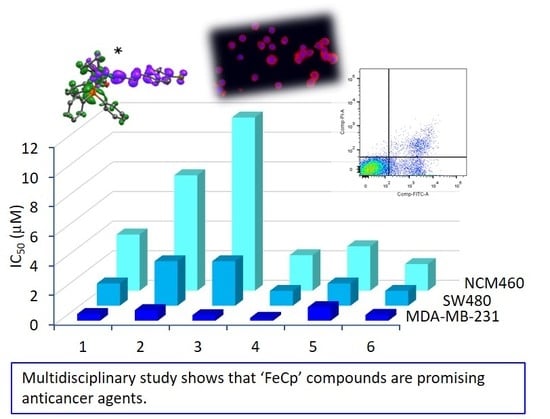
A New Family of Iron(II)-Cyclopentadienyl Compounds Shows Strong Activity against Colorectal and Triple Negative Breast Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
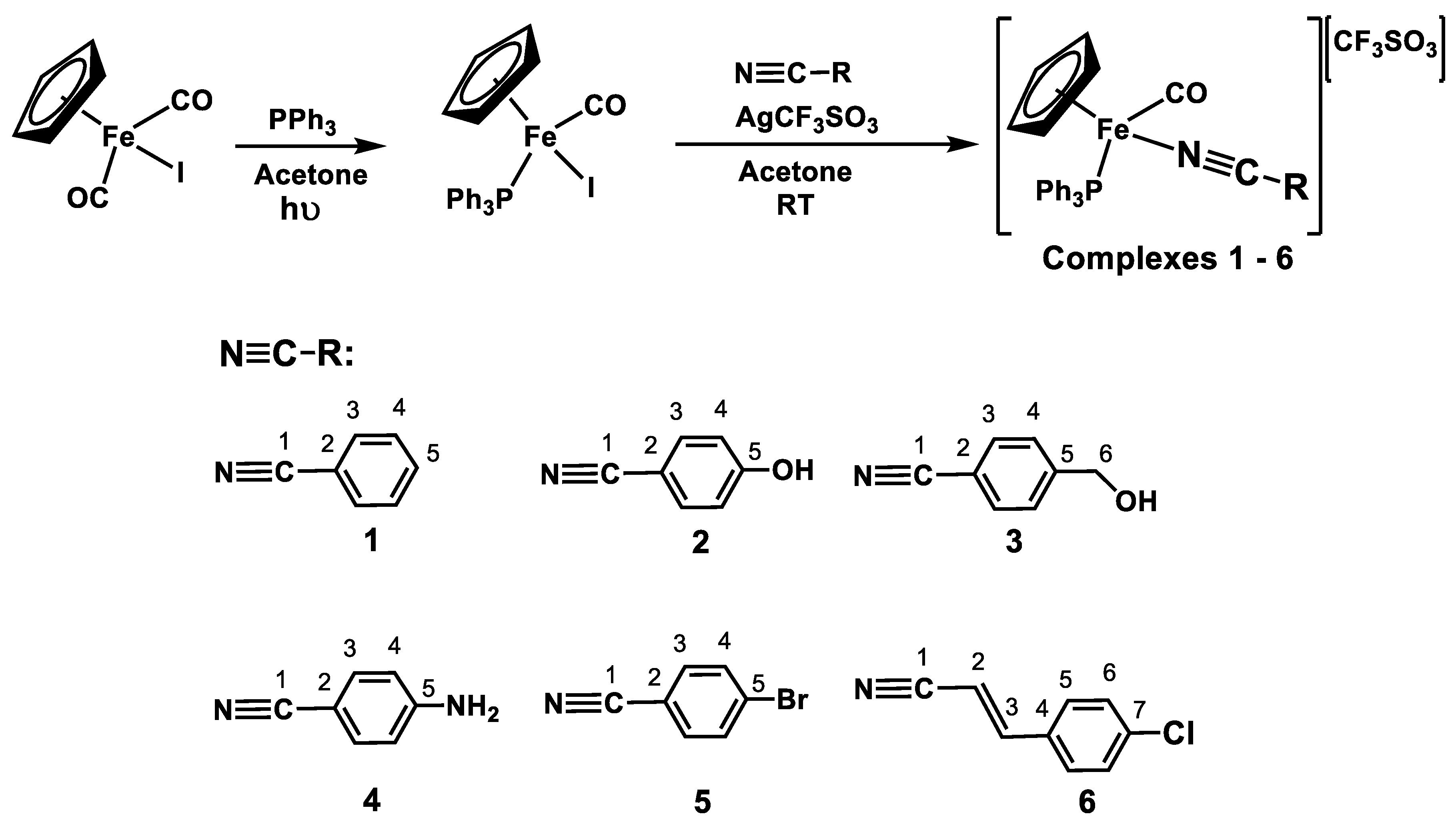
2.1. Synthesis
2.2. NMR Spectroscopy
2.3. FTIR Spectroscopy
2.4. UV-VIS Spectroscopy
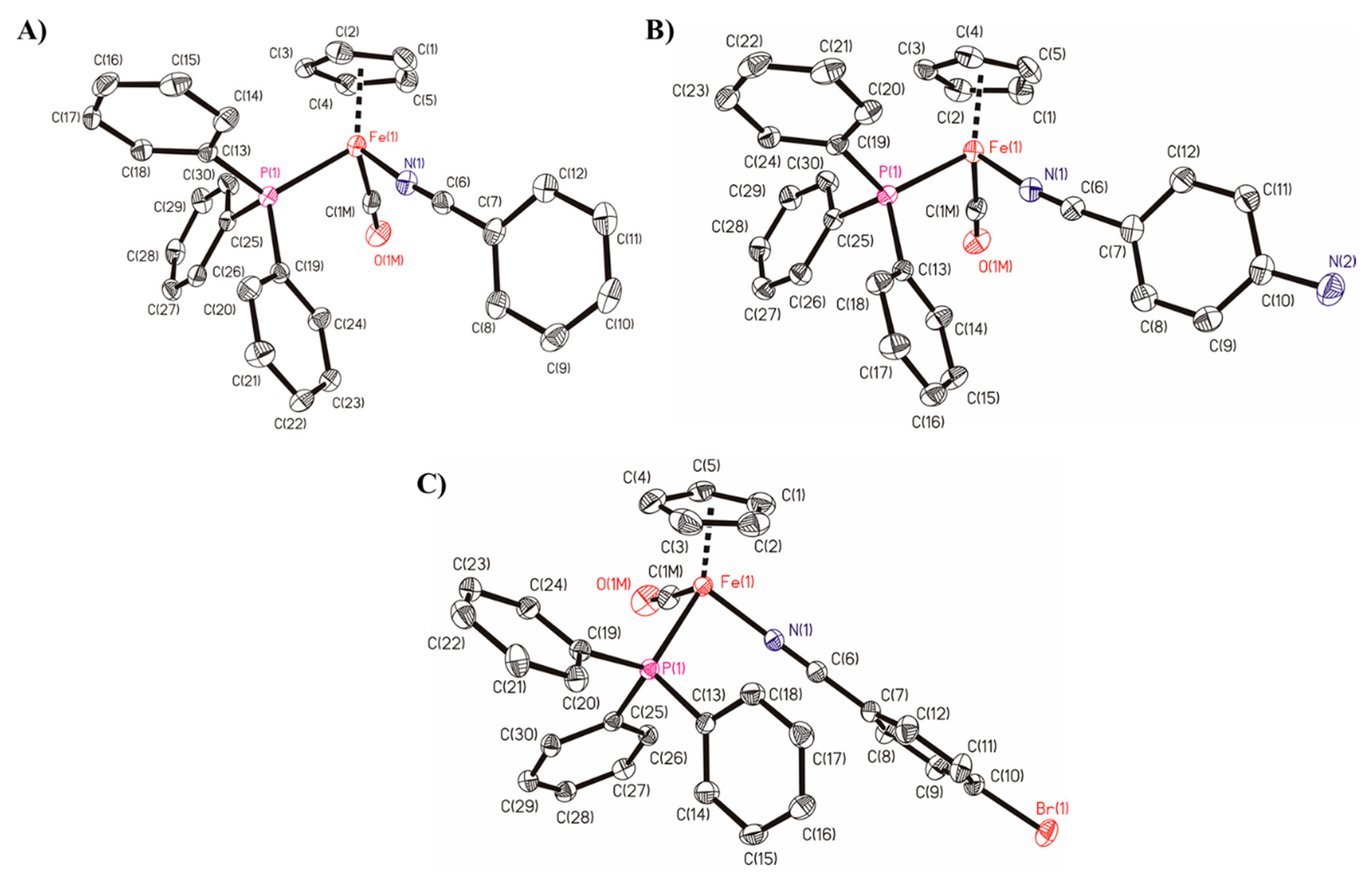
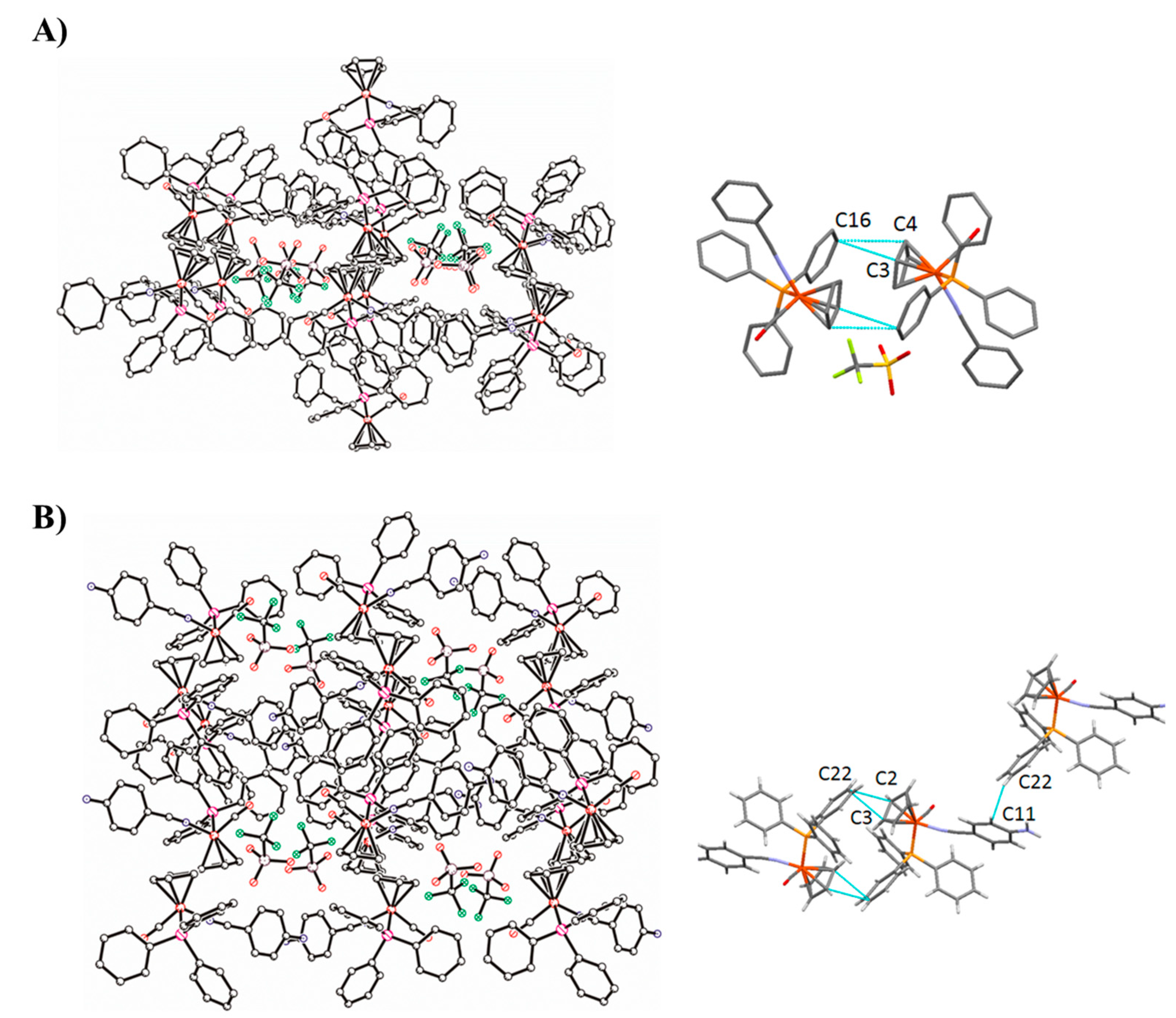
2.5. Single-Crystal Structures of Compounds 1, 4 and 5
2.6. TD-DFT Calculations
2.7. Biological Assays
2.7.1. Stability Assays
2.7.2. In Vitro Cytotoxicity Evaluation and IC50 Determination
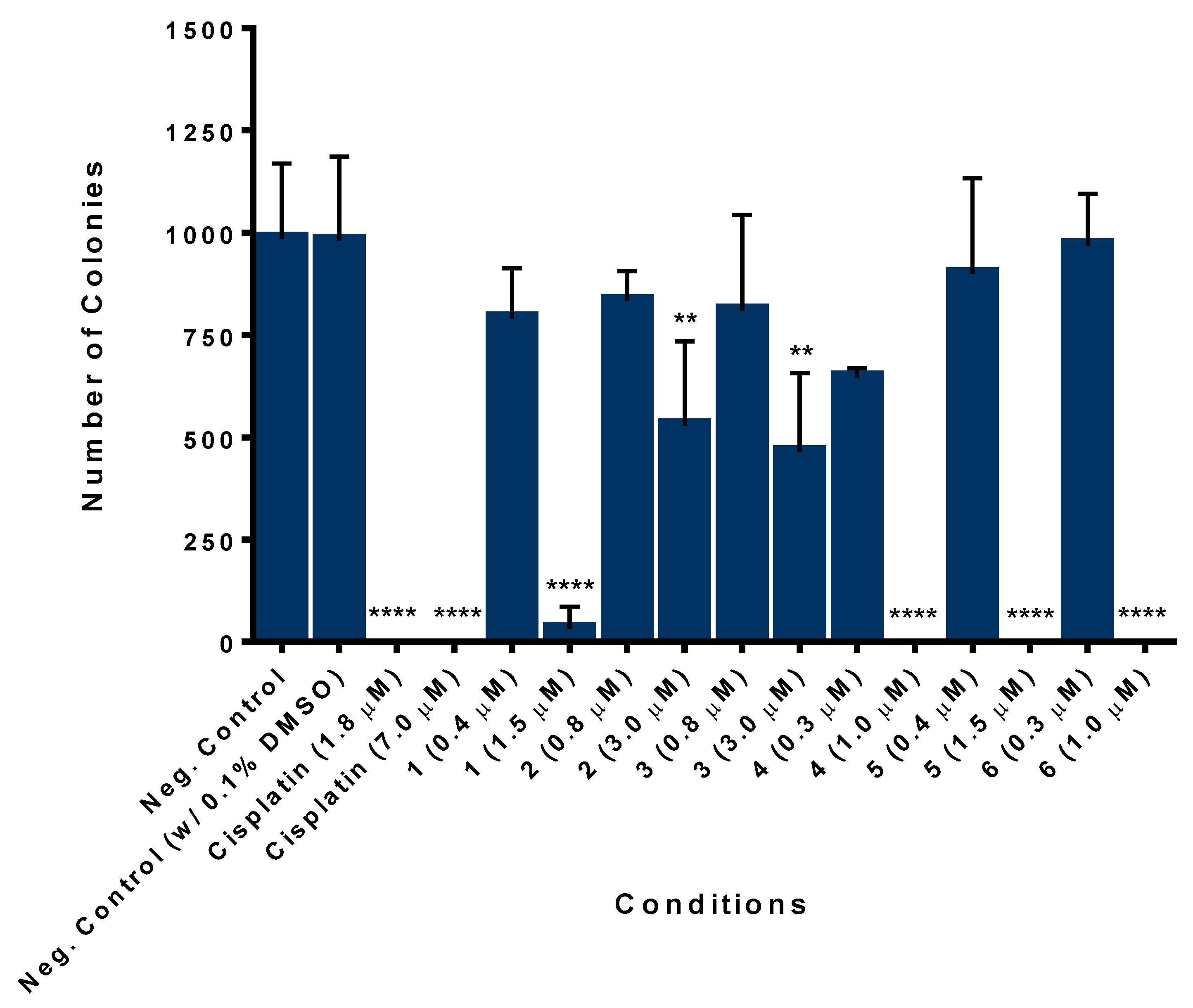
2.7.3. Iron Compounds Reduce the Colony Formation Ability of SW480 Colorectal Cancer Cells
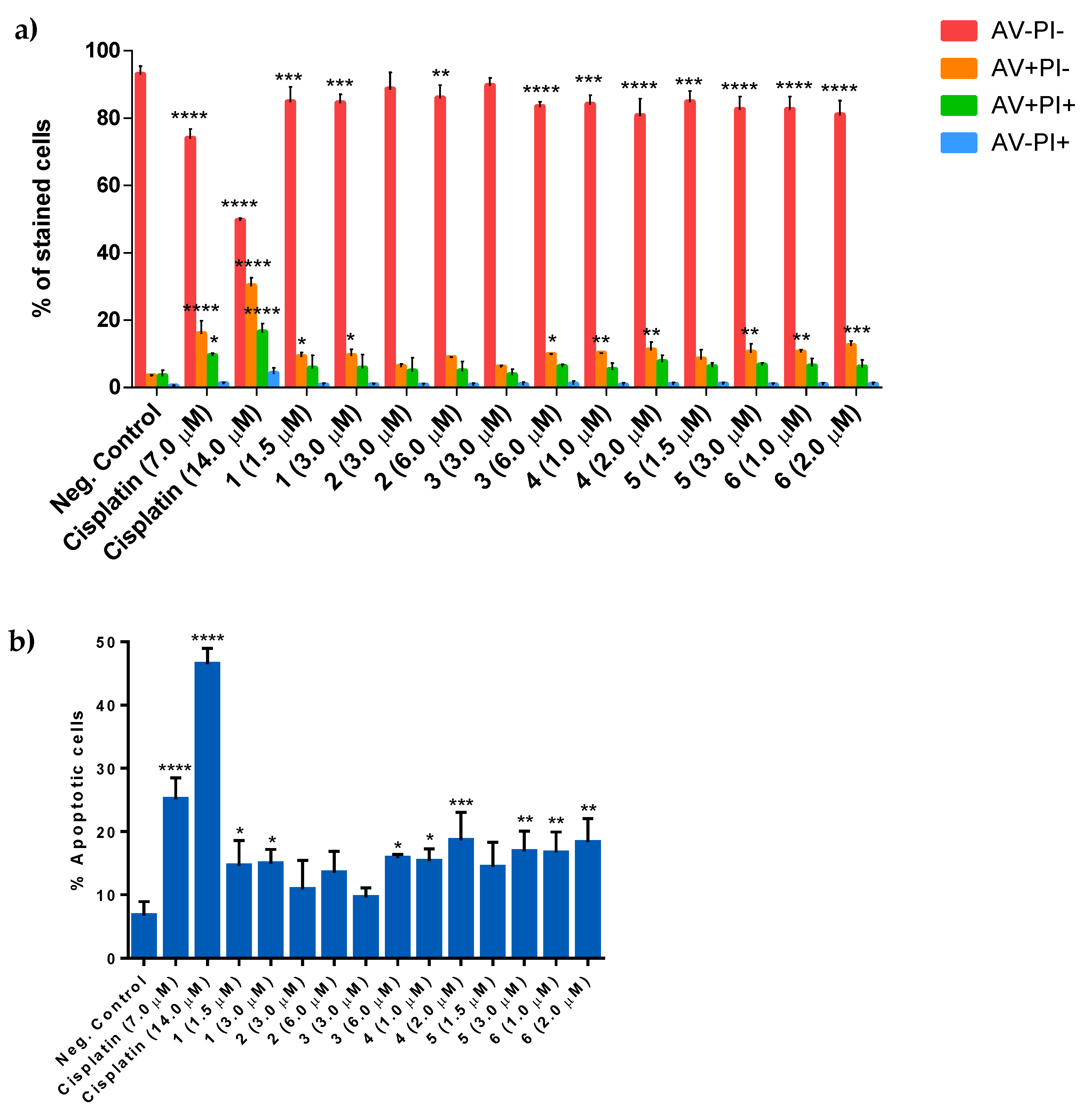
2.7.4. [Fe(η5-C5H5)(CO)(PPh3)(NCR)][CF3SO3] Compounds Induce Apoptosis in SW480 Colorectal Cancer-Derived Cell Line
2.7.5. [Fe(η5-C5H5)(CO)(PPh3)(NCR)][CF3SO3] Compounds Affect the Actin Cytoskeleton of SW480 Colorectal Cancer Cells
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of [Fe(η5-C5H5)(CO)(PPh3)(I)]
3.1.2. General Procedure for the Synthesis of [Fe(η5-C5H5)(CO)(PPh3)(NCR)][CF3SO3] Complexes 1–6
3.1.3. [Fe(η5-C5H5)(CO)(PPh3)(NCPh)][CF3SO3]—Complex 1
3.1.4. [Fe(η5-C5H5)(CO)(PPh3)(p-NCPhOH)][CF3SO3]—Complex 2
3.1.5. [Fe(η5-C5H5)(CO)(PPh3)(p-NCPhCH2OH)][CF3SO3]—Complex 3
3.1.6. [Fe(η5-C5H5)(CO)(PPh3)(p-NCPhNH2)][CF3SO3]—Complex 4
3.1.7. [Fe(η5-C5H5)(CO)(PPh3)(p-NCPhBr)][CF3SO3]—Complex 5
3.1.8. [Fe(η5-C5H5)(CO)(PPh3)(p-NCCH=CHPhCl)][CF3SO3]—Complex 6
3.1.9. [Fe(η5-C5H5)(CO)(PPh3)(trans-NCCH=CHPhCl)][CF3SO3]
