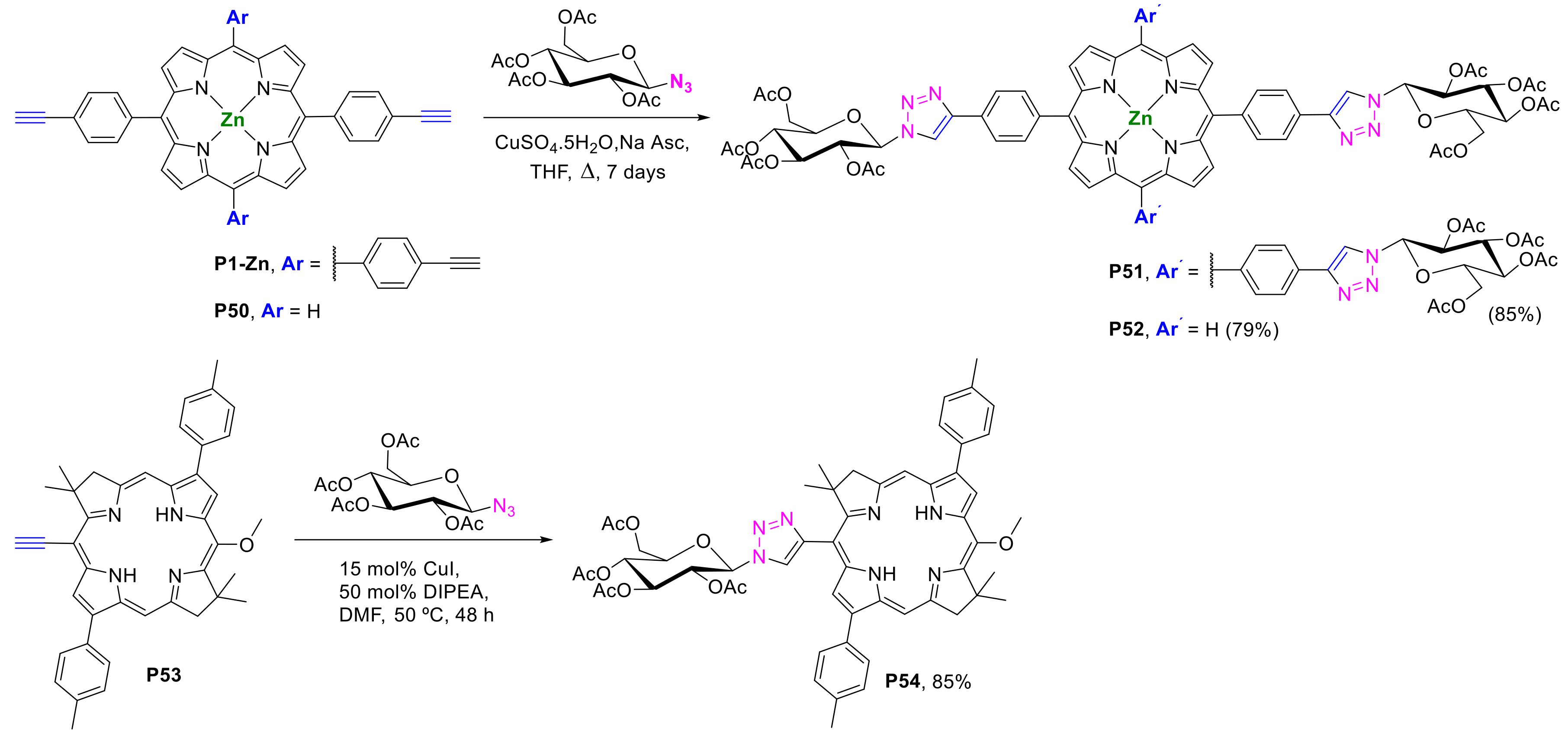
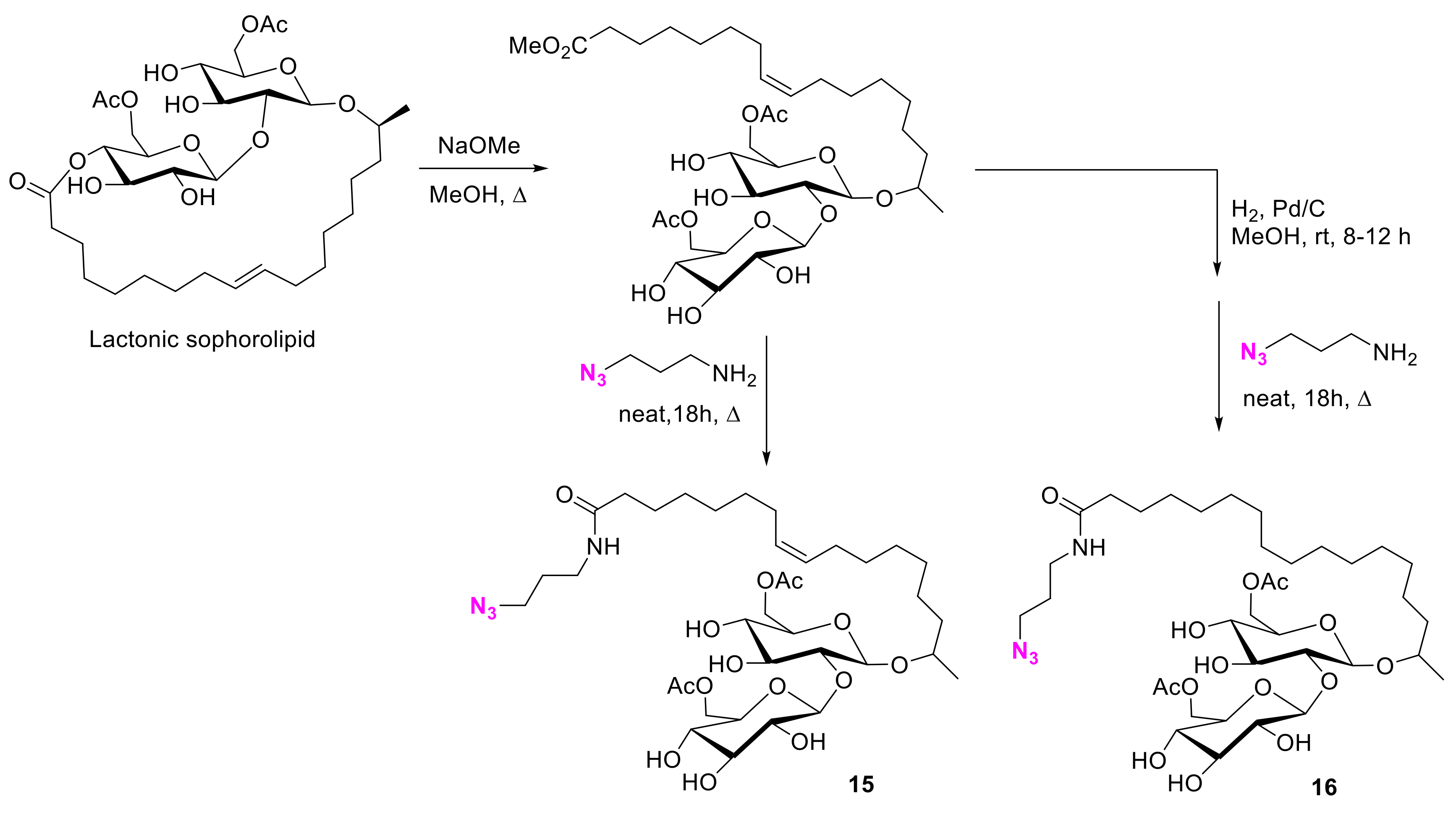
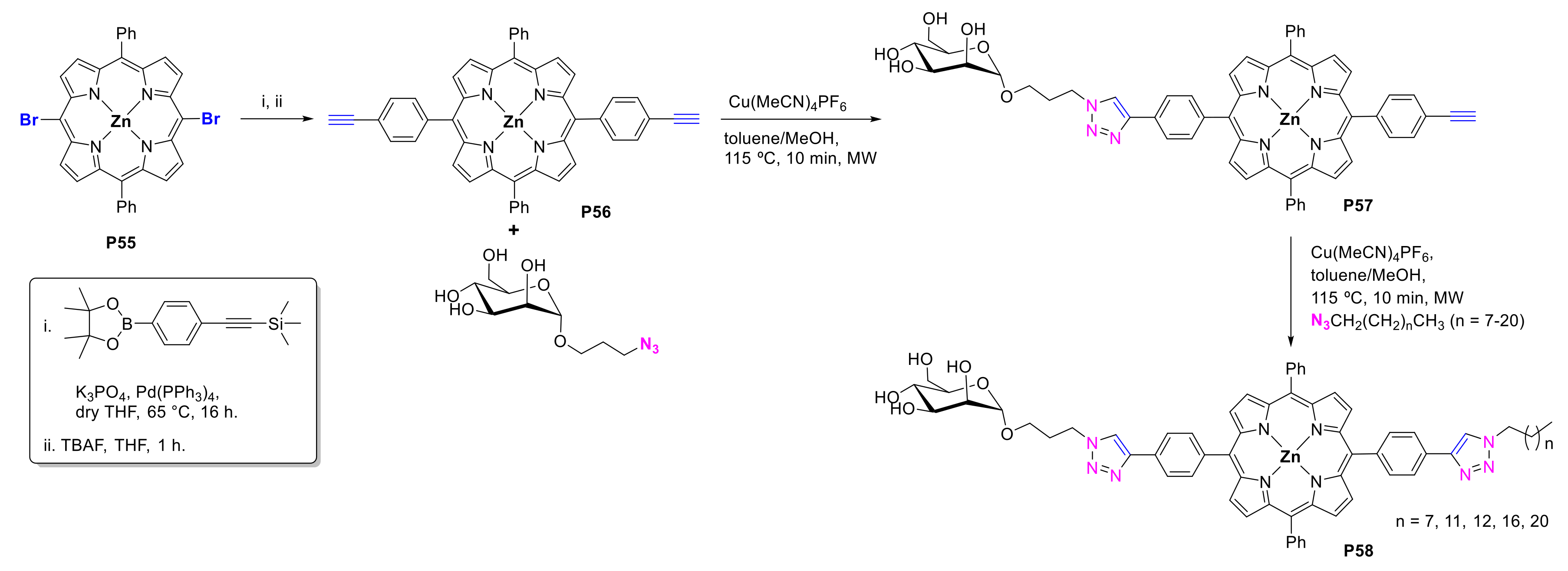
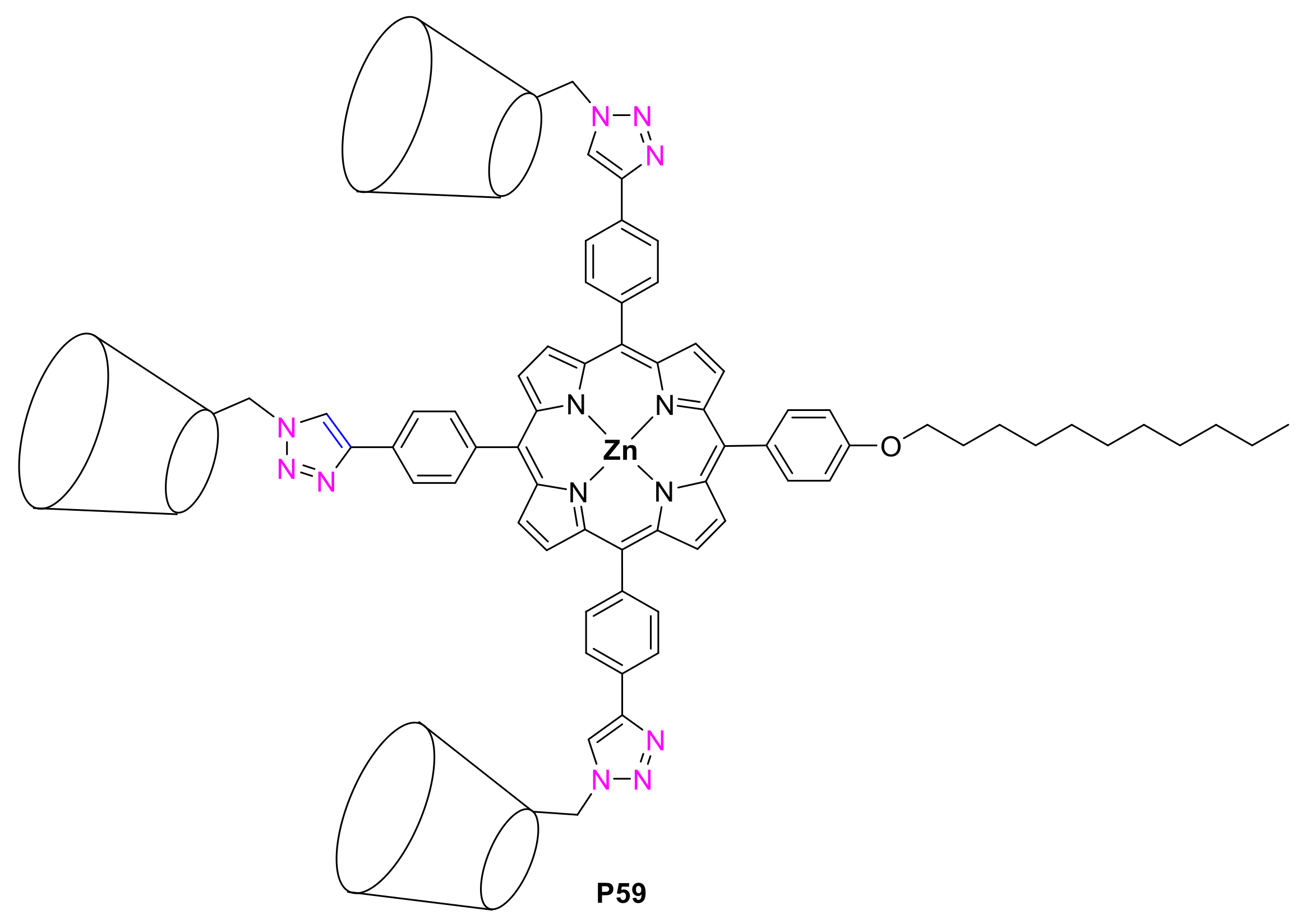
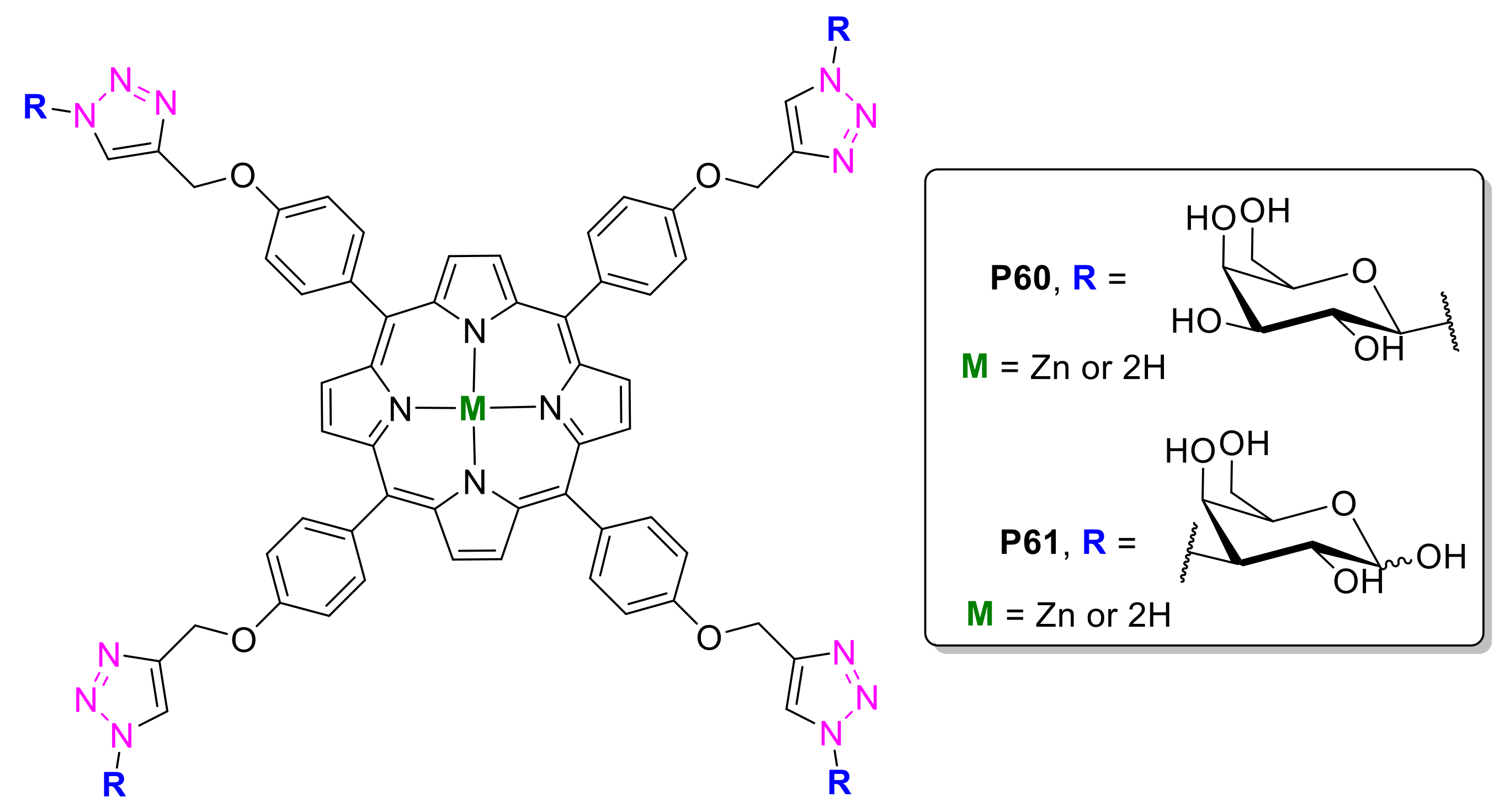
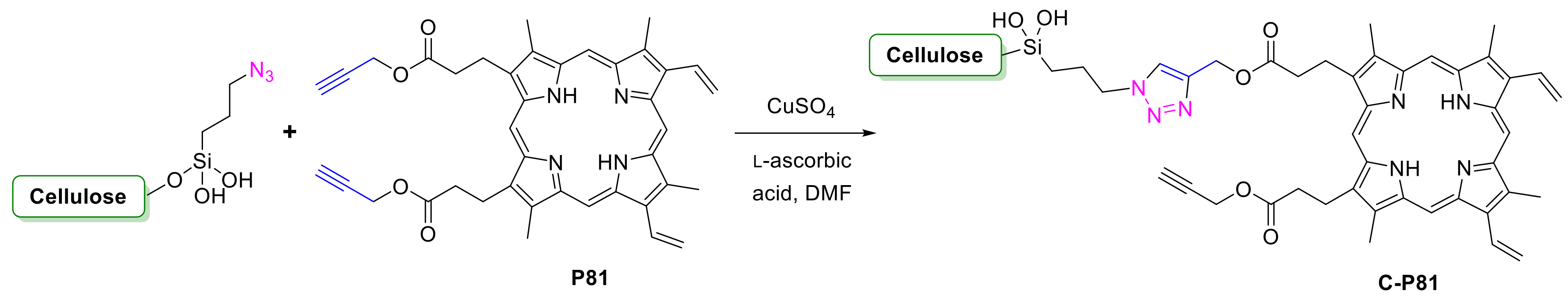
2.1.2. Carbohydrate-Porphyrin Conjugates
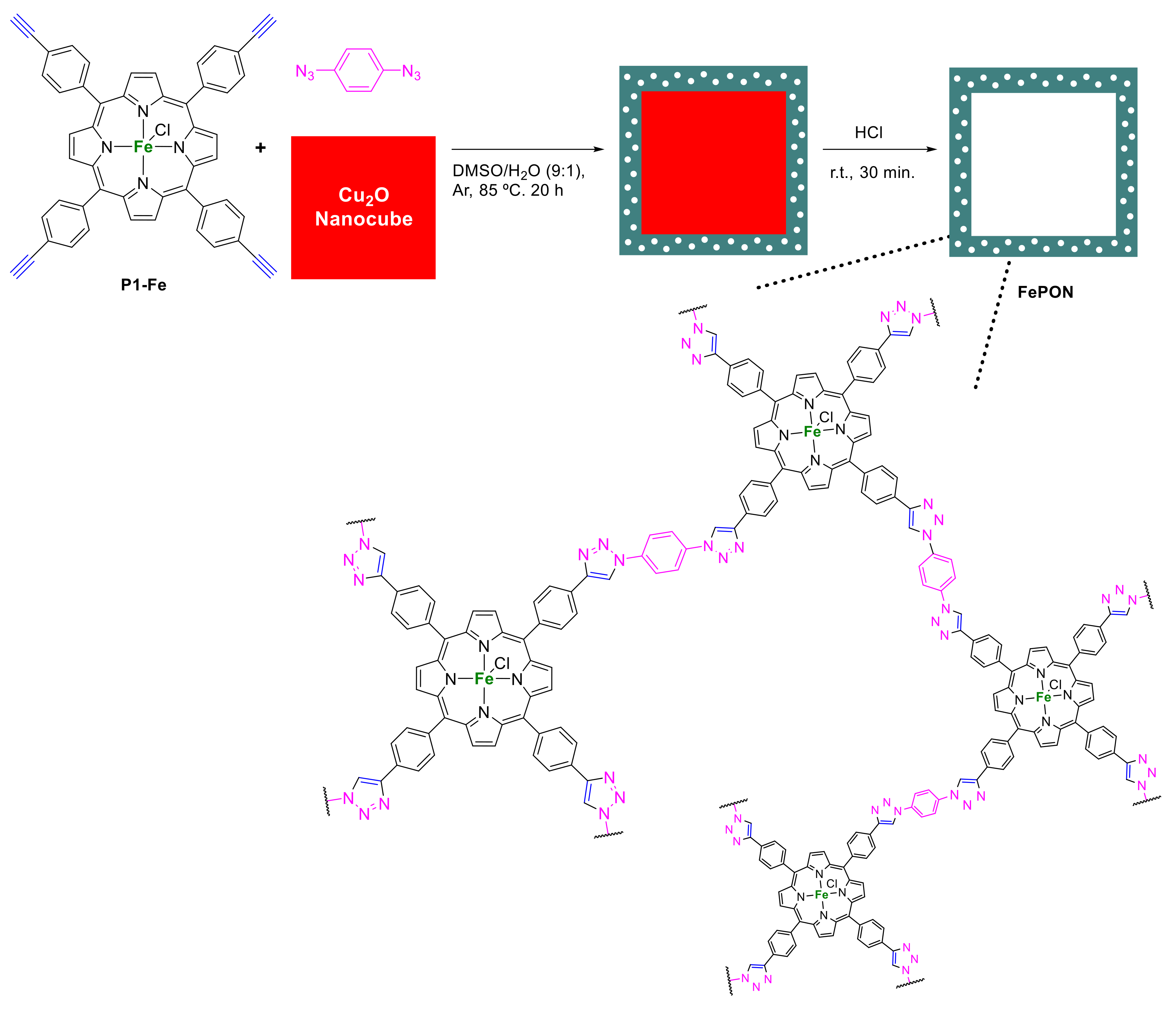
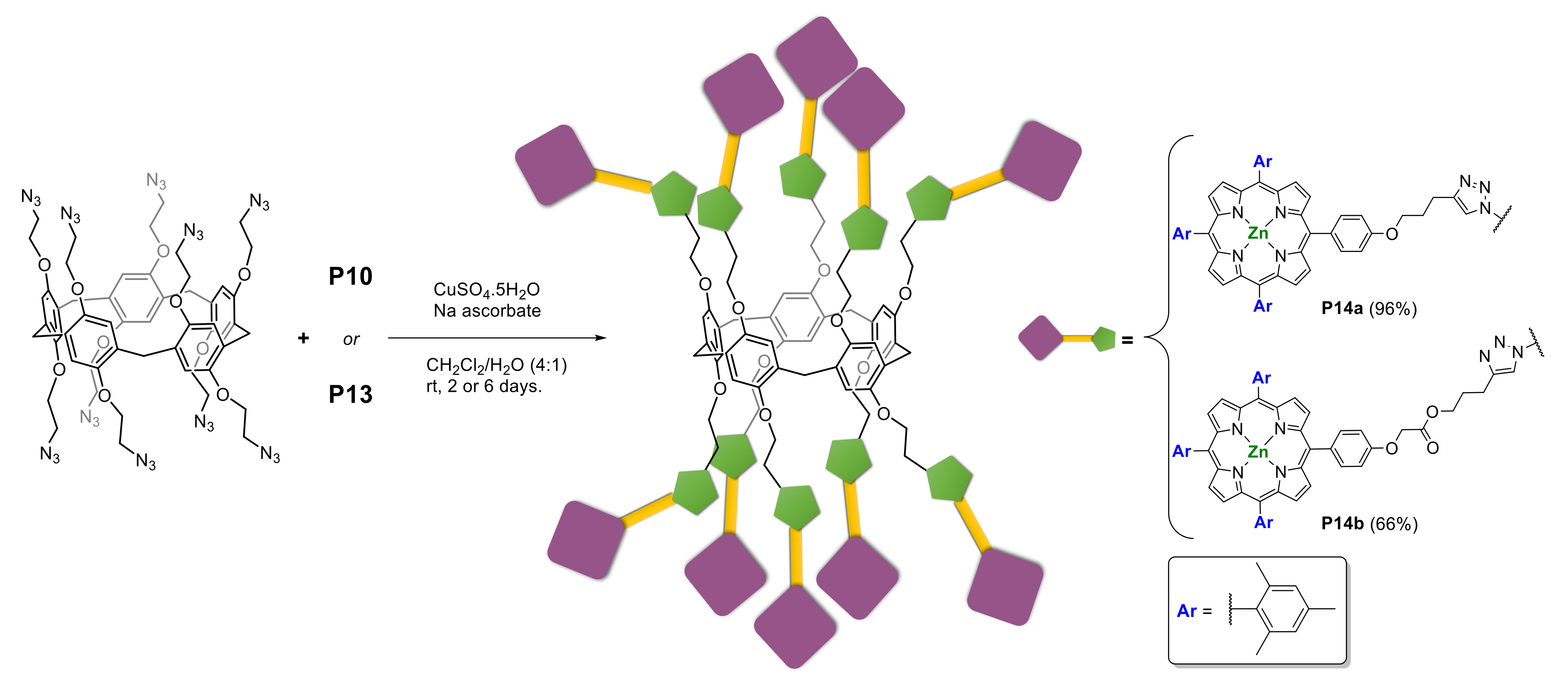
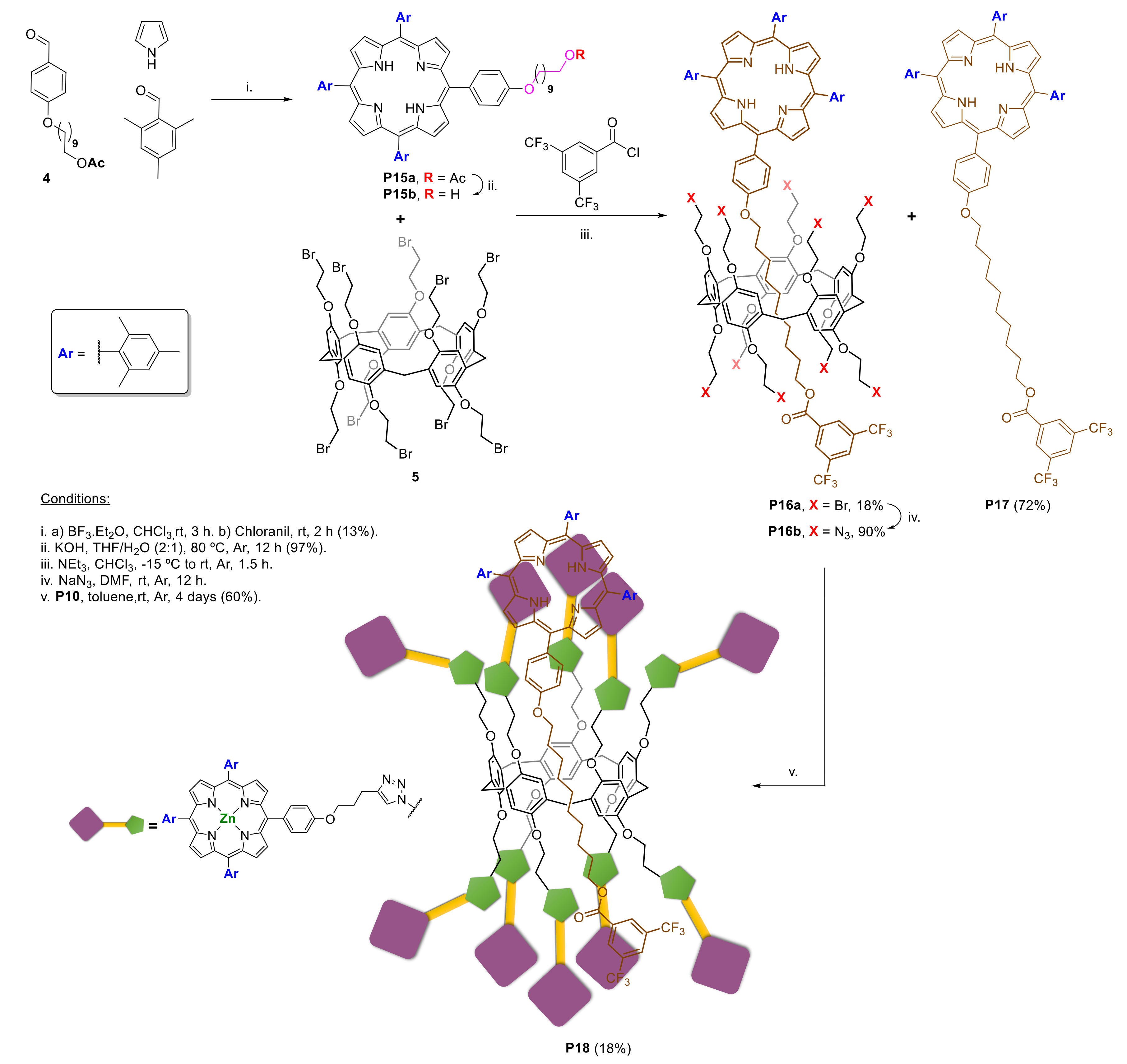
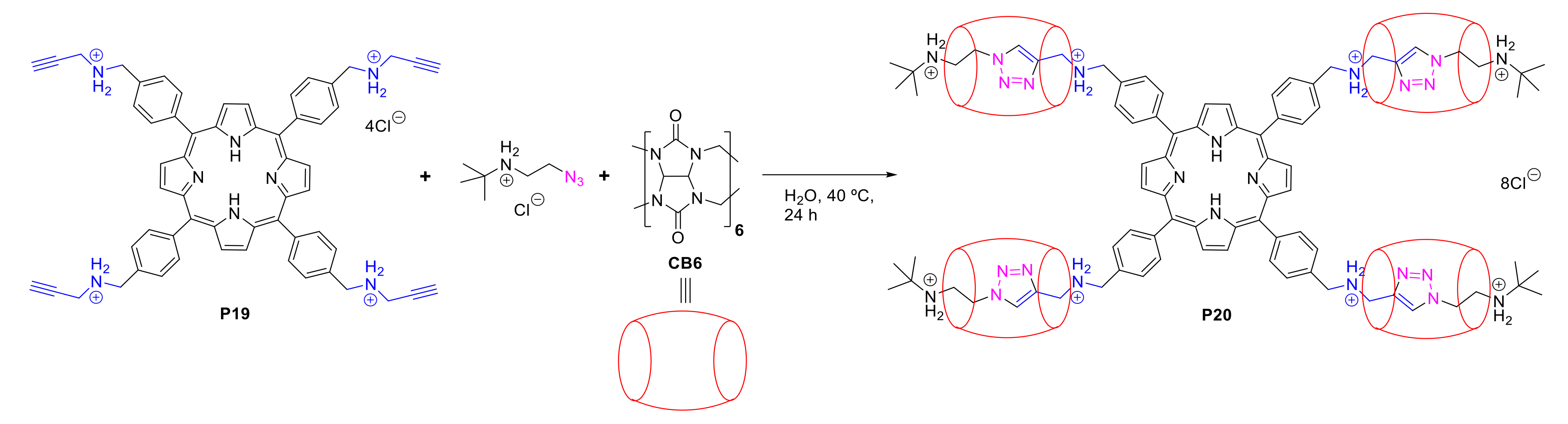
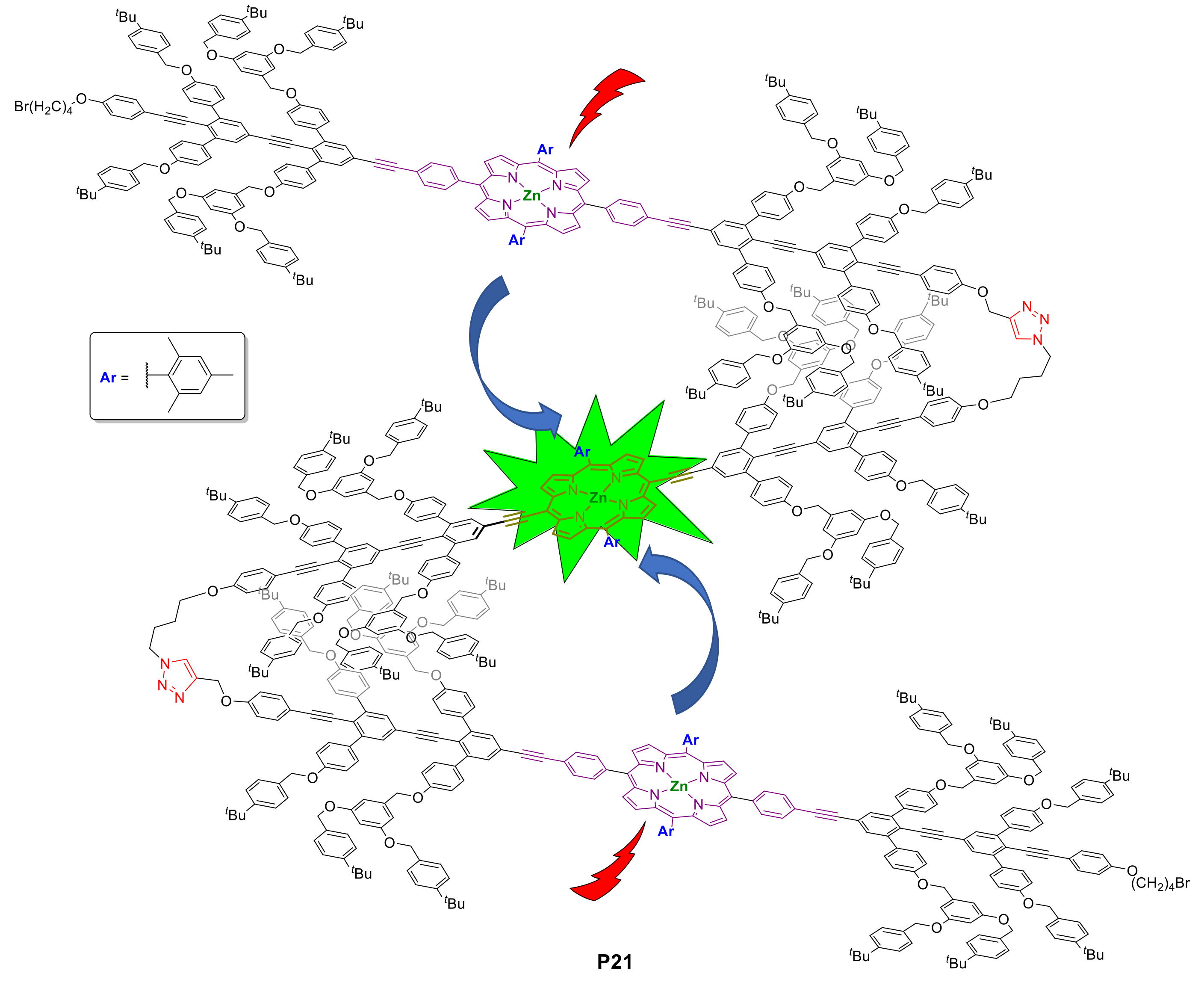
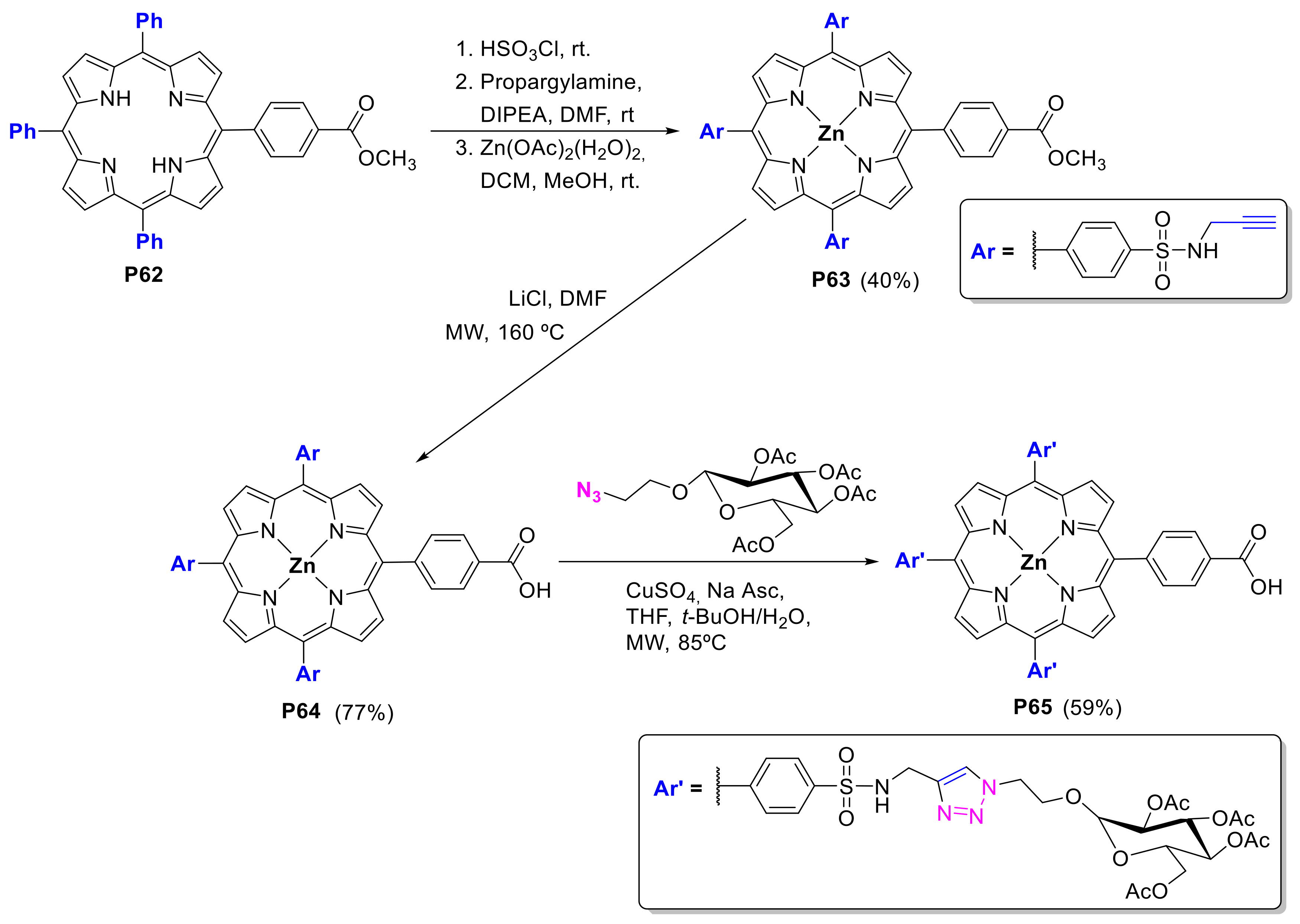
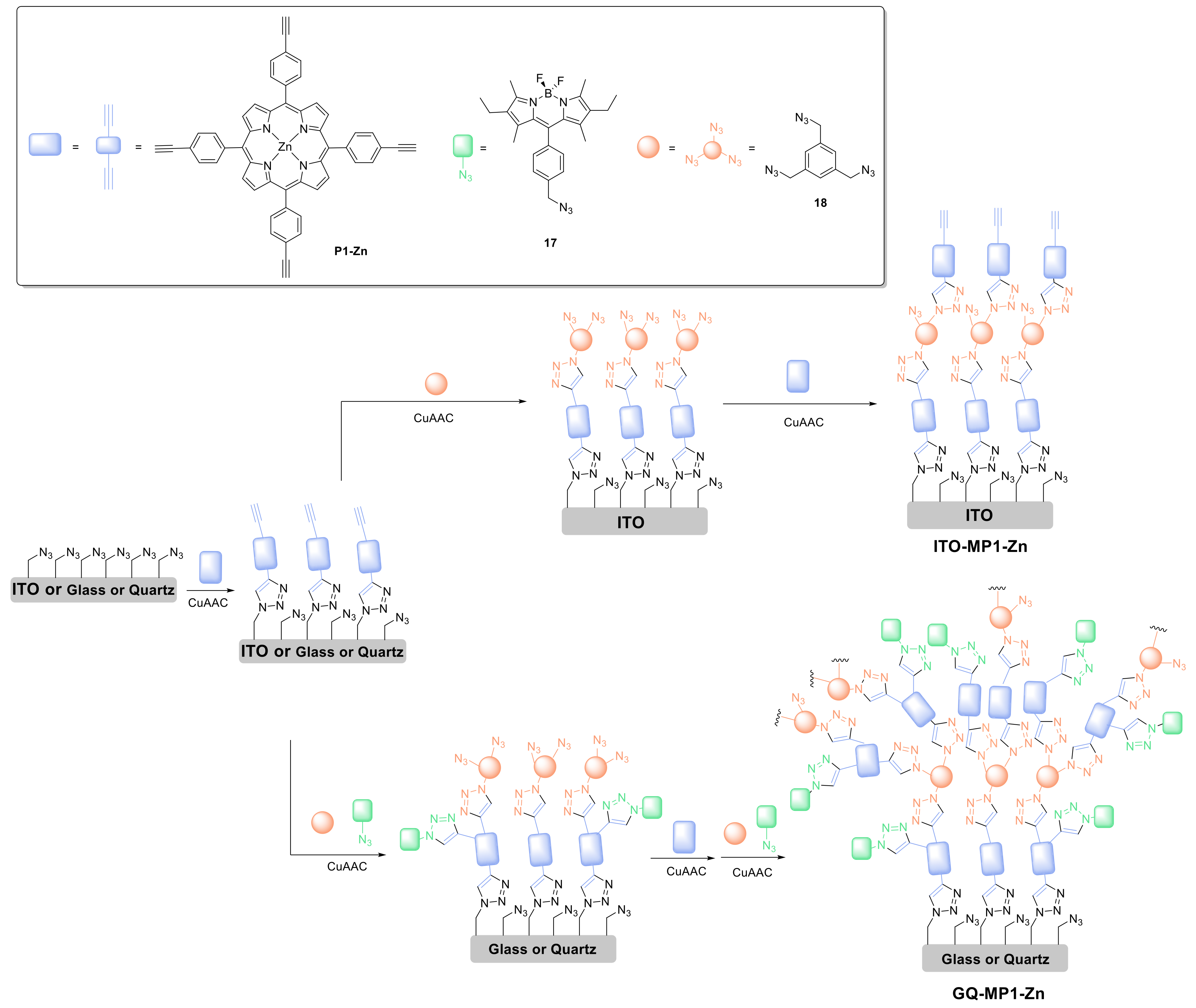
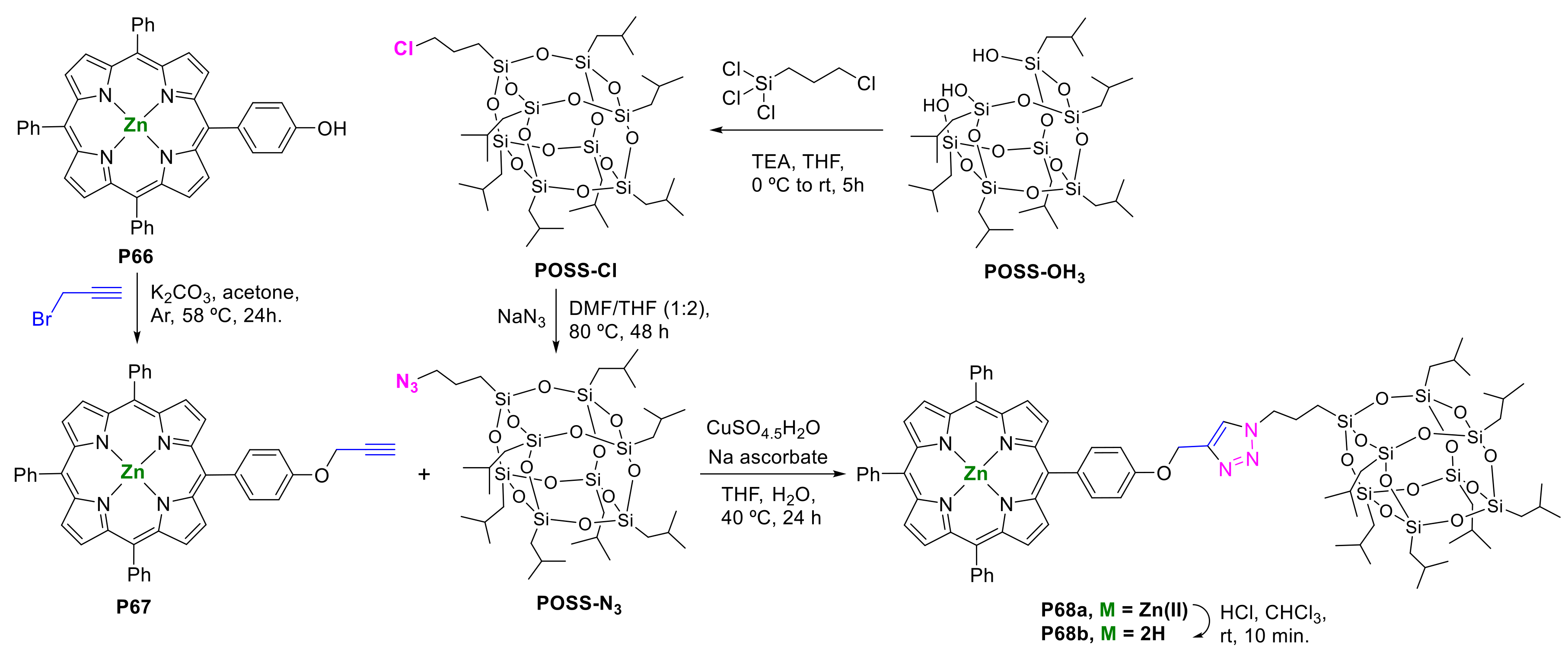
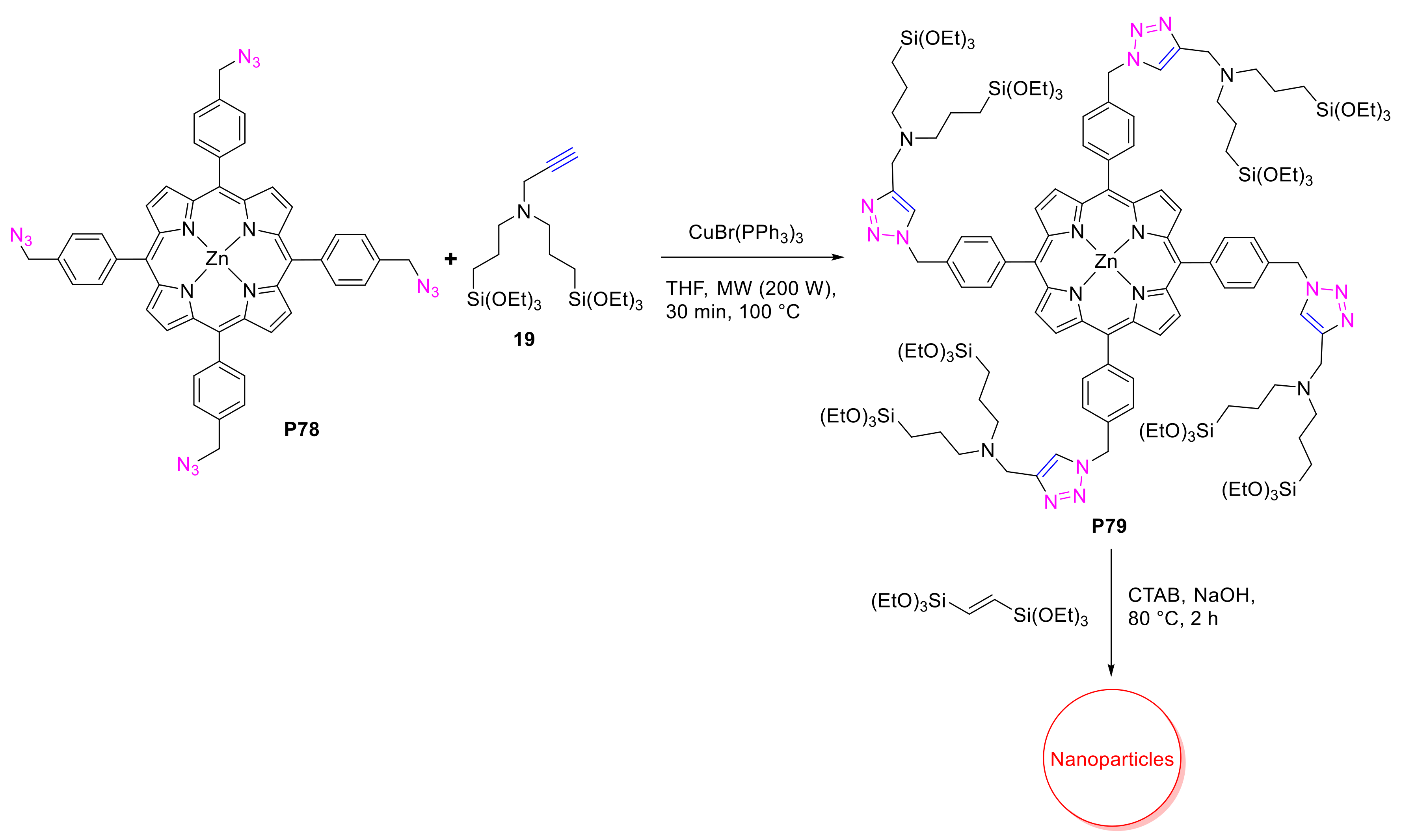
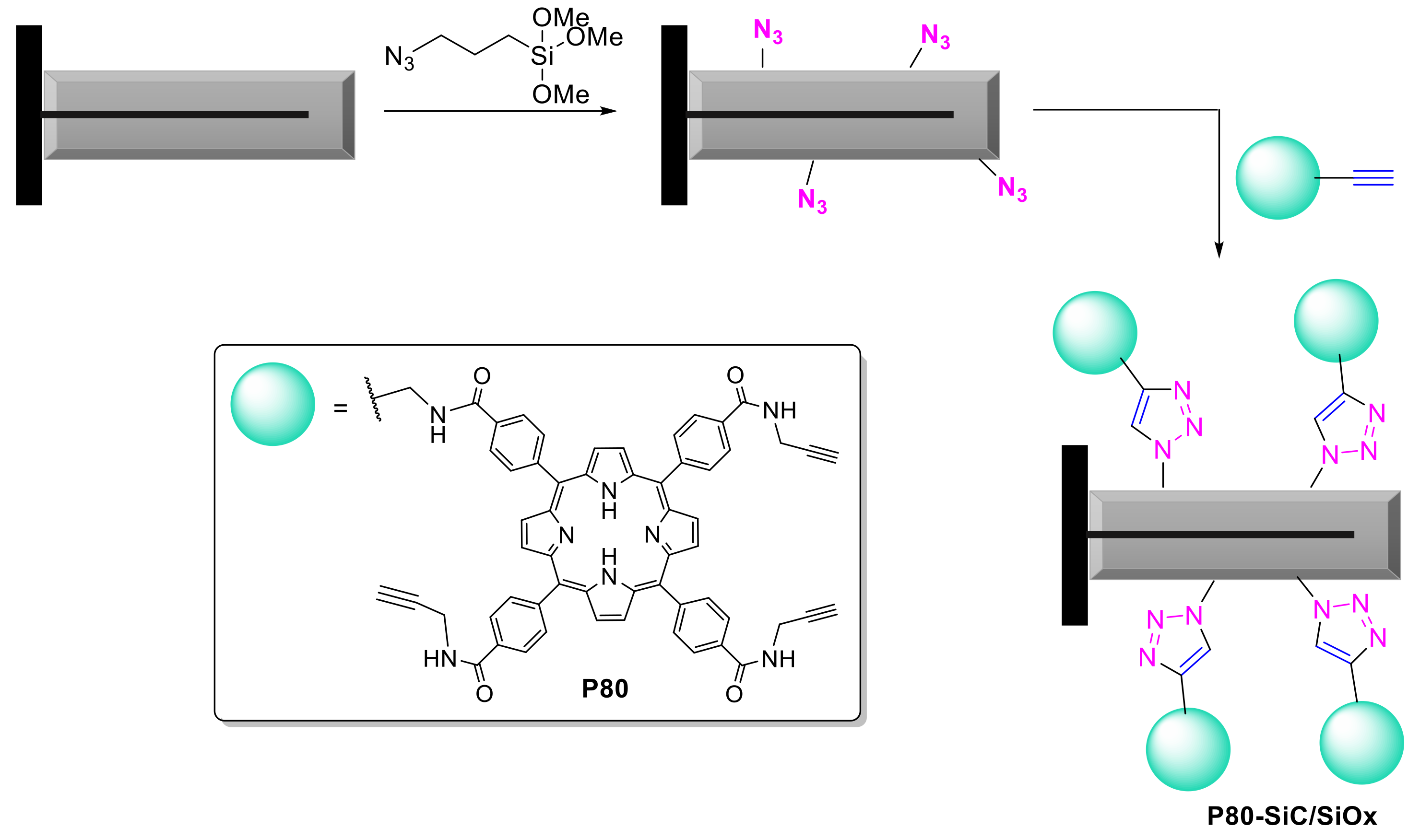
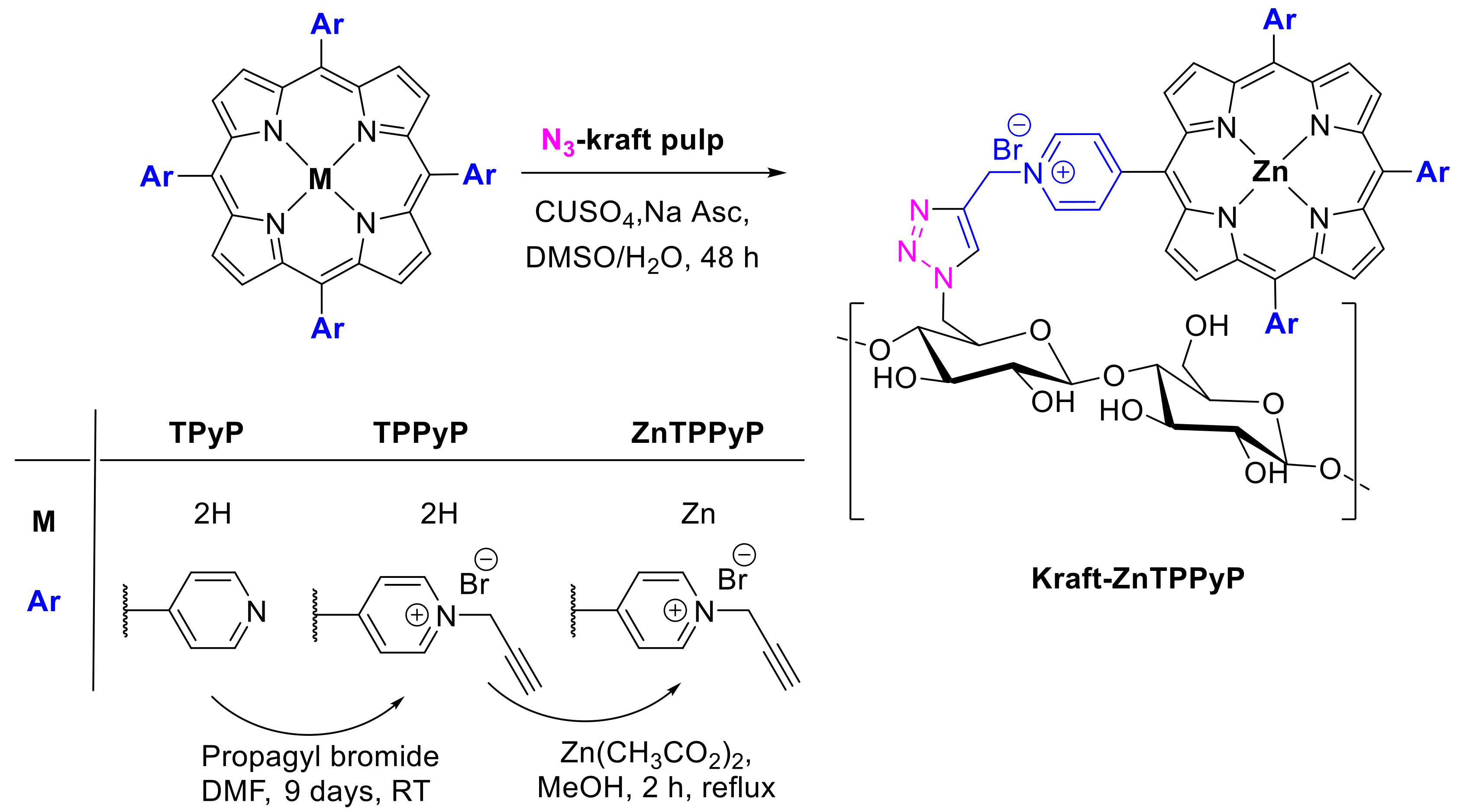
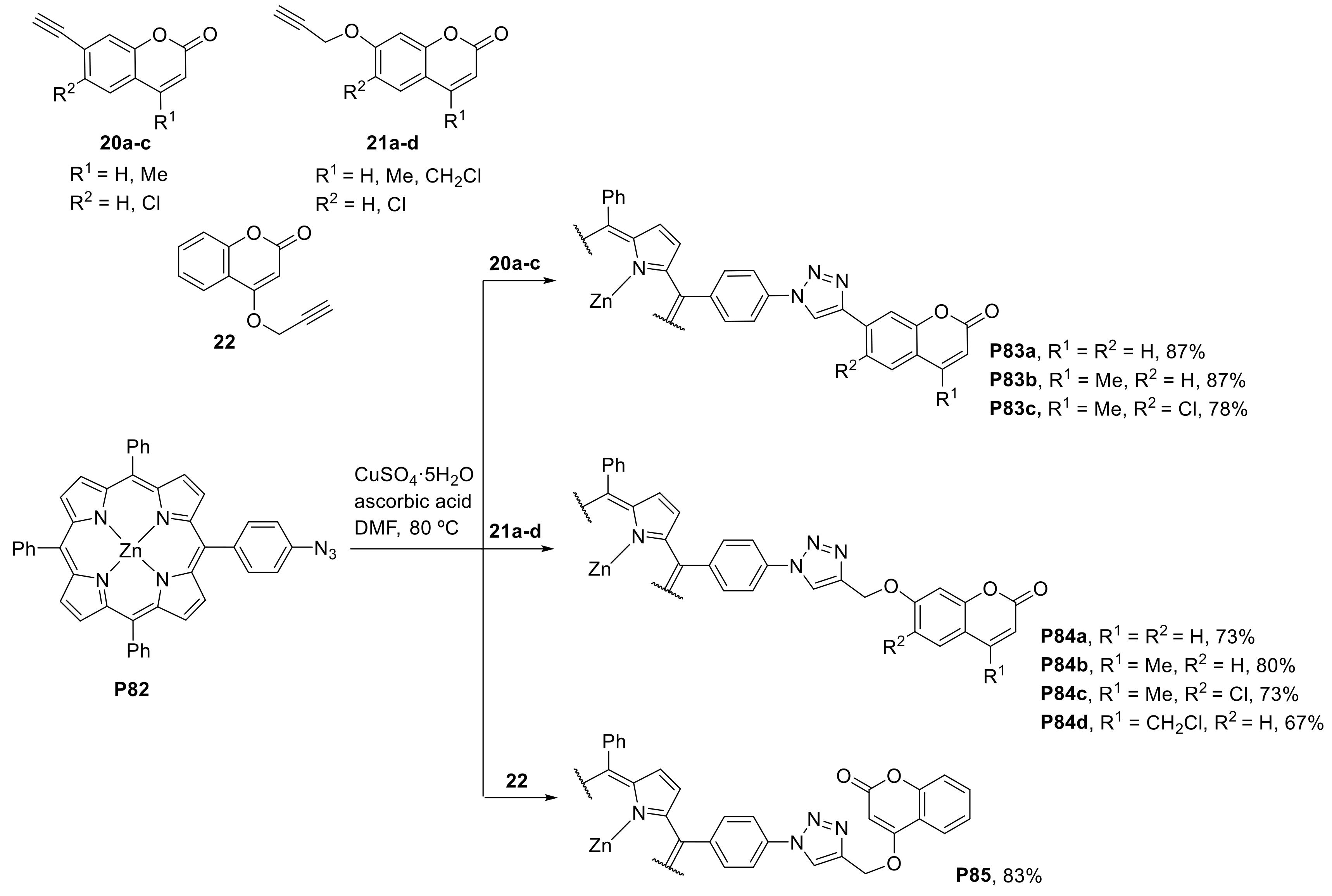
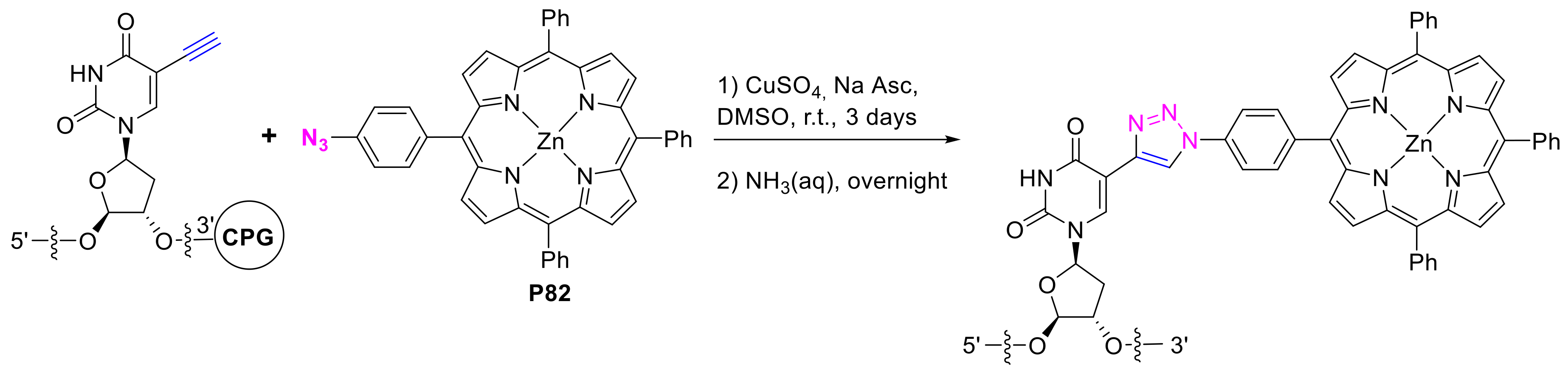
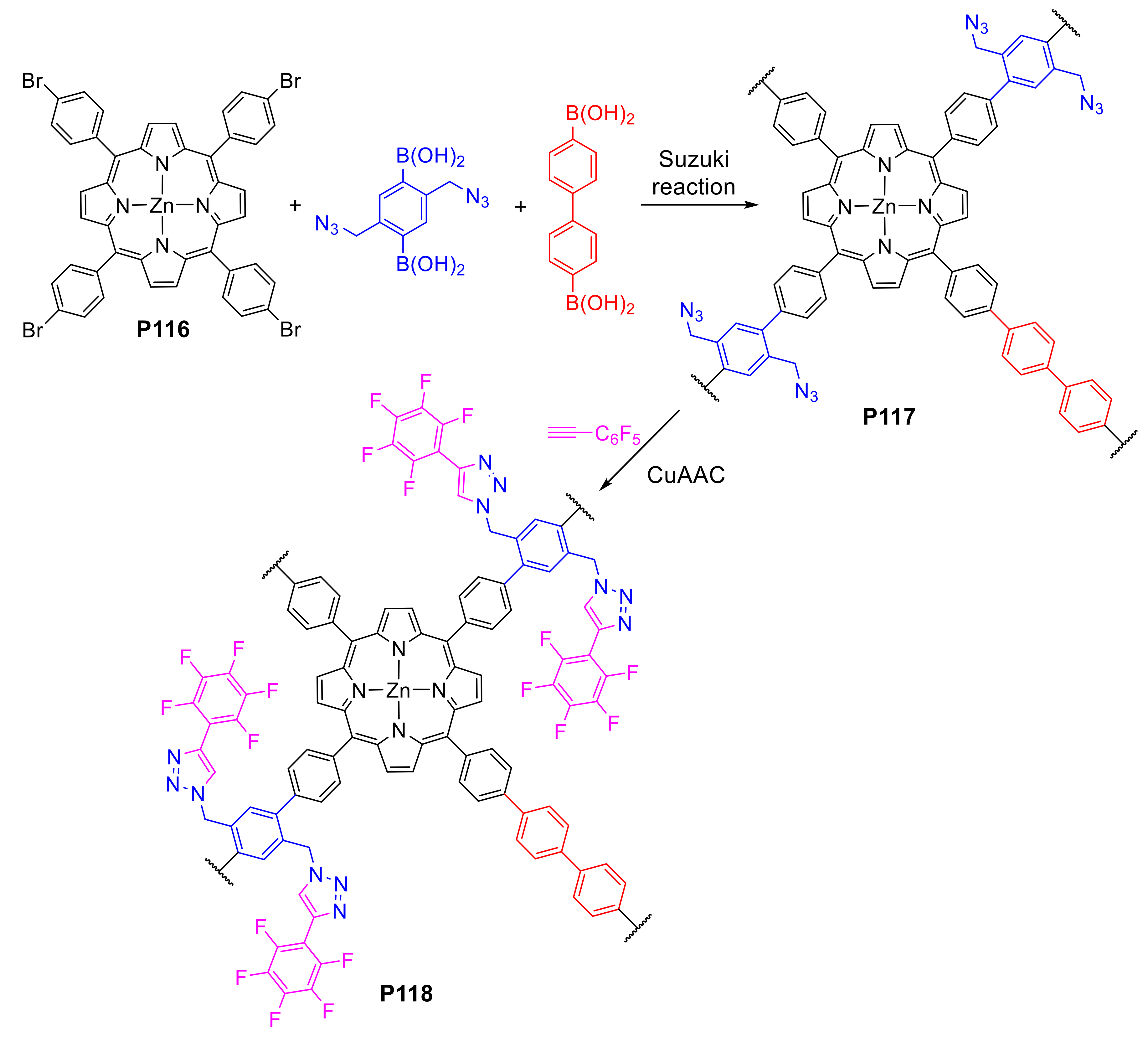
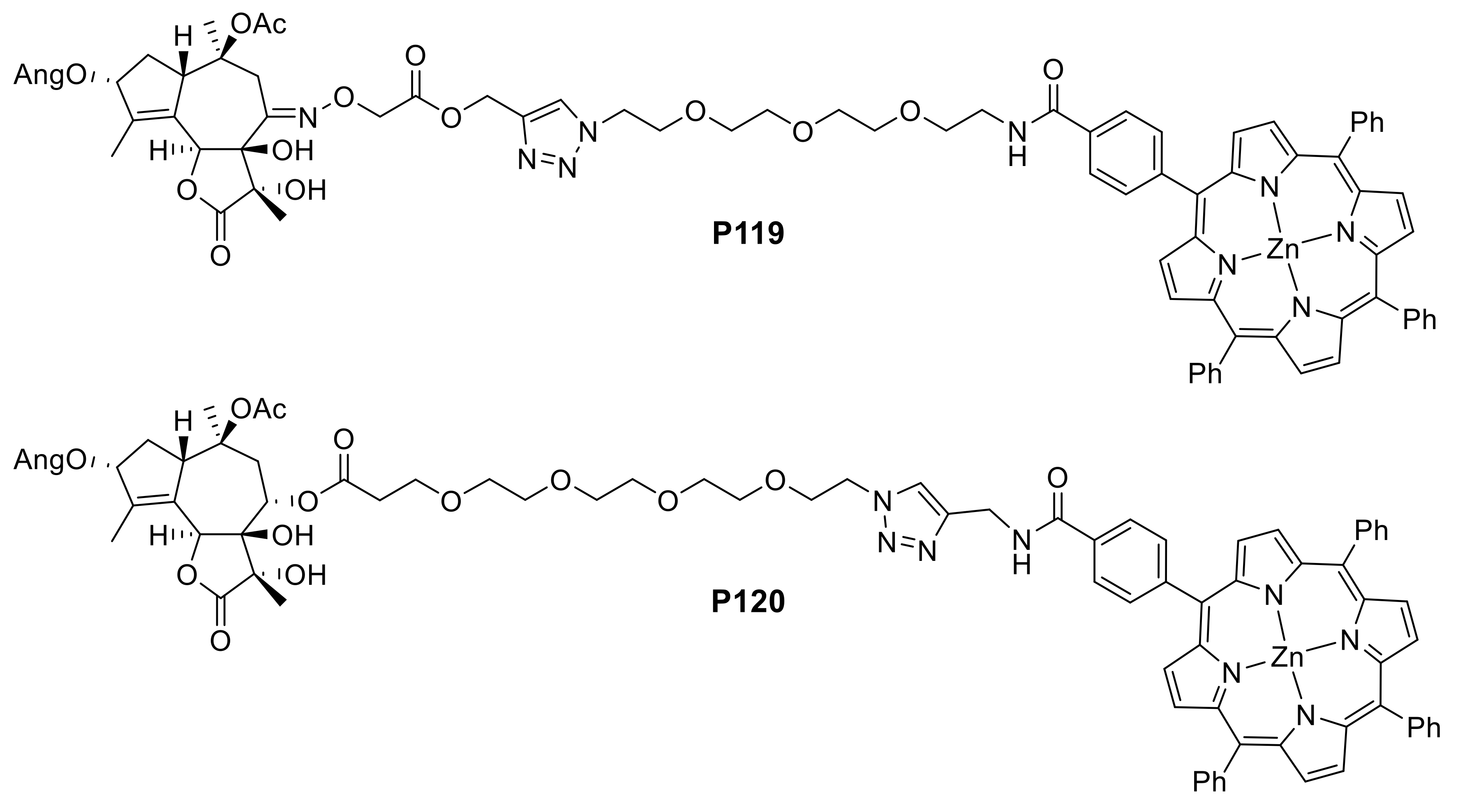
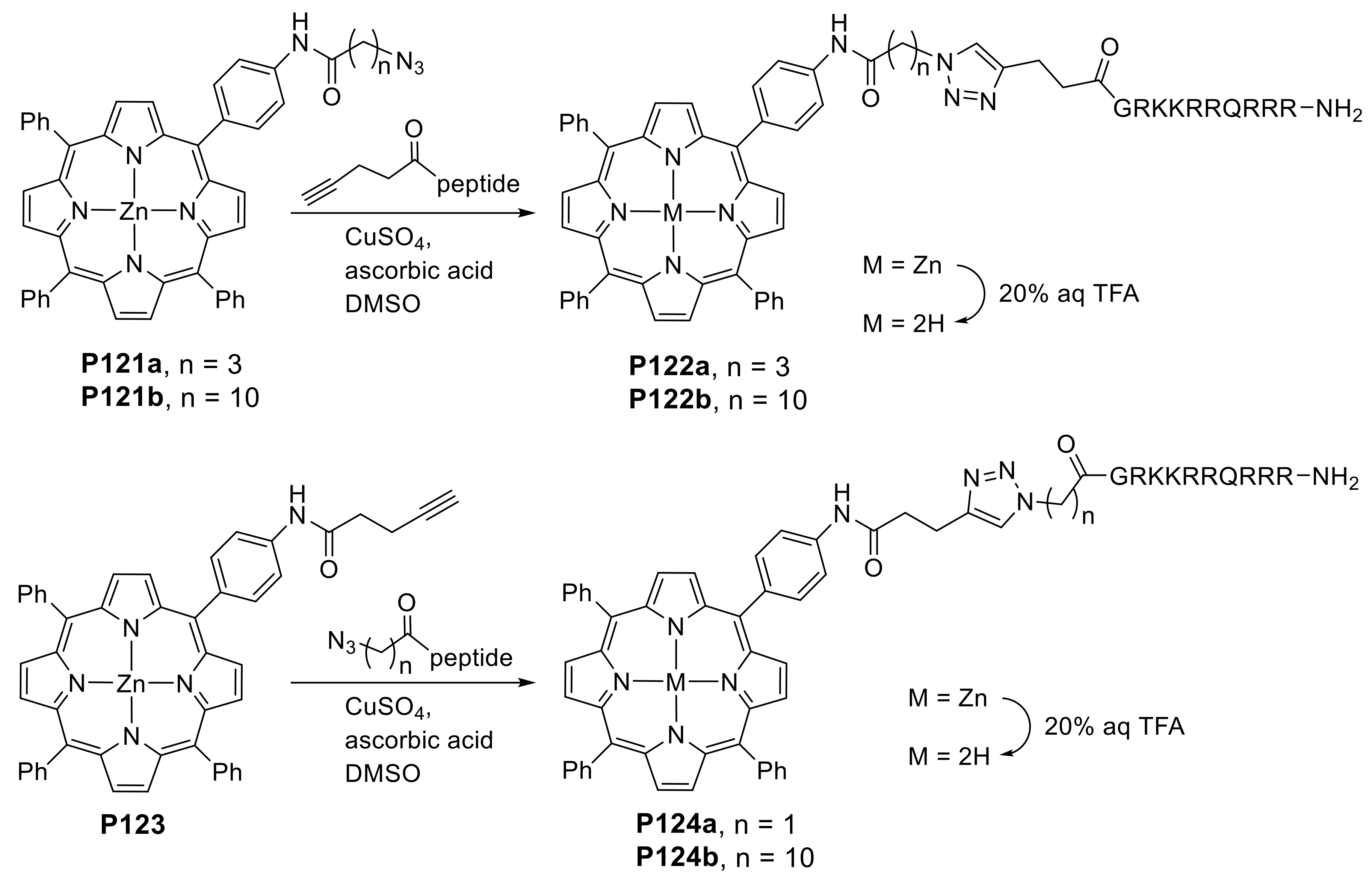
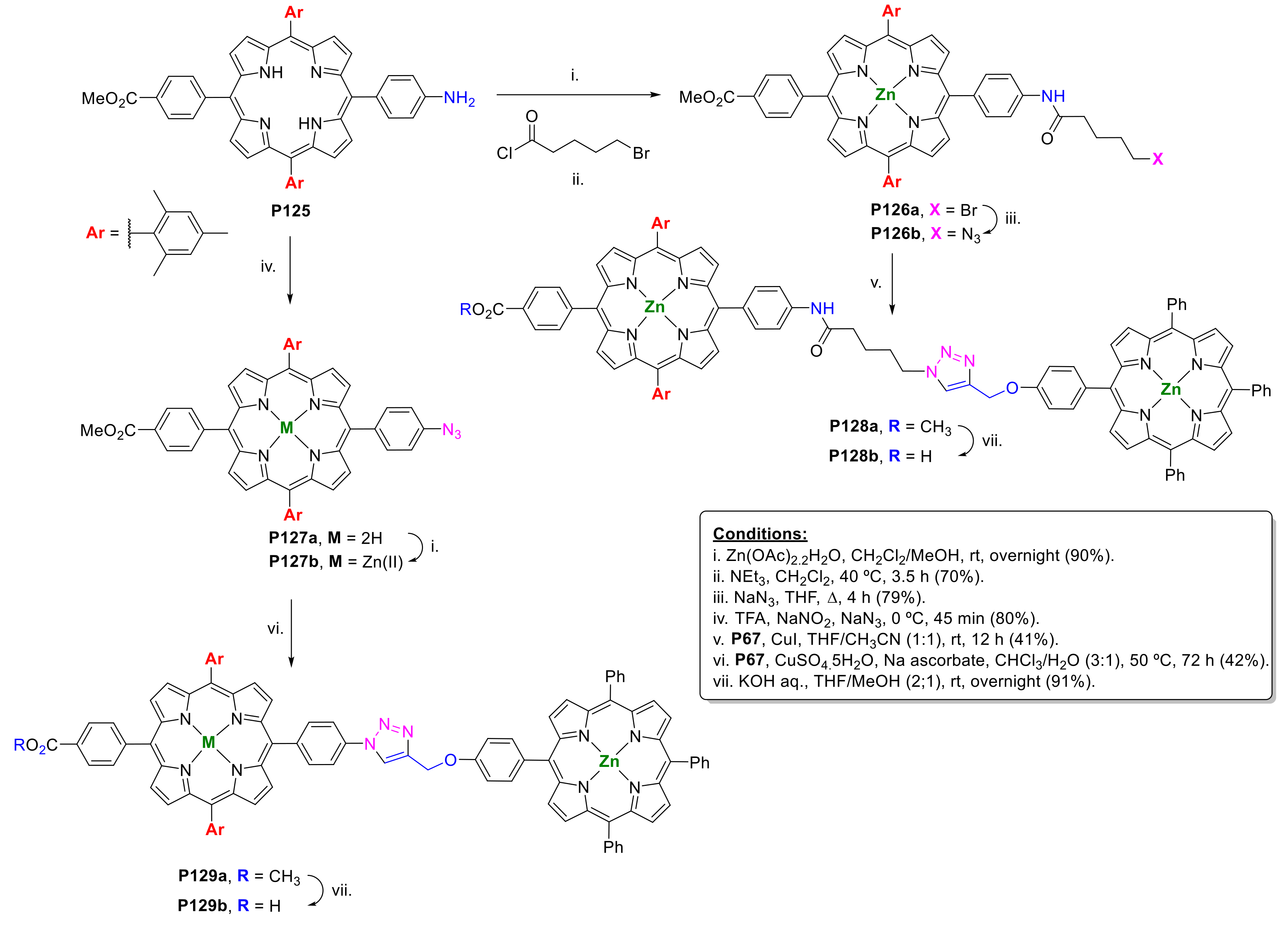
2.1.3. Immobilization of Porphyrins in Different Matrixes
2.2. Porphyrins Bearing Azido Groups
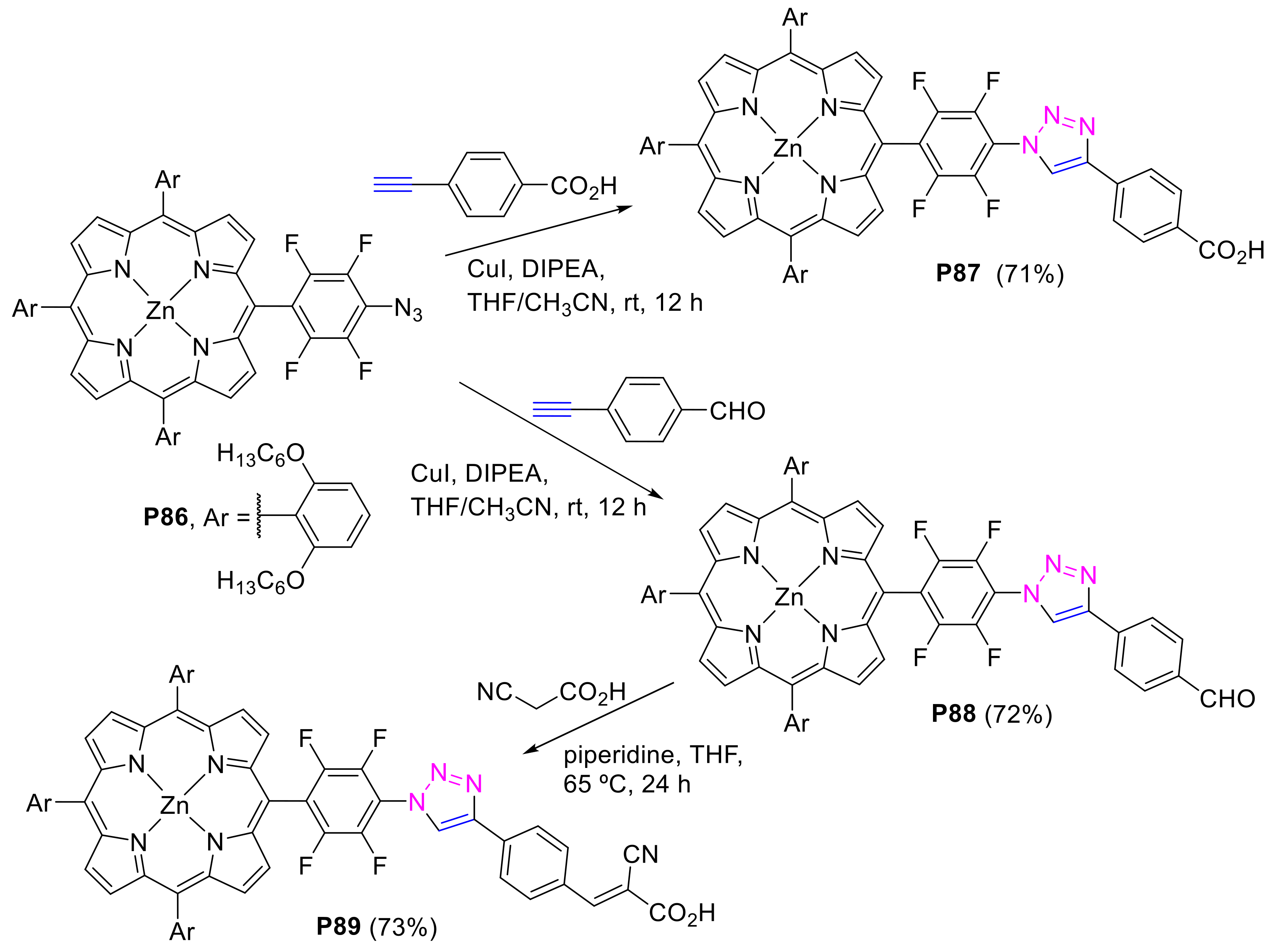
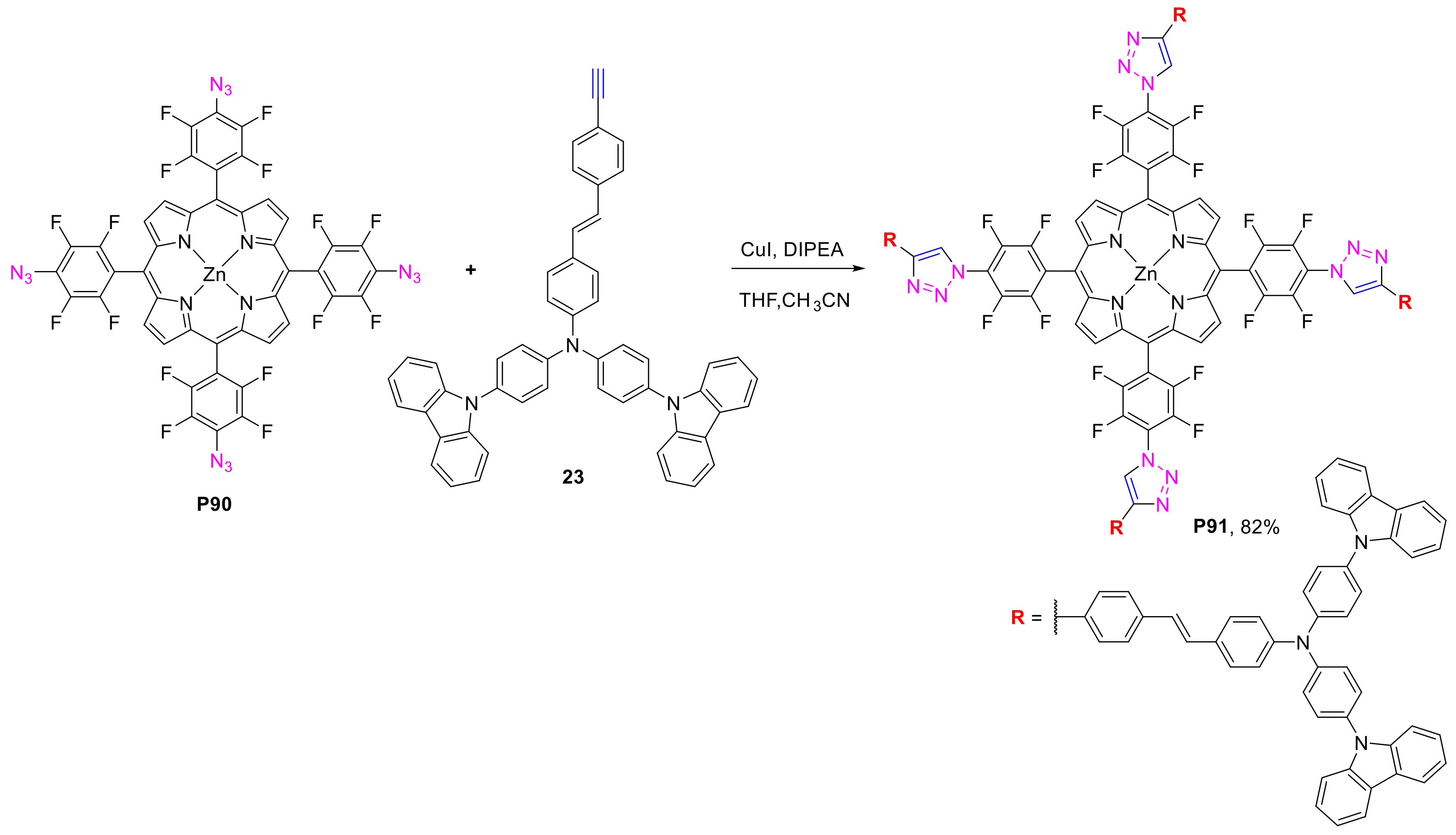
2.2.1. meso-(p-Azidophenyl)porphyrins
2.2.2. meso-(p-Azidomethylphenyl)porphyrins
2.2.3. 2-Azido- or 2-Azidomethylporphyrins
2.2.4. Other Azido-Substituted Porphyrins
2.3. Other Reactions Involving Azides
3. Catalytic Applications and Azide Transformations
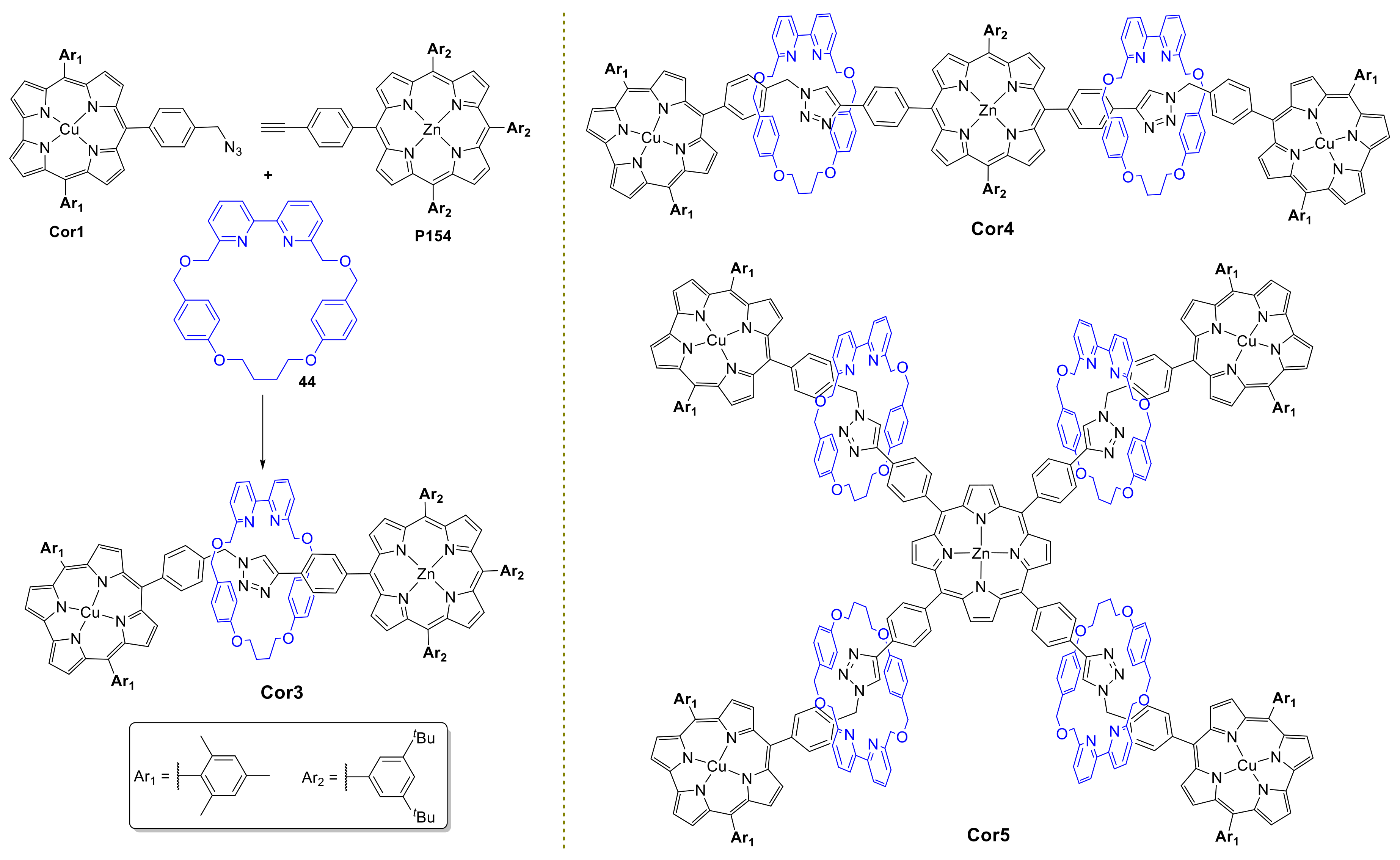
4. Corrole Macrocycles
5. Final Remarks
Funding
Conflicts of Interest
Abbreviations
| A-253 | Human Epidermoid Carcinoma Cells |
| CB6 | cucurbit[6]uril |
| CMPs | Conjugated Microporous Polymers |
| Conc. | concentrated |
| Co(TMP) | (5,10,15,20-tetramesitylporphyrinato)cobalt(II) |
| Co(TPFPP) | [5,10,15,20-tetrakis(pentafluorophenyl)porphyrinato]cobalt(II) |
| Co(TPP) | (5,10,15,20-tetraphenylporphyinato)cobalt(II) |
| CuAAC | copper(I)-catalyzed alkyne-azide cycloaddition |
| CuPPh | [5,10,15,20-tetrakis(4-aminophenyl)porphyrinato]copper(II) |
| DABCO | 1,4-diazabicyclo[2.2.2]octane |
| DCC | N,N’-dicyclohexylcarbodiimide |
| DIPEA | N,N-Diisopropylethylamine |
| DMAP | 4-(dimethylamino)pyridine |
| DMF | N,N-dimethylformamide |
