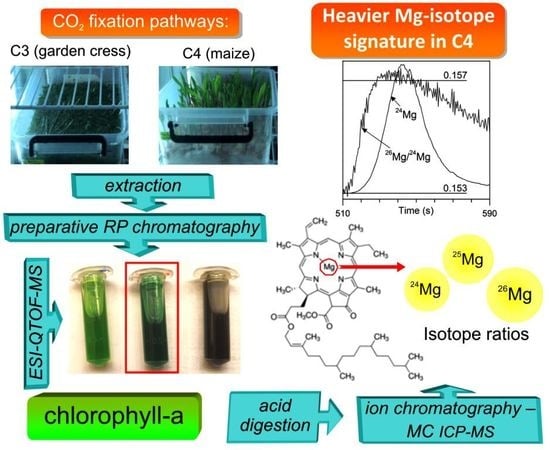
Magnesium–Isotope Fractionation in Chlorophyll-a Extracted from Two Plants with Different Pathways of Carbon Fixation (C3, C4)
Abstract
1. Introduction
2. Results and Discussion
2.1. Separation and Characterization of Chlorophyll-a
2.2. Mg-isotope Fractionation in Chlorophyll-a
3. Materials and Methods
3.1. Reagents and Standards
3.2. Plants Growth and Chlorophyll Extraction
3.3. Purification of Chlorophyll-a and High-resolution Mass Spectrometry Analysis
3.4. Samples Digestion and ICP-MS Determination of Elements
3.5. Ion Chromatography-dry Plasma MC ICP-MS Analysis
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmitt, A.D.; Vigier, N.; Lemarchand, D.; Millot, R.; Stille, P.; Chabaux, F. Processes controlling the stable isotope compositions of Li, B, Mg and Ca in plants, solis and waters: A review. C. R. Geosci. 2012, 344, 704–722. [Google Scholar] [CrossRef]
- Caldelas, C.; Weiss, D.J. Zinc homeostasis and isotopic fractionation in plants: a review. Plant Soil. 2017, 411, 17–46. [Google Scholar] [CrossRef]
- Albarède, F.; Télouk, P.; Balter, V. Medical applications of isotope metallomics. Rev. Mineral. Geochem. 2017, 82, 851–885. [Google Scholar] [CrossRef]
- Hajj, F.; Poszwa, A.; Bouchez, J.; Guérold, F. Radiogenic and “stable” strontium isotopes in provenance studies: A review and first results on archaeological wood from shipwrecks. J. Archeol. Sci. 2017, 86, 24–49. [Google Scholar] [CrossRef]
- Teng, F.Z. Magnesium isotope geochemistry. Rev. Mineral. Geochem. 2017, 82, 219–287. [Google Scholar] [CrossRef]
- Irrgeher, J.; Prohaska, T. Application of non-traditional stable isotopes in analytical ecogeochemistry assessed by MC ICP-MS-A critical review. Anal. Bioanal. Chem. 2016, 408, 369–385. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, T.; Yang, X.; Su, P.; Liu, Q.; Jiang, G. Recent advances in the analysis of non-traditional stable isotopes by multi-collector inductively coupled plasma mass spectrometry. J. Anal. At. Spectrom. 2017, 32, 1848–1861. [Google Scholar] [CrossRef]