3.1.10. [Fe(η5-C5H5)(CO)(PPh3)(cis-NCCH=CHPhCl)][CF3SO3]
3.2. X-Ray Crystal Structure Determination
3.3. TD-DFT Calculations
3.4. Biological Studies
3.4.1. Cell Lines and Culture Conditions
3.4.2. Compounds Dilution and Storage
3.4.3. Sulforhodamine B (SRB) Assay
3.4.4. MTT Assay
3.4.5. Colony Formation Assay
3.4.6. Annexin V/Propidium Iodide Assay
3.4.7. F-Actin Staining Assay with Phalloidin 568
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mjos, K.D.; Orvig, C. Metallodrugs in medicinal inorganic chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Wani, W.A.; Baig, U.; Shreaz, S.; Shiekh, R.A.; Iqbal, P.F.; Jameel, E.; Ahmad, A.; Mohd-Setapar, S.H.; Mushtaque, M.; Ting Hun, L. Recent advances in iron complexes as potential anticancer agents. New J. Chem. 2016, 40, 1063–1090. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Heinemann, F.; Top, S.; Dazzi, A.; Policar, C.; Henry, L.; Lambert, F.; Jaouen, G.; Salmain, M.; Vessieres, A. Ferrocifens labelled with an infrared rhenium tricarbonyl tag: Synthesis, antiproliferative activity, quantification and nano IR mapping in cancer cells. Dalton Trans. 2018, 47, 9824–9833. [Google Scholar] [CrossRef]
- Resnier, P.; Galopin, N.; Sibiril, Y.; Clavreul, A.; Cayon, J.; Briganti, A.; Legras, P.; Vessières, A.; Montier, T.; Jaouen, G.; et al. Efficient ferrocifen anticancer drug and Bcl-2 gene therapy using lipid nanocapsules on human melanoma xenograft in mouse. Pharmacol. Res. 2017, 126, 54–65. [Google Scholar] [CrossRef]