| DMSO | Dimethyl Sulfoxide |
| DNA | Deoxyribonucleic Acid |
| DSSC | Dye-Sensitized Solar Cell |
| E. coli | Escherichia coli |
| EDC | 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| FeTSPP | [5,10,15,20-tetrakis-(4-sulfonatophenyl)porphyrinato]iron(III) chloride |
| FRET | Förster Resonance Energy Transfer |
| GCE | Glassy Carbon Electrodes |
| H2TPPP | 5,10,15,20-tetrakis(4-(3-(pyrazin-2-yl)-1H-pyrazo-1-yl)phenyl)porphyrin |
| HOBt | 1-hydroxybenzotriazole |
| hPG | Hyperbranched Polyglycerol |
| IME | 1,3-dimethylimidazol-2-ylidene |
| ITO | Indium Tin Oxide |
| LbL | Layer-by-Layer |
| LNCaP | Prostate Carcinoma Cell Line |
| MCF7 | Breast Cancer Cell Line |
| MiaPaCa-2 | Pancreatic Carcinoma Cell Line |
| MOF | Metal–Organic Framework |
| mPEG | methoxypoly(ethylene glycol) |
| MRI | Magnetic Resonance Imaging |
| MW | Microwave Irradiation |
| PA-1 | Human Ovarian Teratocarcinoma Cell |
| Pc | Phthalocyanine |
| PDI | Photodynamic Inactivation of Microorganisms |
| PDT | Photodynamic Therapy |
| PEG | Polyethylene Glycol |
| PET | Photoinduced Electron Transfer |
| PET | Positron Emission Tomography |
| PMEDTA | N,N´,N′,N″,N″-pentamethyl-diethylenetriamine |
| POSS | Polyhedral Oligomeric Silsesquioxane |
| rt | Room Temperature |
| Si-PAMAM | siloxane-poly(amido amine) |
| TBAF | Tetrabutylammonium fFuoride |
| TDCPP | 5,10,15,20-tetrakis(2,6-dichlorophenyl)porphyrin |
| TFA | Trifluoroacetic Acid |
| THF | Tetrahydrofuran |
| TIPS | Triisopropylsilyl Ether |
| TPP | 5,10,15,20-tetraphenylporphyrin |
| U-2 OS | Osteosarcoma Cell Line |
| UV | Ultraviolet |
| Vis | Visible |
References
- Griess, J.P.X.X. On a new class of compounds in which nitrogen is substituted for hydrogen. Proc. R. Soc. Lond. 1864, 13, 375–384. [Google Scholar]
- Curtius, T. Ueber Stickstoffwasserstoffsäure (Azoimid) N3H. Ber. Dtsch. Chem. Ges. 1890, 23, 3023–3033. [Google Scholar] [CrossRef]
- Lerner, L. Small-Scale Synthesis of Laboratory Reagents with Reaction Modeling, 1st ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Pinho e Melo, T.M.V.D. Synthesis of Azides. In Organic Azides: Syntheses and Applications; Bräse, S., Banert, K., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2009; pp. 53–94. [Google Scholar]