- Karasinski, J.; Bulska, E.; Wojciechowski, M.; Krata, A.A.; Halicz, L. On-line separation of strontium from a matrix and determination of the 87 Sr/86 Sr ratio by Ion Chromatography/Multicollector-ICPMS. J. Anal. At. Spectrom. 2016, 31, 1459–1463. [Google Scholar] [CrossRef]
- Karasinski, J.; Bulska, E.; Wojciechowski, M.; Halicz, L.; Krata, A.A. High precision direct analysis of magnesium isotope ratio by ion chromatography/multicollector-ICPMS using wet and dry plasma conditions. Talanta 2017, 165, 64–68. [Google Scholar] [CrossRef]
- Karasinski, J.; Bulska, E.; Halicz, L.; Wojciechowski, M.; Krata, A.A. Direct determination of δ44/42Ca isotope ratio by ion chromatography/low-resolution multicollector ICPMS. J. Mass Spectrom. 2018, 53, 78–82. [Google Scholar] [CrossRef]
- Berglund, M.; Wieser, M.E. Isotopic compositions of the elements 2009 (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 397–410. [Google Scholar] [CrossRef]
- Bolou-Bi, B.E.; Vigier, N.; Brenot, A.; Poszwa, A. Magnesium isotope compositions of natural reference materials. Geostand. Geoanal. Res. 2009, 33, 95–109. [Google Scholar] [CrossRef]
- Bolou-Bi, B.E.; Poszwa, A.; Leyval, C.; Vigier, N. Experimental determination of magnesium isotope fractionation during higher plant growth. Geochim. Cosmochim. Acta 2010, 74, 2523–2537. [Google Scholar] [CrossRef]
- Black, J.R.; Epstein, E.; Rains, W.D.; Yin, Q.Z.; Casey, W.H. Magnesium-isotope fractionation during plant growth. Environ. Sci. Technol. 2008, 42, 7831–7836. [Google Scholar] [CrossRef]
- Black, J.R.; Yin, Q.Z.; Casey, W.H. An experimental study of magnesium-isotope fractionation in chlorophyll-a photosynthesis. Geochim. Cosmochim. Acta 2006, 70, 4072–4079. [Google Scholar] [CrossRef]
- Black, J.R.; Yin, Q.Z.; Rustad, J.R.; Casey, W.H. Magnesium isotopic equilibrium in chlorophylls. J. Am. Chem. Soc. 2007, 129, 8690–8691. [Google Scholar] [CrossRef]
- Isaji, Y.; Yoshimura, T.; Araoka, D.; Kuroda, J.; Ogawa, N.O.; Kawahata, H.; Ohkouchi, N. Magnesium isotope fractionation during synthesis of chlorophyll a and bacteriochlorophyll a of benthic phototrophs in hypersaline environments. ACS Earth Space Chem. 2019, 3, 1073–1079. [Google Scholar] [CrossRef]