- Top, S.; Vessières, A.; Cabestaing, C.; Laios, I.; Leclercq, G.; Provot, C.; Jaouen, G. Studies on organometallic selective estrogen receptor modulators. (SERMs) Dual activity in the hydroxy-ferrocifen series. J. Organomet. Chem. 2001, 637, 500–506. [Google Scholar] [CrossRef]
- Top, S.; Dauer, B.; Vaissermann, J.; Jaouen, G. Facile route to ferrocifen, 1-[4-(2-dimethylaminoethoxy)]-1-(phenyl-2-ferrocenyl-but-1-ene), first organometallic analogue of tamoxifen, by the McMurry reaction. J. Organomet. Chem. 1997, 541, 355–361. [Google Scholar] [CrossRef]
- Bruyère, C.; Mathieu, V.; Vessières, A.; Pigeon, P.; Top, S.; Jaouen, G.; Kiss, R. Ferrocifen derivatives that induce senescence in cancer cells: Selected examples. J. Inorg. Biochem. 2014, 141, 144–151. [Google Scholar] [CrossRef]
- Allard, E.; Huynh, N.T.; Vessières, A.; Pigeon, P.; Jaouen, G.; Benoit, J.P.; Passirani, C. Dose effect activity of ferrocifen-loaded lipid nanocapsules on a 9L-glioma model. Int. J. Pharm. 2009, 379, 317–323. [Google Scholar] [CrossRef]
- Morais, T.S.; Valente, A.; Tomaz, A.I.; Marques, F.; Garcia, M.H. Tracking antitumor metallodrugs: Promising agents with the Ru (II)- and Fe (II)-cyclopentadienyl scaffolds. Future Med. Chem. 2016, 8, 527–544. [Google Scholar] [CrossRef]
- Côrte-Real, L.; Karas, B.; Brás, A.R.; Pilon, A.; Avecilla, F.; Marques, F.; Preto, A.; Buckley, B.T.; Cooper, K.R.; Doherty, C.; et al. Ruthenium-Cyclopentadienyl Bipyridine-Biotin Based Compounds: Synthesis and Biological Effect. Inorg. Chem. 2019, 58, 9135–9149. [Google Scholar] [CrossRef]
