Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis
Abstract
1. Introduction
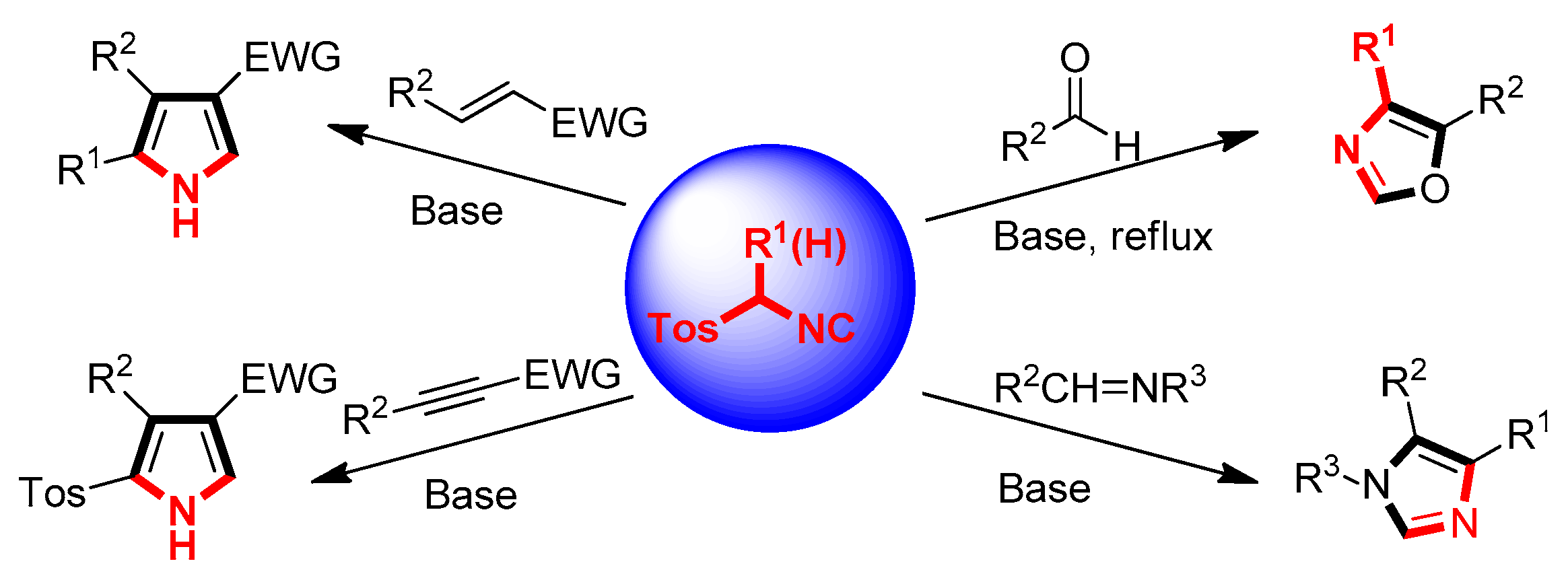
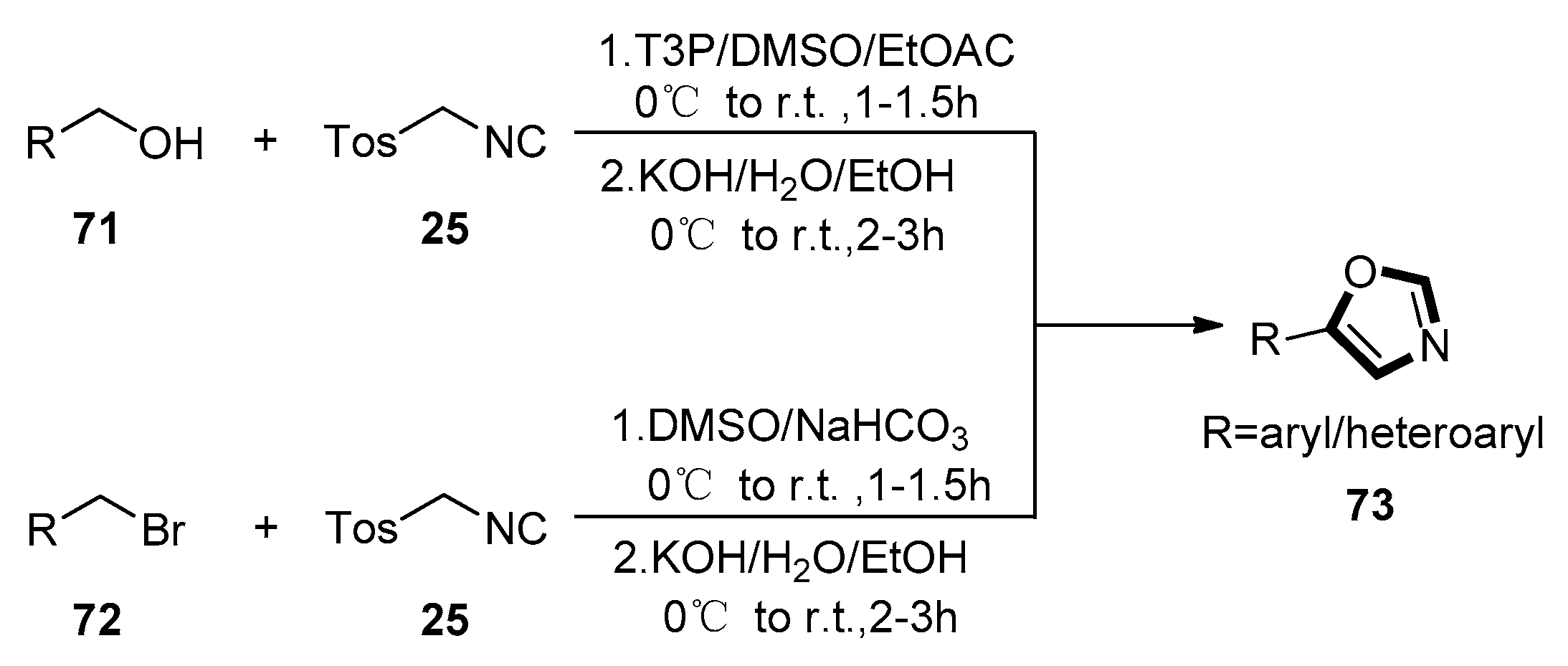
2. General van Leusen Oxazole Synthesis
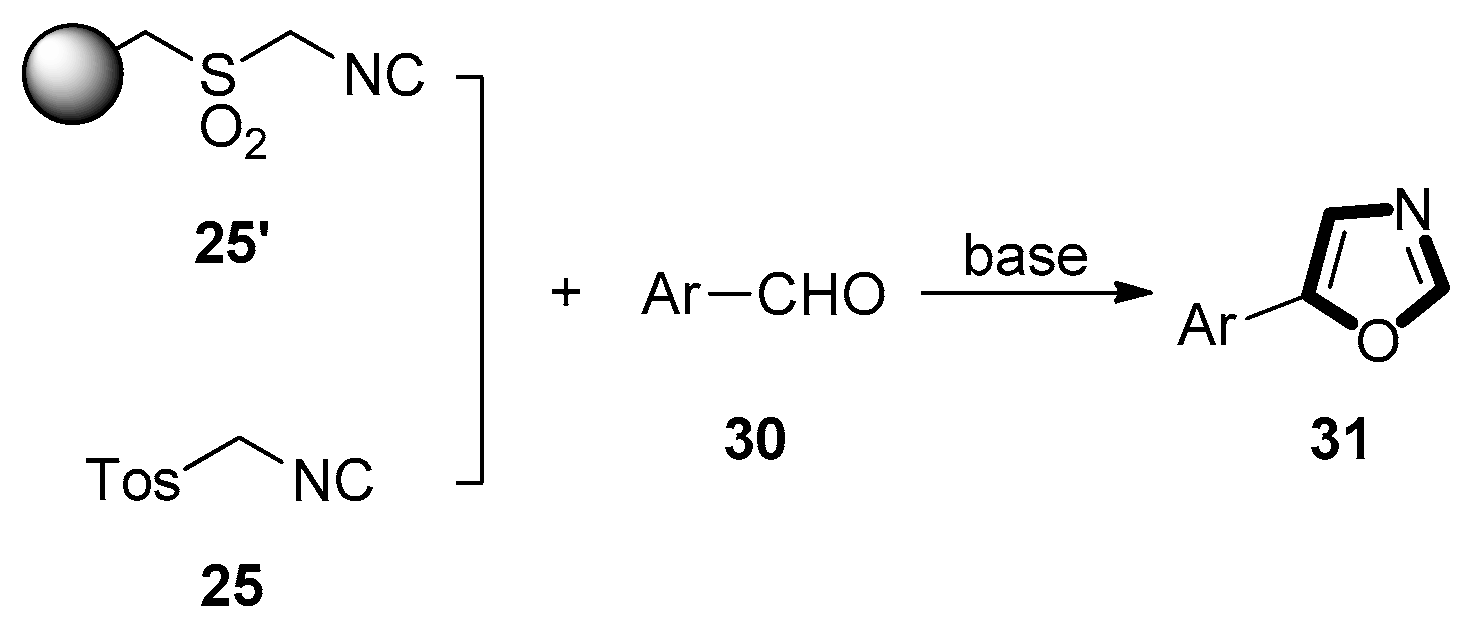
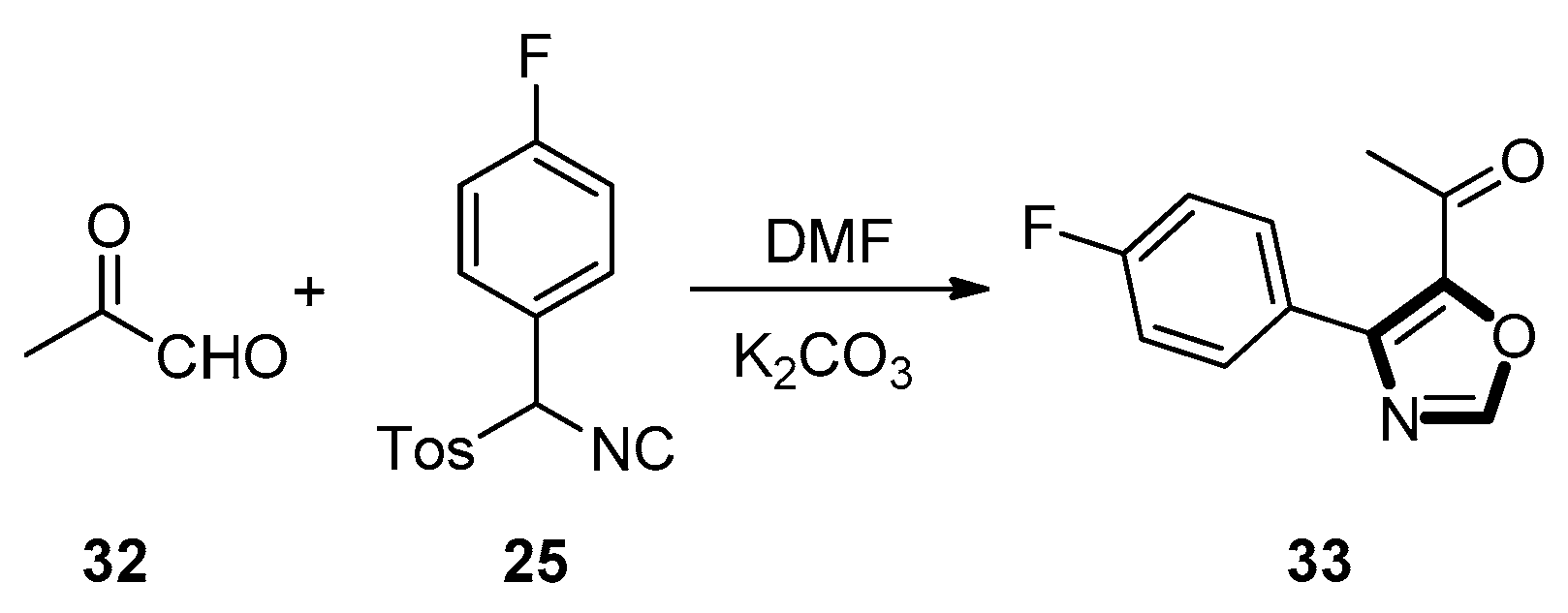
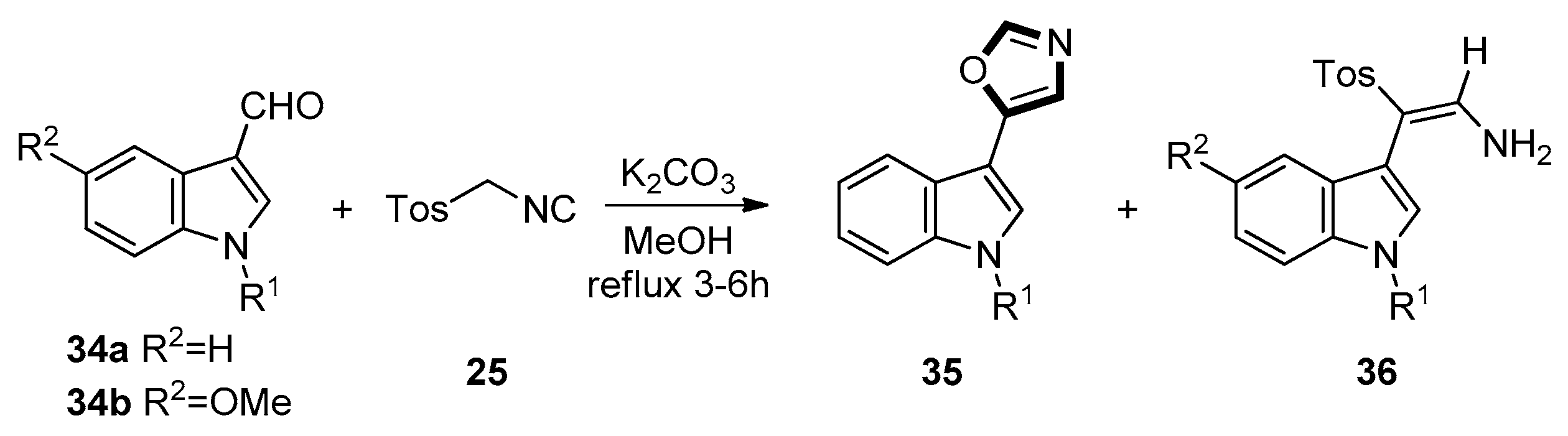
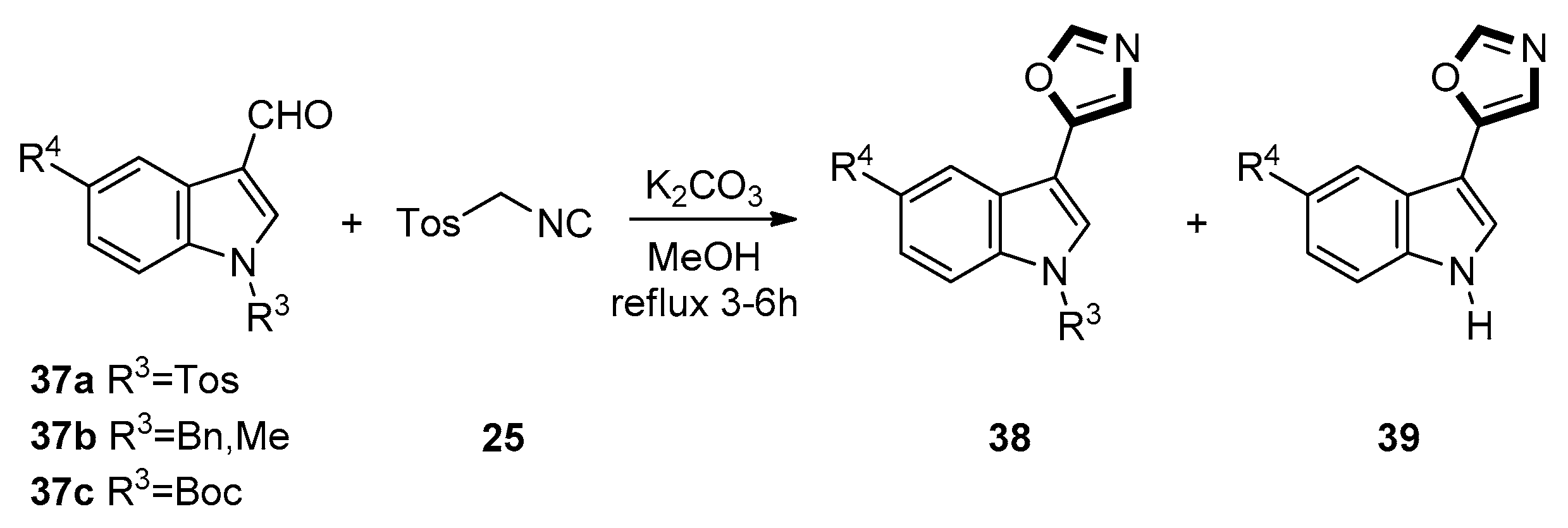
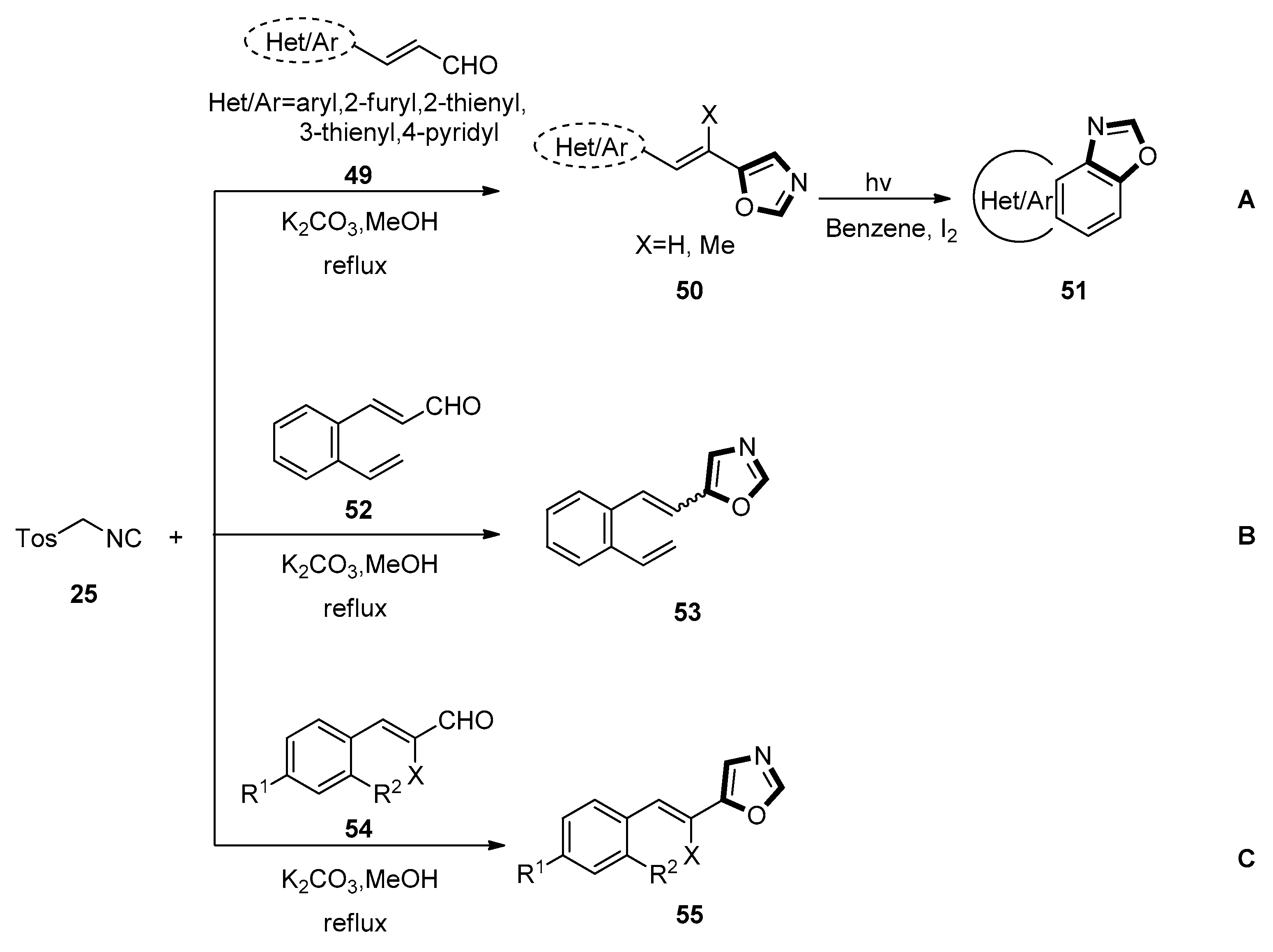
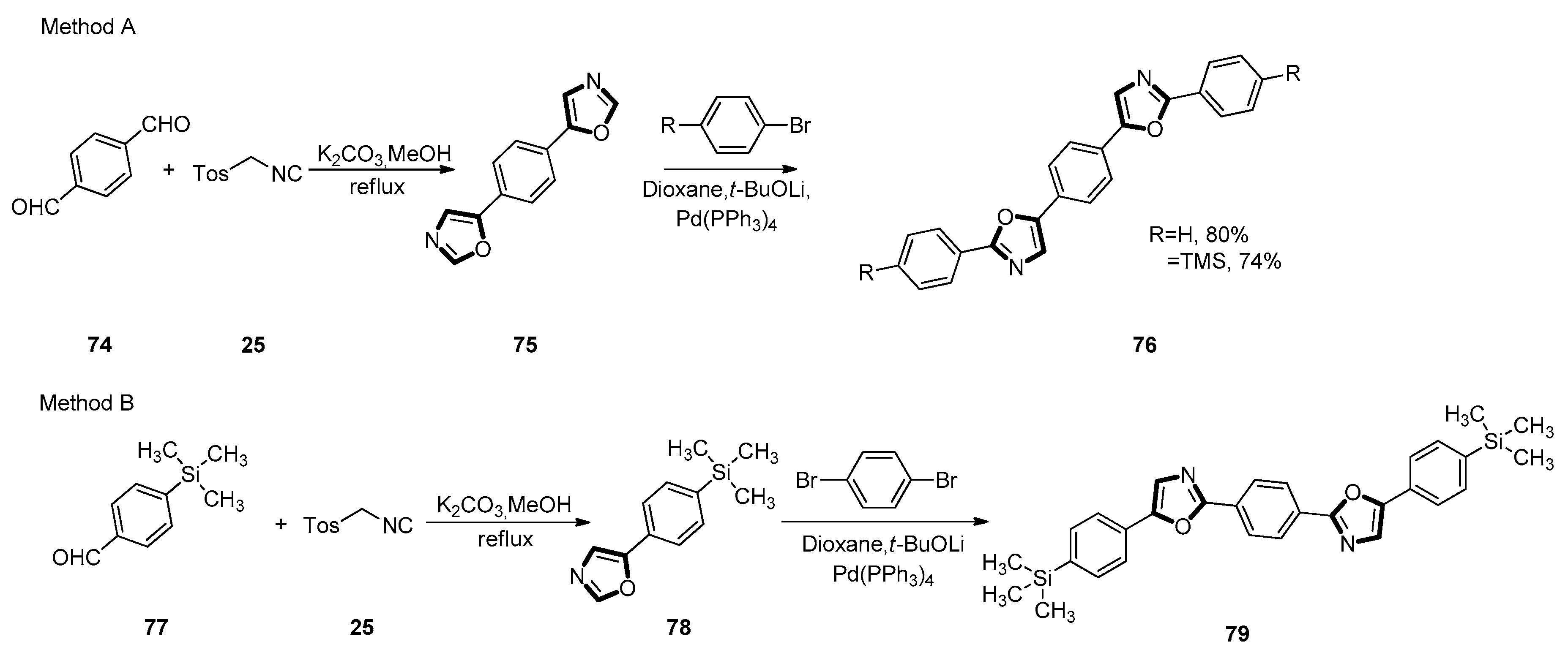
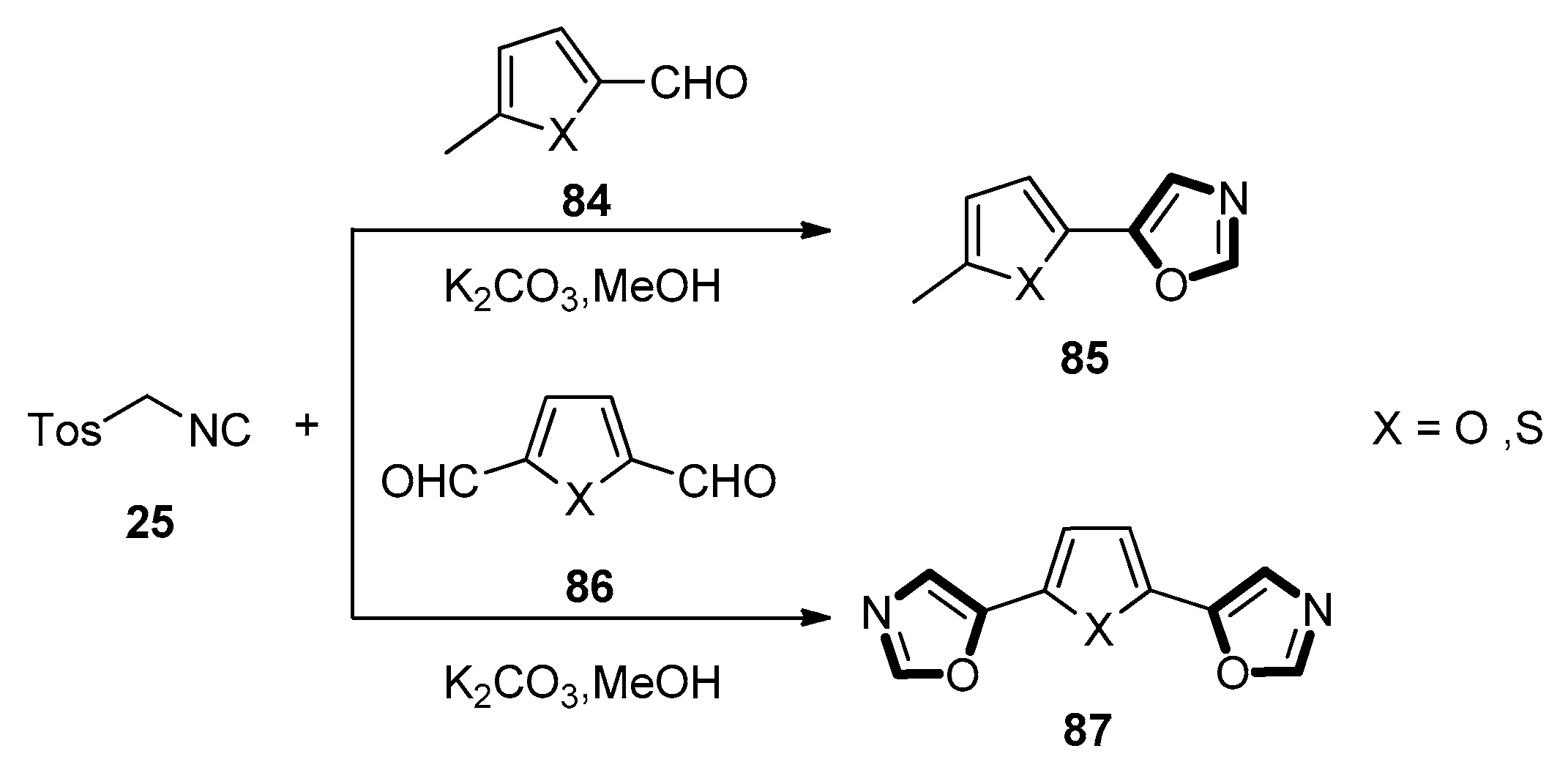
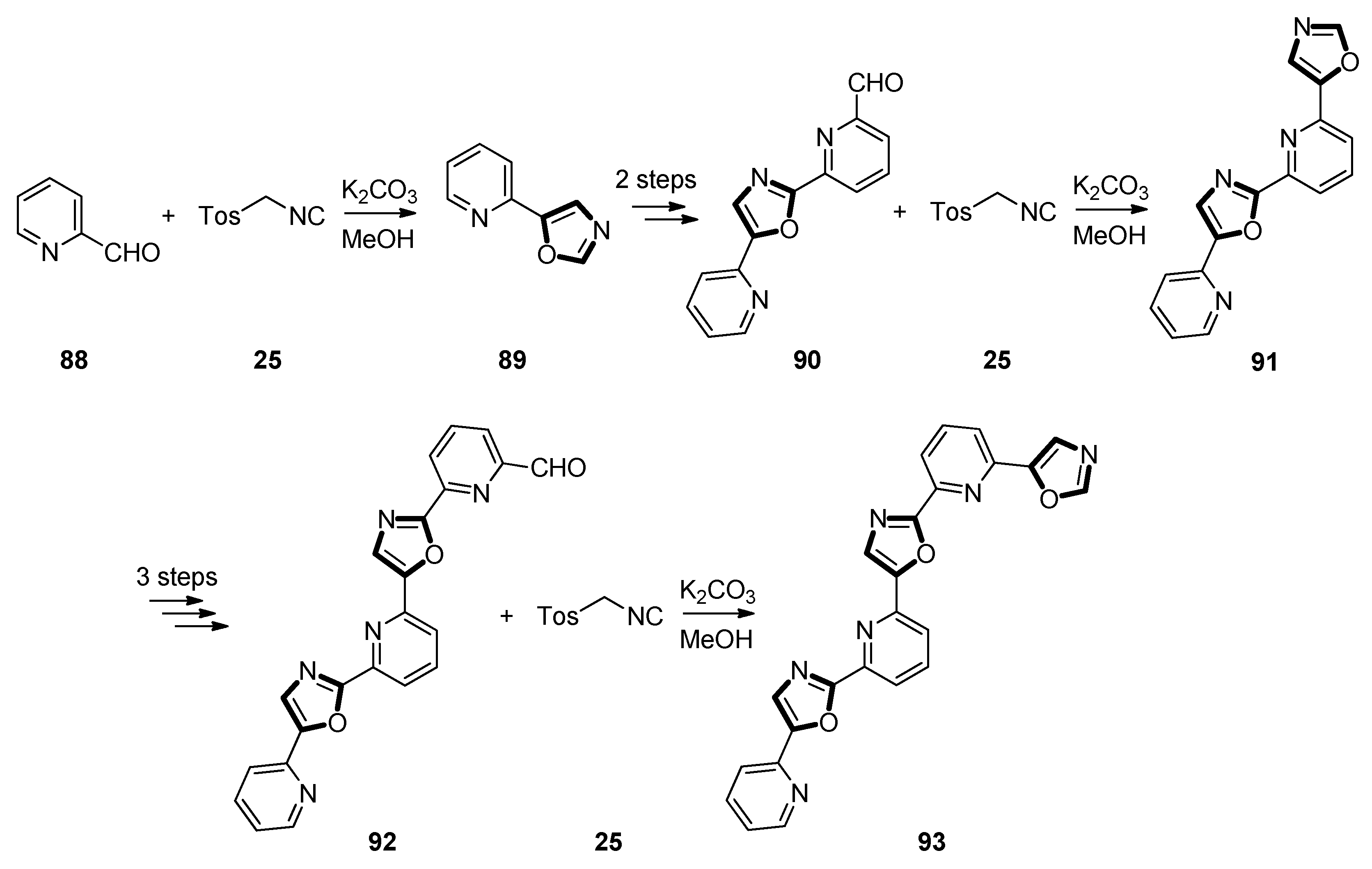
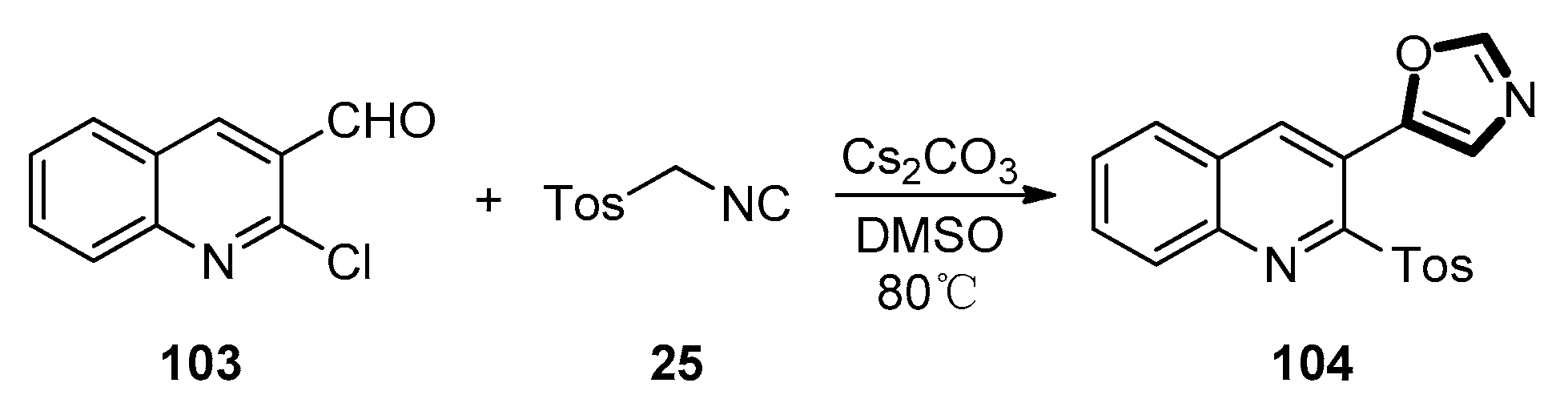
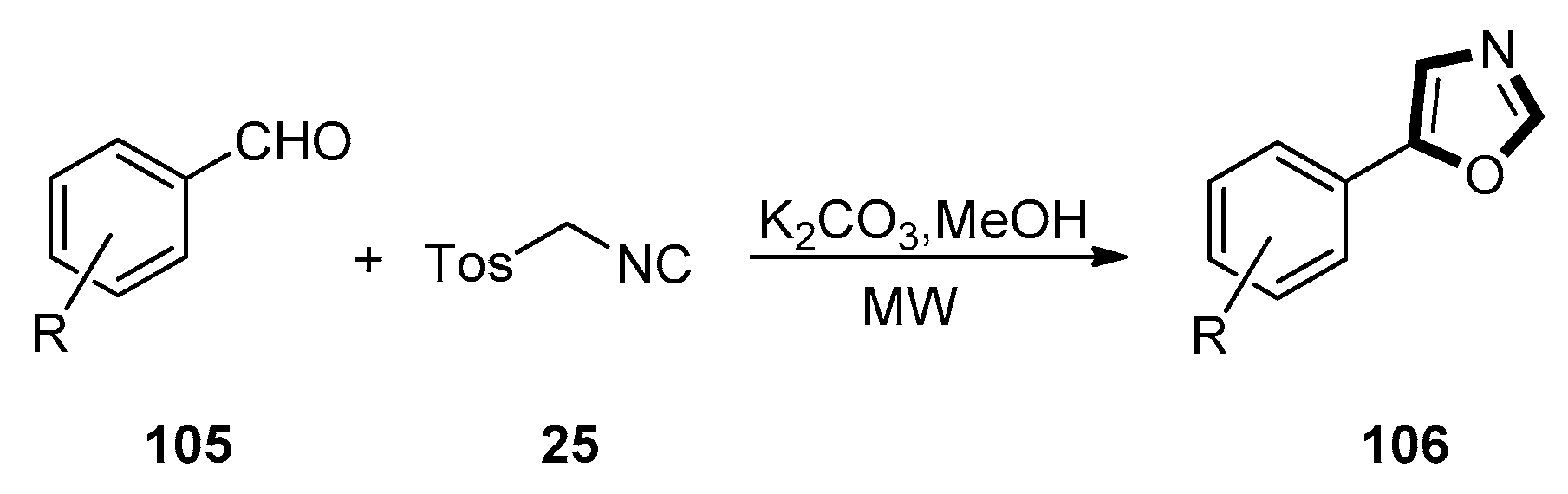
3. Developments of the van Leusen Oxazole Synthesis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, H.Z.; Zhao, Z.L.; Zhou, C.H. Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem. 2018, 144, 444–492. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Palta, K.; Kumar, M.; Bhargava, M.; Dahiya, L. Therapeutic potential of oxazole scaffold: A patent review (2006–2017). Expert Opin. Ther. Pat. 2018, 28, 783–812. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, S.; Narasimhan, B. A comprehensive review on biological activities of oxazole derivatives. BMC Chem. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Chiacchio, M.A.; Lanza, G.; Chiacchio, U.; Giofre, S.V.; Romeo, R.; Iannazzo, D.; Legnani, L. Oxazole-based compounds as anticancer agents. Curr. Med. Chem. 2019, 26, 7337–7371. [Google Scholar] [CrossRef]
- Aljaar, N.; Gujjarappa, R.; Al-Refai, M.; Shtaiwi, M.; Malakar, C.C. Overview on recent approaches towards synthesis of 2-keto-annulated oxazole derivatives. J. Heterocycl. Chem. 2019, 56, 2730–2743. [Google Scholar] [CrossRef]
- Škedelj, V.; Perdih, A.; Brvar, M.; Kroflič, A.; Dubbée, V.; Savage, V.; O’Neill, A.J.; Solmajer, T.; Bešter-Rogač, M.; Blanot, D.; et al. Discovery of the first inhibitors of bacterial enzyme d-aspartate ligase from Enterococcus faecium (Aslfm). Eur. J. Med. Chem. 2013, 67, 208–220. [Google Scholar] [CrossRef]
- Li, N.N.; Xu, Y.T.; Xia, Q.; Bai, C.G.; Wang, T.Y.; Wang, L.; He, D.D.; Xie, N.N.; Li, L.X.; Wang, J.; et al. Simplified captopril analogues as NDM-1 inhibitors. Bioorgan. Med. Chem. Lett. 2014, 24, 386–389. [Google Scholar] [CrossRef]