- Schilling, C.; Jung, N.; Bräse, S. Cycloaddition Reactions with Azides: An Overview. In Organic Azides: Syntheses and Applications; Bräse, S., Banert, K., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2009; pp. 269–284. [Google Scholar]
- Czernecki, S.; Valéry, J.-M. An Efficient Synthesis of 3′-Azido-3′-deoxythymidine (AZT). Synthesis 1991, 1991, 239–240. [Google Scholar] [CrossRef]
- Abrecht, S.; Harrington, P.; Iding, H.; Karpf, M.; Trussardi, R.; Wirz, B.; Zutter, U. The Synthetic Development of the Anti-Influenza Neuraminidase Inhibitor Oseltamivir Phosphate (Tamiflu®): A Challenge for Synthesis & Process Research. Chim. Int. J. Chem. 2004, 58, 621–629. [Google Scholar]
- Haase, J. Large-Scale Preparation and Usage of Azides. In Organic Azides: Syntheses and Applications; Bräse, S., Banert, K., Eds.; John Wiley & Sons, Ltd: Chichester, UK, 2009; pp. 29–51. [Google Scholar]
- Scriven, E.F.V.; Turnbull, K. Azides: Their preparation and synthetic uses. Chem. Rev. 1988, 88, 297–368. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Abu-Orabi, S.T. 1,3-Dipolar Cycloaddition Reactions of Substituted Benzyl Azides with Acetylenic Compounds. Molecules 2002, 7, 302–314. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Xue, P.; Sun, H.H.Y.; Williams, I.D.; Sharpless, K.B.; Fokin, V.V.; Jia, G. Ruthenium-Catalyzed Cycloaddition of Alkynes and Organic Azides. J. Am. Chem. Soc. 2005, 127, 15998–15999. [Google Scholar] [CrossRef]
- Meng, G.; Guo, T.; Ma, T.; Zhang, J.; Shen, Y.; Sharpless, K.B.; Dong, J. Modular click chemistry libraries for functional screens using a diazotizing reagent. Nature 2019, 574, 86–89. [Google Scholar] [CrossRef]
- Kadish, K.M.; Smith, K.M.; Guilard, R. Handbook of Porphyrin Science; World Scientific Publishing Company Co: Singapore, 2010. [Google Scholar]
- Araújo, A.R.L.; Tomé, A.C.; Santos, C.I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Moura, N.M.M.; Abu-Orabi, S.T.; Cavaleiro, J.A.S. Azides and porphyrinoids: Synthetic approaches and applications. Part 2—Azides, phthalocyanines, subphthalocyanines and porphyrazines. Molecules. under review.
