Glutamine Analogues Impair Cell Proliferation, the Intracellular Cycle and Metacyclogenesis in Trypanosoma cruzi
Abstract
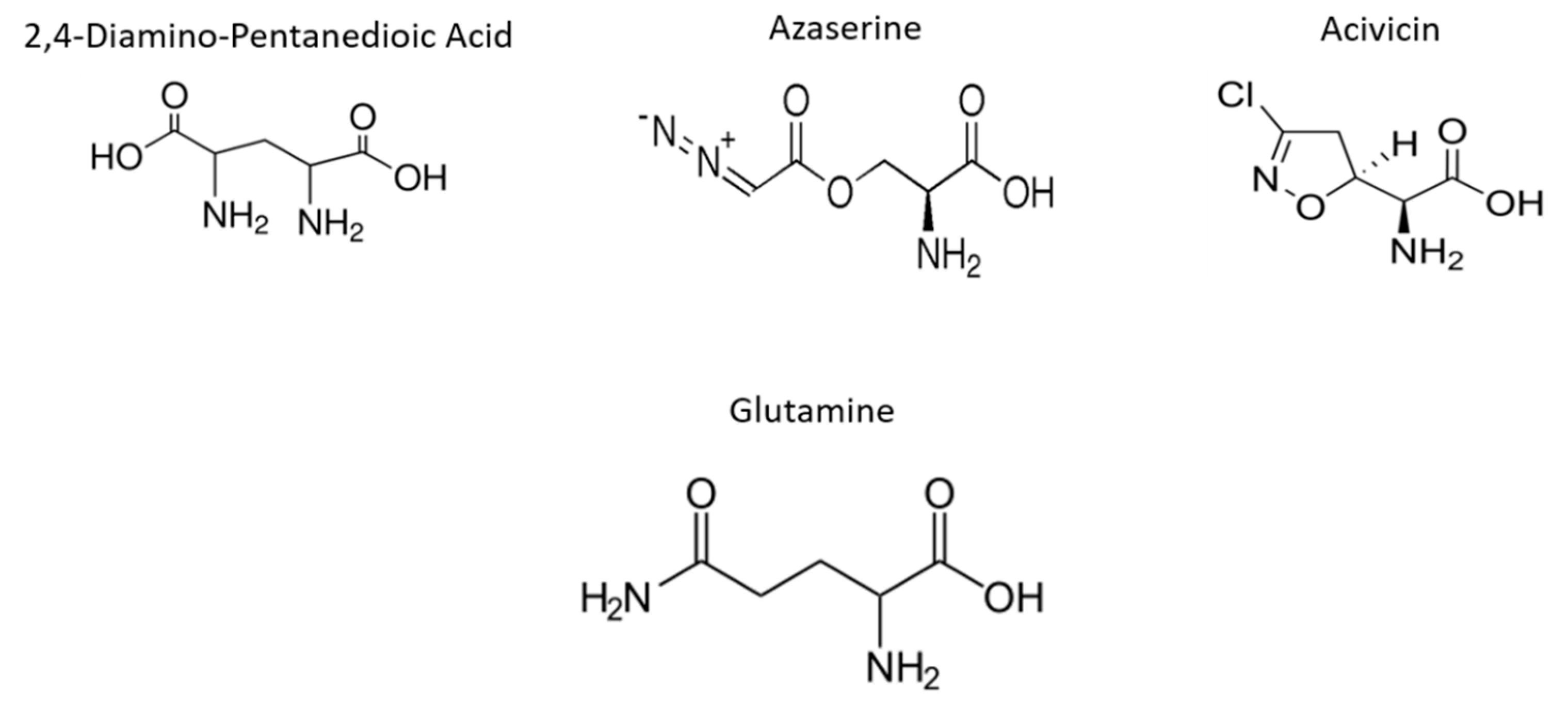
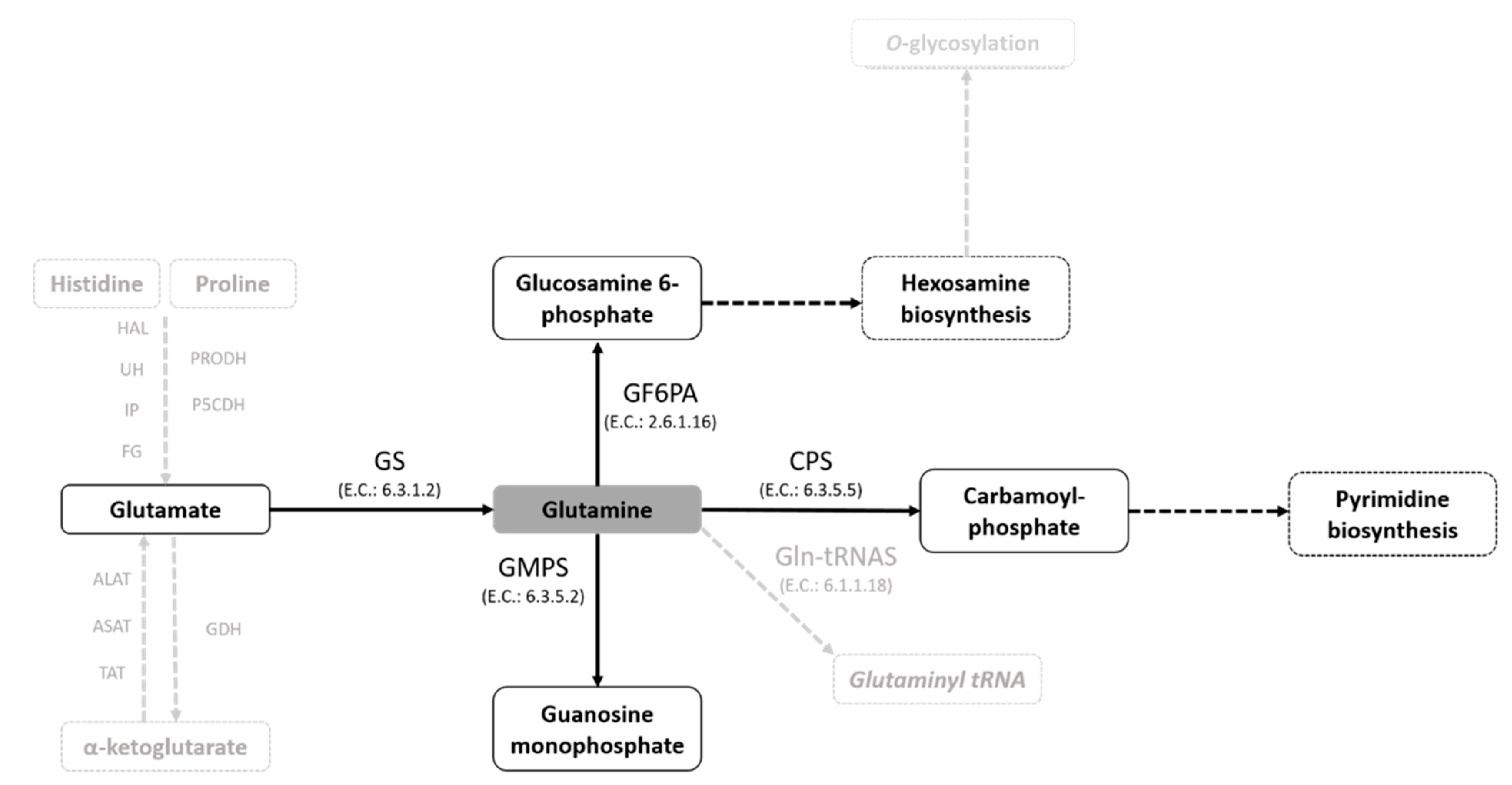
1. Introduction
2. Results
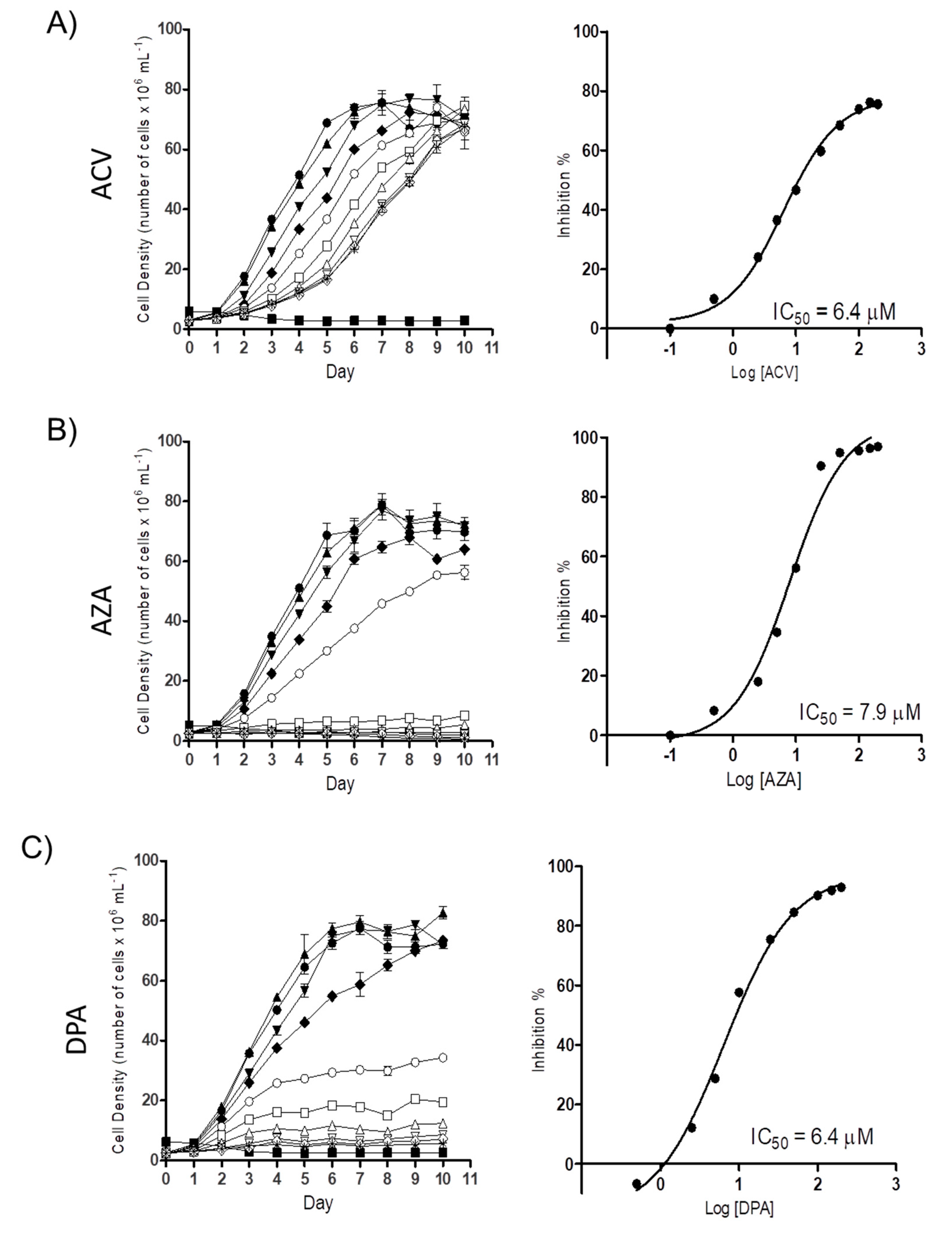