- Patil, P.C.; Tan, J.; Demuth, D.R.; Luzzio, F.A. 1,2,3-Triazole-based inhibitors of Porphyromonas gingivalis adherence to oral streptococci and biofilm formation. Bioorgan. Med. Chem. 2016, 24, 5410–5417. [Google Scholar] [CrossRef]
- Tomi, I.H.R.; Tomma, J.H.; Al-Daraji, A.H.R.; Al-Dujaili, A.H. Synthesis, characterization and comparative study the microbial activity of some heterocyclic compounds containing oxazole and benzothiazole moieties. J. Saudi Chem. Soc. 2015, 19, 392–398. [Google Scholar] [CrossRef]
- Pedras, M.S.; Abdoli, A. Metabolism of the phytoalexins camalexins, their bioisosteres and analogues in the plant pathogenic fungus Alternaria brassicicola. Bioorgan. Med. Chem. 2013, 21, 4541–4549. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Jia, C.Y.; Gu, Y.C.; Mulholland, N.; Turner, S.; Beattie, D.; Zhang, W.H.; Yang, G.F.; Clough, J. Synthesis and antifungal activity of novel indole-replaced streptochlorin analogues. Eur. J. Med. Chem. 2017, 126, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Matsushima, Y.; Hamaguchi, H.; Nagata, H.; Kontani, T.; Moritomo, A.; Koshika, T.; Takeuchi, M. Novel quinuclidinyl heteroarylcarbamate derivatives as muscarinic receptor antagonists. Bioorgan. Med. Chem. 2014, 22, 3478–3487. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Vitale, P.; Panella, A.; Fortuna, C.G.; Scilimati, A. General role of the amino and methylsulfamoyl groups in selective cyclooxygenase(COX)-1 inhibition by 1,4-diaryl-1,2,3-triazoles and validation of a predictive pharmacometric PLS model. Eur. J. Med. Chem. 2015, 94, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Otrubova, K.; Cravatt, B.F.; Boger, D.L. Design, Synthesis, and Characterization of α-Ketoheterocycles That additionally target the cytosolic port Cys269 of fatty acid amide hydrolase. J. Med. Chem. 2014, 57, 1079–1089. [Google Scholar] [CrossRef]
- Draffan, A.G.; Frey, B.; Fraser, B.H.; Pool, B.; Gannon, C.; Tyndall, E.M.; Cianci, J.; Harding, M.; Lilly, M.; Hufton, R.; et al. Derivatives of imidazotriazine and pyrrolotriazine C-nucleosides as potential new anti-HCV agents. Bioorgan. Med. Chem. Lett. 2014, 24, 4984–4988. [Google Scholar] [CrossRef]
- Zhong, Z.J.; Zhang, D.J.; Peng, Z.G.; Li, Y.H.; Shan, G.Z.; Zuo, L.M.; Wu, L.T.; Li, S.Y.; Gao, R.M.; Li, Z.R. Synthesis and antiviral activity of a novel class of (5-oxazolyl)phenyl amines. Eur. J. Med. Chem. 2013, 69, 32–43. [Google Scholar] [CrossRef]
