Biological Evaluation and Molecular Docking with In Silico Physicochemical, Pharmacokinetic and Toxicity Prediction of Pyrazolo[1,5-a]pyrimidines
Abstract
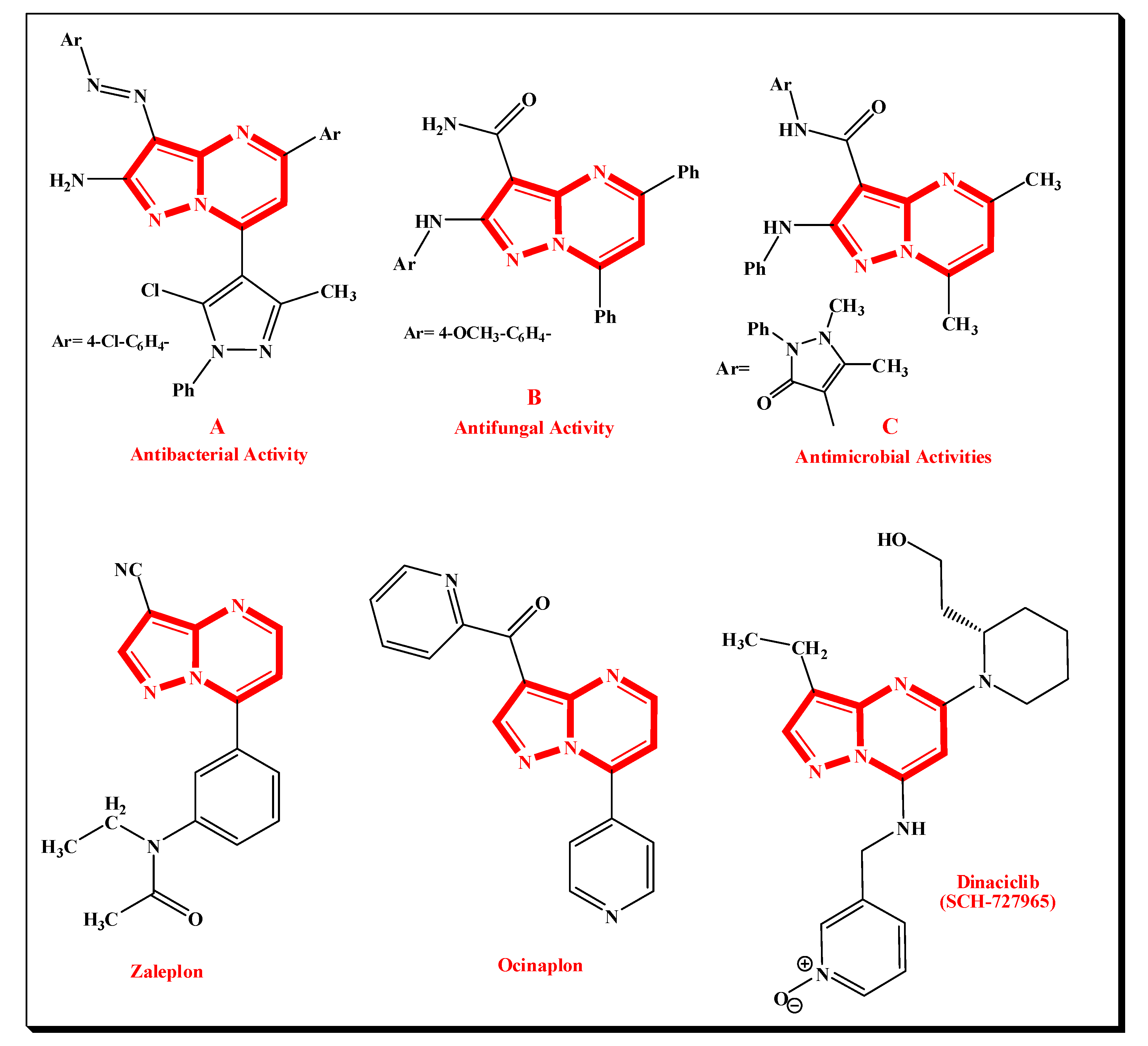
1. Introduction
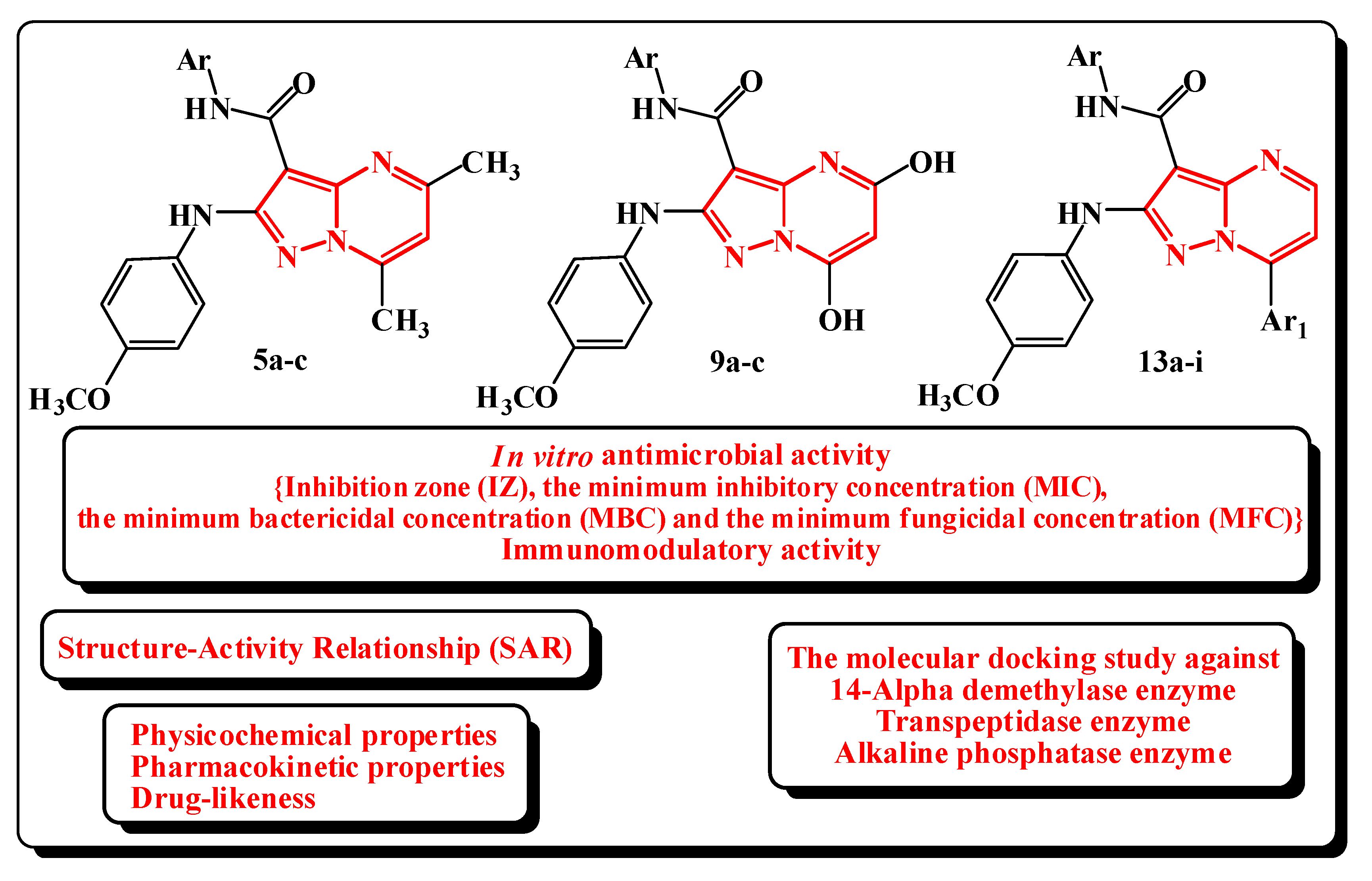
2. Results and Discussion
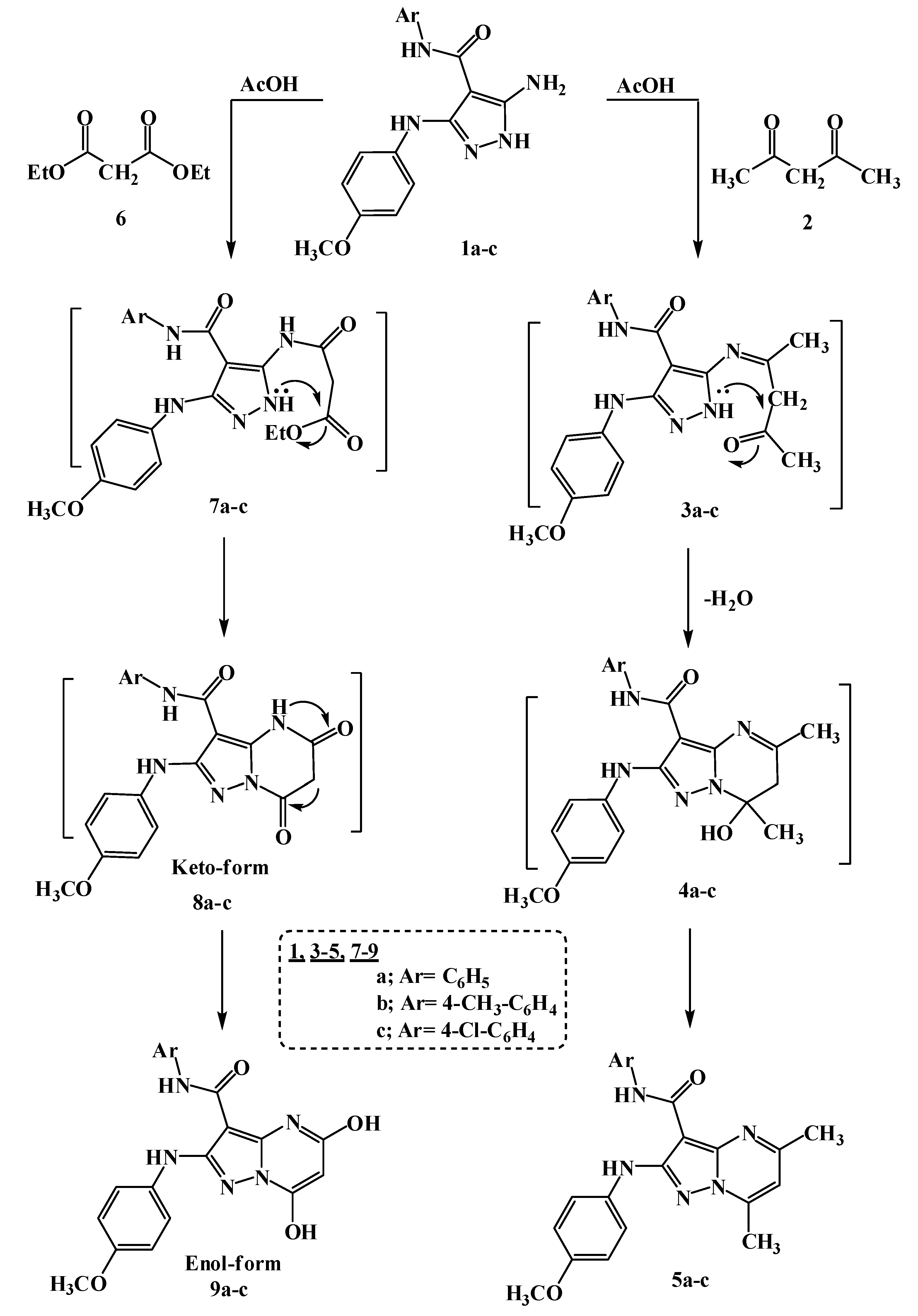
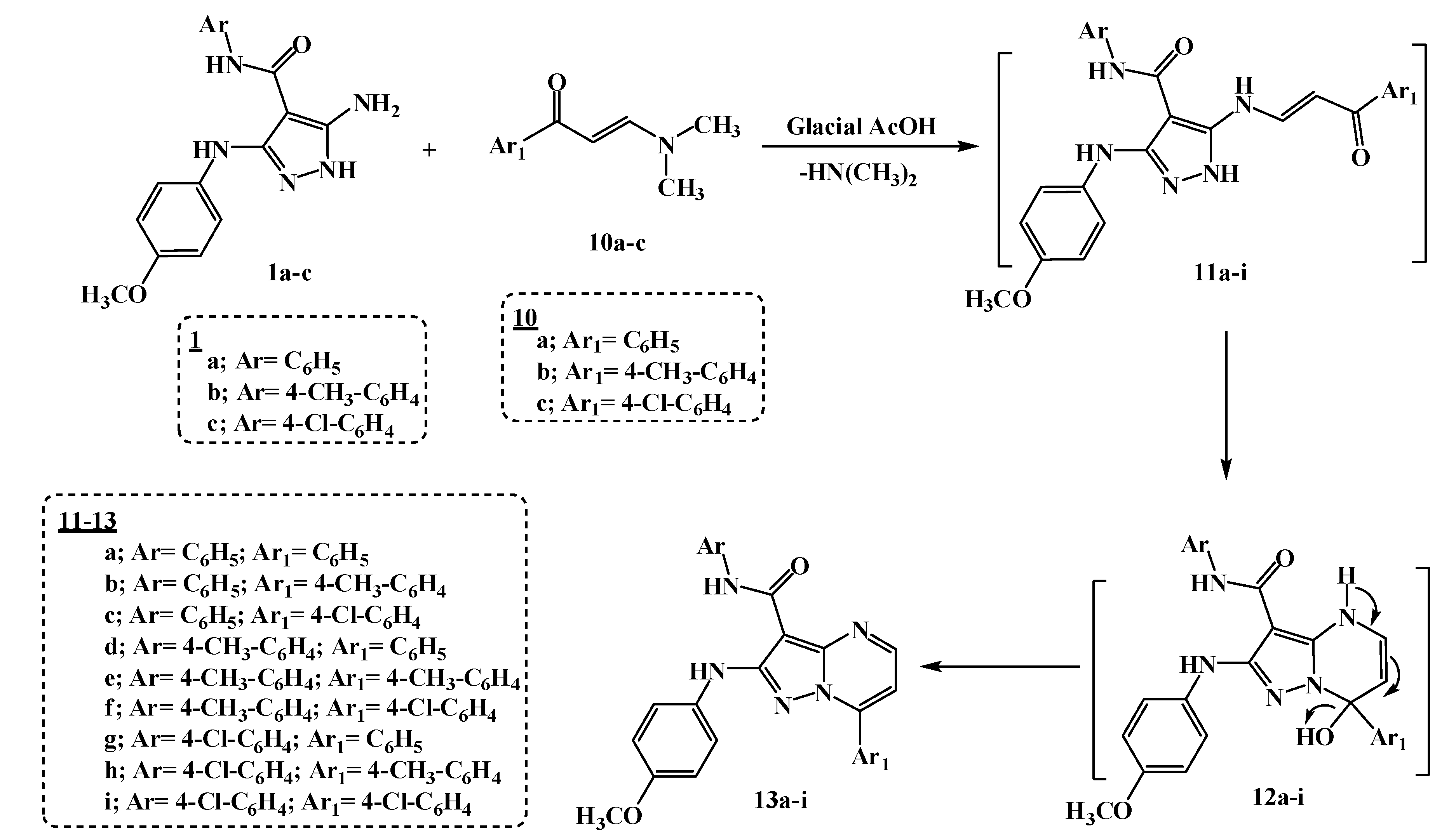
2.1. Chemistry
2.2. Biological Evaluation
2.2.1. In Vitro Antimicrobial Evaluation
2.2.2. Immunomodulatory Activity for Active Compounds
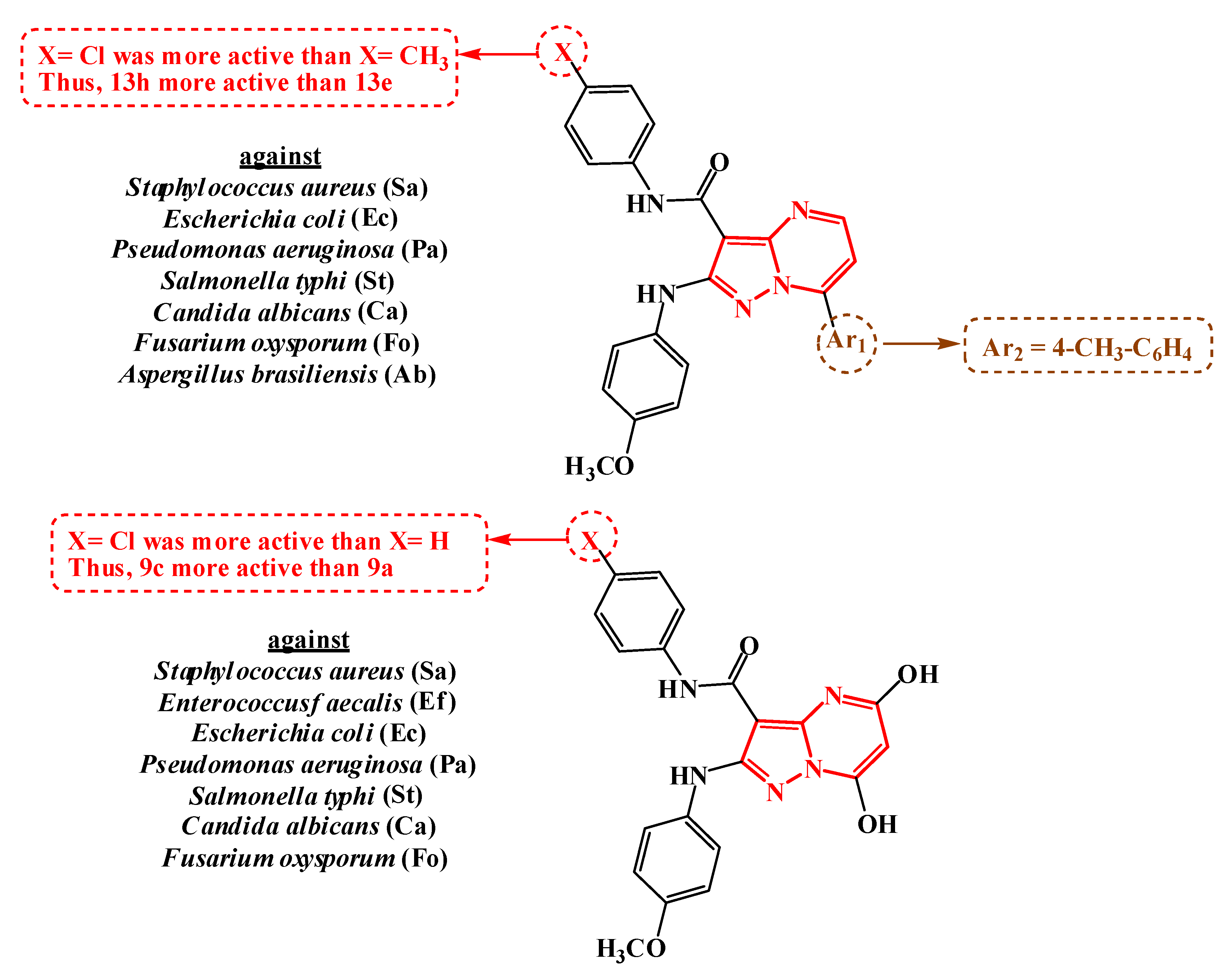