2.1. Gln analogues Impair the Proliferation of the Epimastigote Form
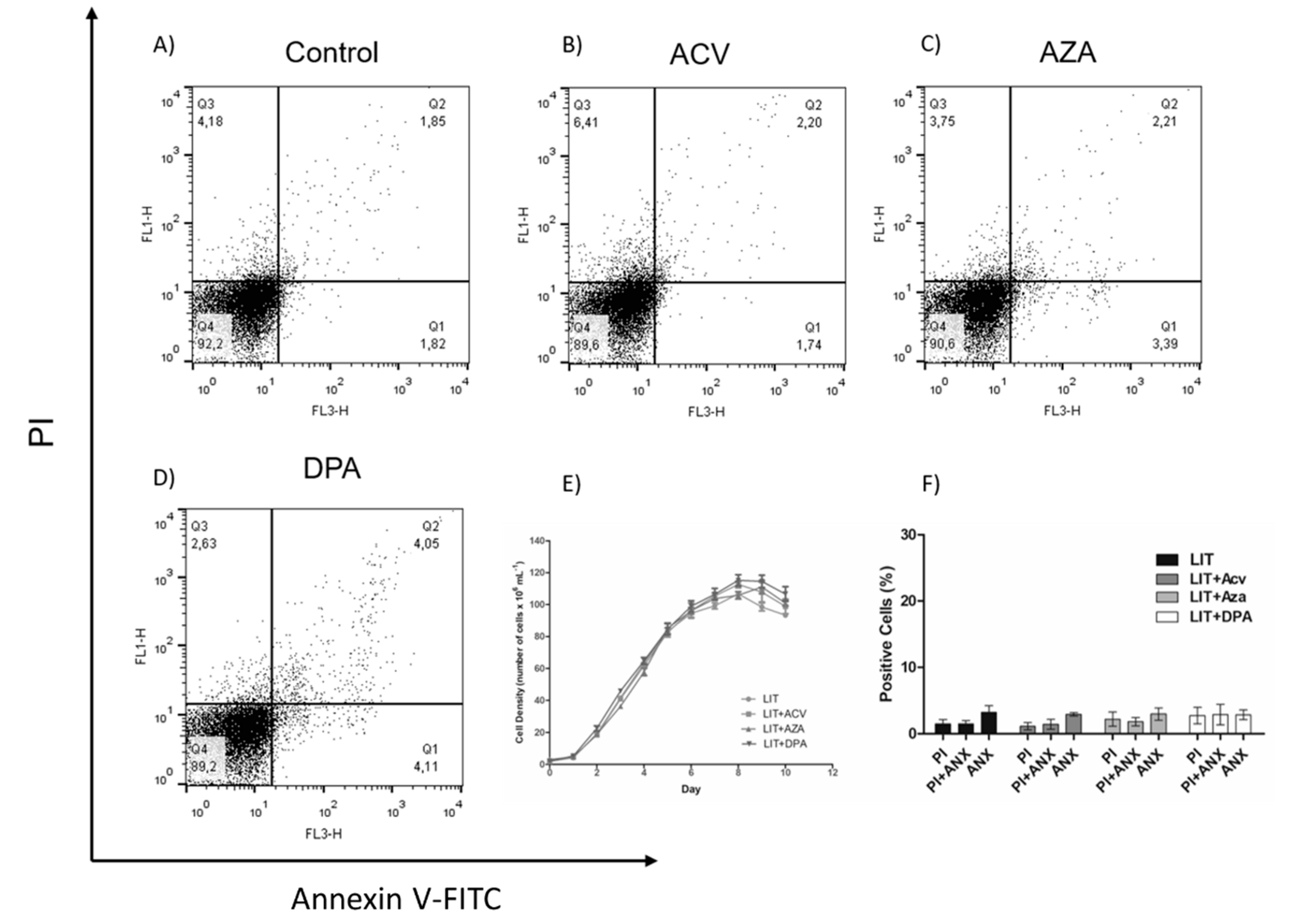
2.2. Gln analogue ACV Induces Extracellular Phosphatidylserine Exposure
2.3. Recovery of Parasites after Treatment with Gln analogues
2.4. Effect of Gln analogues on Metacyclogenesis
2.5. Effect of the Gln analogues on the Intracellular Infection of T. cruzi
3. Discussion
4. Materials and Methods
4.1. Parasites and Mammalian Cells
4.2. Proliferation Inhibition Assays
4.3. Extracellular Phosphatidylserine Exposure Analysis
4.4. Effect of the Gln analogues on Metacyclogenesis
4.5. Effect of the Gln analogues on Mammalian Cell Viability
4.6. Effect of the Gln analogues on Trypomastigote Bursting
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- WHO. WHO|Chagas Disease (American Trypanosomiasis); WHO: Geneva, Switzerland, 2018; Available online: http://www.who.int/chagas/en (accessed on 29 January 2019).