- Kang, D.; Ko, J.H.; Choi, J.; Cho, K.; Lee, S.M.; Kim, H.J.; Ko, Y.-J.; Park, K.H.; Son, S.U. Dual role of Cu2O nanocubes as templates and networking catalysts for hollow and microporous Fe-porphyrin networks. Chem. Commun. 2017, 53, 2598–2601. [Google Scholar] [CrossRef]
- Anderson, N.T.; Dinolfo, P.H.; Wang, X. Synthesis and characterization of porphyrin–DNA constructs for the self-assembly of modular energy transfer arrays. J. Mater. Chem. C 2018, 6, 2452–2459. [Google Scholar] [CrossRef]
- Staegemann, M.H.; Gräfe, S.; Haag, R.; Wiehe, A. A toolset of functionalized porphyrins with different linker strategies for application in bioconjugation. Org. Biomol. Chem. 2016, 14, 9114–9132. [Google Scholar] [CrossRef][Green Version]
- Mohanraj, J.; Barbieri, A.; Armaroli, N.; Vizuete, M.; Langa, F.; Delavaux-Nicot, B.; Vartanian, M.; Iehl, J.; Hahn, U.; Nierengarten, J.-F. Efficient Photoinduced Energy and Electron Transfer in ZnII–Porphyrin/Fullerene Dyads with Interchromophoric Distances up to 2.6 nm and No Wire-like Connectivity. Chem. Eur. J. 2017, 23, 14200–14212. [Google Scholar] [CrossRef]
- Hahn, U.; Nierengarten, J.-F. The copper–catalyzed alkyne-azide cycloaddition for the construction of fullerene–porphyrin conjugates. J. Porphyr. Phthalocyanines 2016, 20, 918–934. [Google Scholar] [CrossRef]
- Ladomenou, K.; Nikolaou, V.; Charalambidis, G.; Coutsolelos, A.G. “Click”-reaction: An alternative tool for new architectures of porphyrin based derivatives. Coord. Chem. Rev. 2016, 306, 1–42. [Google Scholar] [CrossRef]
- Trinh, T.M.N.; Nierengarten, I.; Ben Aziza, H.; Meichsner, E.; Holler, M.; Chessé, M.; Abidi, R.; Bijani, C.; Coppel, Y.; Maisonhaute, E.; et al. Coordination-Driven Folding in Multi-ZnII-Porphyrin Arrays Constructed on a Pillar[5]arene Scaffold. Chem. Eur. J. 2017, 23, 11011–11021. [Google Scholar] [CrossRef]
- Lindsey, J.S.; Wagner, R.W. Investigation of the synthesis of ortho-substituted tetraphenylporphyrins. J. Org. Chem. 1989, 54, 828–836. [Google Scholar] [CrossRef]
- Delavaux-Nicot, B.; Ben Aziza, H.; Nierengarten, I.; Minh Nguyet Trinh, T.; Meichsner, E.; Chessé, M.; Holler, M.; Abidi, R.; Maisonhaute, E.; Nierengarten, J.-F. A Rotaxane Scaffold for the Construction of Multiporphyrinic Light-Harvesting Devices. Chem. Eur. J. 2018, 24, 133–140. [Google Scholar] [CrossRef]
- Özkan, M.; Keser, Y.; Hadi, S.E.; Tuncel, D. A [5]Rotaxane-Based Photosensitizer for Photodynamic Therapy. Eur. J. Org. Chem. 2019, 2019, 3534–3541. [Google Scholar] [CrossRef]
- Kimura, R.; Suzuki, S.; Okada, K.; Kozaki, M. Trimeric Assembly of Dendritic Light-Harvesting Antenna with Two Kinds of Porphyrin Cores. J. Org. Chem. 2017, 82, 8917–8926. [Google Scholar] [CrossRef]
- Gazzali, A.M.; Colombeau, L.; Arnoux, P.; Wahab, H.A.; Frochot, C.; Vanderesse, R.; Acherar, S. Synthesis of mono-, di- and triporphyrin building blocks by click chemistry for photodynamic therapy application. Tetrahedron 2017, 73, 532–541. [Google Scholar] [CrossRef]
- Rezazgui, O.; Trouillas, P.; Qiu, S.-h.; Siegler, B.; Gierschner, J.; Leroy-Lhez, S. Synthesis and conformation of a novel fluorescein–Zn-porphyrin dyad and intramolecular energy transfer. New J. Chem. 2016, 40, 3843–3856. [Google Scholar] [CrossRef]
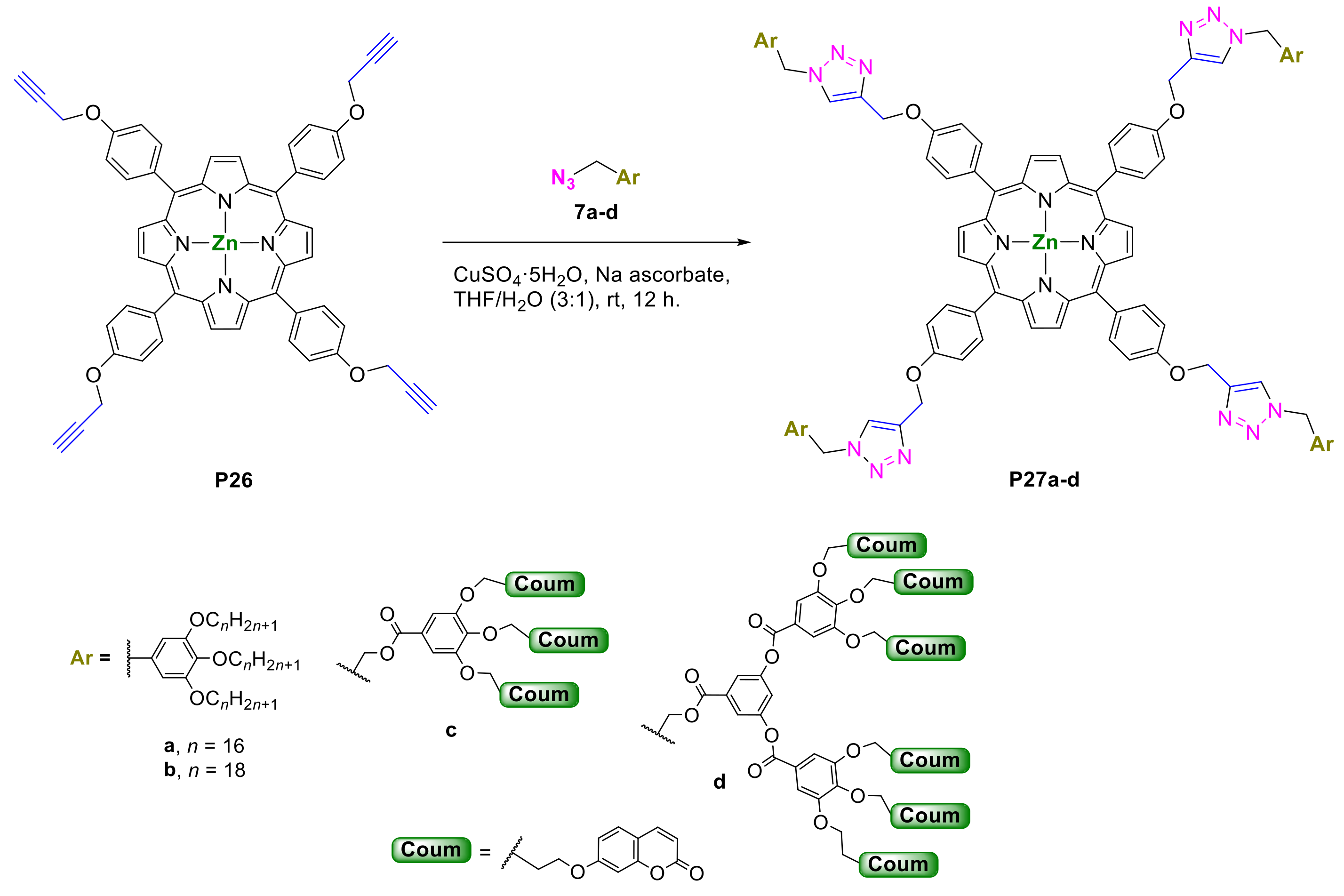
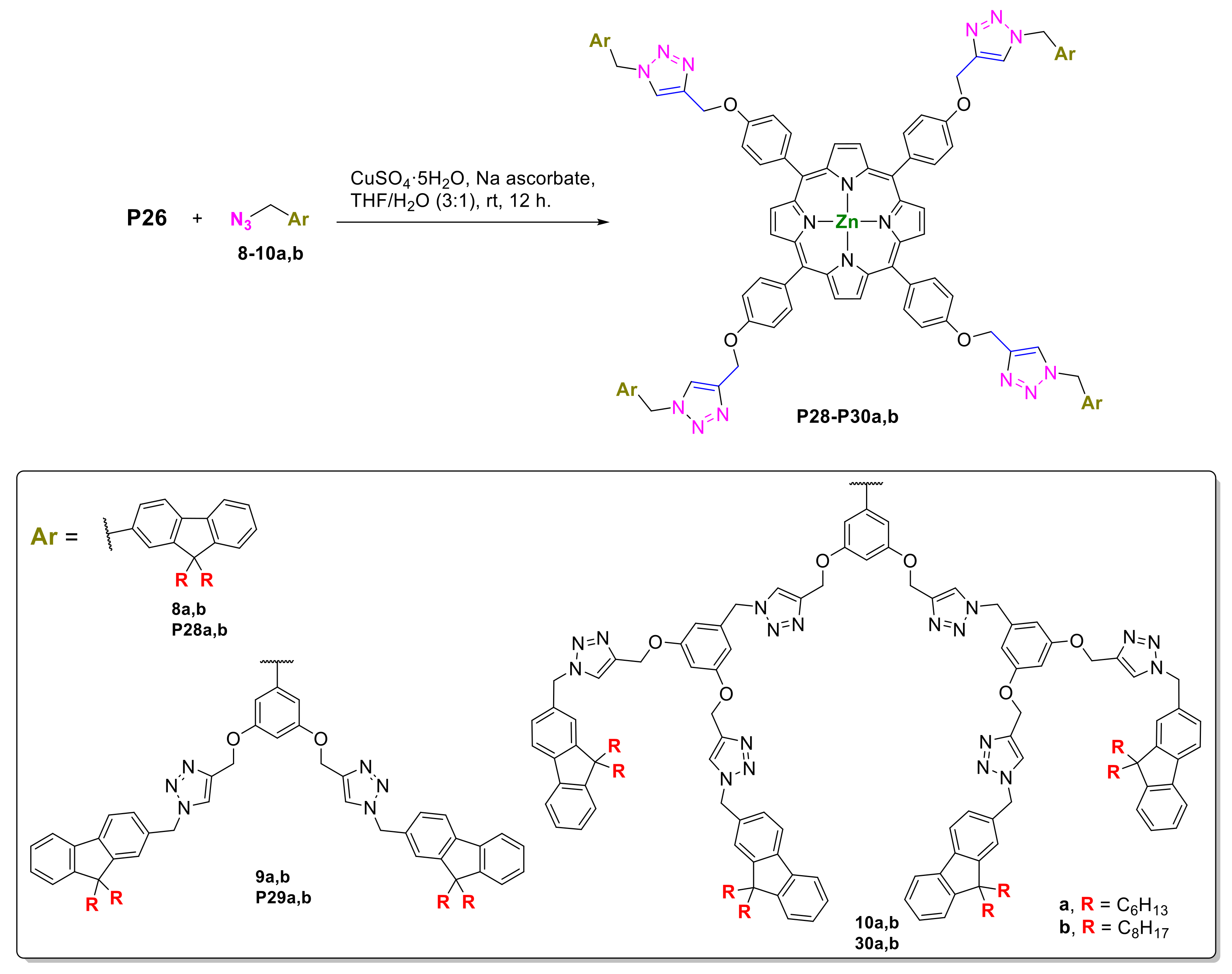
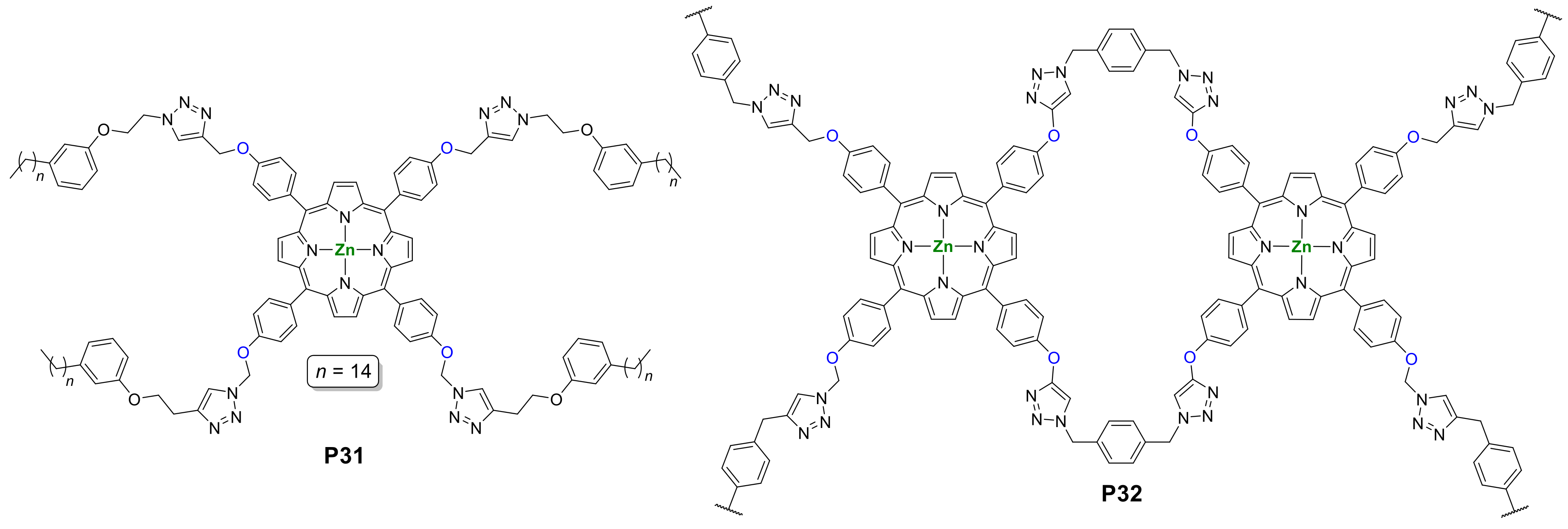
- Zhang, R.; Gao, H.; Ren, Y.; Xiao, Y.; Hu, J.; Cheng, X. Syntheses and Properties of meso-Substituted Porphyrin Mesogens with Triazole Linkages and Peripheral Alkyl Chains. Chem. Asian J. 2018, 13, 536–544. [Google Scholar] [CrossRef]
- Concellón, A.; Termine, R.; Golemme, A.; Romero, P.; Marcos, M.; Serrano, J.L. High hole mobility and light-harvesting in discotic nematic dendrimers prepared via ‘click’ chemistry. J. Mater. Chem. C 2019, 7, 2911–2918. [Google Scholar] [CrossRef]
- Anandkumar, D.; Rajakumar, P. Photophysical and Electrochemical Properties and Anticancer Activities of Porphyrin-Cored Fluorenodendrimers Synthesized by Click Chemistry. Synlett. 2018, 29, 1995–2000. [Google Scholar]
- Prakash Rao, H.S.; Kamalraj, M.; Prabakaran, M. Synthesis and physico-chemical properties of a H-cardanol triazole zinc porphyrin conjugate. RSC Adv. 2019, 9, 4499–4506. [Google Scholar] [CrossRef]
- Zhu, D.; Qin, C.; Ao, S.; Su, Q.; Sun, X.; Jiang, T.; Pei, K.; Ni, H.; Ye, P. Metalloporphyrin-based porous polymers prepared via click chemistry for size-selective adsorption of protein. J. Biomater. Sci. Polym. Ed. 2018, 29, 1250–1264. [Google Scholar] [CrossRef]
- Zheng, Z.; Ayhan, M.M.; Liao, Y.-Y.; Calin, N.; Bucher, C.; Andraud, C.; Bretonnière, Y. Design of two-photon absorbing fluorophores for FRET antenna-core oxygen probes. New J. Chem. 2018, 42, 7914–7930. [Google Scholar] [CrossRef]
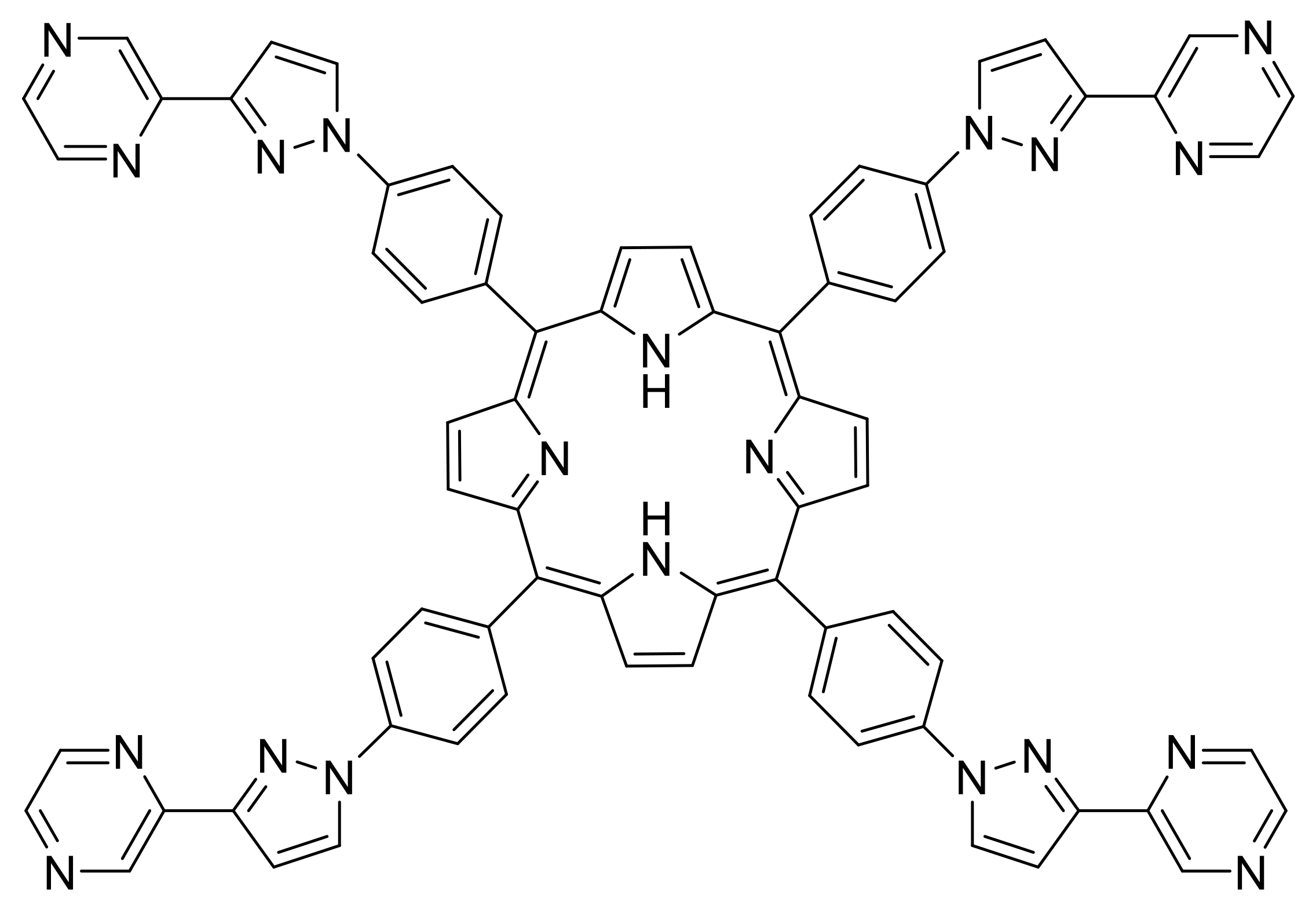
- Yoneyama, K.; Suzuki, R.; Kuramochi, Y.; Satake, A. A Candidate for Multitopic Probes for Ligand Discovery in Dynamic Combinatorial Chemistry. Molecules 2019, 24, 2166. [Google Scholar] [CrossRef]
- Nyuchev, A.V.; Otvagin, V.F.; Gavryushin, A.E.; Romanenko, Y.I.; Koifman, O.I.; Belykh, D.V.; Schmalz, H.-G.; Fedorov, A.Y. Synthesis of Chlorin–(Arylamino)quinazoline Hybrids as Models for Multifunctional Drug Development. Synthesis 2015, 47, 3717–3726. [Google Scholar]
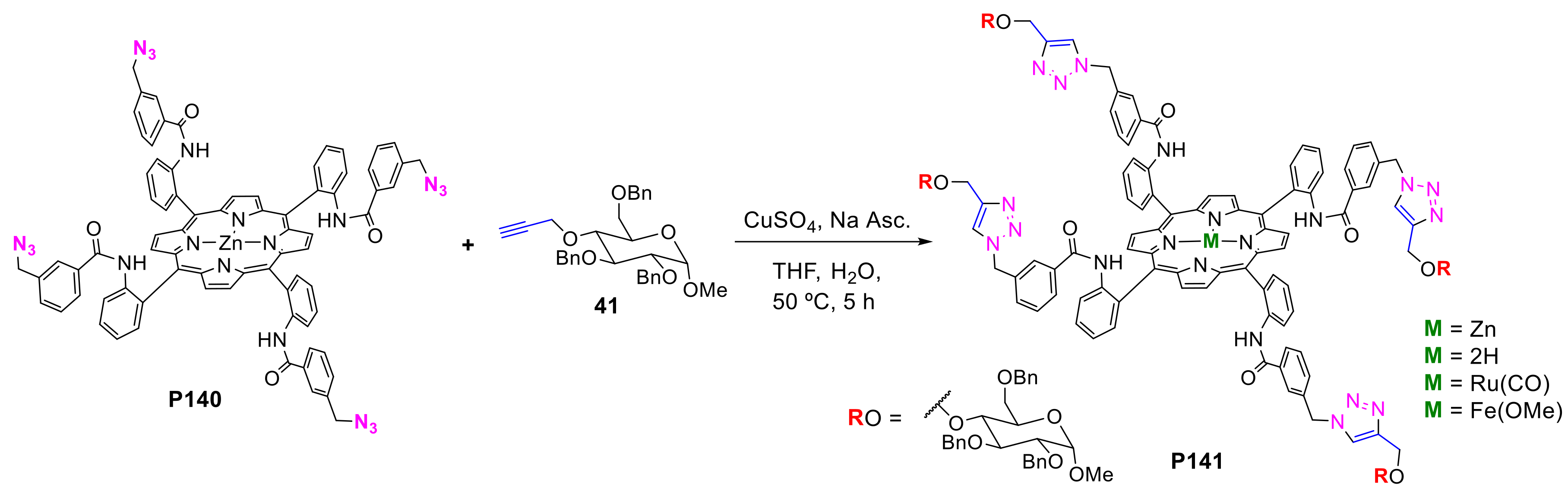
- Mukosera, G.T.; Adams, T.P.; Rothbarth, R.F.; Langat, H.; Akanda, S.; Barkley, R.G.; Dolewski, R.D.; Ruppel, J.V.; Snyder, N.L. Synthesis of glycosylated zinc (II) 5,15-diphenylporphyrin and zinc (II) 5,10,15,20-tetraphenylporphyrin analogs using Cu-catalyzed azide-alkyne 1,3-dipolar cycloaddition reactions. Tetrahedron Lett. 2015, 56, 73–77. [Google Scholar] [CrossRef]
- Bennion, M.C.; Burch, M.A.; Dennis, D.G.; Lech, M.E.; Neuhaus, K.; Fendler, N.L.; Parris, M.R.; Cuadra, J.E.; Dixon, C.F.; Mukosera, G.T.; et al. Synthesis of Porphyrin and Bacteriochlorin Glycoconjugates through CuAAC Reaction Tuning. Eur. J. Org. Chem. 2019, 2019, 6496–6503. [Google Scholar] [CrossRef]
- Mekala, S.; Peters, K.C.; Singer, K.D.; Gross, R.A. Biosurfactant-functionalized porphyrin chromophore that forms J-aggregates. Org. Biomol. Chem. 2018, 16, 7178–7190. [Google Scholar] [CrossRef]