- Ra, K.; Kitagawa, H. Magnesium isotope analysis of different chlorophyll forms in marine phytoplancton using multi-collector ICP-MS. J. Anal. At. Spectrom. 2007, 22, 817–821. [Google Scholar] [CrossRef]
- Moynier, F.; Fujii, T. Theoretical isotopic fractionation of magnesium between chlorophylls. Sci Rep. 2017, 7, 6973. [Google Scholar] [CrossRef]
- Gowik, U.; Westhoff, P. The path from C3 to C4 photosynthesis. Plant Physiol. 2011, 155, 56–63. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr. Opin. Biotechnol. 2008, 19, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Chen, S.; Dai, S. C4 photosynthetic machinery: insights from maize chloroplast proteomics. Front. Plant Sci. 2013, 4, 85. [Google Scholar] [CrossRef] [PubMed]
- Bidigare, R.R.; Kennicutt, M.C.; Keeney-Kennicutt, W.L.; Macko, S.A. Isolation and purification of chlorophylls a and b for the determination of stable carbon and nitrogen isotope compositions. Anal. Chem. 1991, 63, 130–133. [Google Scholar] [CrossRef]
- Milenković, S.M.; Zvezdanović, J.B.; Anđelković, T.D.; Marković, D.Z. The identification of chlorophyll and its derivatives in the pigment mixtures: HPLC-chromatography, visible and mass spectroscopy studies. Adv. Technol. 2012, 1, 16–24. [Google Scholar]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; De Colstoun, E.B.; McMurtrey III, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Galy, A.; Belshaw, N.S.; Halicz, L.; O’Nions, R.K. High precision measurement of magnesium isotopes by multiple-collector inductively coupled plasma mass spectrometry. Int. J. Mass Spectrom. 2001, 208, 89–98. [Google Scholar] [CrossRef]
- Young, E.D.; Galy, A. The isotope geochemistry and cosmochemistry of magnesium. Rev. Mineral. Geochem. 2004, 55, 197–230. [Google Scholar] [CrossRef]
- Hermans, C.; Conn, S.J.; Chen, J.; Xiao, Q.; Verbruggen, N. An update on magnesium homeostasis mechanisms in plants. Metallomics 2013, 5, 1170–1183. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Kuznetsov, D.A.; Breslavskaya, N.N. Chemistry of enzymatic ATP synthesis: an insight through the isotope window. Chem. Rev. 2012, 112, 2042–2058. [Google Scholar] [CrossRef]