- Valente, A.; Garcia, M.H.; Marques, F.; Miao, Y.; Rousseau, C.; Zinck, P. First polymer “ruthenium-cyclopentadienyl” complex as potential anticancer agent. J. Inorg. Biochem. 2013, 127, 79–81. [Google Scholar] [CrossRef]
- Côrte-Real, L.; Paula Robalo, M.; Marques, F.; Nogueira, G.; Avecilla, F.; Silva, T.J.L.; Santos, F.C.; Isabel Tomaz, A.; Helena Garcia, M.; Valente, A. The key role of coligands in novel ruthenium (II)-cyclopentadienyl bipyridine derivatives: Ranging from non-cytotoxic to highly cytotoxic compounds. J. Inorg. Biochem. 2015, 150, 148–159. [Google Scholar] [CrossRef]
- Moreira, T.; Francisco, R.; Comsa, E.; Duban-Deweer, S.; Labas, V.; Teixeira-Gomes, A.P.; Combes-Soia, L.; Marques, F.; Matos, A.; Favrelle, A.; et al. Polymer “ruthenium-cyclopentadienyl” conjugates—New emerging anti-cancer drugs. Eur. J. Med. Chem. 2019, 168, 373–384. [Google Scholar] [CrossRef]
- Côrte-Real, L.; Karas, B.; Gírio, P.; Moreno, A.; Avecilla, F.; Marques, F.; Buckley, B.T.; Cooper, K.R.; Doherty, C.; Falson, P.; et al. Unprecedented inhibition of P-gp activity by a novel ruthenium- cyclopentadienyl compound bearing a bipyridine-biotin ligand. Eur. J. Med. Chem. 2019, 163, 853–863. [Google Scholar]
- Côrte-Real, L.; Mendes, F.; Coimbra, J.; Morais, T.S.; Tomaz, A.I.; Valente, A.; Garcia, M.H.; Santos, I.; Bicho, M.; Marques, F. Anticancer activity of structurally related ruthenium(II) cyclopentadienyl complexes. J. Biol. Inorg. Chem. 2014, 19, 853–867. [Google Scholar] [CrossRef]