2.3. Structure–Activity Relationship (SAR)
2.4. Physicochemical, Pharmacokinetic, ADME, Toxicity Prediction and Drug-Likeness
2.4.1. Lipinski’s Rule of Five for Pyrazolo[1,5-a]pyrimidines 5a–c, 9a–c and 13a–i
2.4.2. Pharmacokinetic Properties of Pyrazolo[1,5-a]pyrimidines 5a–c, 9a–c and 13a–i
2.4.3. In Silico ADME and Toxicity Prediction of Pyrazolo[1,5-a]pyrimidines 5a–c, 9a–c and 13a–i
2.4.4. Drug Likeness Calculations of Pyrazolo[1,5-a]pyrimidines 5a–c, 9a–c and 13a–i
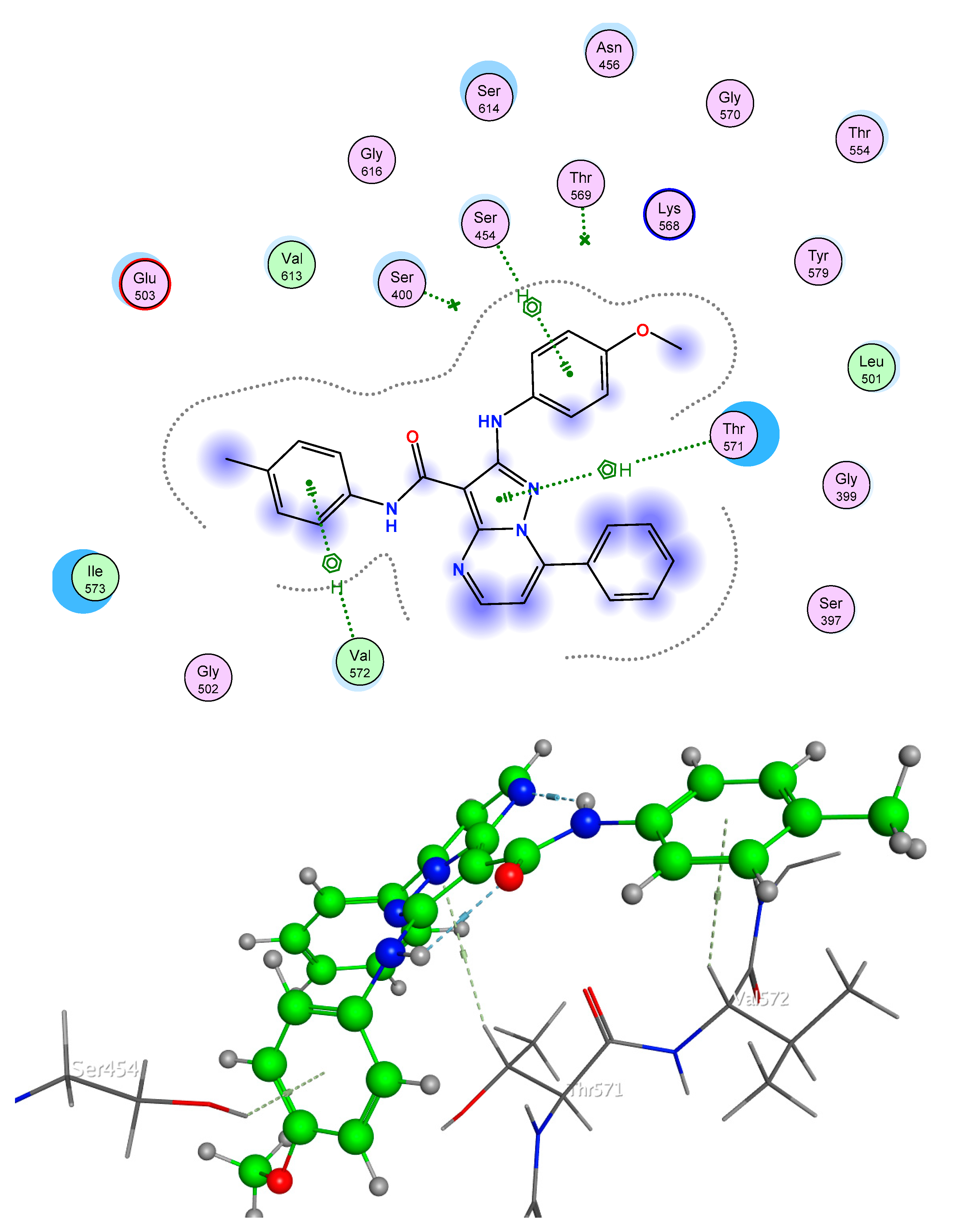
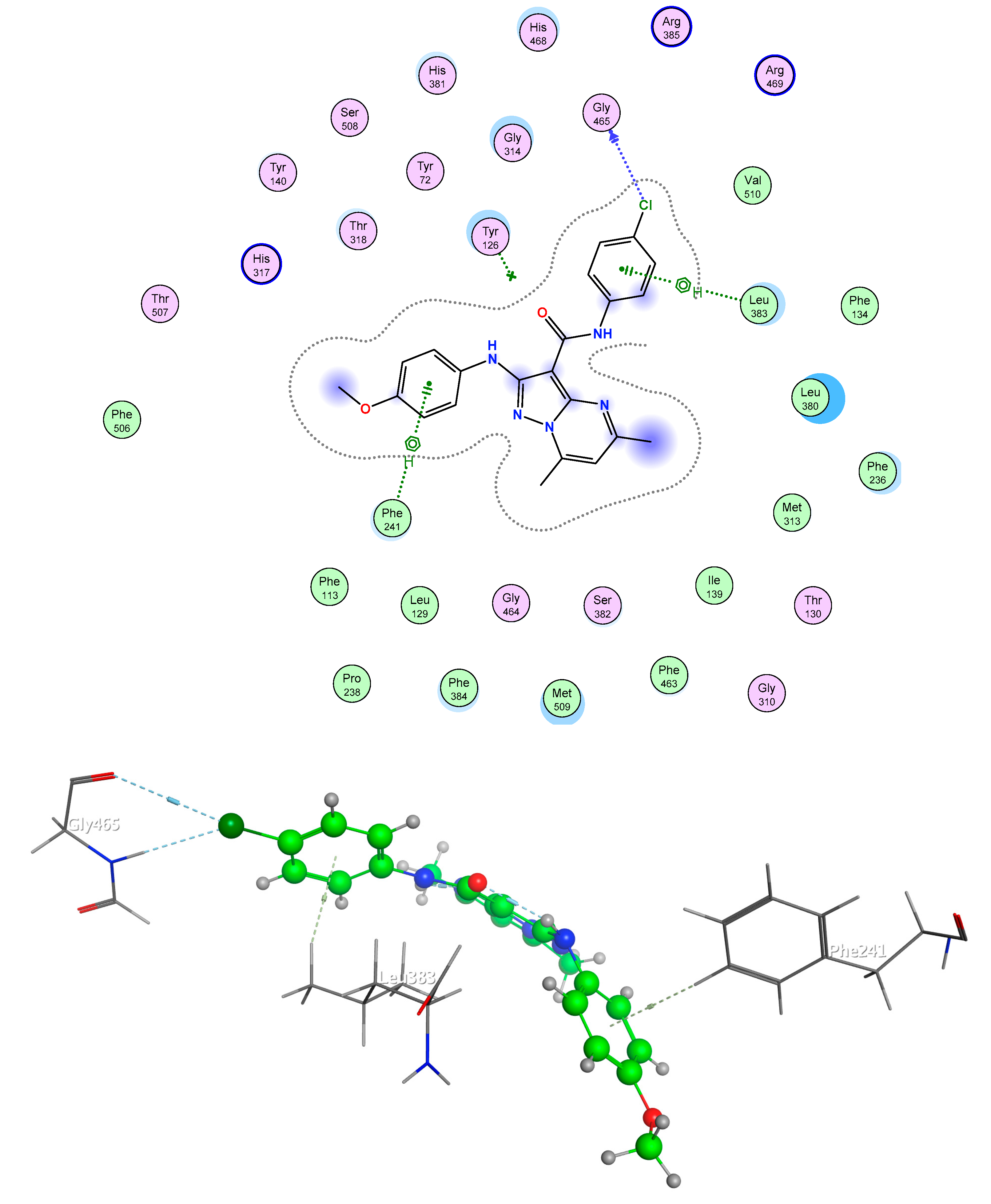