- Kim, S.H.; Markovitz, B.; Trovato, R.; Murphy, B.R.; Austin, H.; Willardsen, A.J.; Baichwal, V.; Morham, S.; Bajji, A. Discovery of a new HIV-1 inhibitor scaffold and synthesis of potential prodrugs of indazoles. Bioorgan. Med. Chem. Lett. 2013, 23, 2888–2892. [Google Scholar] [CrossRef]
- Meissner, A.; Boshoff, H.I.; Vasan, M.; Duckworth, B.P.; Barry, C.E., III; Aldrich, C.C. Structure-activity relationships of 2-aminothiazoles effective against Mycobacterium tuberculosis. Bioorgan. Med. Chem. Lett. 2013, 21, 6385–6397. [Google Scholar] [CrossRef]
- Abhale, Y.K.; Sasane, A.V.; Chavan, A.P.; Shekh, S.H.; Deshmukh, K.K.; Bhansali, S.; Nawale, L.; Sarkar, D.; Mhaske, P.C. Synthesis and antimycobacterial screening of new thiazolyl-oxazole derivatives. Eur. J. Med. Chem. 2017, 132, 333–340. [Google Scholar] [CrossRef]
- Li, D.S.; Gao, N.N.; Zhu, N.Y.; Lin, Y.; Li, Y.; Chen, M.H.; You, X.F.; Lu, Y.; Wan, K.L.; Jiang, J.D.; et al. Discovery of the disubstituted oxazole analogues as a novel class anti-tuberculotic agents against MDR- and XDR-MTB. Bioorgan. Med. Chem. Lett. 2015, 25, 5178–5181. [Google Scholar] [CrossRef]
- Lu, X.W.; Wu, Y.K. On the structure of aspongopusin recently isolated from Aspongopus chinensis. Fitoterapia 2013, 84, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Liang, Z.W.; Xu, Y.Y.; He, W.M.; Xiang, J.N. Gold-catalyzed oxazoles synthesis and their relevant antiproliferative activities. Chin. Chem. Lett. 2013, 24, 1064–1066. [Google Scholar] [CrossRef]
- Maini, R.; Dedkova, L.M.; Paul, R.; Madathil, M.M.; Chowdhury, S.R.; Chen, S.; Hecht, S.M. Ribosome-mediated incorporation of dipeptides and dipeptide analogues into proteins in vitro. J. Am. Chem. Soc. 2015, 137, 11206–11209. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; Ismail, N.H.; Imran, S.; Anouar, E.H.; Selvaraj, M.; Jamil, W.; Ali, M.; Kashif, S.M.; Rahim, F.; Khan, K.M.; et al. Synthesis and molecular modelling studies of phenyl linked oxadiazole-phenylhydrazone hybrids as potent antileishmanial agents. Eur. J. Med. Chem. 2017, 126, 1021–1033. [Google Scholar] [CrossRef] [PubMed]