- Teixeira, R.G.; Brás, A.R.; Côrte-Real, L.; Tatikonda, R.; Sanches, A.; Robalo, M.P.; Avecilla, F.; Moreira, T.; Garcia, M.H.; Haukka, M.; et al. Novel ruthenium methylcyclopentadienyl complex bearing a bipyridine perfluorinated ligand shows strong activity towards colorectal cancer cells. Eur. J. Med. Chem. 2018, 143, 503–514. [Google Scholar] [CrossRef]
- Gonçalves, A.C.; Morais, T.S.; Robalo, M.P.; Marques, F.; Avecilla, F.; Matos, C.P.; Santos, I.; Tomaz, A.I.; Garcia, M.H. Important cytotoxicity of novel iron(II) cyclopentadienyl complexes with imidazole based ligands. J. Inorg. Biochem. 2013, 129, 1–8. [Google Scholar] [CrossRef]
- Valente, A.; Santos, A.M.; Côrte-Real, L.; Robalo, M.P.; Moreno, V.; Font-Bardia, M.; Calvet, T.; Lorenzo, J.; Garcia, M.H. New iron (II) cyclopentadienyl derivative complexes: Synthesis and antitumor activity against human leukemia cancer cells. J. Organomet. Chem. 2014, 756, 52–60. [Google Scholar] [CrossRef]
- Pilon, A.; Gírio, P.; Nogueira, G.; Avecilla, F.; Adams, H.; Lorenzo, J.; Garcia, M.H.; Valente, A. New iron cyclopentadienyl complexes bearing different phosphane co-ligands: Structural factors vs. cytotoxicity. J. Organomet. Chem. 2017, 852, 34–42. [Google Scholar] [CrossRef]
- Poh, H.T.; Ho, P.C.; Fan, W.Y. Cyclopentadienyl iron dicarbonyl (CpFe (CO)2) derivatives as apoptosis-inducing agents. RSC Adv. 2016, 6, 18814–18823. [Google Scholar] [CrossRef]
- Rocco, D.; Batchelor, L.K.; Agonigi, G.; Braccini, S.; Chiellini, F.; Schoch, S.; Biver, T.; Funaioli, T.; Zacchini, S.; Biancalana, L.; et al. Anticancer Potential of Diiron Vinyliminium Complexes. Chem. A Eur. J. 2019, 25, 14801–14816. [Google Scholar] [CrossRef]