- Browne, A.J.; Guerra, C.A.; Alves, R.V.; da Costa, V.M.; Wilson, A.L.; Pigott, D.M.; Hay, S.I.; Lindsay, S.W.; Golding, N.; Moyes, C.L. The contemporary distribution of Trypanosoma cruzi infection in humans, alternative hosts and vectors. Sci. Data 2017, 4, 170050. [Google Scholar] [CrossRef]
- Lisvane Silva, P.; Mantilla, B.S.; Barison, M.J.; Wrenger, C.; Silber, A.M. The uniqueness of the Trypanosoma cruzi mitochondrion: Opportunities to identify new drug target for the treatment of Chagas disease. Curr. Pharm. Des. 2011, 17, 2074–2099. [Google Scholar] [CrossRef] [PubMed]
- Perez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Lidani, K.C.F.; Andrade, F.A.; Bavia, L.; Damasceno, F.S.; Beltrame, M.H.; Messias-Reason, I.J.; Sandri, T.L. Chagas disease: From discovery to a worldwide health problem. Front. Public Health 2019, 7, 166. [Google Scholar] [CrossRef]
- Urbina, J.A. Specific chemotherapy of Chagas disease: Relevance, current limitations and new approaches. Acta Trop. 2010, 115, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Marcondes de Rezende, J. American trypanosomiasis (Chagas disease). Infect. Dis. Clin. N. Am. 2012, 26, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Bern, C. Chagas’ Disease. N. Engl. J. Med. 2015, 373, 1882. [Google Scholar] [CrossRef] [PubMed]
- Marchese, L.; Nascimento, J.; Damasceno, F.; Bringaud, F.; Michels, P.; Silber, A. The uptake and metabolism of amino acids, and their unique role in the biology of pathogenic trypanosomatids. Pathogens 2018, 7, 36. [Google Scholar] [CrossRef]
- Cazzulo, J.J. Energy metabolism in Trypanosoma cruzi. Subcell Biochem. 1992, 18, 235–257. [Google Scholar]
- Silber, A.M.; Colli, W.; Ulrich, H.; Alves, M.J.; Pereira, C.A. Amino acid metabolic routes in Trypanosoma cruzi: Possible therapeutic targets against Chagas’ disease. Curr. Drug Targets Infect. Disord. 2005, 5, 53–64. [Google Scholar] [CrossRef][Green Version]
- Barison, M.J.; Rapado, L.N.; Merino, E.F.; Furusho Pral, E.M.; Mantilla, B.S.; Marchese, L.; Nowicki, C.; Silber, A.M.; Cassera, M.B. Metabolomic profiling reveals a finely tuned, starvation-induced metabolic switch in Trypanosoma cruzi epimastigotes. J. Biol. Chem. 2017, 292, 8964–8977. [Google Scholar] [CrossRef] [PubMed]
- Damasceno, F.S.; Barison, M.J.; Crispim, M.; Souza, R.O.O.; Marchese, L.; Silber, A.M. L-Glutamine uptake is developmentally regulated and is involved in metacyclogenesis in Trypanosoma cruzi. Mol. Biochem. Parasitol. 2018, 224, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Paes, L.S.; Suarez Mantilla, B.; Zimbres, F.M.; Pral, E.M.; Diogo de Melo, P.; Tahara, E.B.; Kowaltowski, A.J.; Elias, M.C.; Silber, A.M. Proline dehydrogenase regulates redox state and respiratory metabolism in Trypanosoma cruzi. PLoS ONE 2013, 8, e69419. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.M.; Covarrubias, C.; Rojas, R.G.; Silber, A.M.; Yoshida, N. Use of L-proline and ATP production by Trypanosoma cruzi metacyclic forms as requirements for host cell invasion. Infect. Immun. 2009, 77, 3023–3032. [Google Scholar] [CrossRef] [PubMed]