- Galy, A.; Yoffe, O.; Janney, P.E.; Williams, R.W.; Cloquet, C.; Alard, O.; Halicz, L.; Wadhwa, M.; Hutcheon, I.D.; Ramon, E.; et al. Magnesium isotope heterogeneity of the isotopic standard SRM980 and new reference materials for magnesium-isotope-ratio measurements. J. Anal. At. Spectrom. 2003, 18, 1352–1356. [Google Scholar] [CrossRef]
- Torres Elguera, J.C.; Yanez Barrientos, E.; Wrobel, K.; Wrobel, K. Effect of cadmium (Cd (II)), selenium (Se (IV)) and their mixtures on phenolic compounds and antioxidant capacity in Lepidium sativum. Acta Physiol. Plant. 2013, 35, 431–441. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
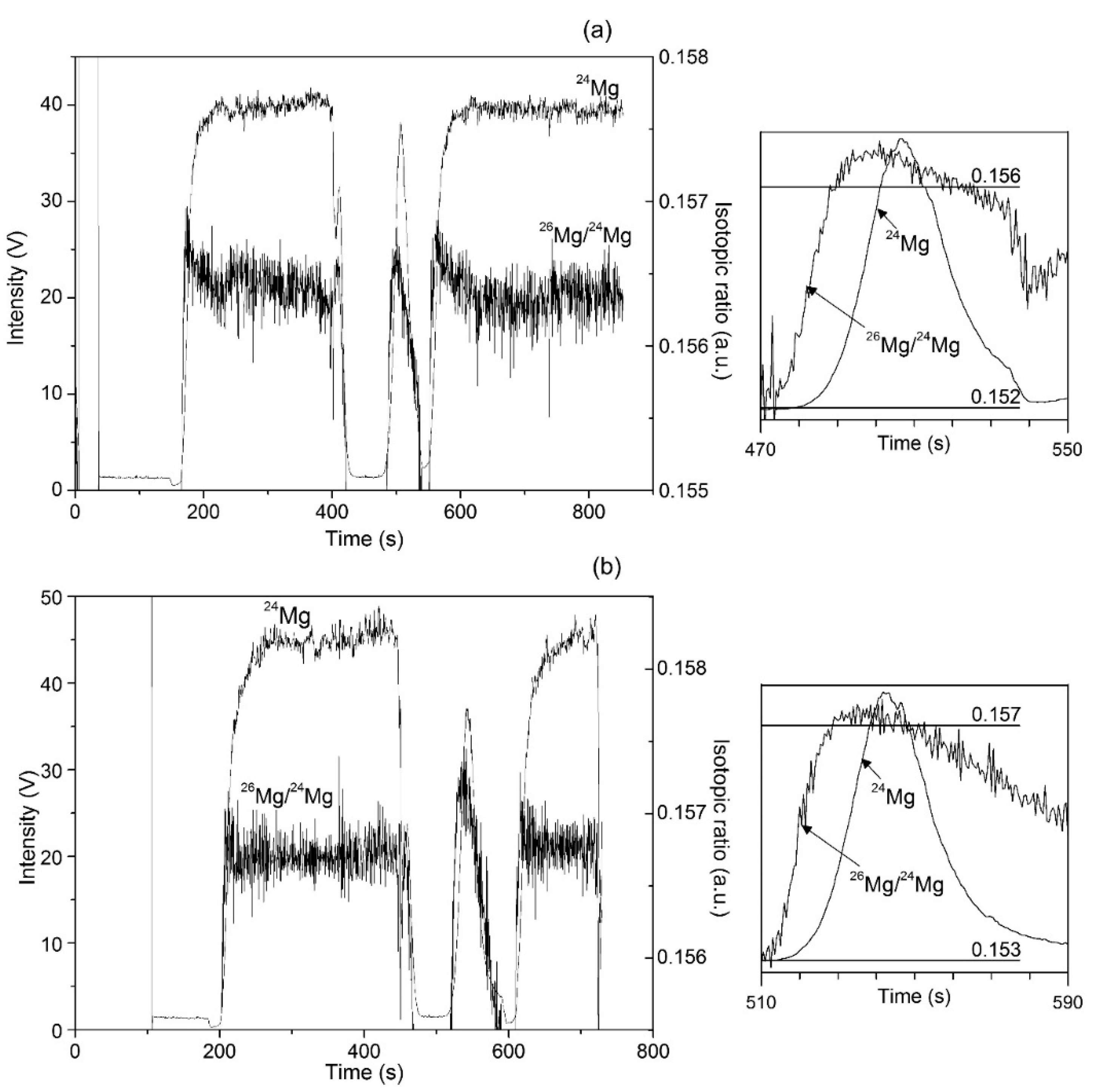
| Sample | Peak Area (mean) µS min | RSD, % | Recovery, % |
|---|---|---|---|
| DSM-3 (directly) | 1.62 | 1.04 | - |
| DSM-3 | 1.39 | 1.06 | 85.4 |
| Cambridge-1 | 1.68 | 0.06 | 104 |
| Maize chlorophyll-a | 1.28 | 0.23 | 78.9 |
| Garden cress chlorophyll-a | 1.50 | 0.42 | 92.2 |
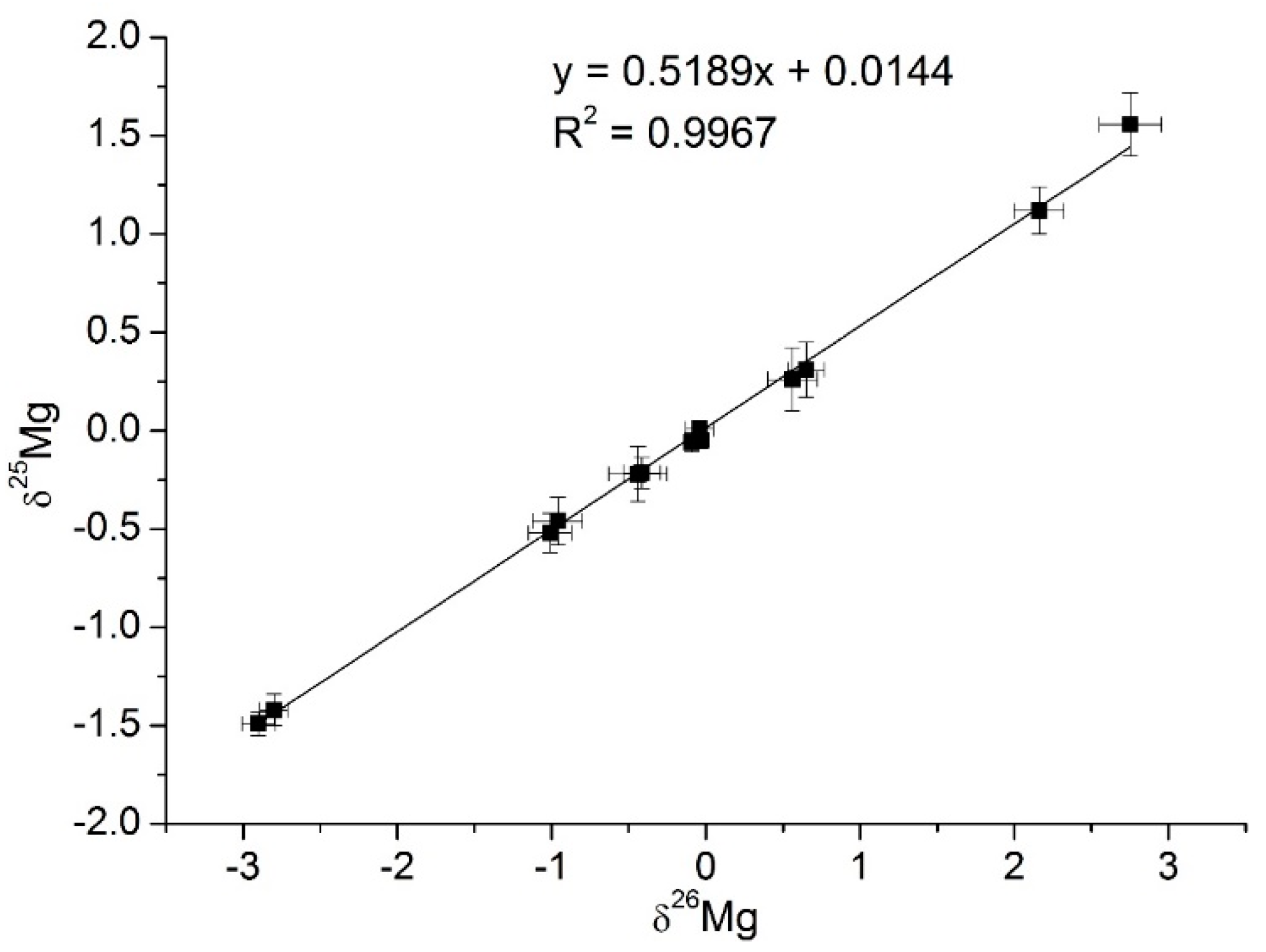
| Sample | δ26Mg, ‰ | δ25Mg, ‰ |
|---|---|---|
| This Work | ||
| Maize-June | 0.65 ± 0.12 | 0.31 ± 0.14 |
| Maize-September | 0.56 ± 0.16 | 0.26 ± 0.10 |
| Garden cress-June | −0.96 ± 0.6 | −0.46 ± 0.12 |
| Garden cress-September | −1.01 ± 0.14 | −0.52 ± 0.10 |
| MgCl2 (nutrient solution) | −0.09 ± 0.06 | −0.05 ± 0.05 |
| Maize chlorophyll-a-June | 2.16 ± 0.16 | 1.12 ± 0.12 |
| Maize chlorophyll-a-September | 2.75 ± 0.20 | 1.56 ± 0.16 |
| Garden cress chlorophyll-a-June | −0.414 ± 0.12 | −0.21 ± 0.08 |
| Garden cress chlorophyll-a-September | −0.440 ± 0.19 | −0.22 ± 0.14 |
| DSM-3-June | −0.04 ± 0.01 | 0.09 ± 0.02 |
| DSM-3-September | −0.03 ± 0.02 | 0.05 ±0.04 |
| Cambridge-1-June | −2.80 ±0.09 | −1.42 ± 0.08 |
| Cambridge-1-September | −2.89 ± 0.11 | −1.49 ± 0.06 |
| Reported Values | ||
| DSM-3 [12] | 0.01 ± 0.14 | 0.00 ± 0.09 |
| DSM-3 [9] | −0.04 ± 0.17 | |
| Cambridge-1 [14] | −2.60 ± 0.14 | −1.34 ± 0.07 |
| Cambridge [12] | −2.71 ± 0.18 | −1.39 ± 0.08 |
| Clover leaf [13] | −0.61 ± 0.14 | −0.31 ± 0.07 |