- Treichel, P.M.; Shubkin, R.L.; Barnett, K.W.; Reichard, D. Chemistry of the Cyclopentadienylmetal Carbonyls. II. Cyclopentadienyliron Carbonyl Derivatives. Inorg. Chem. 1966, 5, 1177–1181. [Google Scholar] [CrossRef]
- Bibler, J.P.; Wojcicki, A. Reactions of Cyclopentadienyl (methyl)iron Dicarbonyl with Various Ligands. Cyclopentadienyl (acetyl)iron Carbonyl Phosphine and Phosphite Complexes. Inorg. Chem. 1966, 5, 889–892. [Google Scholar] [CrossRef]
- Makunya, N.M.; Meijboom, R.; Muller, A.; Roodt, A. Tertiary phosphine induced migratory carbonyl insertion in cyclopentadienyl complexes of iron (II). J. Organomet. Chem. 2005, 690, 4159–4167. [Google Scholar] [CrossRef]
- De Oliveira, P.F.; Alves, J.M.; Damasceno, J.L.; Oliveira, R.A.M.; Dias, H.J.; Crotti, A.E.M.; Tavares, D.C. Cytotoxicity screening of essential oils in cancer cell lines. Rev. Bras. Farmacogn. 2015, 25, 183–188. [Google Scholar] [CrossRef]
- Florindo, P.R.; Pereira, D.M.; Borralho, P.M.; Rodrigues, C.M.P.; Piedade, M.F.M.; Fernandes, A.C. Cyclopentadienyl-ruthenium (II) and iron (II) organometallic compounds with carbohydrate derivative ligands as good colorectal anticancer agents. J. Med. Chem. 2015, 58, 4339–4347. [Google Scholar] [CrossRef]
- Piper, T.S.; Wilkinson, G. Cyclopentadienyl-nitric oxide compounds of chromium and manganese. J. Inorg. Nucl. Chem. 1956, 2, 38–45. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SADABS, Version 2.10; University of Göttingen: Göttingen, Germany, 2004. [Google Scholar]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalman, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Feller, D. The role of databases in support of computational chemistry calculations. J. Comput. Chem. 1996, 17, 1571–1586. [Google Scholar] [CrossRef]