- Moylan, C.; Sweed, A.M.K.; Shaker, Y.M.; Scanlan, E.M.; Senge, M.O. Lead structures for applications in photodynamic therapy 7. Efficient synthesis of amphiphilic glycosylated lipid porphyrin derivatives: Refining linker conjugation for potential PDT applications. Tetrahedron 2015, 71, 4145–4153. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, H.-Y.; Sun, H.-L.; Liu, Y. Supramolecular Nanoassemblies of an Amphiphilic Porphyrin–Cyclodextrin Conjugate and Their Morphological Transition from Vesicle to Network. Chem. Eur. J. 2015, 21, 4457–4464. [Google Scholar] [CrossRef]
- Pereira, P.M.R.; Rizvi, W.; Bhupathiraju, N.V.S.D.K.; Berisha, N.; Fernandes, R.; Tomé, J.P.C.; Drain, C.M. Carbon-1 versus Carbon-3 Linkage of d-Galactose to Porphyrins: Synthesis, Uptake, and Photodynamic Efficiency. Bioconjugate Chem. 2018, 29, 306–315. [Google Scholar] [CrossRef]
- Anandkumar, D.; Raja, R.; Rajakumar, P. Synthesis, photophysical properties and anticancer activity of micro-environment sensitive amphiphilic bile acid dendrimers. RSC Adv. 2016, 6, 25808–25818. [Google Scholar] [CrossRef]
- Aguilar-Ortíz, E.; Lévaray, N.; Vonlanthen, M.; Morales-Espinoza, E.G.; Rojas-Aguirre, Y.; Zhu, X.X.; Rivera, E. Synthesis and characterization of novel dendritic compounds bearing a porphyrin core and cholic acid units using “click chemistry”. Dye. Pigment. 2016, 132, 110–120. [Google Scholar] [CrossRef]
- Arja, K.; Elgland, M.; Nilsson, K.P.R. Synthesis and Characterization of Oligothiophene–Porphyrin-Based Molecules That Can Be Utilized for Optical Assignment of Aggregated Amyloid-β Morphotypes. Front. Chem. 2018, 6, 391. [Google Scholar] [CrossRef] [PubMed]
- Arja, K.; Elgland, M.; Appelqvist, H.; Konradsson, P.; Lindgren, M.; Nilsson, K.P.R. Synthesis and Characterization of Novel Fluoro-glycosylated Porphyrins that can be Utilized as Theranostic Agents. ChemistryOpen 2018, 7, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Topka, M.R.; Dinolfo, P.H. Synthesis, Characterization, and Fluorescence Properties of Mixed Molecular Multilayer Films of BODIPY and Zn(II) Tetraphenylporphyrins. ACS Appl. Mater. Interfaces 2015, 7, 8053–8060. [Google Scholar] [CrossRef]
- Civic, M.R.; Dinolfo, P.H. Electrochemical Rectification of Redox Mediators Using Porphyrin-Based Molecular Multilayered Films on ITO Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 20465–20473. [Google Scholar] [CrossRef]
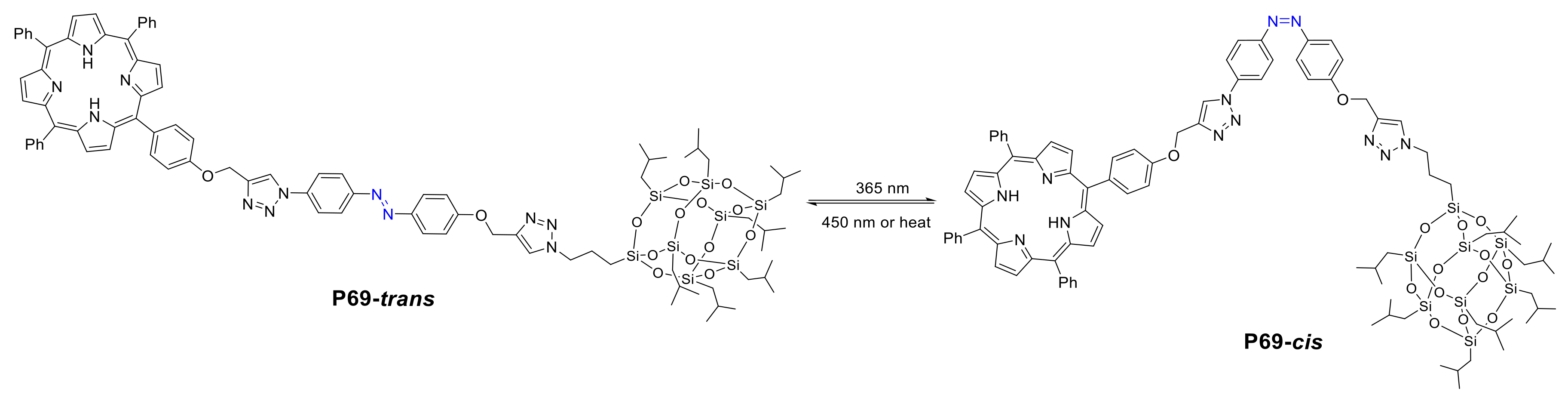
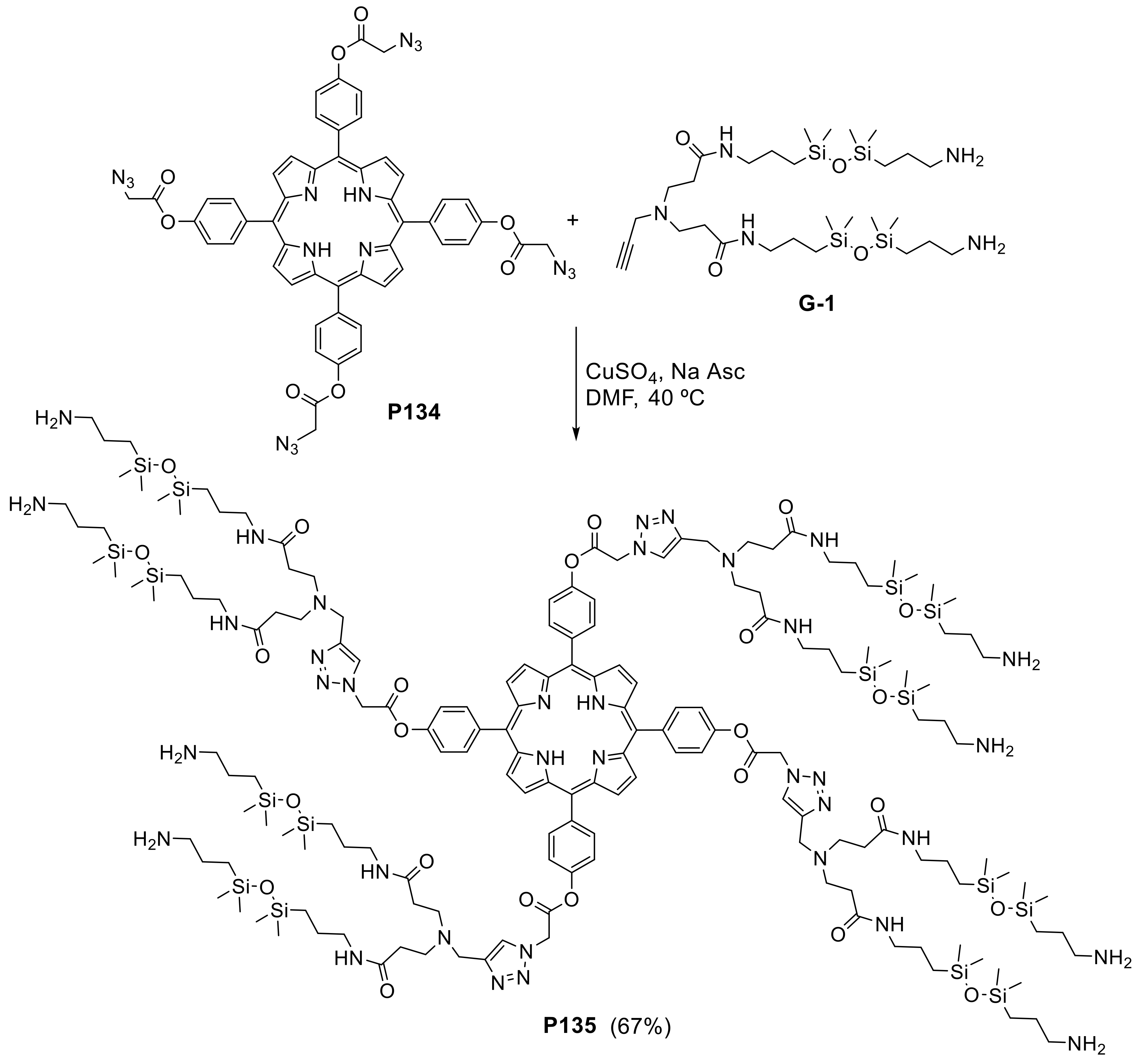
- Liu, F.; Zhang, Y.; Xu, L.; Zhang, W. Morphology-Controlled Self-Assembly of an Organic/Inorganic Hybrid Porphyrin Derivative Containing Polyhedral Oligomeric Silsesquioxane (POSS). Chem. Eur. J. 2015, 21, 5540–5547. [Google Scholar] [CrossRef]
- Zhang, Y.; Yue, T.; Cao, H.; Gao, Y.; Zhang, W. Photocontrollable Supramolecular Self-Assembly of a Porphyrin Derivative that Contains a Polyhedral Oligomeric Silsesquioxane (POSS). Asian J. Org. Chem. 2017, 6, 1034–1042. [Google Scholar] [CrossRef]
- Zhang, W.; Müller, A.H.E. Synthesis of tadpole-shaped POSS-containing hybrid polymers via “click chemistry”. Polymer 2010, 51, 2133–2139. [Google Scholar] [CrossRef]
- Li, X.; Li, J.L.; Huang, W.G.; Zhang, X.-Z.; Zhang, B.; Cai, T. Metalloporphyrin-bound Janus nanocomposites with dual stimuli responsiveness for nanocatalysis in living radical polymerization. Nanoscale 2018, 10, 19254–19261. [Google Scholar] [CrossRef]
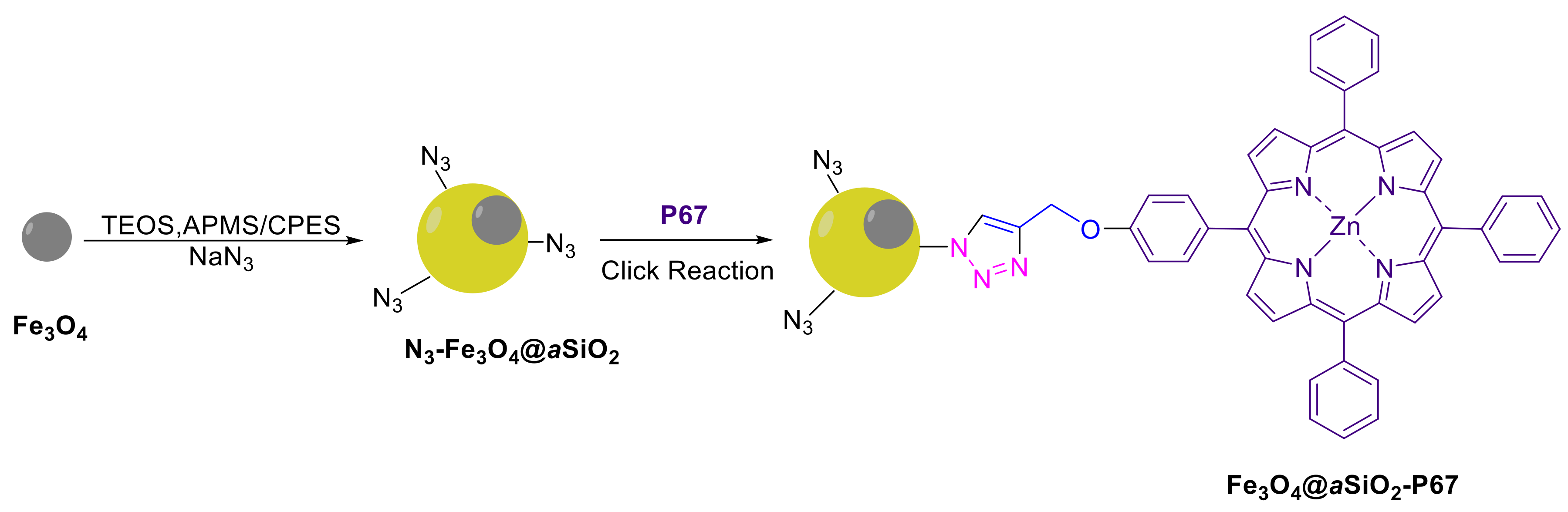
- Hollingsworth, J.V.; Bhupathiraju, N.V.S.D.K.; Sun, J.; Lochner, E.; Vicente, M.G.H.; Russo, P.S. Preparation of Metalloporphyrin-Bound Superparamagnetic Silica Particles via “Click” Reaction. ACS Appl. Mater. Interfaces 2016, 8, 792–801. [Google Scholar] [CrossRef]
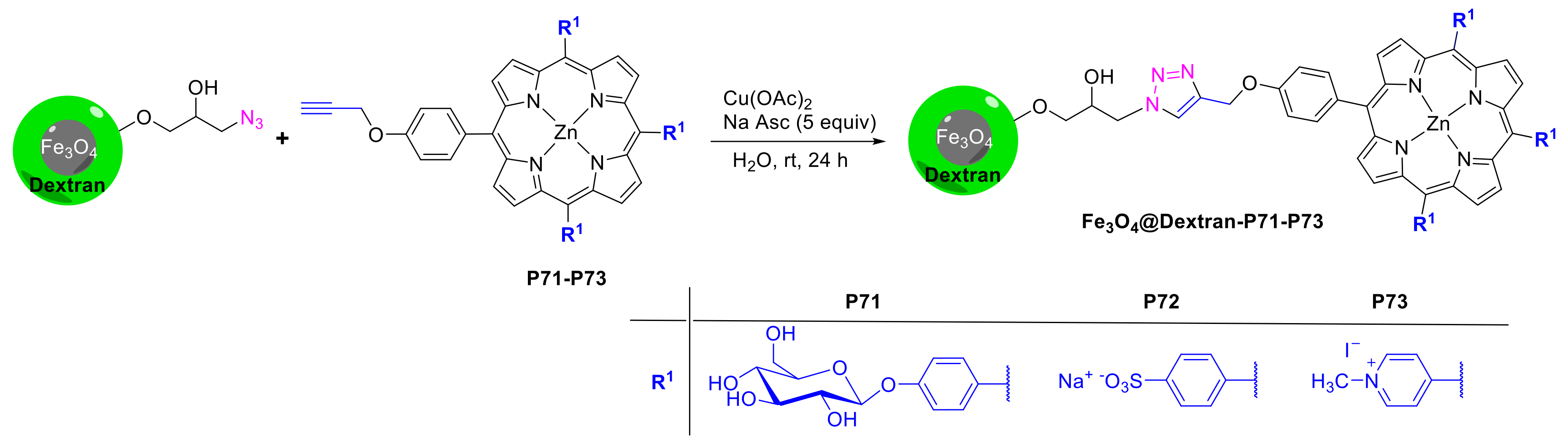
- Mbakidi, J.-P.; Brégier, F.; Ouk, T.-S.; Granet, R.; Alves, S.; Rivière, E.; Chevreux, S.; Lemercier, G.; Sol, V. Magnetic Dextran Nanoparticles That Bear Hydrophilic Porphyrin Derivatives: Bimodal Agents for Potential Application in Photodynamic Therapy. ChemPlusChem 2015, 80, 1416–1426. [Google Scholar] [CrossRef] [PubMed]
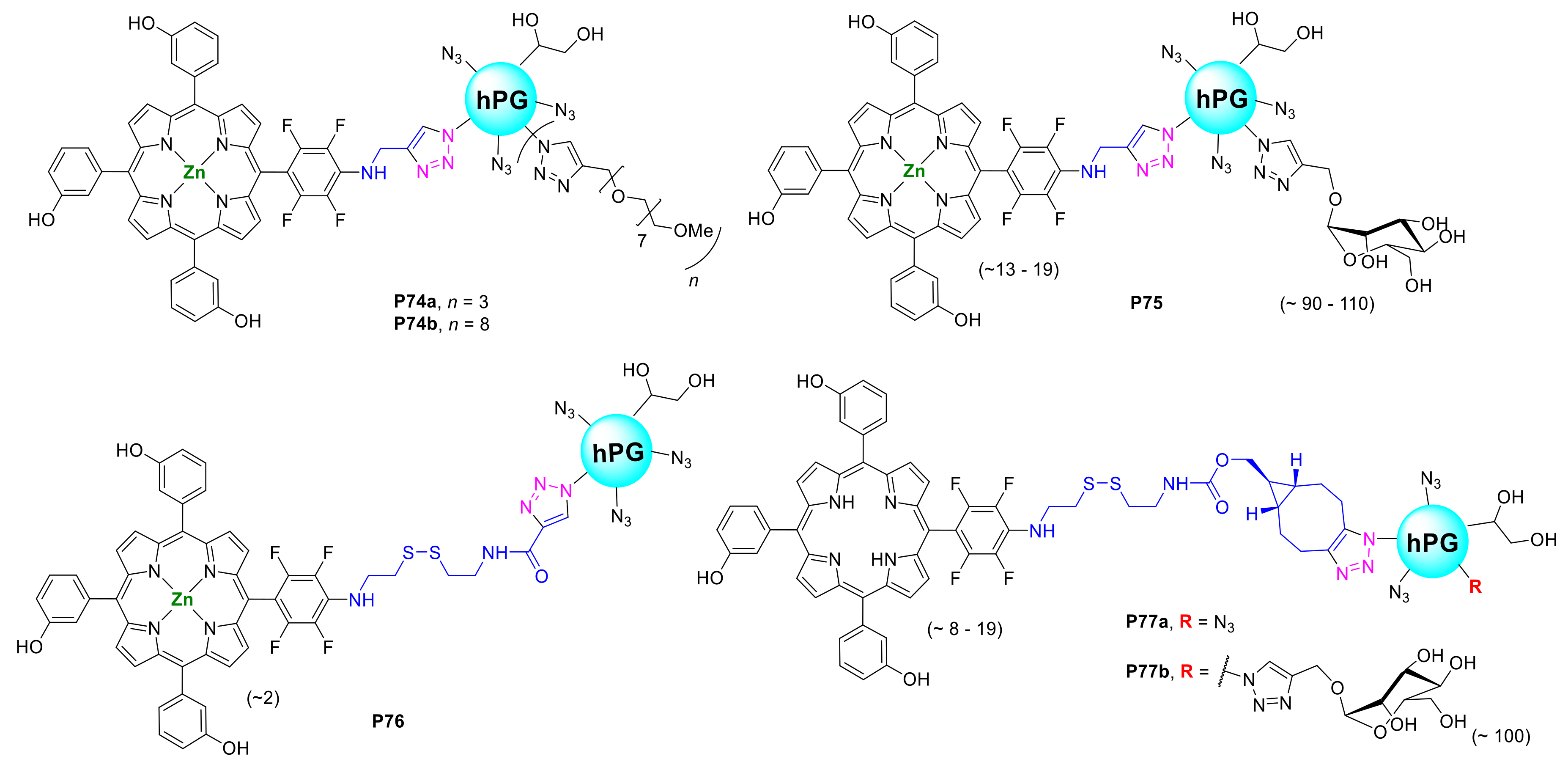
- Staegemann, M.H.; Gräfe, S.; Gitter, B.; Achazi, K.; Quaas, E.; Haag, R.; Wiehe, A. Hyperbranched Polyglycerol Loaded with (Zinc-)Porphyrins: Photosensitizer Release Under Reductive and Acidic Conditions for Improved Photodynamic Therapy. Biomacromolecules 2018, 19, 222–238. [Google Scholar] [CrossRef] [PubMed]
- Staegemann, M.H.; Gitter, B.; Dernedde, J.; Kuehne, C.; Haag, R.; Wiehe, A. Mannose-Functionalized Hyperbranched Polyglycerol Loaded with Zinc Porphyrin: Investigation of the Multivalency Effect in Antibacterial Photodynamic Therapy. Chem. Eur. J. 2017, 23, 3918–3930. [Google Scholar] [CrossRef] [PubMed]
- Mauriello-Jimenez, C.; Croissant, J.; Maynadier, M.; Cattoën, X.; Wong Chi Man, M.; Vergnaud, J.; Chaleix, V.; Sol, V.; Garcia, M.; Gary-Bobo, M.; et al. Porphyrin-functionalized mesoporous organosilica nanoparticles for two-photon imaging of cancer cells and drug delivery. J. Mater. Chem. B 2015, 3, 3681–3684. [Google Scholar] [CrossRef]
- Rossi, F.; Bedogni, E.; Bigi, F.; Rimoldi, T.; Cristofolini, L.; Pinelli, S.; Alinovi, R.; Negri, M.; Dhanabalan, S.C.; Attolini, G.; et al. Porphyrin conjugated SiC/SiOx nanowires for X-ray-excited photodynamic therapy. Sci. Rep. 2015, 5, 7606. [Google Scholar] [CrossRef] [PubMed]
- Fadavi, F.; Abdulkhani, A.; Hamzeh, Y.; Bacher, M.; Gorfer, M.; Bandian, D.; Rosenau, T.; Hettegger, H. Photodynamic Antimicrobial Cellulosic Material Through Covalent Linkage of Protoporphyrin IX onto Lyocell Fibers. J. Wood Chem. Technol. 2019, 39, 57–74. [Google Scholar] [CrossRef]
- Khaldi, Z.; Takeki, J.K.N.; Ouk, T.-S.; Lucas, R.; Zerrouki, R. Synthesis and photo-bactericidal properties of a cationic porphyrin grafted onto kraft pulp fibers. J. Porphyr. Phthalocyanines 2019, 23, 489–496. [Google Scholar] [CrossRef]
- Pereira, M.A.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Almeida, A. Influence of external bacterial structures on the efficiency of photodynamic inactivation by a cationic porphyrin. Photochem. Photobiol. Sci. 2014, 13, 680–690. [Google Scholar] [CrossRef]
- Almeida, J.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Costa, L.; Faustino, M.A.F.; Almeida, A. Photodynamic inactivation of multidrug-resistant bacteria in hospital wastewaters: Influence of residual antibiotics. Photochem. Photobiol. Sci. 2014, 13, 626–633. [Google Scholar] [CrossRef]