- Yamamuro, D.; Uchida, R.; Ohtawa, M.; Arima, S.; Futamura, Y.; Katane, M.; Homma, H.; Nagamitsu, T.; Osada, H.; Tomoda, H. Synthesis and biological activity of 5-(4-methoxyphenyl)-oxazole derivatives. Bioorgan. Med. Chem. Lett. 2015, 25, 313–316. [Google Scholar] [CrossRef]
- Da Rosa, R.; de Moraes, M.H.; Zimmermann, L.A.; Schenkel, E.P.; Steindel, M.; Bernardes, L.S.C. Design and synthesis of a new series of 3,5-disubstituted isoxazoles active against Trypanosoma cruzi and Leishmania amazonensis. Eur. J. Med. Chem. 2017, 128, 25–35. [Google Scholar] [CrossRef]
- Yoon, D.S.; Wu, S.C.; Seethala, R.; Golla, R.; Nayeem, A.; Everlof, J.G.; Gordon, D.A.; Hamann, L.G.; Robl, J.A. Discovery of pyridyl sulfonamide 11-beta-hydroxysteroid dehydrogenase type 1 (11β-HSD1) inhibitors for the treatment of metabolic disorders. Bioorgan. Med. Chem. Lett. 2014, 24, 5045–5049. [Google Scholar] [CrossRef]
- Kalwat, M.A.; Huang, Z.; Wichaidit, C.; McGlynn, K.; Earnest, S.; Savoia, C.; Dioum, E.M.; Schneider, J.W.; Hutchison, M.R.; Cobb, M.H. Isoxazole alters metabolites and gene expression, decreasing proliferation and promoting a neuroendocrine phenotype in β-Cells. ACS Chem. Biol. 2016, 11, 1128–1136. [Google Scholar] [CrossRef]
- Zahanich, I.; Kondratov, I.; Naumchyk, V.; Kheylik, Y.; Platonov, M.; Zozulya, S.; Krasavin, M. Phenoxymethyl 1,3-oxazoles and 1,2,4-oxadiazoles as potent and selective agonists of free fatty acid receptor 1 (GPR40). Bioorgan. Med. Chem. Lett. 2015, 25, 3105–3111. [Google Scholar] [CrossRef]
- Van Leusen, A.M.; Hoogenboom, B.E.; Siderius, H. A novel and efficient synthesis of oxazoles from tosylmethylisocyanide and carbonyl compounds. Tetrahedron Lett. 1972, 13, 2369–2372. [Google Scholar] [CrossRef]
- Cornforth, J.W.; Huang, H.T. Synthesis of a 4-cyano-oxazole. J. Chem. Soc. 1948, 1969–1971. [Google Scholar] [CrossRef]
- Fischer, E. Neue bildungsweise der oxazole. Eur. J. Inorg. Chem. 1896, 29, 205–214. [Google Scholar] [CrossRef]
- Doyle, M.P.; Buhro, W.E.; Davidson, J.G.; Elliott, R.C.; Hoekstra, J.W.; Oppenhuizen, M. Lewis acid promoted reactions of diazocarbonyl compounds. 3. Synthesis of oxazoles from nitriles through intermediate. beta-imidatoalkenediazonium salts. J. Org. Chem. 1980, 45, 3657–3664. [Google Scholar] [CrossRef]