- Schuchardt, K.L.; Didier, B.T.; Elsethagen, T.; Sun, L.; Gurumoorthi, V.; Chase, J.; Li, J.; Windus, T.L. Basis set exchange: A community database for computational sciences. J. Chem. Inf. Model. 2007, 47, 1045–1052. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Tenderholt, A.L.; Langner, K.M. Software News and Updates cclib: A Library for Package-Independent Computational Chemistry Algorithms. J. Comput. Chem. 2009, 29, 839–845. [Google Scholar] [CrossRef]
- Moyer, M.P.; Manzano, L.A.; Merriman, R.L.; Stauffer, J.S.; Tanzer, L.R. NCM460, a normal human colon mucosal epithelial cell line. In Vitro Cell. Dev. Biol. Anim. 1996, 32, 315–317. [Google Scholar] [CrossRef]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–6 are available from the authors. |





| Complex | (η5-C5H5) | H3 |
|---|---|---|
| [Fe(η5-C5H5)(CO)(PPh3)I] | 4.57 | - |
| 1 | 5.21 (+0.64) | 7.37 (−0.37) |
| 2 | 5.17 (+0.60) | 7.19 (−0.43) |
| 3 | 5.20 (+0.63) | 7.48 (−0.26) |
| 4 | 5.13 (+0.56) | 6.97 (−0.42) |
| 5 | 5.21 (+0.64) | 7.31 (−0.45) |
| Complex | υN≡C | υCO |
|---|---|---|
| [Fe(η5-C5H5)(CO)(PPh3)I] | - | 1935 |
| 1 | 2247 (+19) | 1977 (+42) |
| 2 | 2249 (+16) | 1986 (+51) |
| 3 | 2250 (+23) | 1984 (+49) |
| 4 | 2237 (+23) | 1980 (+45) |
| 5 | 2245 (+22) | 1996 (+61) |
| 6 | 2241 (+25) | 1956 (+21) |
| IC50 (μM) | ||||
|---|---|---|---|---|
| Compounds | MDA-MB-231 | SW480 | NCM460 | Selectivity Index |
| 1 | 0.45 ± 0.02 | 1.5 ± 0.2 | 3.8 ± 0.6 | 2.5 |