- Sylvester, D.; Krassner, S.M. Proline metabolism in Trypanosoma cruzi epimastigotes. Comp. Biochem. Physiol. B 1976, 55, 443–447. [Google Scholar] [CrossRef]
- Barisón, M.J.; Damasceno, F.S.; Mantilla, B.S.; Silber, A.M. The active transport of histidine and its role in ATP production in Trypanosoma cruzi. J. Bioenerg. Biomembr. 2016, 48, 437–449. [Google Scholar]
- Mantilla, B.S.; Paes, L.S.; Pral, E.M.F.; Martil, D.E.; Thiemann, O.H.; Fernández-Silva, P.; Bastos, E.L.; Silber, A.M. Role of Δ1-pyrroline-5-carboxylate dehydrogenase supports mitochondrial metabolism and host-cell invasion of Trypanosoma cruzi. J. Biol. Chem. 2015, 290, 7767–7790. [Google Scholar] [CrossRef]
- Pereira, C.A.; Alonso, G.D.; Paveto, M.C.; Flawia, M.M.; Torres, H.N. L-arginine uptake and L-phosphoarginine synthesis in Trypanosoma cruzi. J. Eukaryot. Microbiol. 1999, 46, 566–570. [Google Scholar] [CrossRef]
- Pereira, C.A.; Alonso, G.D.; Ivaldi, S.; Silber, A.; Alves, M.J.M.; Bouvier, L.A.; Flawiá, M.M.; Torres, H.N. Arginine metabolism in Trypanosoma cruzi is coupled to parasite stage and replication. FEBS Lett. 2002, 526, 111–114. [Google Scholar] [CrossRef]
- Pereira, C.A.; Alonso, G.D.; Ivaldi, S.; Silber, A.M.; Alves, M.J.M.; Torres, H.N.; Flawiá, M.M. Arginine kinase overexpression improves Trypanosoma cruzi survival capability. FEBS Lett. 2003, 554, 201–205. [Google Scholar] [CrossRef]
- Contreras, V.T.; Salles, J.M.; Thomas, N.; Morel, C.M.; Goldenberg, S. In vitro differentiation of Trypanosoma cruzi under chemically defined conditions. Mol. Biochem Parasitol. 1985, 16, 315–327. [Google Scholar] [CrossRef]
- Tonelli, R.R.; Silber, A.M.; Almeida-de-Faria, M.; Hirata, I.Y.; Colli, W.; Alves, M.J. L-proline is essential for the intracellular differentiation of Trypanosoma cruzi. Cell Microbiol. 2004, 6, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Homsy, J.J.; Granger, B.; Krassner, S.M. Some factors inducing formation of metacyclic stages of Trypanosoma cruzi. J. Protozool. 1989, 36, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Silber, A.M.; Tonelli, R.R.; Lopes, C.G.; Cunha-e-Silva, N.; Torrecilhas, A.C.; Schumacher, R.I.; Colli, W.; Alves, M.J. Glucose uptake in the mammalian stages of Trypanosoma cruzi. Mol. Biochem Parasitol. 2009, 168, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Rohloff, P.; Rodrigues, C.O.; Docampo, R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol. Biochem Parasitol. 2003, 126, 219–230. [Google Scholar] [CrossRef]
- Magdaleno, A.; Suarez Mantilla, B.; Rocha, S.C.; Pral, E.M.; Silber, A.M. The Involvement of Glutamate Metabolism in the Resistance to Thermal, Nutritional, and Oxidative Stress in Trypanosoma cruzi. Enzym. Res. 2011, 2011, 486928. [Google Scholar] [CrossRef]
- Magdaleno, A.; Ahn, I.Y.; Paes, L.S.; Silber, A.M. Actions of a proline analogue, L-thiazolidine-4-carboxylic acid (T4C), on Trypanosoma cruzi. PLoS ONE 2009, 4, e4534. [Google Scholar] [CrossRef]
- Saye, M.; Miranda, M.R.; di Girolamo, F.; de los Milagros Camara, M.; Pereira, C.A. Proline modulates the Trypanosoma cruzi resistance to reactive oxygen species and drugs through a novel D, L-proline transporter. PLoS ONE 2014, 9, e92028. [Google Scholar] [CrossRef]