- Dalla Vechia, L.; de Souza, R.O.M.A.; Miranda, L.S.D.E. The Dakin-West reaction: Past, present and future. Tetrahedron 2018, 74, 4359–4371. [Google Scholar] [CrossRef]
- Wasserman, H.H.; Vinick, F.J. Mechanism of the Robinson-Gabriel synthesis of oxazoles. J. Org. Chem. 1973, 38, 2407–2408. [Google Scholar] [CrossRef]
- Tandon, V.K.; Rai, S. p-Toluenesulfonylmethyl isocyanide: A versatile synthon in organic chemistry. Sulfur Rep. 2003, 24, 307–385. [Google Scholar]
- Akritopoulou-Zanze, I. Isocyanide-based multicomponent reactions in drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 324–331. [Google Scholar] [CrossRef]
- Lujan-Montelongo, J.A.; Estevez, A.O.; Fleming, F.F. Alkyl sulfinates: Formal nucleophiles for synthesizing TosMIC analogs. Eur. J. Org. Chem. 2015, 2015, 1602–1605. [Google Scholar] [CrossRef]
- Mathiyazhagan, A.D.; Anilkumar, G. Recent advances and applications of p-toluenesulfonylmethyl isocyanide (TosMIC). Org. Biomol. Chem. 2019, 17, 6735–6747. [Google Scholar] [CrossRef]
- Van Leusen, D.; van Leusen, A.M. Synthetic uses of tosylmethyl isocyanide (TosMIC). Org. React. 2001, 57, 417–666. [Google Scholar]
- Ma, Z.N.; Ma, Z.C.; Zhang, D.W. Synthesis of multi-substituted pyrrole derivatives through [3+2] cycloaddition with tosylmethylisocyanides (TosMICs) and electron-deficient compounds. Molecules 2018, 23, 2666. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.N.; Ma, Z.N.; Zhang, D.W. Synthesis of imidazole-based medicinal molecules utilizing the van leusen imidazole synthesis. Pharmaceuticals 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, B.A.; Ganesan, A. A solid-phase equivalent of van Leusen’s TosMIC, and its application in oxazole synthesis. Tetrahedron Lett. 1999, 40, 5633–5636. [Google Scholar] [CrossRef]
- Kulkarni, B.A.; Ganesan, A. Solution-phase parallel oxazole synthesis with TosMIC. Tetrahedron Lett. 1999, 40, 5637–5638. [Google Scholar] [CrossRef]
- Sisko, J.; Kassick, A.J.; Mellinger, R.; Filan, J.J.; Allen, A.; Olsen, M.A. An investigation of imidazole and oxazole syntheses using aryl-substituted TosMIC reagents. J. Org. Chem. 2000, 65, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarty, M.; Basak, R.; Harigaya, Y.; Takayanagi, H. Reaction of 3/2-formylindoles with TosMIC: Formation of indolyloxazoles and stable indolyl primary enamines. Tetrahedron 2005, 61, 1793–1801. [Google Scholar] [CrossRef]