| 2 | 0.71 ± 0.05 | 3.0 ± 0.3 | 7.8 ± 1.2 | 2.6 |
| 3 | 0.37 ± 0.04 | 3.0 ± 0.2 | 11.7 ± 1.8 | 3.9 |
| 4 | 0.19 ± 0.01 | 1.0 ± 0.1 | 2.4 ± 0.5 | 2.4 |
| 5 | 0.91 ± 0.05 | 1.5 ± 0.1 | 3.0 ± 0.4 | 2.0 |
| 6 | 0.39 ± 0.02 | 1.0 ± 0.1 | 1.8 ± 0.1 | 1.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pilon, A.; Brás, A.R.; Côrte-Real, L.; Avecilla, F.; Costa, P.J.; Preto, A.; Garcia, M.H.; Valente, A. A New Family of Iron(II)-Cyclopentadienyl Compounds Shows Strong Activity against Colorectal and Triple Negative Breast Cancer Cells. Molecules 2020, 25, 1592. https://doi.org/10.3390/molecules25071592
Pilon A, Brás AR, Côrte-Real L, Avecilla F, Costa PJ, Preto A, Garcia MH, Valente A. A New Family of Iron(II)-Cyclopentadienyl Compounds Shows Strong Activity against Colorectal and Triple Negative Breast Cancer Cells. Molecules. 2020; 25(7):1592. https://doi.org/10.3390/molecules25071592
Chicago/Turabian StylePilon, Adhan, Ana Rita Brás, Leonor Côrte-Real, Fernando Avecilla, Paulo J. Costa, Ana Preto, M. Helena Garcia, and Andreia Valente. 2020. "A New Family of Iron(II)-Cyclopentadienyl Compounds Shows Strong Activity against Colorectal and Triple Negative Breast Cancer Cells" Molecules 25, no. 7: 1592. https://doi.org/10.3390/molecules25071592
APA StylePilon, A., Brás, A. R., Côrte-Real, L., Avecilla, F., Costa, P. J., Preto, A., Garcia, M. H., & Valente, A. (2020). A New Family of Iron(II)-Cyclopentadienyl Compounds Shows Strong Activity against Colorectal and Triple Negative Breast Cancer Cells. Molecules, 25(7), 1592. https://doi.org/10.3390/molecules25071592