- Soberon, M.; Gonzalez, A. Physiological role of glutaminase activity in Saccharomyces cerevisiae. J. Gen. Microbiol. 1987, 133, 1–8. [Google Scholar] [CrossRef]
- Ames, T.D.; Breaker, R.R. Bacterial aptamers that selectively bind glutamine. RNA Biol. 2011, 8, 82–89. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Cheng, T. Q’s next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene 2010, 29, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Lou, L. Biochemical characterization of human GMP synthetase. J. Biol. Chem. 1995, 270, 7347–7353. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, J.; Straub, K.; Wu, J.; Lou, L. The glutamine hydrolysis function of human GMP synthetase. Identification of an essential active site cysteine. J. Biol. Chem. 1995, 270, 23450–23455. [Google Scholar] [CrossRef] [PubMed]
- Raushel, F.M.; Thoden, J.B.; Holden, H.M. The amidotransferase family of enzymes: Molecular machines for the production and delivery of ammonia. Biochemistry 1999, 38, 7891–7899. [Google Scholar] [CrossRef]
- Crispim, M.; Damasceno, F.S.; Hernandez, A.; Barison, M.J.; Pretto Sauter, I.; Souza Pavani, R.; Santos Moura, A.; Pral, E.M.F.; Cortez, M.; Elias, M.C.; et al. The glutamine synthetase of Trypanosoma cruzi is required for its resistance to ammonium accumulation and evasion of the parasitophorous vacuole during host-cell infection. PLoS Negl. Trop. Dis. 2018, 12, e0006170. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Aaronson, S.A.; Abrams, J.; Alnemri, E.S.; Andrews, D.W.; Baehrecke, E.H.; Bazan, N.G.; Blagosklonny, M.V.; Blomgren, K.; Borner, C.; et al. Guidelines for the use and interpretation of assays for monitoring cell death in higher eukaryotes. Cell Death Differ. 2009, 16, 1093–1107. [Google Scholar] [CrossRef]
- van Zandbergen, G.; Luder, C.G.; Heussler, V.; Duszenko, M. Programmed cell death in unicellular parasites: A prerequisite for sustained infection? Trends Parasitol. 2010, 26, 477–483. [Google Scholar] [CrossRef]
- O’Rourke, R.W.; Meyer, K.A.; Gaston, G.; White, A.E.; Lumeng, C.N.; Marks, D.L. Hexosamine biosynthesis is a possible mechanism underlying hypoxia’s effects on lipid metabolism in human adipocytes. PLoS ONE 2013, 8, e71165. [Google Scholar]
- Rajapakse, A.G.; Ming, X.F.; Carvas, J.M.; Yang, Z. The hexosamine biosynthesis inhibitor azaserine prevents endothelial inflammation and dysfunction under hyperglycemic condition through antioxidant effects. Am. J. Physiol. Hear. Circ. Physiol. 2009, 296, H815–H822. [Google Scholar] [CrossRef]
- Yoneda, S. Some aspects of the purine metabolism of Trypanosoma cruzi in tissue culture. Rev. Bras. Pesqui. Med. Biol. 1971, 4, 205–218. [Google Scholar]
- Jaffe, J.J. Sensitivity of Trypanosoma equiperdum to the action of tumor-inhibitory antibiotics in vitro. Biochem. Pharmacol. 1965, 14, 1867–1881. [Google Scholar] [CrossRef]
- Momparler, R.L.; Jaffe, J.J. Effect of Azaserine on the Incorporation of 14-C-Labeled Purines and pyrimidines into acid-soluble and nucleic acid fractions of Trypanosoma equiperdum. Biochem. Pharmacol. 1965, 14, 255–262. [Google Scholar] [CrossRef]