- Kotha, S.; Shah, V. Synthesis of bis- and trisoxazole derivatives via Suzuki-Miyaura cross-coupling reaction and van Leusen oxazole synthesis. Synthesis 2007, 23, 3653–3658. [Google Scholar] [CrossRef]
- Yu, X.Q.; Wu, B.; Wen, J.; Zhang, J.; Li, J.; Xiang, Y.Z. One-pot van Leusen synthesis of 4,5-disubstituted oxazoles in ionic liquids. Synlett 2009, 3, 500–504. [Google Scholar] [CrossRef]
- Šagud, I.; Faraguna, F.; Marinić, Ž.; Šindler-Kulyk, M. Photochemical approach to naphthoxazoles and fused heterobenzoxazoles from 5-(phenyl/heteroarylethenyl)oxazoles. J. Org. Chem. 2011, 76, 2904–2908. [Google Scholar] [CrossRef]
- Šagud, I.; Božić, S.; Marinić, Ž.; Šindler-Kulyk, M. Photochemical approach to functionalized benzobicyclo [3.2.1]octene structures via fused oxazoline derivatives from 4- and 5-(o-vinylstyryl)oxazoles. Beilstein J. Org. Chem. 2014, 10, 2222–2229. [Google Scholar] [CrossRef]
- Šagud, I.; Šindler-Kulyk, M.; Škorić, I.; Kelava, V.; Marinić, Ž. Synthesis of naphthoxazoles by photocyclization of 4-/5-(phenylethenyl)oxazoles. Eur. J. Org. Chem. 2018, 2018, 3326–3335. [Google Scholar] [CrossRef]
- Hamon, F.; Largy, E.; Guedin-Beaurepaire, A.; Rouchon-Dagois, M.; Sidibe, A.; Monchaud, D.; Mergny, J.L.; Riou, J.F.; Nguyen, C.H.; Teulade-Fichou, M.P. An acyclic oligoheteroaryle that discriminates strongly between diverse G-quadruplex topologies. Angew. Chem. Int. Ed. 2011, 50, 8745–8749. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Chen, Q.; Mulholland, N.; Beattie, D.; Irwin, D.; Gu, Y.C.; Yang, G.F.; Clough, J. Synthesis and fungicidal activity of novel pimprinine analogues. Eur. J. Med. Chem. 2012, 53, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Shin, J.; Lee, H.S.; Shin, H.J.; Lee, Y.J. A coise and rapid approach to the marine natural product streptochlorin and its analogues. Bull. Korean Chem. Soc. 2013, 34, 357–358. [Google Scholar] [CrossRef]
- Georgiades, S.; Rizeq, N. Synthesis of a ‘propeller-like’ oligoheteroaryl with alternating pyridine and oxazole motifs. Synlett 2015, 26, 489–493. [Google Scholar] [CrossRef]
- Shah, S.; Thakore, R.; Vyas, T.; Sridhar, B. Conformationally flexible C3-symmetric 1,3-oxazoles as molecular scaffolds. Synlett 2016, 27, 294–300. [Google Scholar] [CrossRef]