- Simões, C.; Gomes, M.C.; Neves, M.G.P.M.S.; Cunha, Â.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Faustino, M.A.F. Photodynamic inactivation of Escherichia coli with cationic meso-tetraarylporphyrins – The charge number and charge distribution effects. Catal. Today 2016, 266, 197–204. [Google Scholar] [CrossRef]
- Tavares, A.; Carvalho, C.M.B.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; et al. Antimicrobial Photodynamic Therapy: Study of Bacterial Recovery Viability and Potential Development of Resistance after Treatment. Mar. Drugs 2010, 8, 91–105. [Google Scholar] [CrossRef] [PubMed]
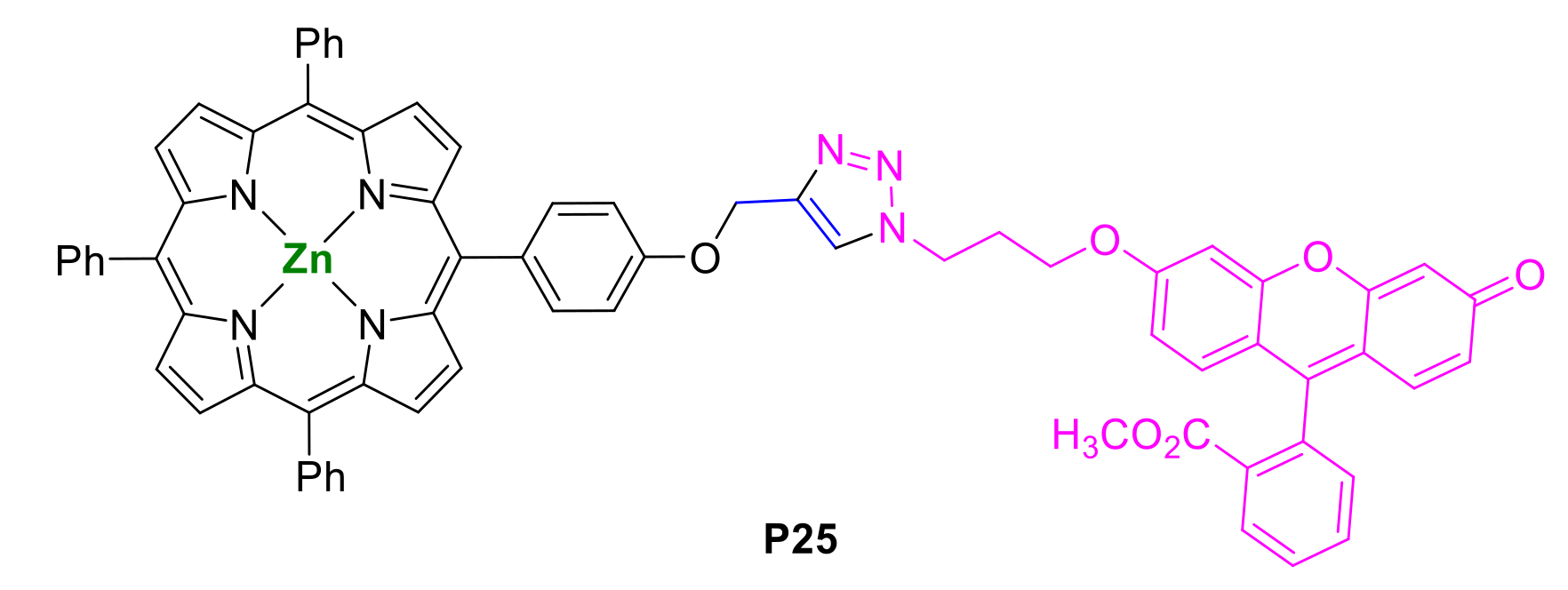
- Singh, D.K.; Nath, M. meso-Phenyl-triazole bridged porphyrin-coumarin dyads: Synthesis, characterization and photophysical properties. Dye. Pigment. 2015, 121, 256–264. [Google Scholar] [CrossRef]
- Takada, T.; Iwaki, T.; Nakamura, M.; Yamana, K. Photoresponsive Electrodes Modified with DNA Duplexes Possessing a Porphyrin Dimer. Chem. Eur. J. 2017, 23, 18258–18263. [Google Scholar] [CrossRef]
- Nikolaou, V.; Charisiadis, A.; Chalkiadaki, S.; Alexandropoulos, I.; Pradhan, S.C.; Soman, S.; Panda, M.K.; Coutsolelos, A.G. Enhancement of the photovoltaic performance in D3A porphyrin-based DSCs by incorporating an electron withdrawing triazole spacer. Polyhedron 2018, 140, 9–18. [Google Scholar] [CrossRef]
- Heredia, D.A.; Martínez, S.R.; Durantini, A.M.; Pérez, M.E.; Mangione, M.I.; Durantini, J.E.; Gervaldo, M.A.; Otero, L.A.; Durantini, E.N. Antimicrobial Photodynamic Polymeric Films Bearing Biscarbazol Triphenylamine End-Capped Dendrimeric Zn(II) Porphyrin. ACS Appl. Mater. Interfaces 2019, 11, 27574–27587. [Google Scholar] [CrossRef] [PubMed]
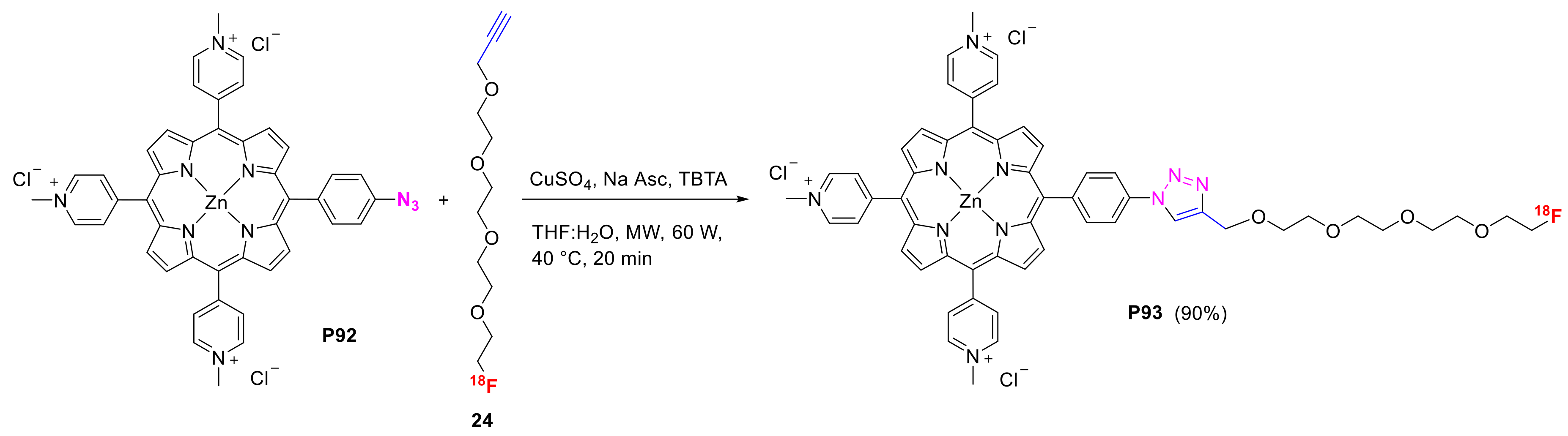
- Entract, G.M.; Bryden, F.; Domarkas, J.; Savoie, H.; Allott, L.; Archibald, S.J.; Cawthorne, C.; Boyle, R.W. Development of PDT/PET Theranostics: Synthesis and Biological Evaluation of an 18F-Radiolabeled Water-Soluble Porphyrin. Mol. Pharm. 2015, 12, 4414–4423. [Google Scholar] [CrossRef] [PubMed]
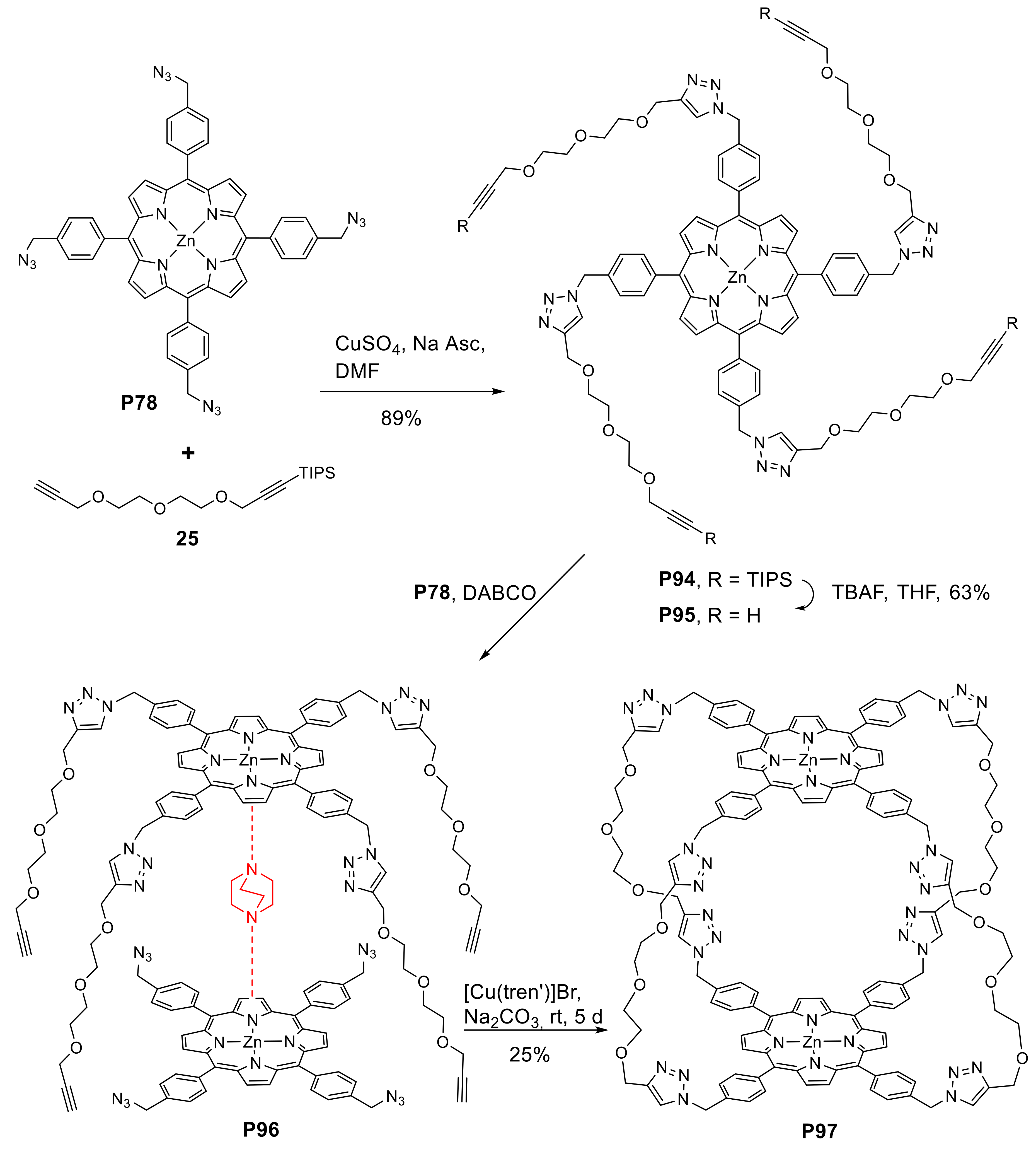
- Kocher, L.; Durot, S.; Heitz, V. Control of the cavity size of flexible covalent cages by silver coordination to the peripheral binding sites. Chem. Commun. 2015, 51, 13181–13184. [Google Scholar] [CrossRef]
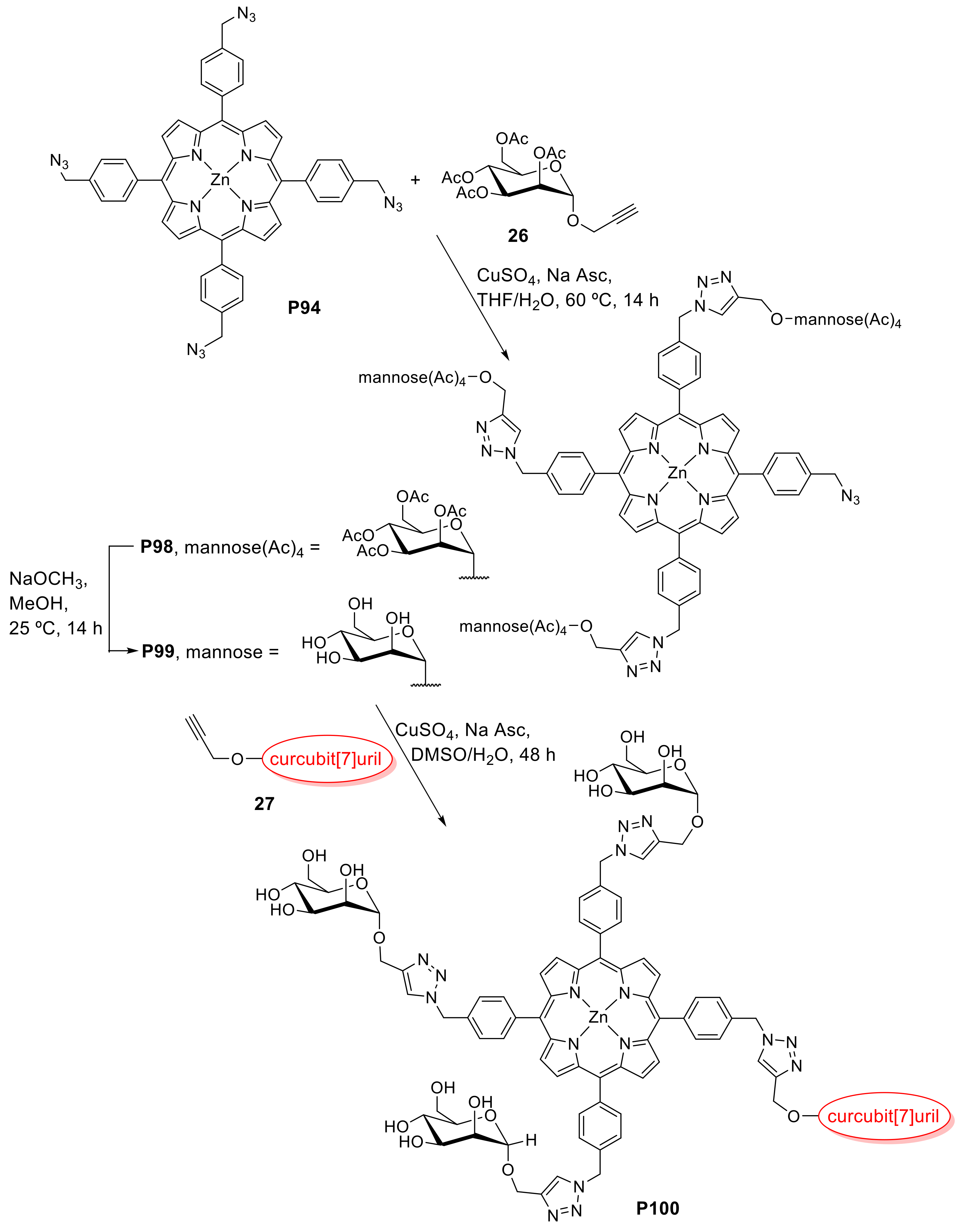
- Koc, A.; Khan, R.; Tuncel, D. “Clicked” Porphyrin-Cucurbituril Conjugate: A New Multifunctional Supramolecular Assembly Based on Triglycosylated Porphyrin and Monopropargyloxycucurbit[7]uril. Chem. Eur. J. 2018, 24, 15550–15555. [Google Scholar] [CrossRef]
- Özkan, M.; Keser, Y.; Koc, A.; Tuncel, D. Glycosylated porphyrin-cucurbituril conjugate for photodynamic inactivation of bacteria and doxorubicin carriage for anticancer drug delivery. J. Porphyr. Phthalocyanines 2019, 23, 1406–1413. [Google Scholar] [CrossRef]
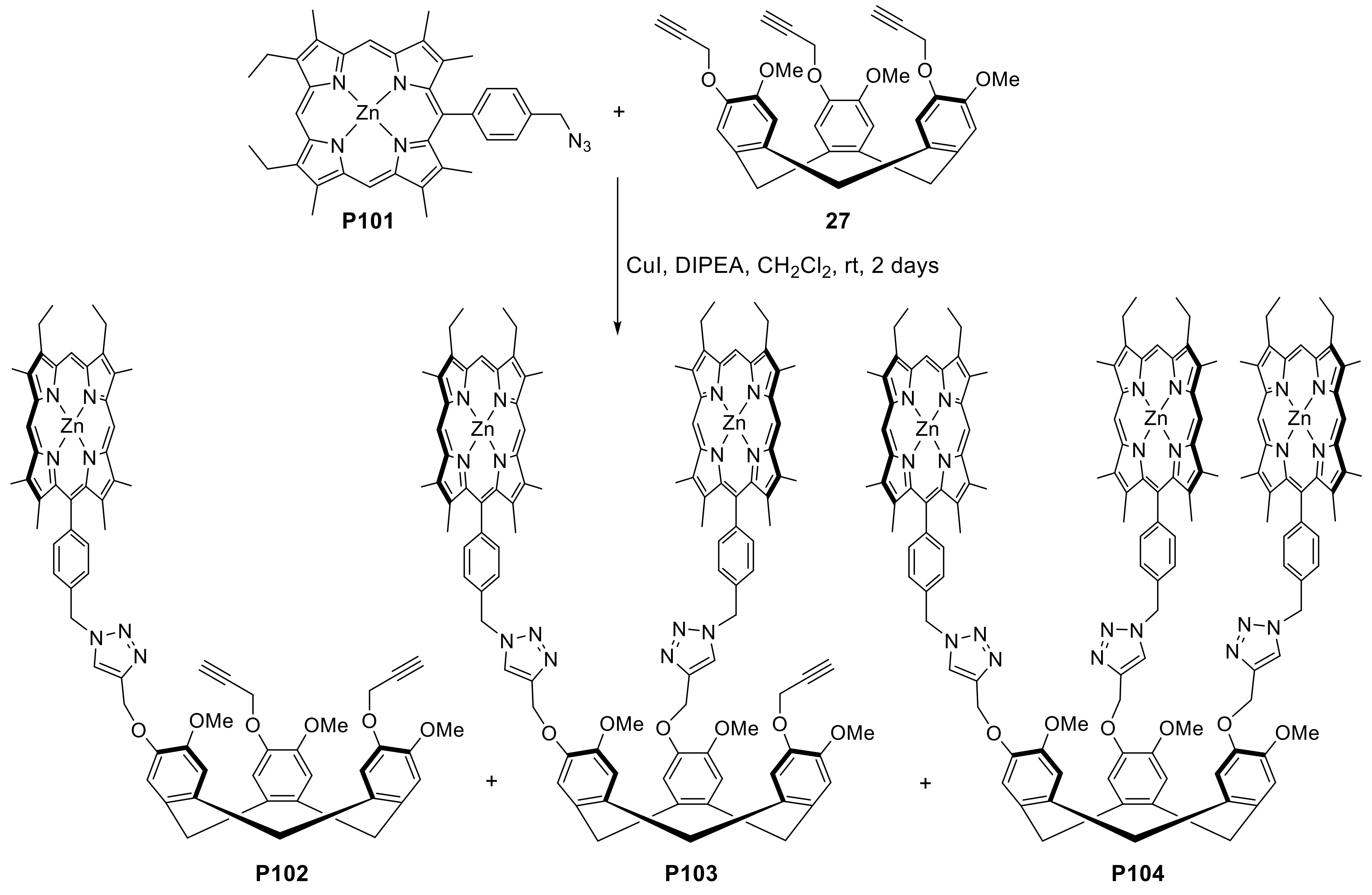
- Deschamps, J.; Langlois, A.; Martin, G.; Bucher, L.; Desbois, N.; Gros, C.P.; Harvey, P.D. Cyclotriveratrylene-Containing Porphyrins. Inorg. Chem. 2016, 55, 9230–9239. [Google Scholar] [CrossRef]
- Singh, D.K.; Nath, M. Synthesis and photophysical properties of β-triazole bridged porphyrin–coumarin dyads. RSC Adv. 2015, 5, 68209–68217. [Google Scholar] [CrossRef]
- Singh, D.K.; Nath, M. Synthesis, characterization and photophysical studies of ß-triazolomethyl-bridged porphyrin-benzo- a-pyrone dyads. J. Chem. Sci. 2016, 128, 545–554. [Google Scholar] [CrossRef]
- Singh, D.K.; Nath, M. Synthesis and spectroscopic properties of β-triazoloporphyrin–xanthone dyads. Beilstein J. Org. Chem. 2015, 11, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
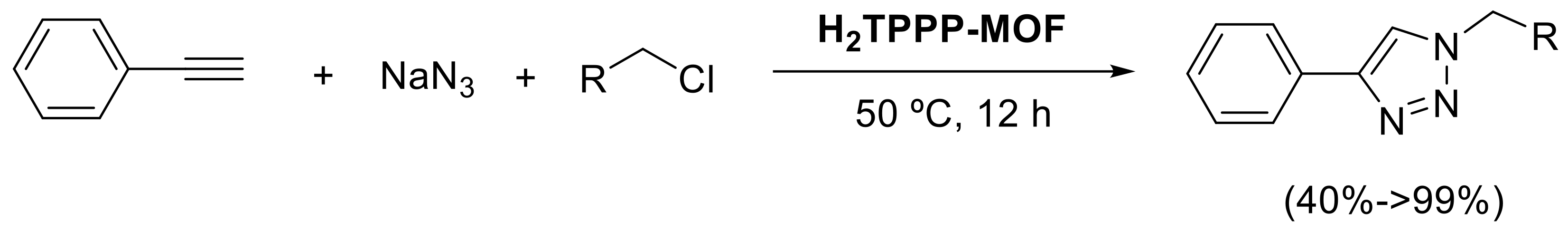
- Cui, D.; Yao, C.; Xu, Y. Conjugated microporous polymers with azide groups: A new strategy for postsynthetic fluoride functionalization and effectively enhanced CO2 adsorption properties. Chem. Commun. 2017, 53, 11422–11425. [Google Scholar] [CrossRef] [PubMed]
- Tomanová, P.; Rimpelová, S.; Jurášek, M.; Buděšínský, M.; Vejvodová, L.; Ruml, T.; Kmoníčková, E.; Drašar, P.B. Trilobolide–porphyrin conjugates: On synthesis and biological effects evaluation. Steroids 2015, 97, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Dondi, R.; Yaghini, E.; Tewari, K.M.; Wang, L.; Giuntini, F.; Loizidou, M.; MacRobert, A.J.; Eggleston, I.M. Flexible synthesis of cationic peptide–porphyrin derivatives for light-triggered drug delivery and photodynamic therapy. Org. Biomol. Chem. 2016, 14, 11488–11501. [Google Scholar] [CrossRef]
- Nikolaou, V.; Angaridis, P.A.; Charalambidis, G.; Sharma, G.D.; Coutsolelos, A.G. A “click-chemistry” approach for the synthesis of porphyrin dyads as sensitizers for dye-sensitized solar cells. Dalton Trans. 2015, 44, 1734–1747. [Google Scholar] [CrossRef]
- Séverac, M.; Pleux, L.L.; Scarpaci, A.; Blart, E.; Odobel, F. Synthesis of new azido porphyrins and their reactivity in copper(I)-catalyzed Huisgen 1,3-dipolar cycloaddition reaction with alkynes. Tetrahedron Lett. 2007, 48, 6518–6522. [Google Scholar] [CrossRef]
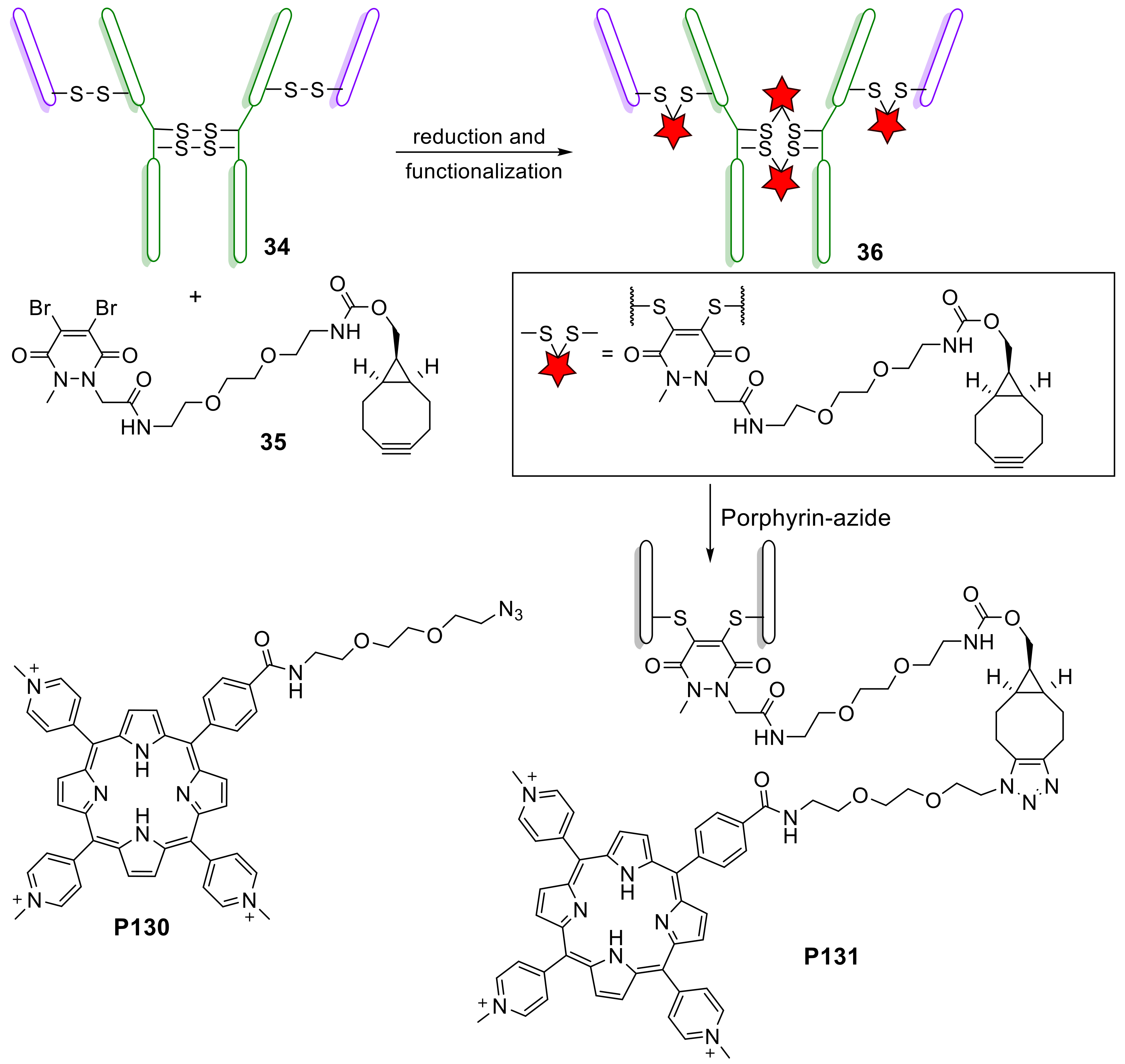
- Maruani, A.; Savoie, H.; Bryden, F.; Caddick, S.; Boyle, R.; Chudasama, V. Site-selective multi-porphyrin attachment enables the formation of a next-generation antibody-based photodynamic therapeutic. Chem. Commun. 2015, 51, 15304–15307. [Google Scholar] [CrossRef]
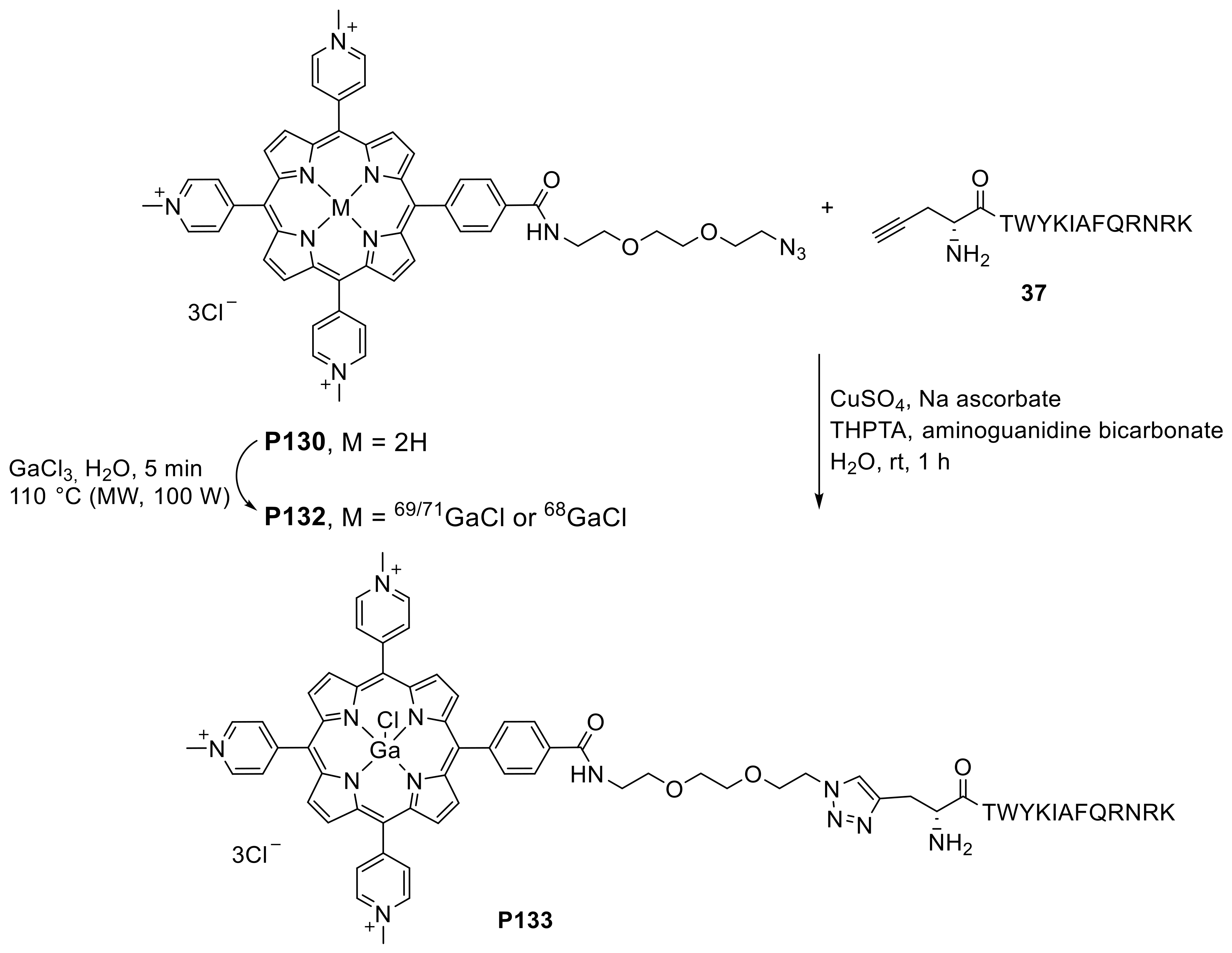
- Bryden, F.; Savoie, H.; Rosca, E.V.; Boyle, R.W. PET/PDT theranostics: Synthesis and biological evaluation of a peptide-targeted gallium porphyrin. Dalton Trans. 2015, 44, 4925–4932. [Google Scholar] [CrossRef]
- Dai, X.-H.; Yang, W.-H.; Yan, W.-L.; Hu, J.-M.; Dai, Y.-R.; Pan, J.-M.; Yan, Y.-S. Porphyrin-cored dendrimers consisting of novel siloxane-poly (amido amine) dendron-like arms: Synthesis, characterization, and photophysical properties. Colloids Surf. A 2017, 520, 222–230. [Google Scholar] [CrossRef]
- Chang, D.-D.; Yang, W.-H.; Dai, X.-H.; Wang, J.-X.; Chen, L.; Pan, J.-M.; Yan, Y.-S.; Dai, Y.-R. Click synthesis of glycosylated porphyrin-cored PAMAM dendrimers with specific recognition and thermosensitivity. J. Polym. Res. 2018, 25, 257. [Google Scholar] [CrossRef]
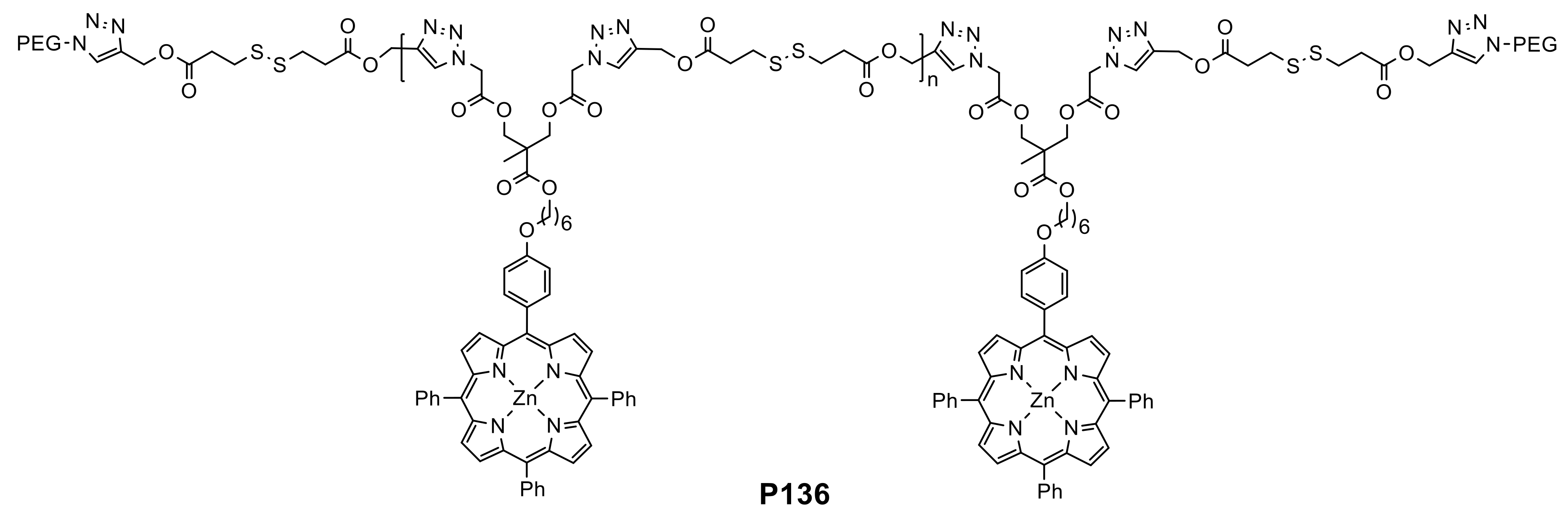