| Wheat leaf [14] | 0.11 | 0.05 |
| Wheat chlorophyll [14] | (−0.34)–(−0.58) | (−0.18)–(−0.27) |
| Cyanobacteria chlorophyll-a [17] | (−0.12)–(−2.13) | (−0.09)−(−0.62) |
| English Ivy leaf [16] | (−0.510)–(−0.644) | (−0.277)–(−0.343) |
| English Ivy chlorophyll [16] | −0.182 ± 0.145 | −0.099 ± 0.082 |
| Commercial chlorophyll-a from different plants and algae [18] | 1.82–2.76 | 0.93–1.72 |
| Spinach chlorophyll-a [27] | −1.451 ± 0.098 | −0.741 ± 0.062 |
| Isotope Fractionation | Maize (C4) | Garden Cress (C3) | ||
|---|---|---|---|---|
| June | September | June | September | |
| ∆26Mgplant-nutrient, ‰ | 0.74 | 0.65 | −0.87 | −0.92 |
| ∆25Mgplant-nutrient, ‰ | 0.36 | 0.31 | −0.41 | −0.47 |
| ∆26Mgchlorophyll-plant, ‰ | 1.51 | 2.19 | 0.55 | 0.52 |
| ∆25Mgchlorophyll-plant, ‰ | 0.81 | 1.30 | 0.25 | 0.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wrobel, K.; Karasiński, J.; Tupys, A.; Arroyo Negrete, M.A.; Halicz, L.; Wrobel, K.; Bulska, E. Magnesium–Isotope Fractionation in Chlorophyll-a Extracted from Two Plants with Different Pathways of Carbon Fixation (C3, C4). Molecules 2020, 25, 1644. https://doi.org/10.3390/molecules25071644
Wrobel K, Karasiński J, Tupys A, Arroyo Negrete MA, Halicz L, Wrobel K, Bulska E. Magnesium–Isotope Fractionation in Chlorophyll-a Extracted from Two Plants with Different Pathways of Carbon Fixation (C3, C4). Molecules. 2020; 25(7):1644. https://doi.org/10.3390/molecules25071644
Chicago/Turabian StyleWrobel, Katarzyna, Jakub Karasiński, Andrii Tupys, Missael Antonio Arroyo Negrete, Ludwik Halicz, Kazimierz Wrobel, and Ewa Bulska. 2020. "Magnesium–Isotope Fractionation in Chlorophyll-a Extracted from Two Plants with Different Pathways of Carbon Fixation (C3, C4)" Molecules 25, no. 7: 1644. https://doi.org/10.3390/molecules25071644
APA StyleWrobel, K., Karasiński, J., Tupys, A., Arroyo Negrete, M. A., Halicz, L., Wrobel, K., & Bulska, E. (2020). Magnesium–Isotope Fractionation in Chlorophyll-a Extracted from Two Plants with Different Pathways of Carbon Fixation (C3, C4). Molecules, 25(7), 1644. https://doi.org/10.3390/molecules25071644