- Sadashiva, M.; Rangappa, K.; Vinay Kumar, K.; Swaroop, T.; Rajeev, N.; Vinayaka, A.; Lingaraju, G. A one-pot tandem approach for the synthesis of 5-(het)aryloxazoles from substituted (het)aryl methyl alcohols and benzyl bromides. Synlett 2016, 27, 1363–1366. [Google Scholar]
- Skorotetcky, M.S.; Borshchev, O.V.; Surin, N.M.; Odarchenko, Y.; Pisarev, S.A.; Peregudova, S.M.; Törnroos, K.W.; Chernyshov, D.; Ivanov, D.A.; Ponomarenko, S.A. Synthesis and photostability of 1,4-bis(5-phenyloxazol-2-yl)benzene (POPOP) structural isomers and their trimethylsilyl derivatives. Dyes Pigments 2017, 141, 128–136. [Google Scholar] [CrossRef]
- Rahimzadeh, G.; Kianmehr, E.; Mahdavi, M. Improvement of the van Leusen reaction in the presence of β-cyclodextrin: A green and efficient synthesis of oxazoles in water. Z. Naturforsch. B 2017, 72, 923–926. [Google Scholar] [CrossRef]
- Kotha, S.; Todeti, S.; Gopal, M.B.; Datta, A. Synthesis and photophysical properties of c 3-symmetric star-shaped molecules containing heterocycles such as furan, thiophene, and oxazole. ACS Omega 2017, 2, 6291–6297. [Google Scholar] [CrossRef]
- Civcir, P.Ü.; Kurtay, G.; Sarıkavak, K. Experimental and theoretical investigation of new furan and thiophene derivatives containing oxazole, isoxazole, or isothiazole subunits. Struct. Chem. 2017, 28, 773–790. [Google Scholar] [CrossRef]
- Rizeq, N.; Georgiades, S.N. Investigation of ‘head-to-tail’-connected oligoaryl N,O-ligands as recognition motifs for cancer-relevant G-quadruplexes. Molecules 2017, 22, 2160. [Google Scholar] [CrossRef] [PubMed]
- Savanur, H.M.; Kalkhambkar, R.G.; Laali, K.K. Libraries of C-5 substituted imidazoles and oxazoles by sequential van Leusen (vL)-Suzuki, vL-Heck and vL-Sonogashira in imidazolium-ILs with piperidine-appended-IL as base. Eur. J. Org. Chem. 2018, 2018, 5285–5288. [Google Scholar] [CrossRef]
- Lechel, T.; Kumar, R.; Bera, M.K.; Zimmer, R.; Reissig, H.U. The LANCA three-component reaction to highly substituted beta-ketoenamides—Versatile intermediates for the synthesis of functionalized pyridine, pyrimidine, oxazole and quinoxaline derivatives. Beilstein J. Org. Chem. 2019, 15, 655–678. [Google Scholar] [CrossRef] [PubMed]