- Xue, Y.; Tian, J.; Xu, L.; Liu, Z.; Shen, Y.; Zhang, W. Ultrasensitive redox-responsive porphyrin-based polymeric nanoparticles for enhanced photodynamic therapy. Eur. Polym. J. 2019, 110, 344–354. [Google Scholar] [CrossRef]
- Kosman, J.; Stanislawska, A.; Gluszynska, A.; Juskowiak, B. Conjugation of hemin to G-quadruplex forming oligonucleotide using click chemistry. Int. J. Biol. Macromol. 2017, 101, 799–804. [Google Scholar] [CrossRef]
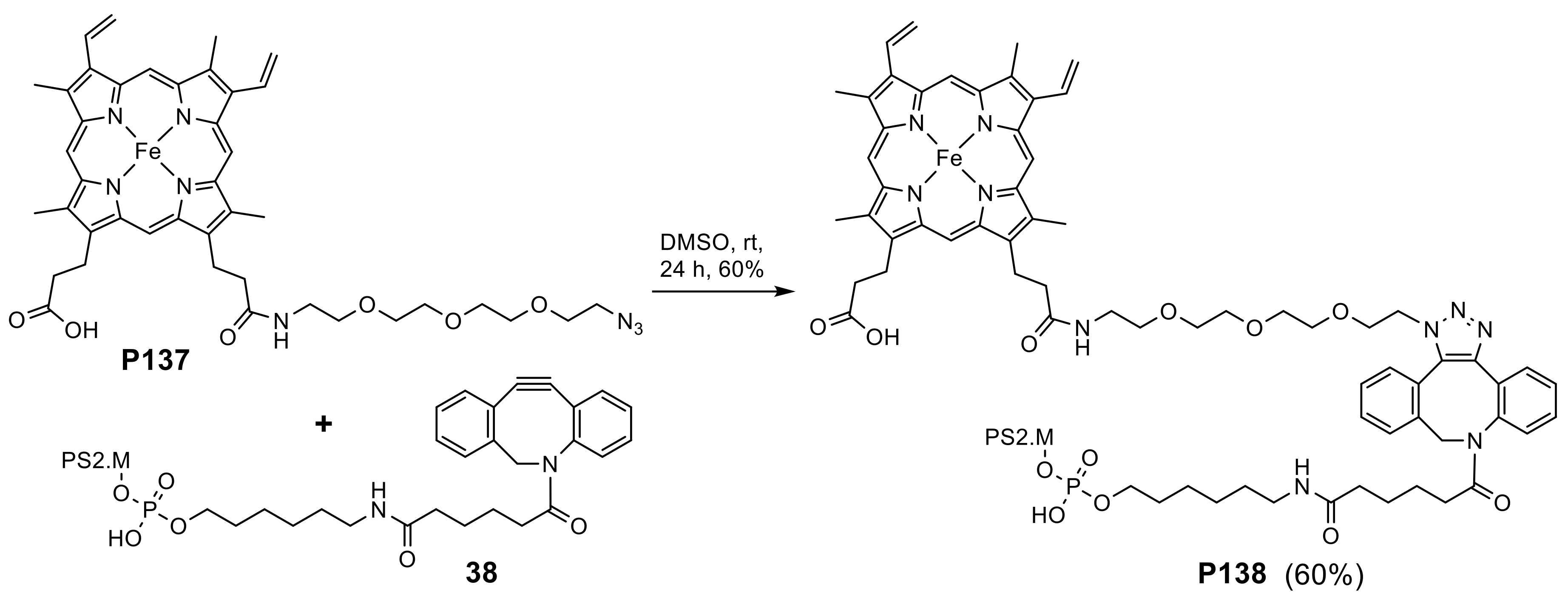
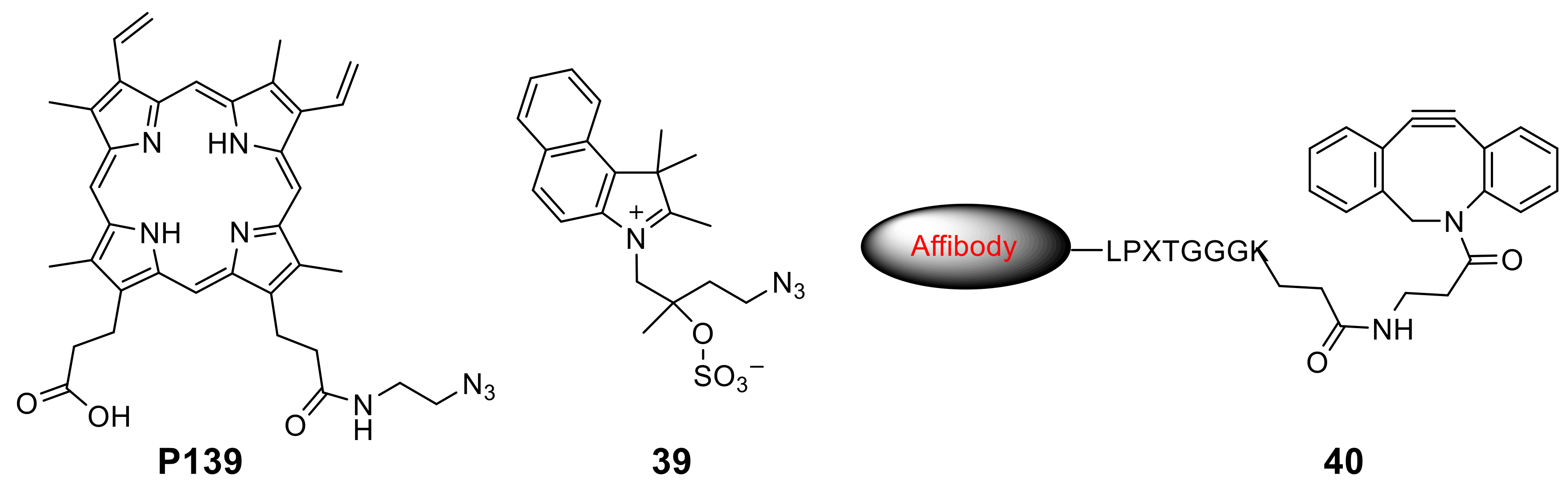
- Amirshaghaghi, A.; Altun, B.; Nwe, K.; Yan, L.; Stein, J.M.; Cheng, Z.; Tsourkas, A. Site-Specific Labeling of Cyanine and Porphyrin Dye-Stabilized Nanoemulsions with Affibodies for Cellular Targeting. J. Am. Chem. Soc. 2018, 140, 13550–13553. [Google Scholar] [CrossRef]
- Damiano, C.; Gadolini, S.; Intrieri, D.; Lay, L.; Colombo, C.; Gallo, E. Iron and Ruthenium Glycoporphyrins: Active Catalysts for the Synthesis of Cyclopropanes and Aziridines. Eur. J. Inorg. Chem. 2019, 2019, 4412–4420. [Google Scholar] [CrossRef]
- Vroemans, R.; Tran, M.T.D.; Sayed, M.G.; Boodts, S.; Dehaen, W. Synthesis and characterization of novel axially chiral β-linked 1,2,3-triazolyl porphyrins. Dye. Pigment. 2018, 156, 61–66. [Google Scholar] [CrossRef]
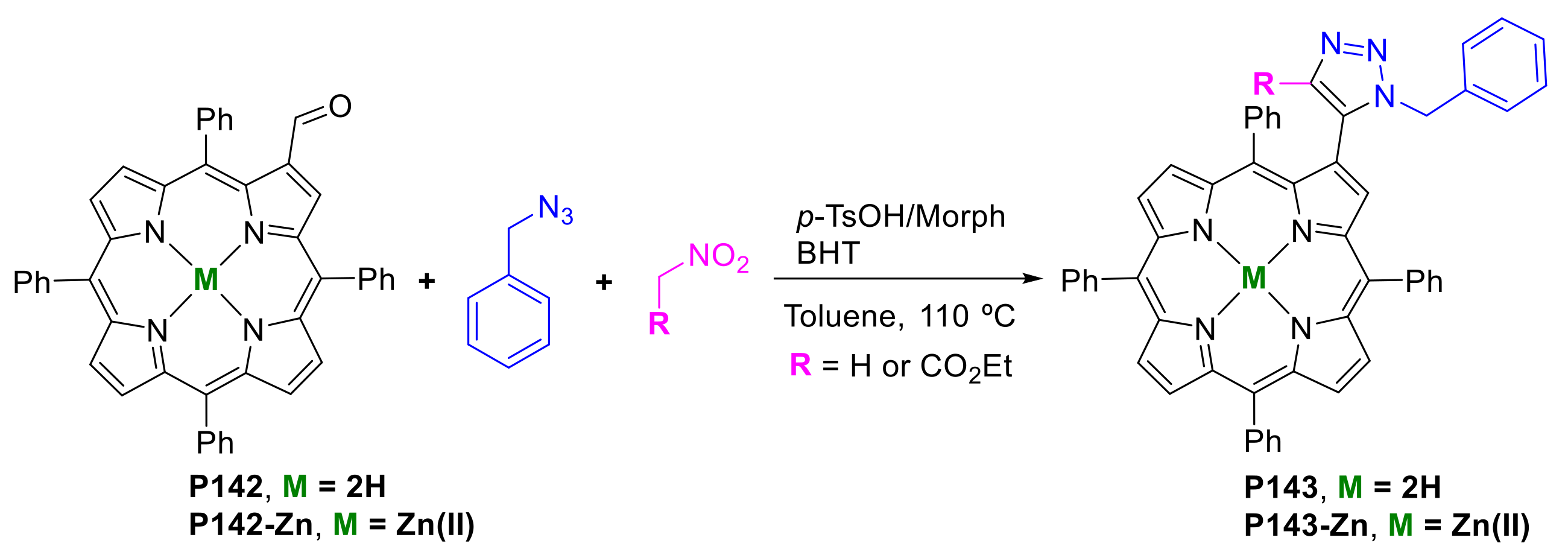
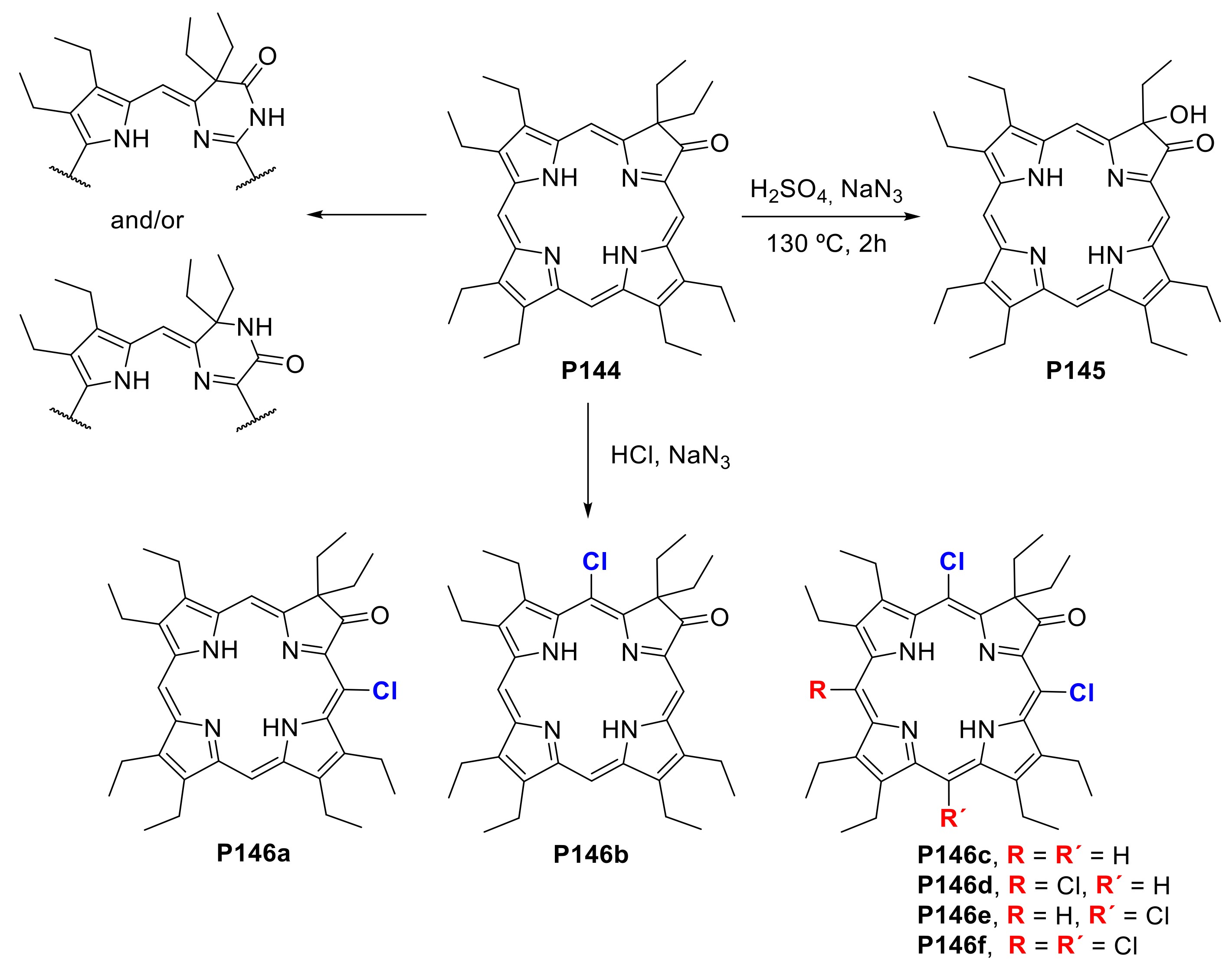
- Li, R.; Zeller, M.; Bruhn, T.; Brückner, C. Surprising Outcomes of Classic Ring-Expansion Conditions Applied to Octaethyloxochlorin, 3. Schmidt-Reaction Conditions. Eur. J. Org. Chem. 2017, 2017, 1835–1842. [Google Scholar] [CrossRef]
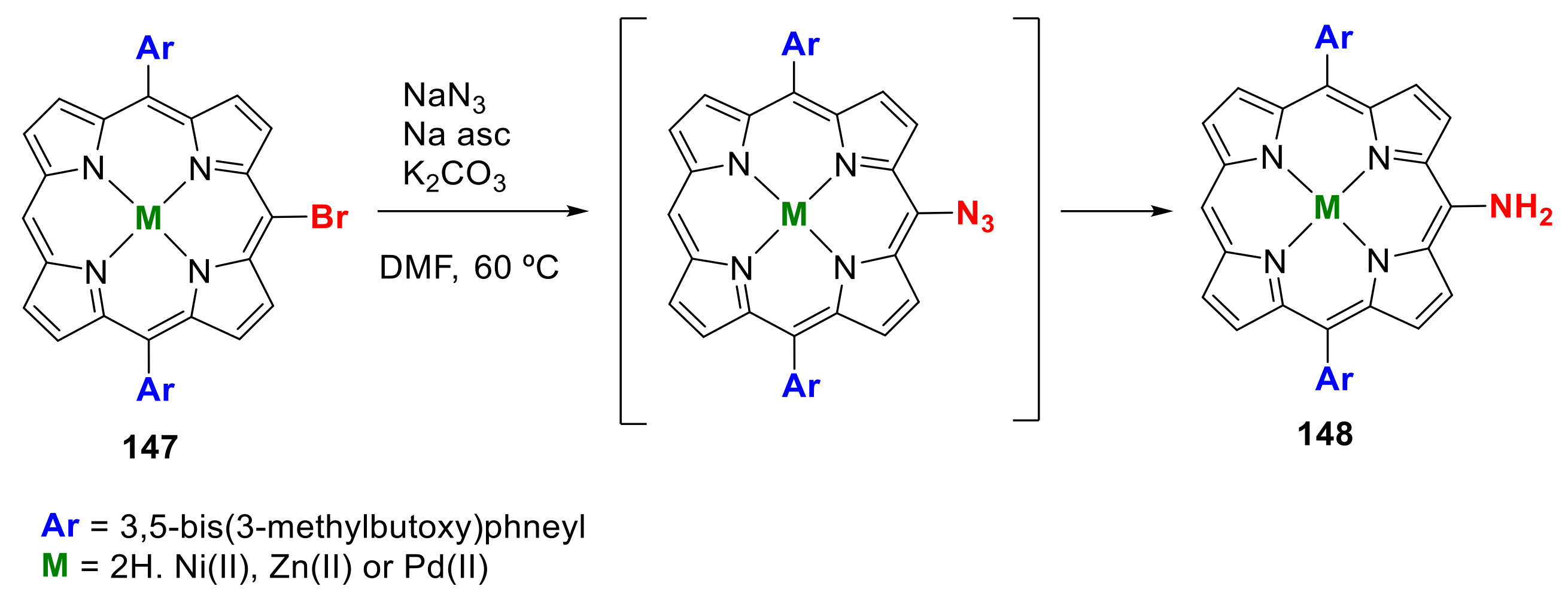
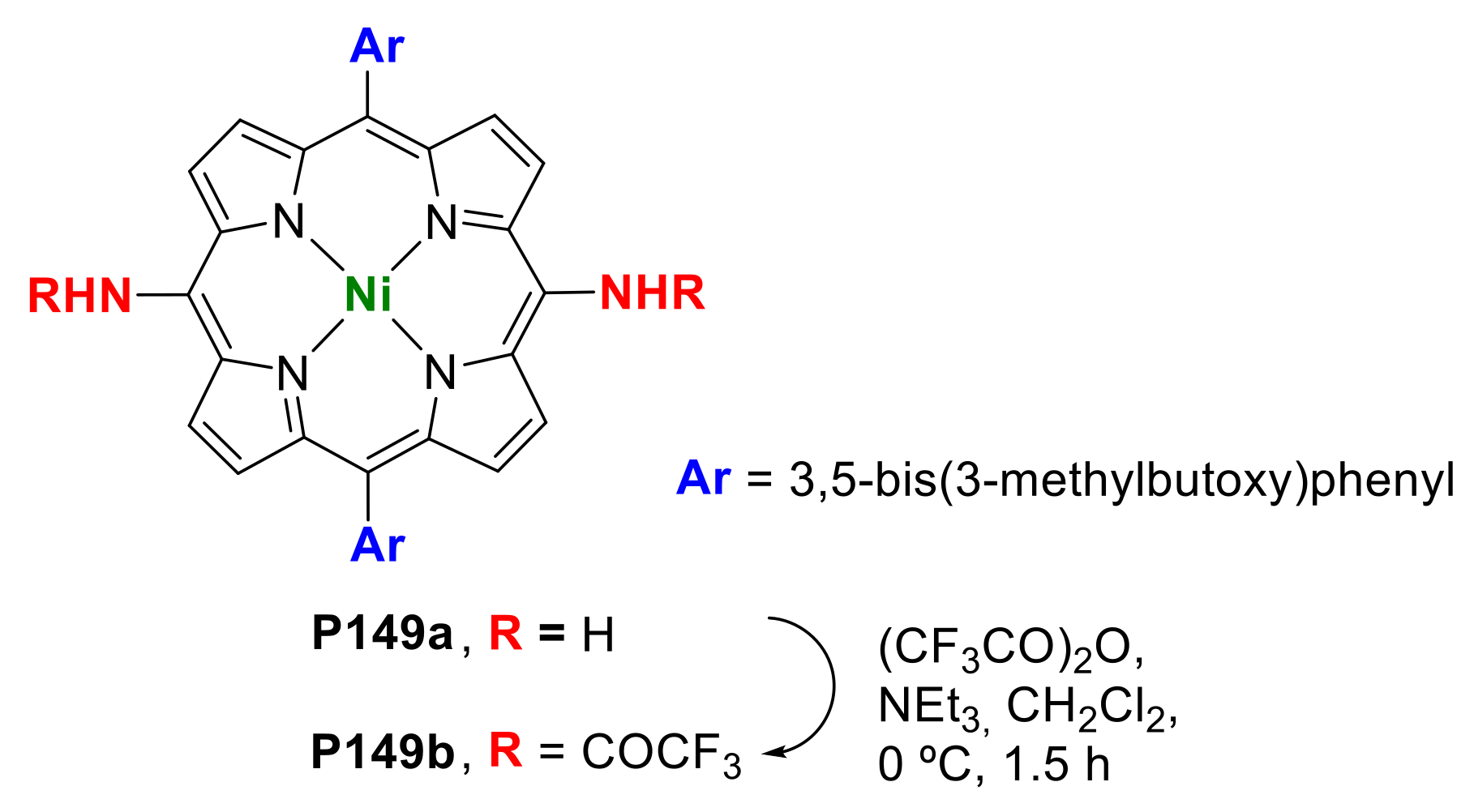
- Yamashita, K.-i.; Kataoka, K.; Takeuchi, S.; Sugiura, K.-i. Metal-Free Synthesis of meso-Aminoporphyrins through Reduction of meso-Azidoporphyrins Generated in Situ by Nucleophilic Substitution Reactions of meso-Bromoporphyrins. J. Org. Chem. 2016, 81, 11176–11184. [Google Scholar] [CrossRef]
- Li, X.; Dong, L.; Liu, Y. Theoretical Study of Iron Porphyrin Nitrene: Formation Mechanism, Electronic Nature, and Intermolecular C-H Amination. Inorg. Chem. 2020, 59, 1622–1632. [Google Scholar] [CrossRef]
- Singh, R.; Mukherjee, A. Metalloporphyrin Catalyzed C–H Amination. ACS Catal. 2019, 9, 3604–3617. [Google Scholar] [CrossRef]
- Liu, Y.; You, T.; Wang, T.-T.; Che, C.-M. Iron-catalyzed C-H amination and its application in organic synthesis. Tetrahedron 2019, 75, 130607. [Google Scholar] [CrossRef]
- Wang, P.; Deng, L. Recent Advances in Iron-Catalyzed C-H Bond Amination via Iron Imido Intermediate. Chin. J. Chem. 2018, 36, 1222–1240. [Google Scholar] [CrossRef]
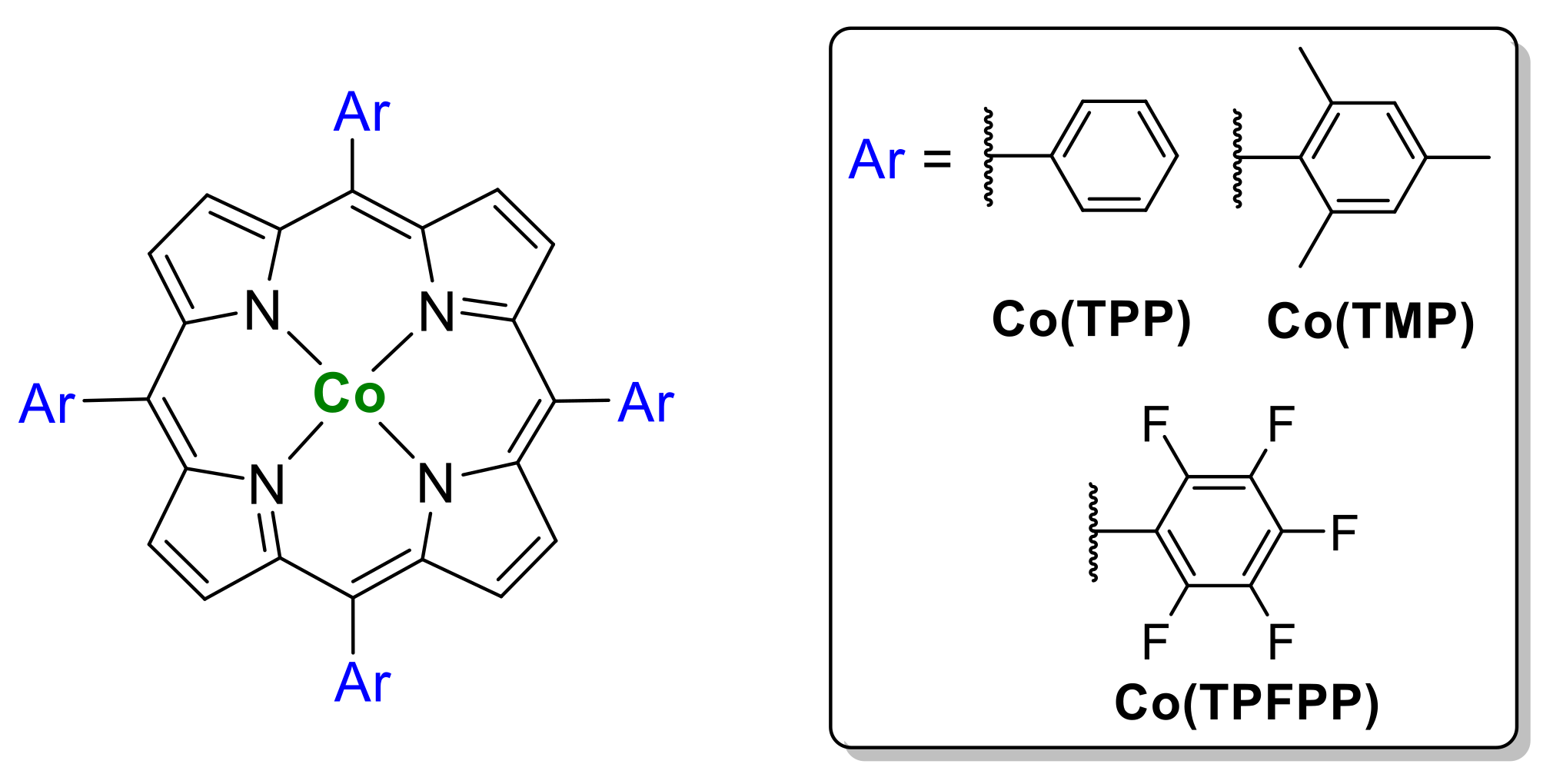
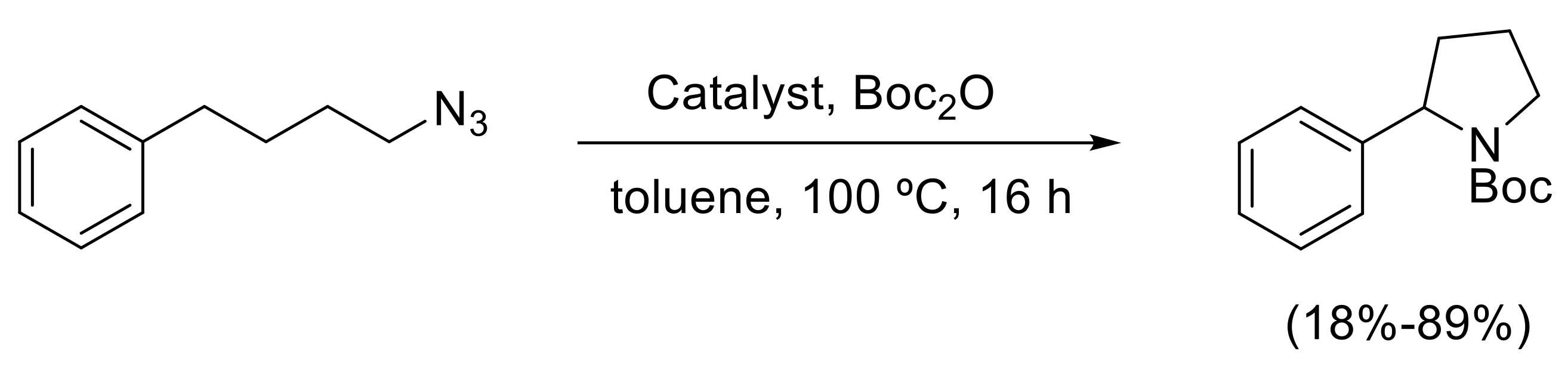
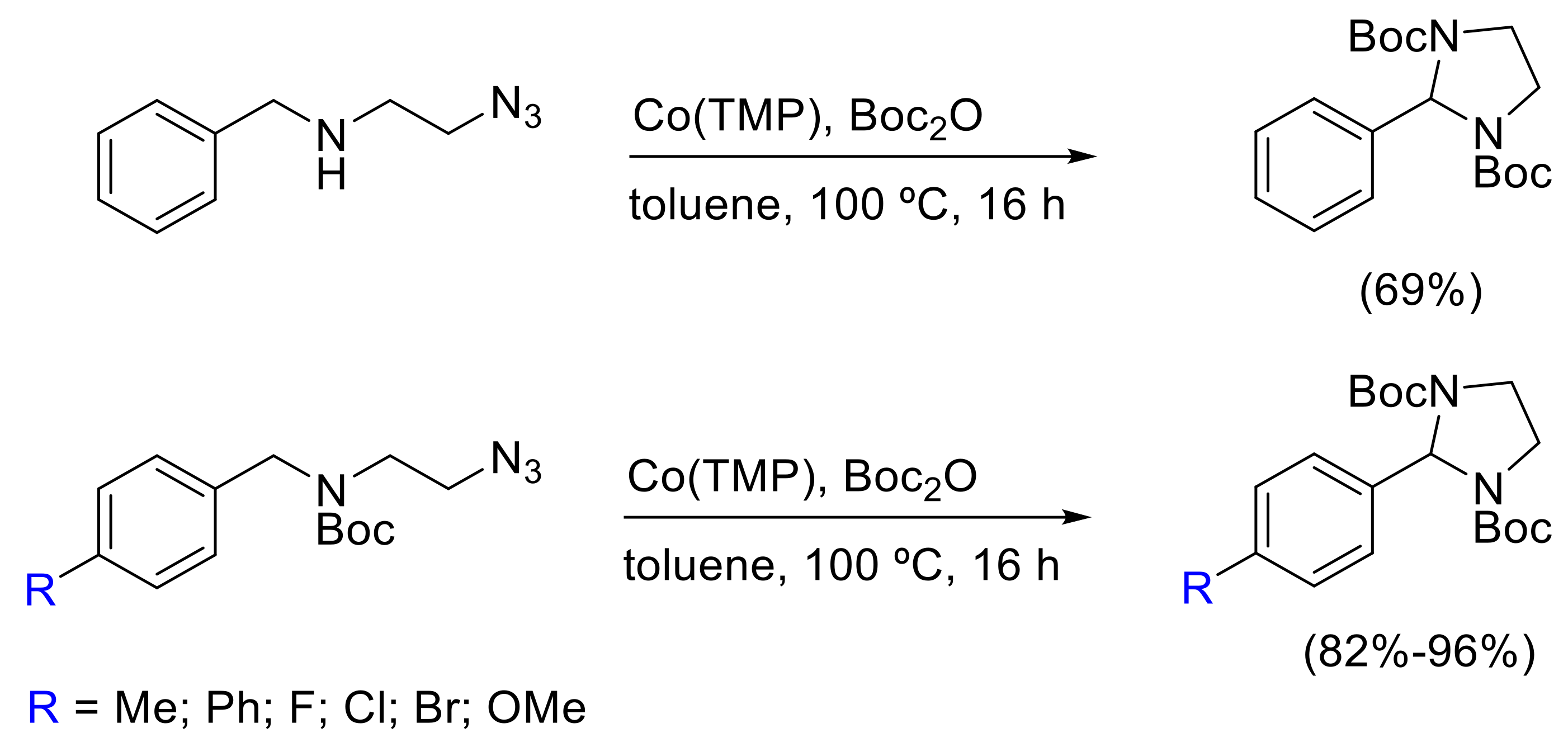
- Kuijpers, P.F.; Tiekink, M.J.; Breukelaar, W.B.; Broere, D.L.J.; van Leest, N.P.; van der Vlugt, J.I.; Reek, J.N.H.; de Bruin, B. Cobalt-Porphyrin-Catalysed Intramolecular Ring-Closing C-H Amination of Aliphatic Azides: A Nitrene-Radical Approach to Saturated Heterocycles. Chem. Eur. J. 2017, 23, 7945–7952. [Google Scholar] [CrossRef] [PubMed]
- Intrieri, D.; Rossi, S.; Puglisi, A.; Gallo, E. Metal-porphyrin catalyzed aziridination of α-methylstyrene: Batch vs. flow process. J. Porphyr. Phthalocyanines 2017, 21, 381–390. [Google Scholar] [CrossRef]
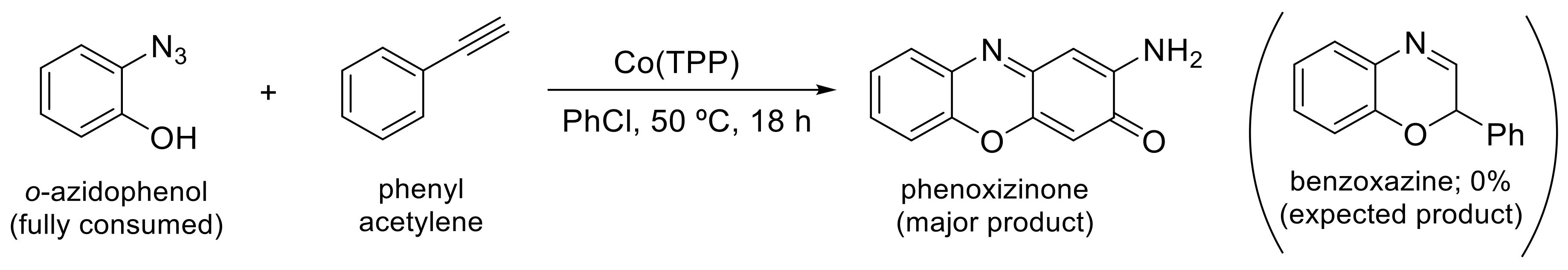
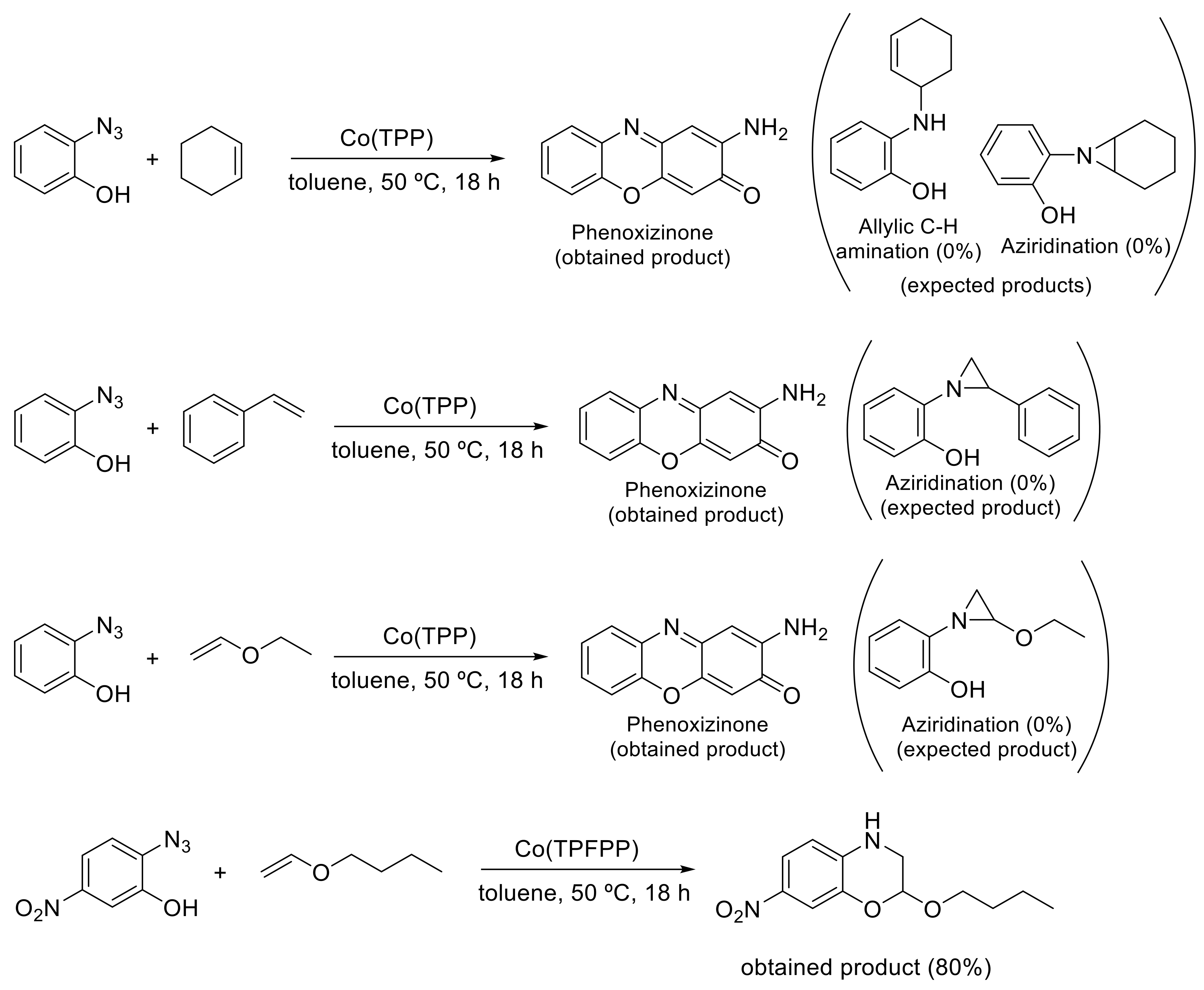
- Goswami, M.; Rebreyend, C.; De Bruin, B. Porphyrin Cobalt(III) “Nitrene Radical” Reactivity; Hydrogen Atom Transfer from Ortho-YH Substituents to the Nitrene Moiety of Cobalt-Bound Aryl Nitrene Intermediates (Y = O, NH). Molecules 2016, 21, 242. [Google Scholar] [CrossRef]
- Goswami, M.; Lyaskovskyy, V.; Domingos, S.R.; Buma, W.J.; Woutersen, S.; Troeppner, O.; Ivanović-Burmazović, I.; Lu, H.; Cui, X.; Zhang, X.P.; et al. Characterization of Porphyrin-Co(III)-‘Nitrene Radical’ Species Relevant in Catalytic Nitrene Transfer Reactions. J. Am. Chem. Soc. 2015, 137, 5468–5479. [Google Scholar] [CrossRef]
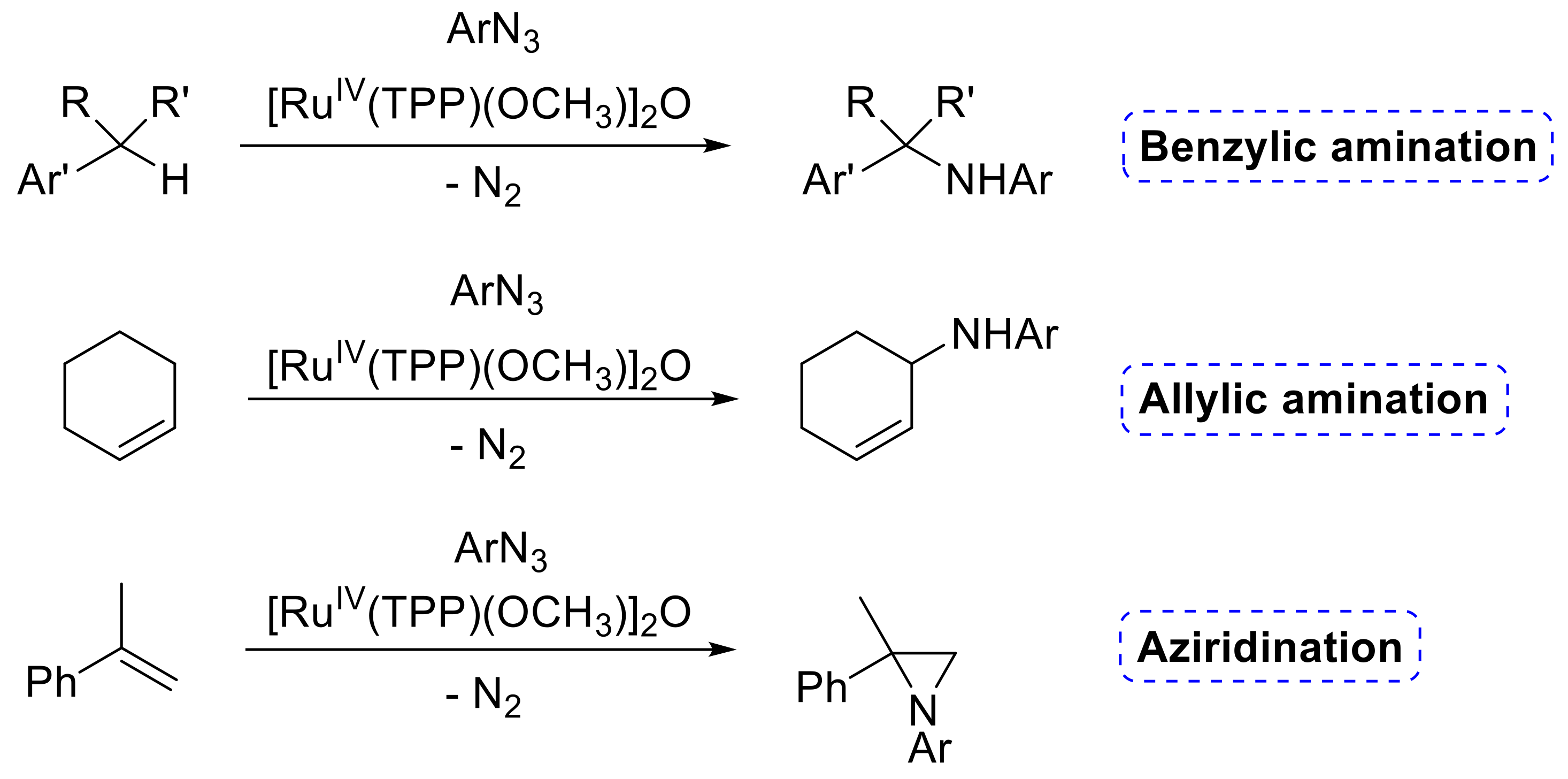
- Manca, G.; Mealli, C.; Carminati, D.M.; Intrieri, D.; Gallo, E. Comparative Study of the Catalytic Amination of Benzylic C–H Bonds Promoted by Ru(TPP)(py)2 and Ru(TPP)(CO). Eur. J. Inorg. Chem. 2015, 2015, 4885–4893. [Google Scholar] [CrossRef]
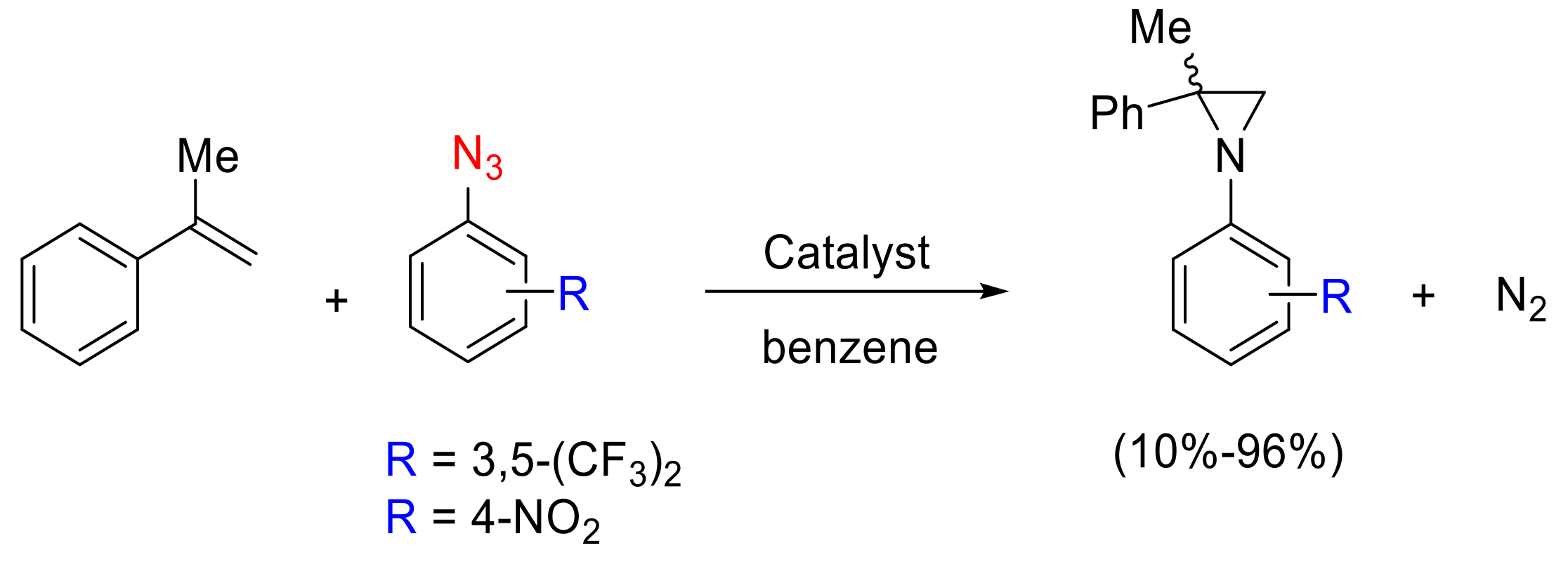
- Zardi, P.; Pozzoli, A.; Ferretti, F.; Manca, G.; Mealli, C.; Gallo, E. A mechanistic investigation of the ruthenium porphyrin catalysed aziridination of olefins by aryl azides. Dalton Trans. 2015, 44, 10479–10489. [Google Scholar] [CrossRef]
- Damiano, C.; Intrieri, D.; Gallo, E. Aziridination of alkenes promoted by iron or ruthenium complexes. Inorg. Chim. Acta 2018, 470, 51–67. [Google Scholar] [CrossRef]
- Rossi, S.; Puglisi, A.; Intrieri, D.; Gallo, E. From Anilines to Aziridines: A Two-Step Synthesis under Continuous-Flow Conditions. J. Flow Chem. 2016, 6, 234–239. [Google Scholar] [CrossRef]
- Rossi, S.; Puglisi, A.; Benaglia, M.; Carminati, D.M.; Intrieri, D.; Gallo, E. Synthesis in mesoreactors: Ru(porphyrin)CO-catalyzed aziridination of olefins under continuous flow conditions. Catal. Sci. Technol. 2016, 6, 4700–4704. [Google Scholar] [CrossRef]
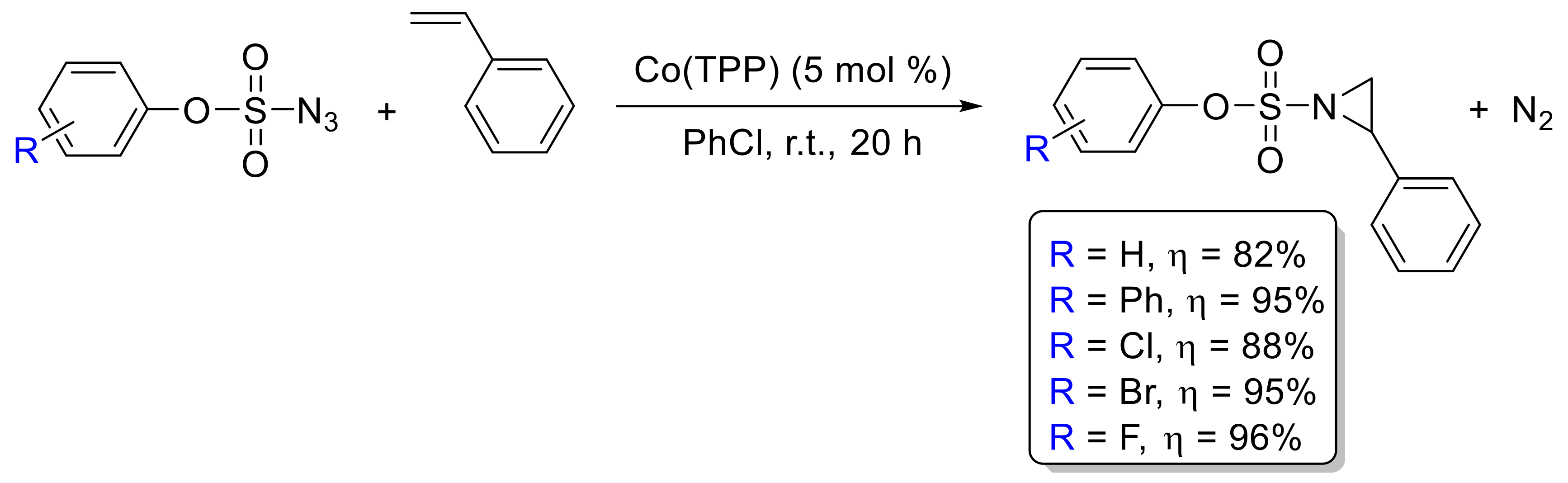
- Subbarayan, V.; Jin, L.-M.; Cui, X.; Zhang, X.P. Room temperature activation of aryloxysulfonyl azides by [Co(II)(TPP)] for selective radical aziridination of alkenes via metalloradical catalysis. Tetrahedron Lett. 2015, 56, 3431–3434. [Google Scholar] [CrossRef] [PubMed]
- Zardi, P.; Intrieri, D.; Carminati, D.M.; Ferretti, F.; Macchi, P.; Gallo, E. Synthesis and catalytic activity of μ-oxo ruthenium(IV) porphyrin species to promote amination reactions. J. Porphyr. Phthalocyanines 2016, 20, 1156–1165. [Google Scholar] [CrossRef]
- Tseberlidis, G.; Zardi, P.; Caselli, A.; Cancogni, D.; Fusari, M.; Lay, L.; Gallo, E. Glycoporphyrin Catalysts for Efficient C-H Bond Aminations by Organic Azides. Organometallics 2015, 34, 3774–3781. [Google Scholar] [CrossRef]
- Khojastehnezhad, A.; Bakavoli, M.; Javid, A.; Khakzad Siuki, M.M.; Shahidzadeh, M. Synthesis, characterization, and investigation of catalytic activity of copper(II) porphyrin graphene oxide for azide–alkyne cycloaddition. Res. Chem. Intermed. 2019, 45, 4473–4485. [Google Scholar] [CrossRef]