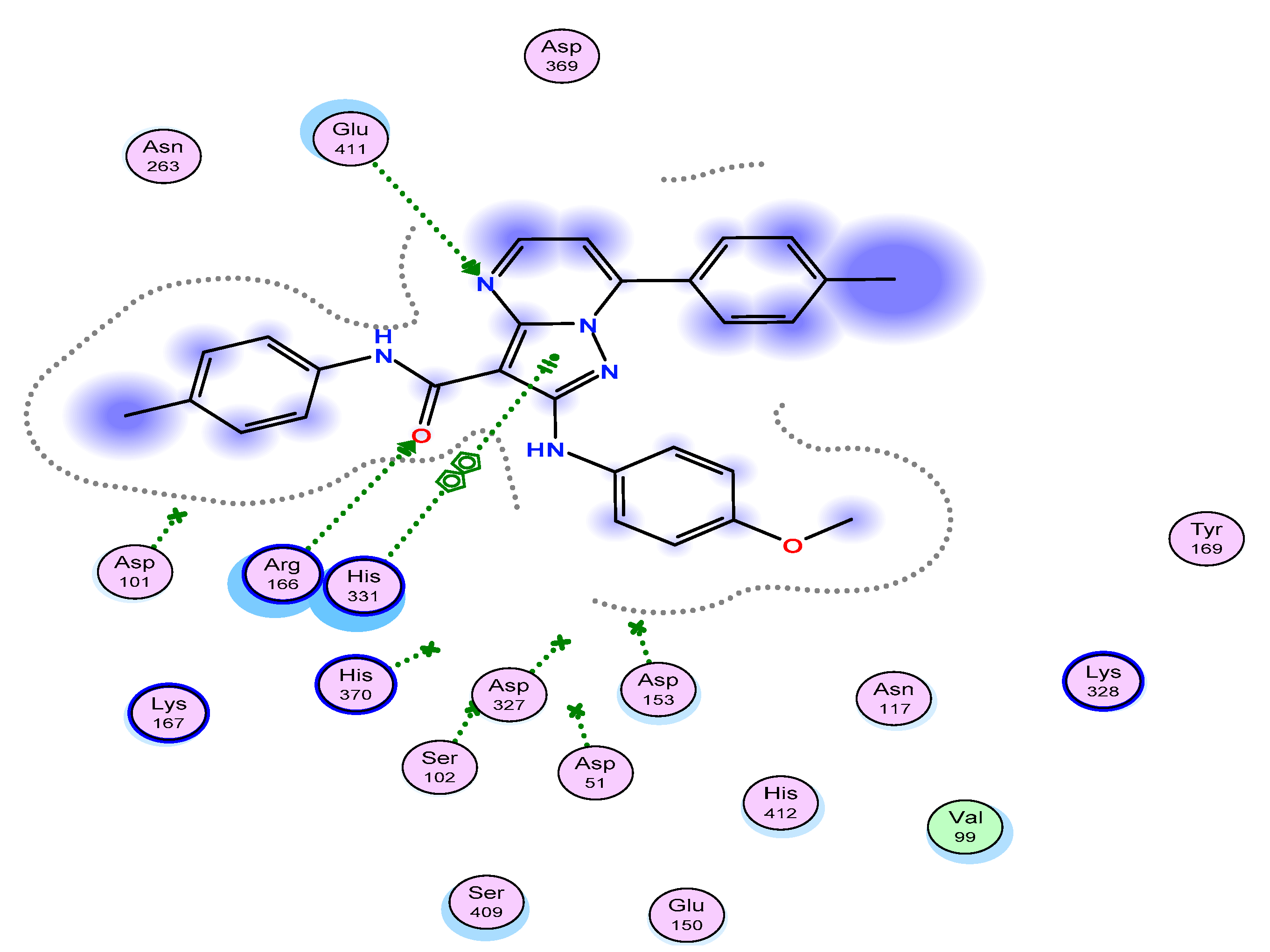
2.5. Molecular Docking
2.5.1. Bacteria
2.5.2. Fungi
2.5.3. Immunity Docking
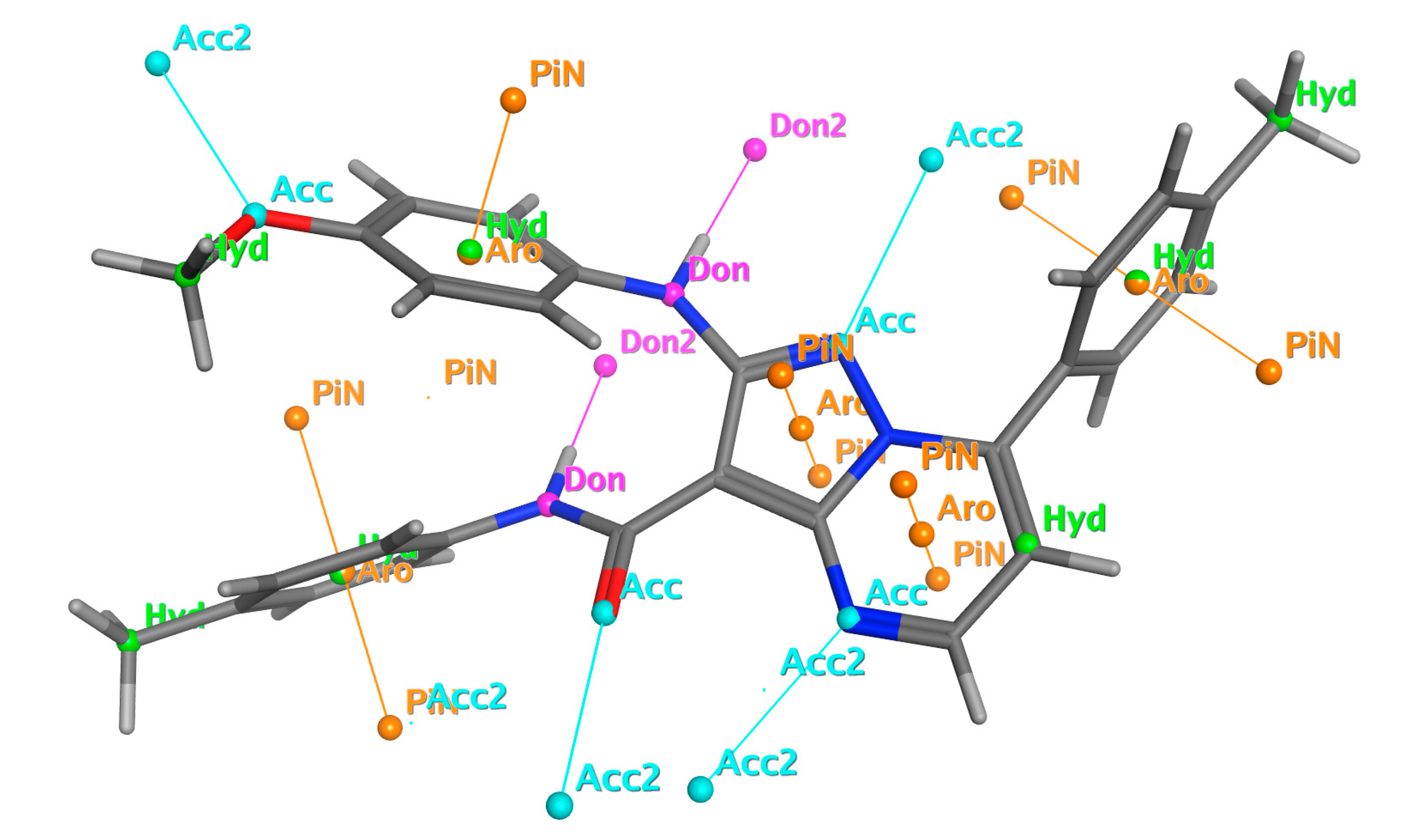
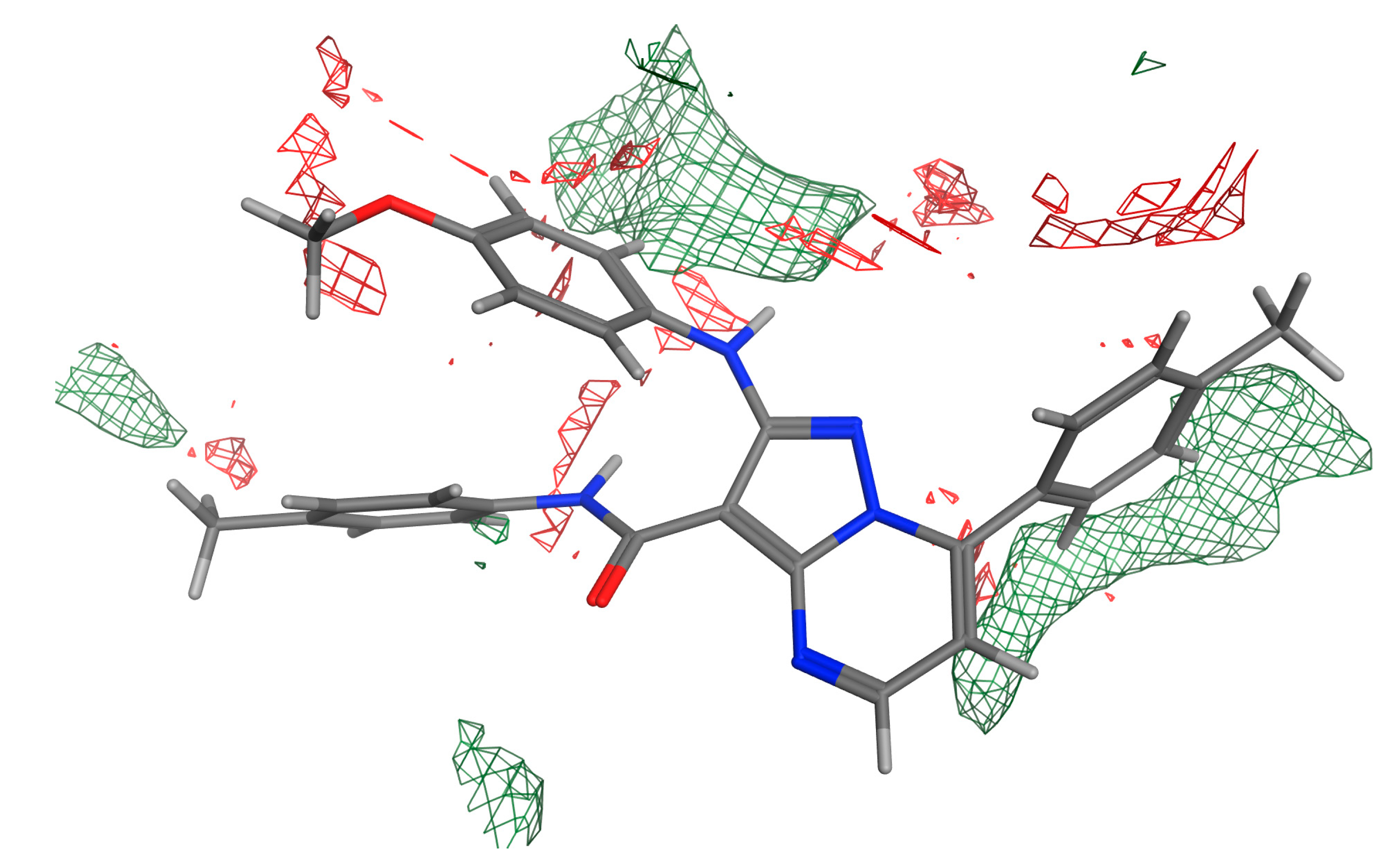
2.5.4. Pharmacophore and Electrostatic Map of 13e
3. Materials and Methods
3.1. Chemicals
3.2. In Vitro Biological Evaluation
3.3. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shaaban, O.G.; Issa, D.A.E.; El-Tombary, A.A.; Abd El Wahab, S.M.; Abdel Wahab, A.E.; Abdelwahab, I.A. Synthesis and molecular docking study of some 3,4-dihydrothieno[2,3-d]pyrimidine derivatives