- Poster, D.S.; Bruno, S.; Penta, J.; Neil, G.L.; McGovren, J.P. Acivicin. An antitumor antibiotic. Cancer Clin. Trials 1981, 4, 327–330. [Google Scholar] [PubMed]
- Kreuzer, J.; Bach, N.C.; Forler, D.; Sieber, S.A. Target discovery of acivicin in cancer cells elucidates its mechanism of growth inhibition. Chem. Sci. 2014, 6, 237–245. [Google Scholar] [CrossRef]
- Oliveira de Souza, J.; Dawson, A.; Hunter, W.N. An Improved Model of the Trypanosoma brucei CTP Synthetase Glutaminase Domain-Acivicin Complex. ChemMedChem 2017, 12, 577–579. [Google Scholar] [CrossRef]
- Li, Q.; Leija, C.; Rijo-Ferreira, F.; Chen, J.; Cestari, I.; Stuart, K.; Tu, B.P.; Phillips, M.A. GMP synthase is essential for viability and infectivity of Trypanosoma brucei despite a redundant purine salvage pathway. Mol. Microbiol. 2015, 97, 1006–1020. [Google Scholar] [CrossRef]
- Damasceno, F.S.; Barison, M.J.; Pral, E.M.; Paes, L.S.; Silber, A.M. Memantine, an antagonist of the NMDA glutamate receptor, affects cell proliferation, differentiation and the intracellular cycle and induces apoptosis in Trypanosoma cruzi. PLoS Negl. Trop Dis. 2014, 8, e2717. [Google Scholar] [CrossRef]
- Duszenko, M.; Figarella, K.; Macleod, E.T.; Welburn, S.C. Death of a trypanosome: A selfish altruism. Trends Parasitol. 2006, 22, 536–542. [Google Scholar] [CrossRef]
- Figarella, K.; Rawer, M.; Uzcategui, N.L.; Kubata, B.K.; Lauber, K.; Madeo, F.; Wesselborg, S.; Duszenko, M. Prostaglandin D2 induces programmed cell death in Trypanosoma brucei bloodstream form. Cell Death Differ. 2005, 12, 335–346. [Google Scholar] [CrossRef]
- Camargo, E.P. Growth and differentiation in Trypanosoma cruzi. I. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. Sao Paulo 1964, 6, 93–100. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds can be purchased by SIGMA. Limited quantities are available from the authors. |



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souza, R.O.O.; Crispim, M.; Silber, A.M.; Damasceno, F.S. Glutamine Analogues Impair Cell Proliferation, the Intracellular Cycle and Metacyclogenesis in Trypanosoma cruzi. Molecules 2020, 25, 1628. https://doi.org/10.3390/molecules25071628
Souza ROO, Crispim M, Silber AM, Damasceno FS. Glutamine Analogues Impair Cell Proliferation, the Intracellular Cycle and Metacyclogenesis in Trypanosoma cruzi. Molecules. 2020; 25(7):1628. https://doi.org/10.3390/molecules25071628
Chicago/Turabian StyleSouza, Rodolpho Ornitz Oliveira, Marcell Crispim, Ariel Mariano Silber, and Flávia Silva Damasceno. 2020. "Glutamine Analogues Impair Cell Proliferation, the Intracellular Cycle and Metacyclogenesis in Trypanosoma cruzi" Molecules 25, no. 7: 1628. https://doi.org/10.3390/molecules25071628
APA StyleSouza, R. O. O., Crispim, M., Silber, A. M., & Damasceno, F. S. (2020). Glutamine Analogues Impair Cell Proliferation, the Intracellular Cycle and Metacyclogenesis in Trypanosoma cruzi. Molecules, 25(7), 1628. https://doi.org/10.3390/molecules25071628