- Jiang, W.; Yang, J.; Liu, Y.-Y.; Ma, J.-F. Porphyrin-based mixed-valent Ag(I)/Ag(II) and Cu(I)/Cu(II) networks as efficient heterogeneous catalysts for the azide–alkyne “click” reaction and promising oxidation of ethylbenzene. Chem. Commun. 2016, 52, 1373–1376. [Google Scholar] [CrossRef]
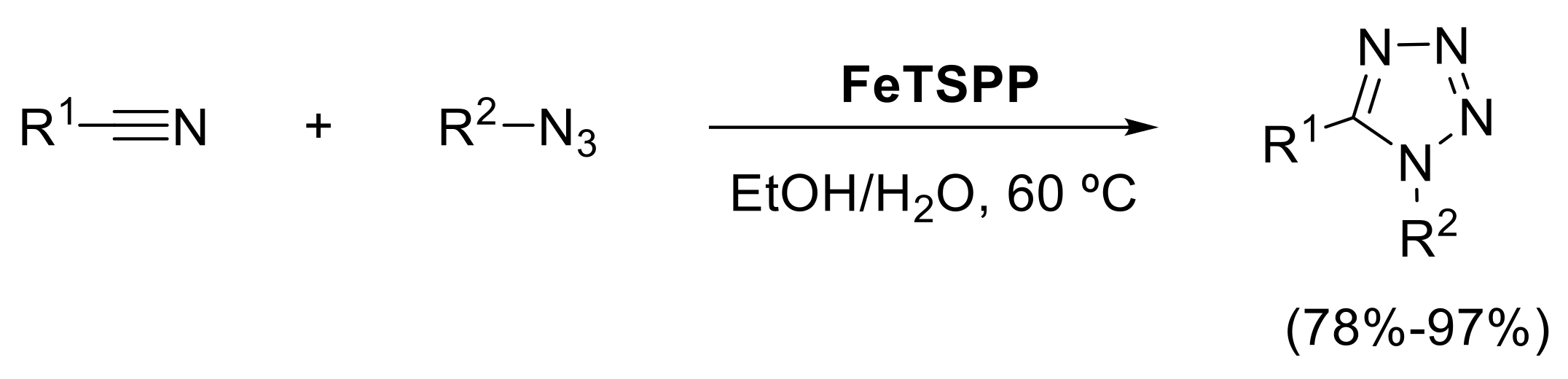
- El-Remaily, M.A.E.A.A.A.; Elhady, O.M. Iron (III)-porphyrin Complex FeTSPP as an efficient catalyst for synthesis of tetrazole derivatives via [2 + 3]cycloaddition reaction in aqueous medium. Appl. Organometal. Chem. 2019, 33, e4989. [Google Scholar] [CrossRef]
- Intrieri, D.; Carminati, D.M.; Gallo, E. Recent advances in C–H bond aminations catalyzed by ruthenium porphyrin complexes. J. Porphyr. Phthalocyanines 2016, 20, 190–203. [Google Scholar] [CrossRef]
- Goswami, M.; Geuijen, P.; Reek, J.N.H.; de Bruin, B. Application of [Co(Corrole)]–Complexes in Ring-Closing C–H Amination of Aliphatic Azides via Nitrene Radical Intermediates. Eur. J. Inorg. Chem. 2018, 2018, 617–626. [Google Scholar] [CrossRef]
- Shing, K.-P.; Liu, Y.; Cao, B.; Chang, X.-Y.; You, T.; Che, C.-M. N-Heterocyclic Carbene Iron(III) Porphyrin-Catalyzed Intramolecular C(sp3)–H Amination of Alkyl Azides. Angew. Chem. Int. Ed. 2018, 57, 11947–11951. [Google Scholar] [CrossRef] [PubMed]
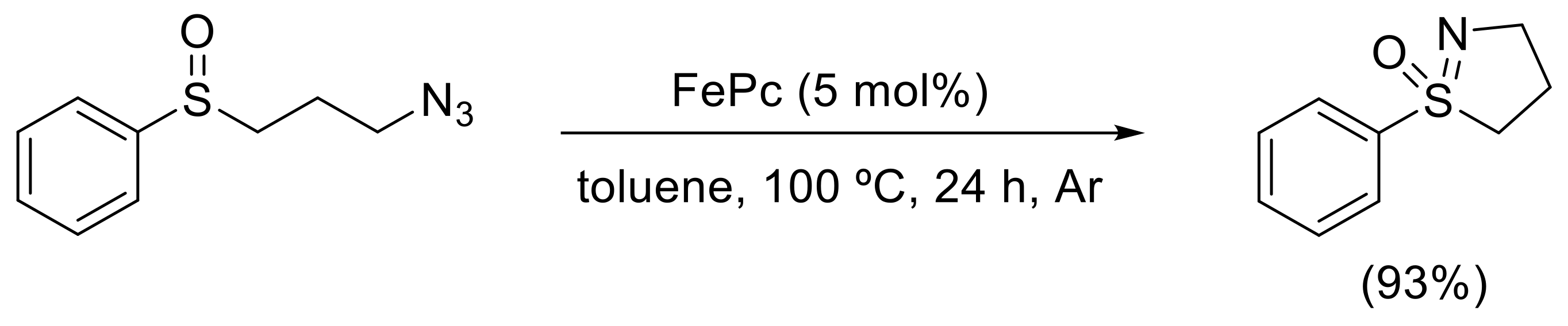
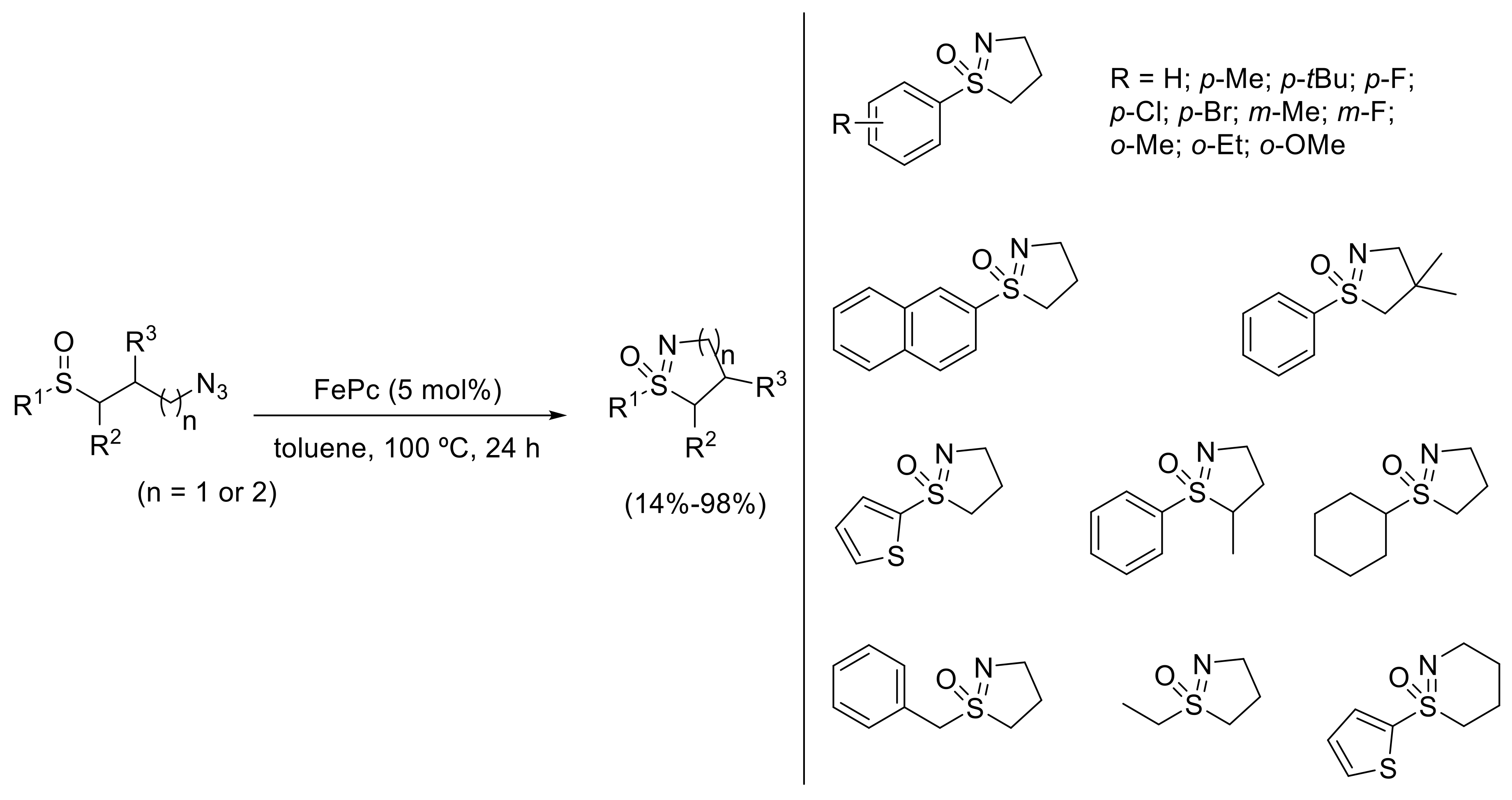
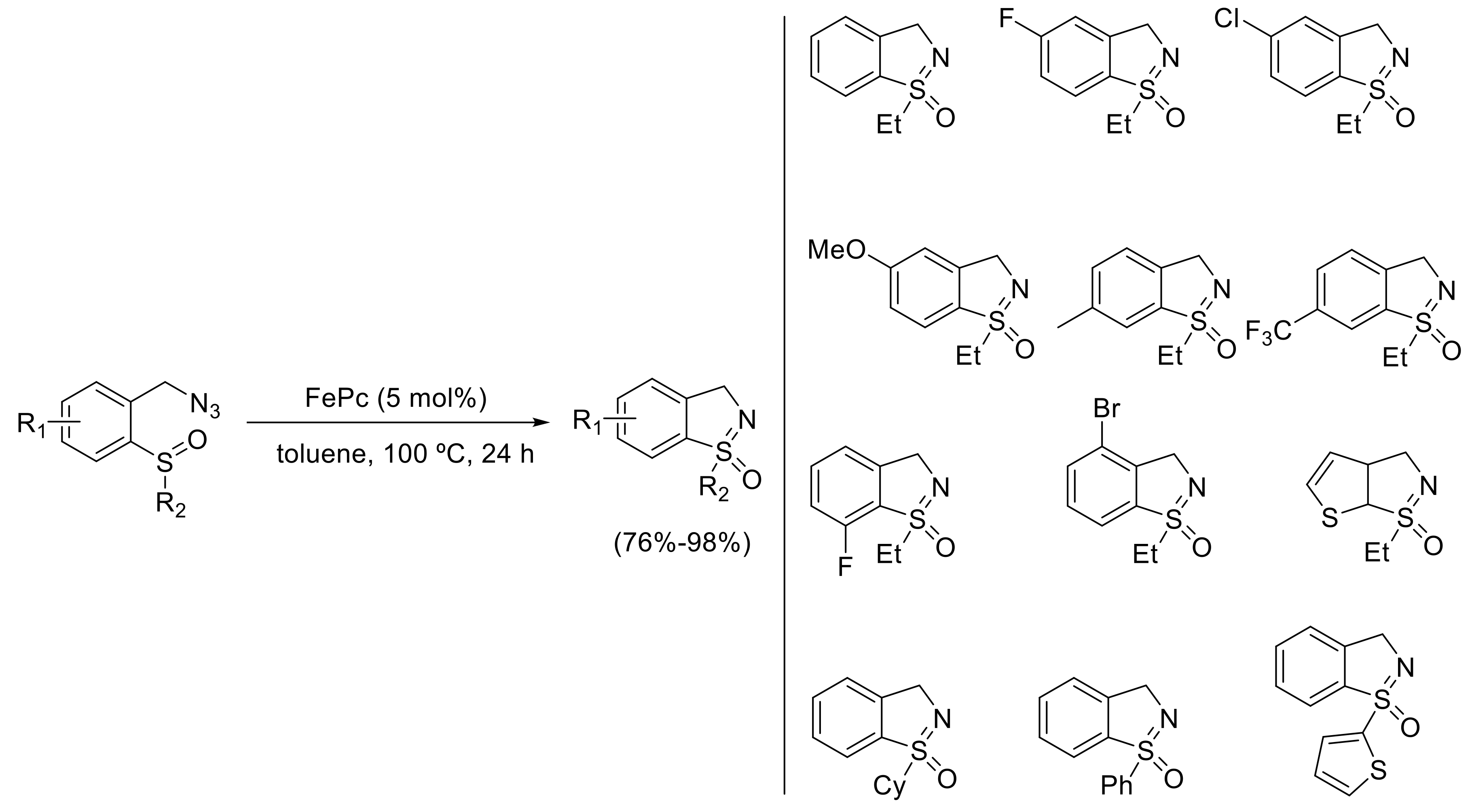
- Yu, H.; Li, Z.; Bolm, C. Three-Dimensional Heterocycles by Iron-Catalyzed Ring-Closing Sulfoxide Imidation. Angew. Chem. Int. Ed. 2018, 57, 12053–12056. [Google Scholar] [CrossRef] [PubMed]
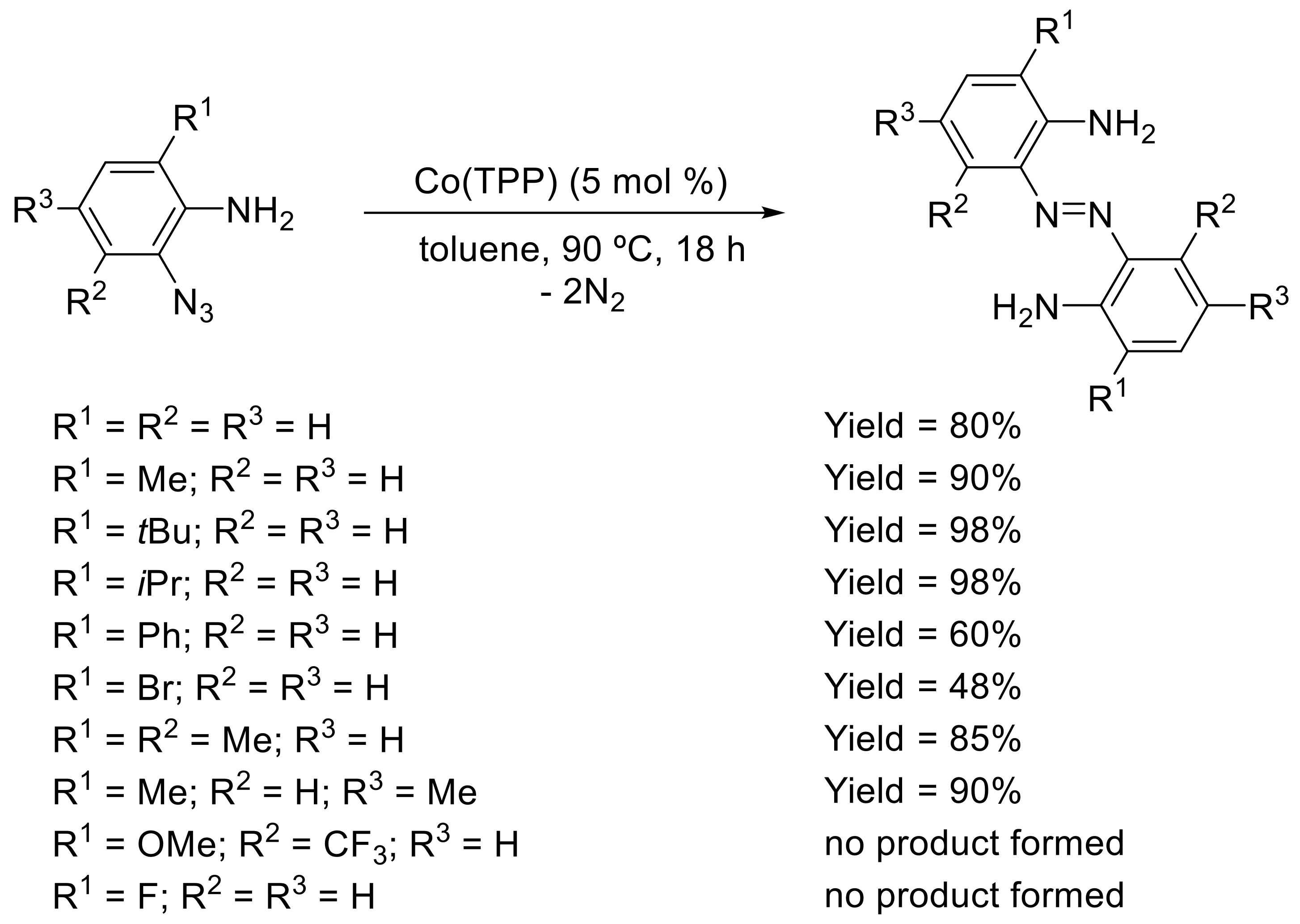
- Goswami, M.; Bruin, B.D. Porphyrin Co(III)-Nitrene Radical Mediated Pathway for Synthesis of o-Aminoazobenzenes. Molecules 2018, 23, 1052. [Google Scholar] [CrossRef] [PubMed]
- Gross, Z.; Galili, N.; Saltsman, I. The First Direct Synthesis of Corroles from Pyrrole. Angew. Chem. Int. Ed. 1999, 38, 1427–1429. [Google Scholar] [CrossRef]
- Koszarna, B.; Gryko, D.T. Efficient Synthesis of meso-Substituted Corroles in a H2O−MeOH Mixture. J. Org. Chem. 2006, 71, 3707–3717. [Google Scholar] [CrossRef]
- Nardis, S.; Monti, D.; Paolesse, R. Novel Aspects of Corrole Chemistry. Mini-Rev. Org. Chem. 2005, 2, 355–374. [Google Scholar] [CrossRef]
- Paolesse, R. Syntheses of Corroles. In The Porphyrin Handbook; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: San Diego, CA, USA, 2000. [Google Scholar]
- Barata, J.F.B.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Tomé, A.C.; Cavaleiro, J.A.S. Strategies for Corrole Functionalization. Chem. Rev. 2017, 117, 3192–3253. [Google Scholar] [CrossRef]
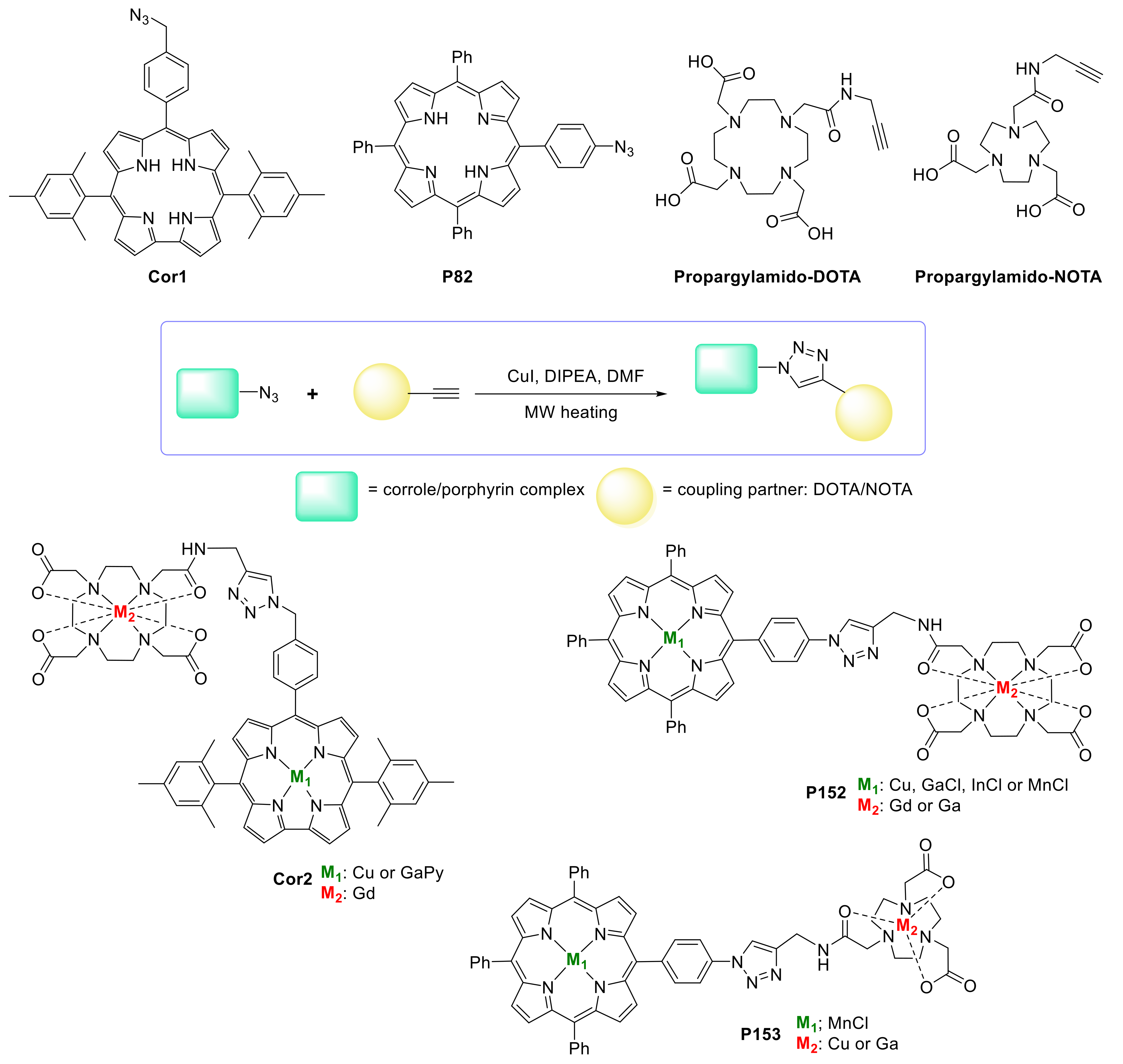
- Buckley, H.L.; Rubin, L.K.; Chromiński, M.; McNicholas, B.J.; Tsen, K.H.Y.; Gryko, D.T.; Arnold, J. Corroles That “Click”: Modular Synthesis of Azido- and Propargyl-Functionalized Metallocorrole Complexes and Convergent Synthesis of a Bis-corrole Scaffold. Inorg. Chem. 2014, 53, 7941–7950. [Google Scholar] [CrossRef]
- Nikolaou, V.; Karikis, K.; Farré, Y.; Charalambidis, G.; Odobel, F.; Coutsolelos, A.G. Click made porphyrin–corrole dyad: A system for photo-induced charge separation. Dalton Trans. 2015, 44, 13473–13479. [Google Scholar] [CrossRef]
- Ngo, T.H.; Zieba, D.; Webre, W.A.; Lim, G.N.; Karr, P.A.; Kord, S.; Jin, S.; Ariga, K.; Galli, M.; Goldup, S.; et al. Engaging Copper(III) Corrole as an Electron Acceptor: Photoinduced Charge Separation in Zinc Porphyrin–Copper Corrole Donor–Acceptor Conjugates. Chem. Eur. J. 2016, 22, 1301–1312. [Google Scholar] [CrossRef]
- Brizet, B.; Desbois, N.; Bonnot, A.; Langlois, A.; Dubois, A.; Barbe, J.-M.; Gros, C.P.; Goze, C.; Denat, F.; Harvey, P.D. Slow and Fast Singlet Energy Transfers in BODIPY-gallium(III)corrole Dyads Linked by Flexible Chains. Inorg. Chem. 2014, 53, 3392–3403. [Google Scholar] [CrossRef] [PubMed]
- Desbois, N.; Pacquelet, S.; Dubois, A.; Michelin, C.; Gros, C.P. Easy access to heterobimetallic complexes for medical imaging applications via microwave-enhanced cycloaddition. Beilstein J. Org. Chem. 2015, 11, 2202–2208. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.H.; Labuta, J.; Lim, G.N.; Webre, W.A.; D’Souza, F.; Karr, P.A.; Lewis, J.E.M.; Hill, J.P.; Ariga, K.; Goldup, S.M. Porphyrinoid rotaxanes: Building a mechanical picket fence. Chem. Sci. 2017, 8, 6679–6685. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Lei, H.; Li, X.; Qi, J.; Zhang, W.; Cao, R. Attaching Cobalt Corroles onto Carbon Nanotubes: Verification of Four-Electron Oxygen Reduction by Mononuclear Cobalt Complexes with Significantly Improved Efficiency. ACS Catal. 2019, 9, 4551–4560. [Google Scholar] [CrossRef]
- Tomé, A.C. 1,2,3-Triazoles (update 2015). In Science of Synthesis: Knowledge Updates 2015/2; Aitken, R.A., Nielsen, M.B., Drabowicz, J., Li, J.J., Plietker, B.J., Wirth, T., Eds.; Thieme: Stuttgart, Germany, 2016. [Google Scholar]










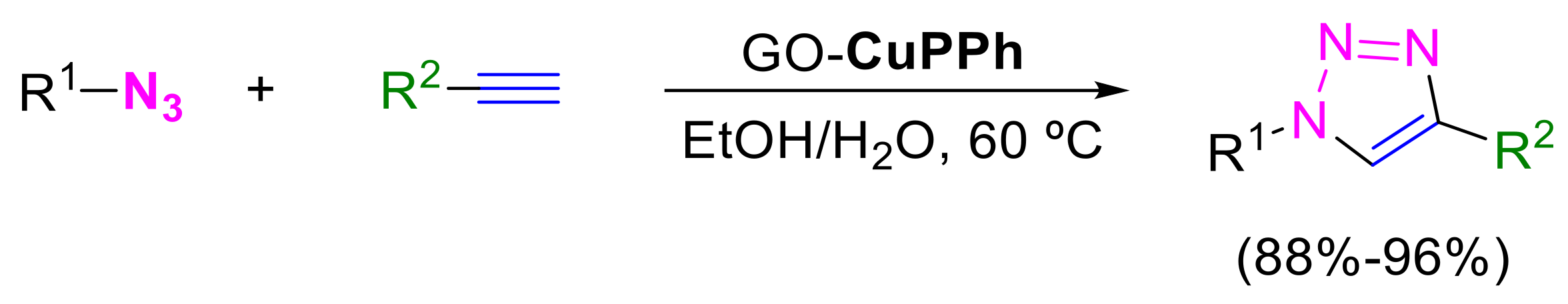



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, A.R.L.; Tomé, A.C.; Santos, C.I.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Simões, M.M.Q.; Moura, N.M.M.; Abu-Orabi, S.T.; Cavaleiro, J.A.S. Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1—Azides, Porphyrins and Corroles. Molecules 2020, 25, 1662. https://doi.org/10.3390/molecules25071662
Araújo ARL, Tomé AC, Santos CIM, Faustino MAF, Neves MGPMS, Simões MMQ, Moura NMM, Abu-Orabi ST, Cavaleiro JAS. Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1—Azides, Porphyrins and Corroles. Molecules. 2020; 25(7):1662. https://doi.org/10.3390/molecules25071662
Chicago/Turabian StyleAraújo, Ana R. L., Augusto C. Tomé, Carla I. M. Santos, Maria A. F. Faustino, Maria G. P. M. S. Neves, Mário M. Q. Simões, Nuno M. M. Moura, Sultan T. Abu-Orabi, and José A. S. Cavaleiro. 2020. "Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1—Azides, Porphyrins and Corroles" Molecules 25, no. 7: 1662. https://doi.org/10.3390/molecules25071662
APA StyleAraújo, A. R. L., Tomé, A. C., Santos, C. I. M., Faustino, M. A. F., Neves, M. G. P. M. S., Simões, M. M. Q., Moura, N. M. M., Abu-Orabi, S. T., & Cavaleiro, J. A. S. (2020). Azides and Porphyrinoids: Synthetic Approaches and Applications. Part 1—Azides, Porphyrins and Corroles. Molecules, 25(7), 1662. https://doi.org/10.3390/molecules25071662