- Zarganes-Tzitzikas, T.; Clemente, G.; Elsinga, P.; Dömling, A. MCR scaffolds get hotter with 18F-labeling. Molecules 2019, 24, 1327. [Google Scholar] [CrossRef]
- Yasaei, Z.; Mohammadpour, Z.; Shiri, M.; Tanbakouchian, Z.; Fazelzadeh, S. Isocyanide reactions toward the synthesis of 3-(oxazol-5-yl)quinoline-2-carboxamides and 5-(2-tosylquinolin-3-yl)oxazole. Front. Chem. 2019, 7, 433. [Google Scholar] [CrossRef]
- Rashamuse, T.J.; Harrison, A.T.; Mosebi, S.; van Vuuren, S.; Coyanis, E.M.; Bode, M.L. Design, synthesis and biological evaluation of imidazole and oxazole fragments as HIV-1 integrase-LEDGF/p75 disruptors and inhibitors of microbial pathogens. Bioorgan. Med. Chem. 2020, 28, 115210. [Google Scholar] [CrossRef]
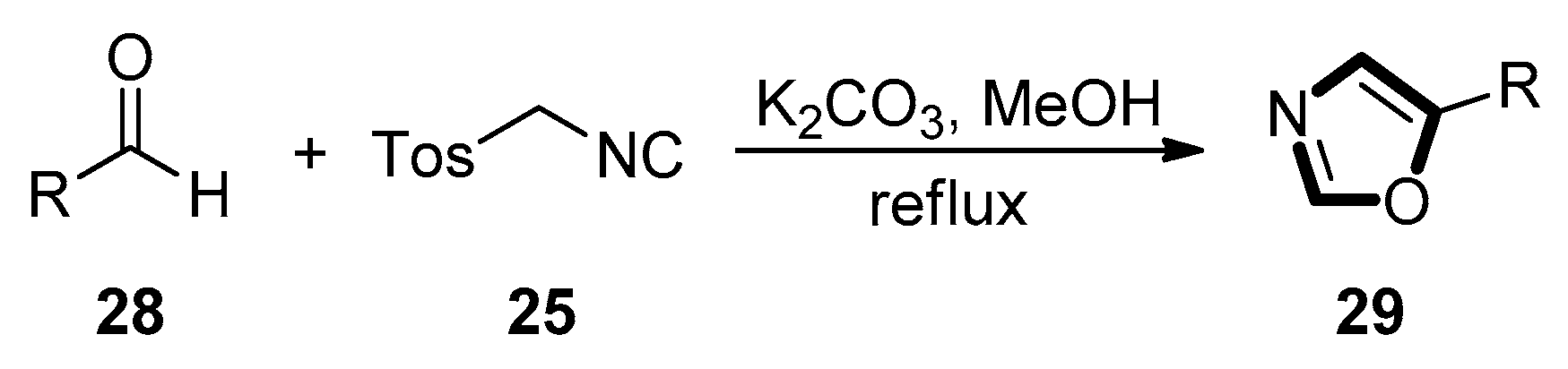


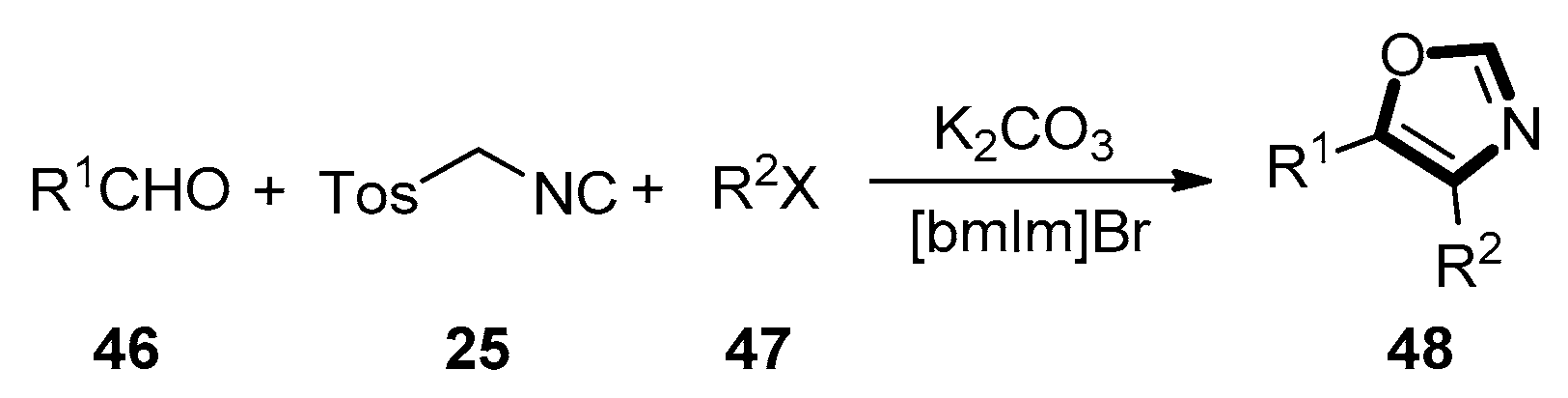

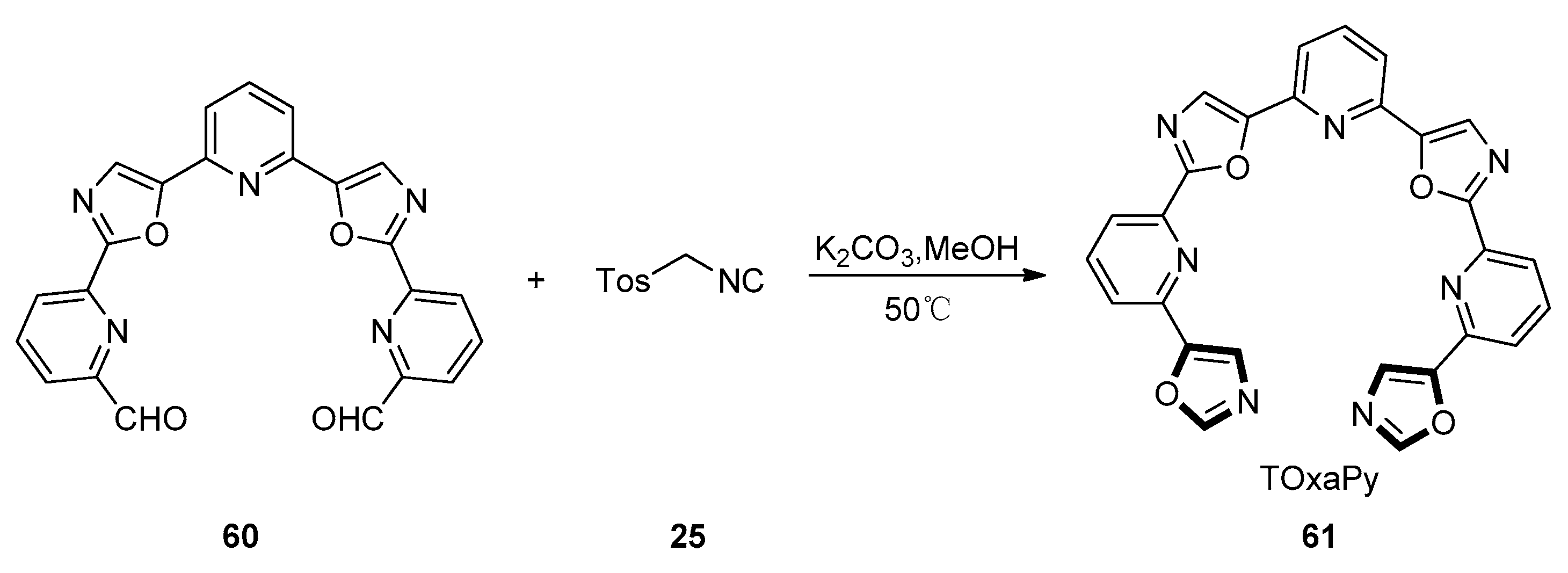
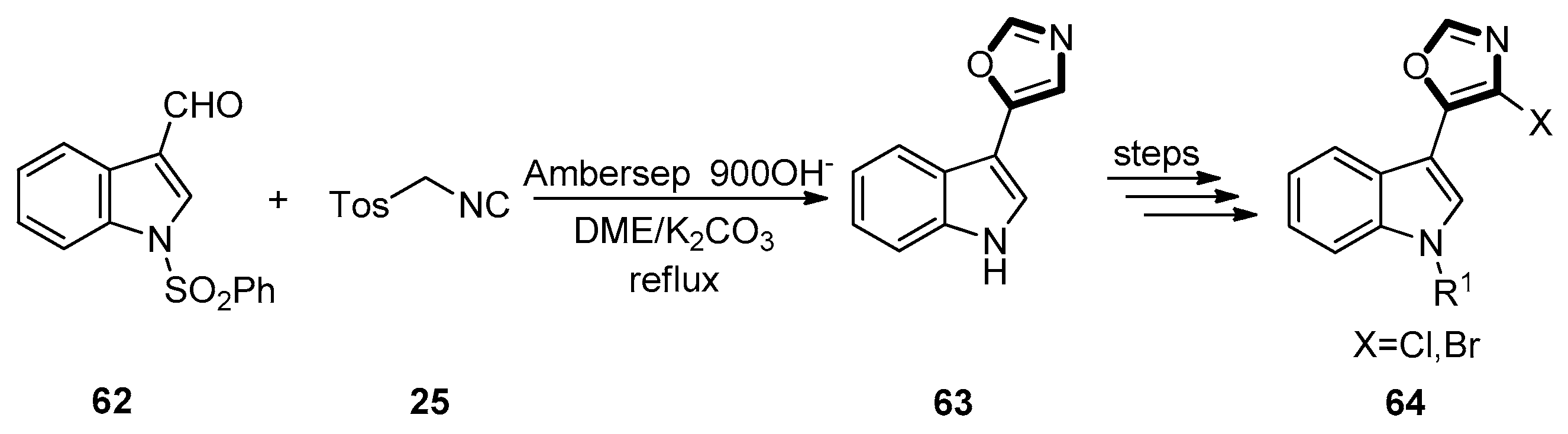
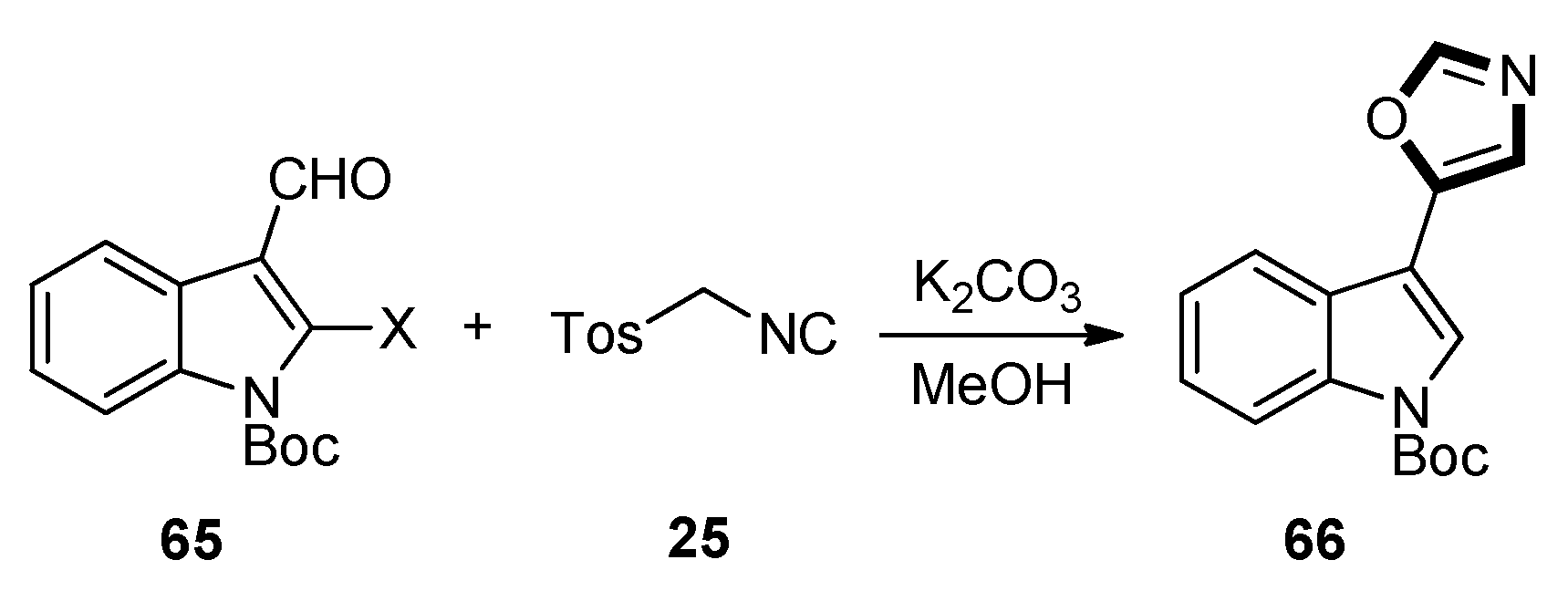
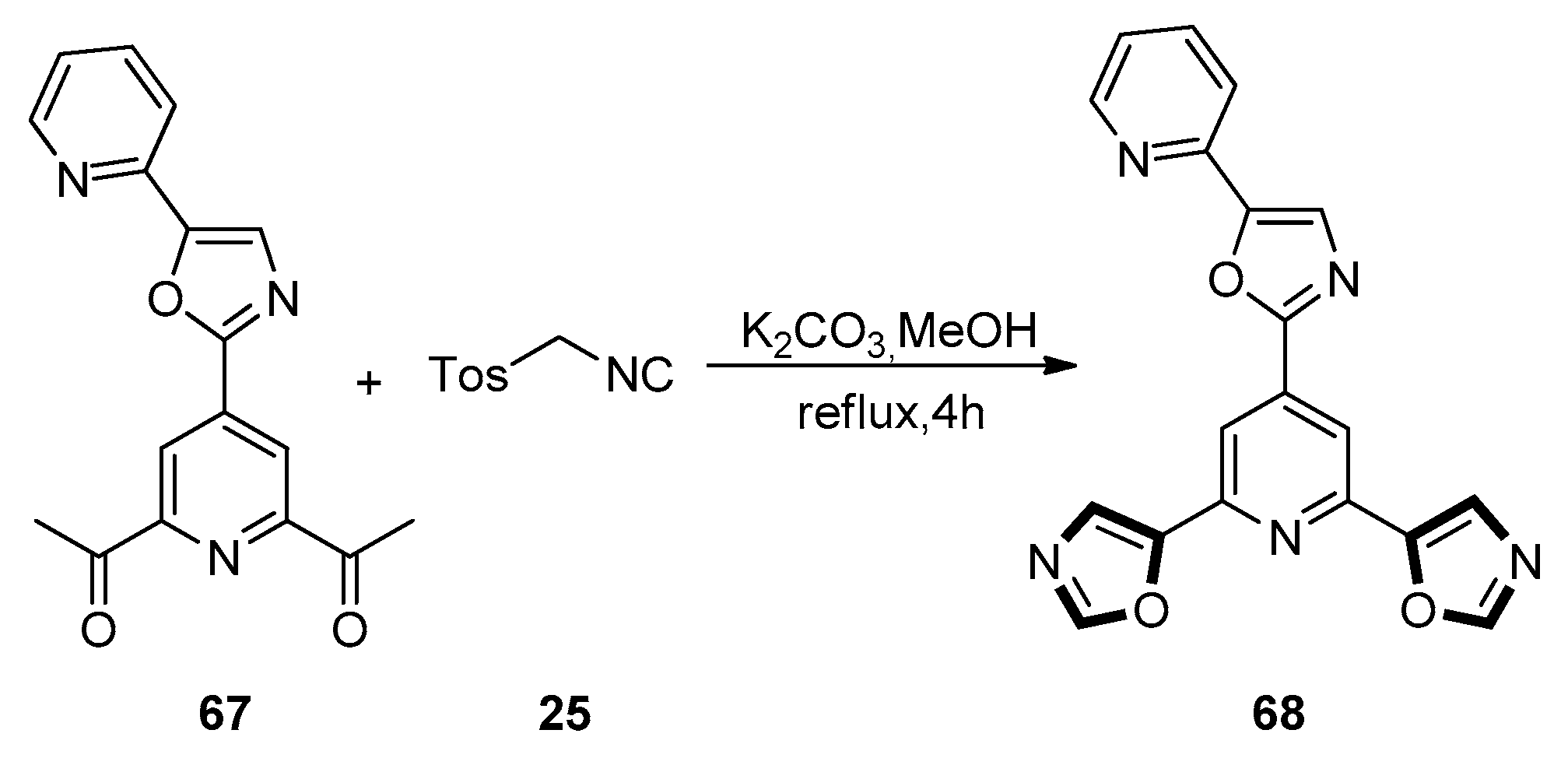

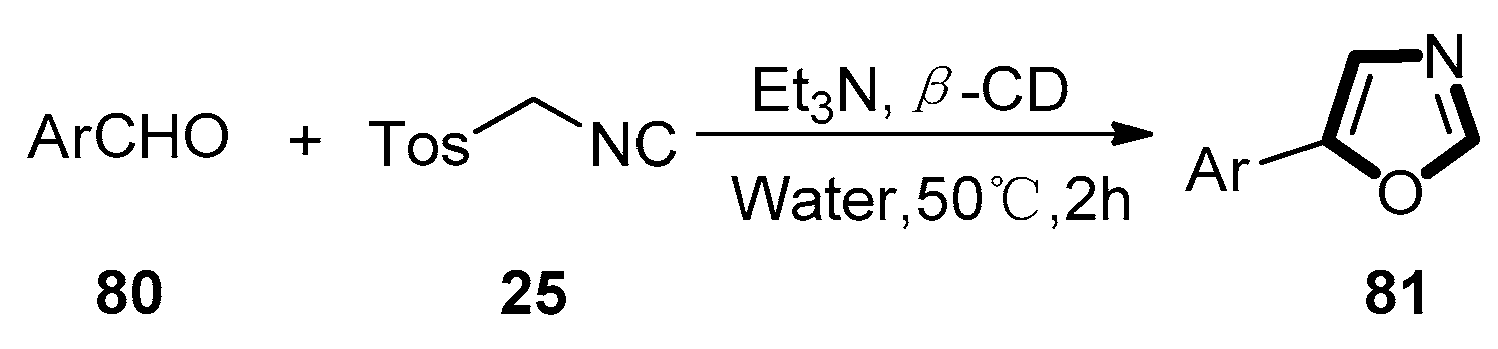



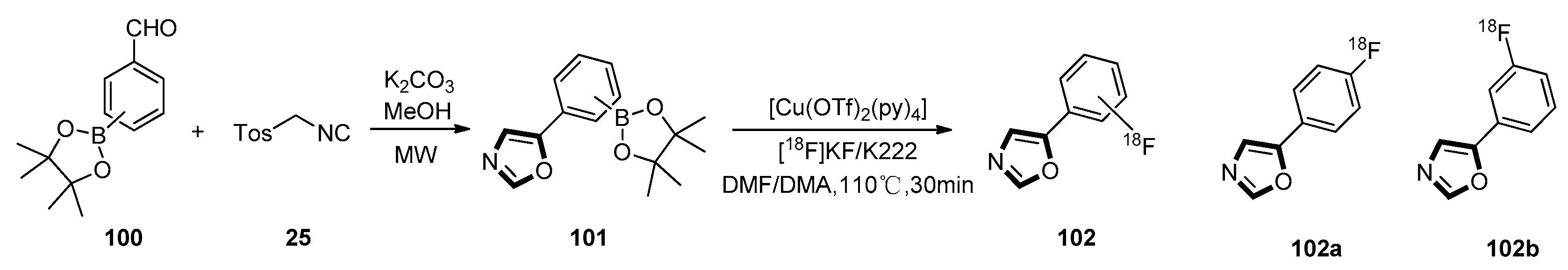
| Pharmacological Activities | Chemical Structures |
|---|---|
| Antibacterial | 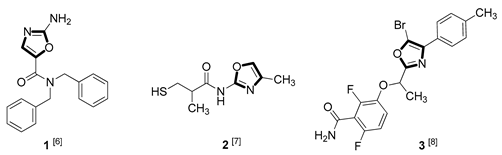 |
| Antifungal | 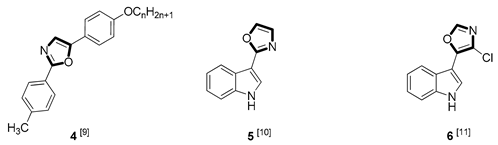 |
| Anti-inflammatory | 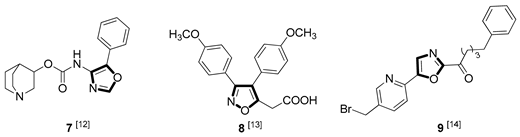 |
| Antiviral | 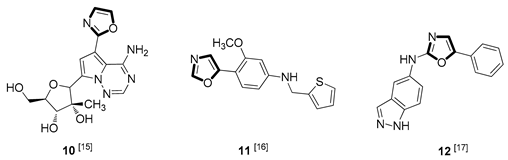 |
| Antitubercular |  |
| Anticancer | 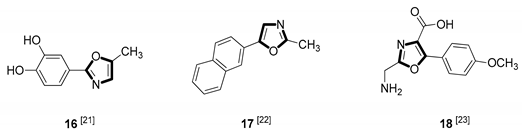 |
| Antiparasitic | 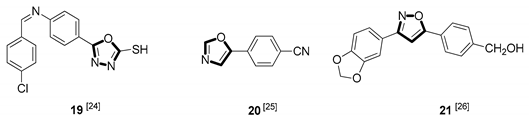 |
| Antidiabetic | 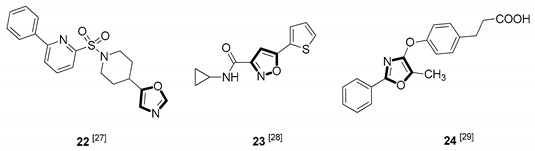 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Liu, W.; Zhang, D. Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis. Molecules 2020, 25, 1594. https://doi.org/10.3390/molecules25071594
Zheng X, Liu W, Zhang D. Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis. Molecules. 2020; 25(7):1594. https://doi.org/10.3390/molecules25071594
Chicago/Turabian StyleZheng, Xunan, Wei Liu, and Dawei Zhang. 2020. "Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis" Molecules 25, no. 7: 1594. https://doi.org/10.3390/molecules25071594
APA StyleZheng, X., Liu, W., & Zhang, D. (2020). Recent Advances in the Synthesis of Oxazole-Based Molecules via van Leusen Oxazole Synthesis. Molecules, 25(7), 1594. https://doi.org/10.3390/molecules25071594