as potential antimicrobial agents. Bioorg. Chem. 2019, 88, 102934. [Google Scholar] [CrossRef] [PubMed]
- Chobe, S.S.; Dawane, B.S.; Tumbi, K.M.; Nandekar, P.P.; Sangamwar, A.T. An ecofriendly synthesis and DNA binding interaction study of some pyrazolo[1,5-a]pyrimidines derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 7566–7572. [Google Scholar] [CrossRef] [PubMed]
- Modi, P.; Patel, S.; Mahesh, C. Structure-based design, synthesis and biological evaluation of a newer series of pyrazolo[1,5-a]pyrimidine analogues as potential anti-tubercular agents. Bioorg. Chem. 2019, 87, 240–251. [Google Scholar] [CrossRef] [PubMed]
- El-Mekabaty, A.; Fadda, A.A. Novel pyrazolo[1,5-a]pyrimidines and pyrazolo[5,1-c][1,2,4]triazines incorporating indole moiety as a new class of antioxidant agents. J. Heterocycl. Chem. 2018, 55, 2303–2308. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moustafa, G.O.; Askar, A.A.; Naglah, A.M.; Al-Omar, M.A. Synthesis and antibacterial evaluation of fused pyrazoles and Schiff bases. Synth. Commun. 2018, 48, 2761–2772. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hafez, T.S.; Osman, S.A.M.; Ali, M.M. Synthesis and in vitro cytotoxic activity of novel pyrazolo[1,5-a]pyrimidines and related Schiff bases. Turk. J. Chem. 2015, 39, 1102–1113. [Google Scholar] [CrossRef]
- Hafez, T.S.; Osman, S.A.; Yosef, H.A.A.; Abd El-All, A.S.; Hassan, A.S.; El-Sawy, A.A.; Abdallah, M.M.; Youns, M. Synthesis, structural elucidation and in vitro antitumor activities of some pyrazolopyrimidines and Schiff bases derived from 5-amino-3-(arylamino)-1H-pyrazole-4-carboxamides. Sci. Pharm. 2013, 81, 339–357. [Google Scholar] [CrossRef]
- Hassan, A.S.; Moustafa, G.O.; Awad, H.M. Synthesis and in vitro anticancer activity of pyrazolo[1,5-a]pyrimidines and pyrazolo[3,4-d][1,2,3]triazines. Synth. Commun. 2017, 47, 1963–1972. [Google Scholar] [CrossRef]
- Dawane, B.S.; Konda, S.G.; Zangade, S.B. Design, Synthesis and Characterization of Some Novel Pyrazolo[1,5-a]pyrimidines as Potent Antimicrobial Agents. J. Heterocycl. Chem. 2010, 47, 1250–1254. [Google Scholar] [CrossRef]
- Hassan, A.S.; Masoud, D.M.; Sroor, F.M.; Askar, A.A. Synthesis and biological evaluation of pyrazolo[1,5-a]pyrimidine-3-carboxamide as antimicrobial agents. Med. Chem. Res. 2017, 26, 2909–2919. [Google Scholar] [CrossRef]
- Bondock, S.; Rabie, R.; Etman, H.A.; Fadda, A.A. Synthesis and antimicrobial activity of some new heterocycles incorporating antipyrine moiety. Eur. J. Med. Chem. 2008, 43, 2122–2129. [Google Scholar] [CrossRef] [PubMed]
- Parry, D.; Guzi, T.; Shanahan, F.; Davis, N.; Prabhavalkar, D.; Wiswell, D.; Seghezzi, W.; Paruch, K.; Dwyer, M.P.; Doll, R.; et al. Dinaciclib (SCH 727965), a novel and potent cyclin-dependent kinase inhibitor. Mol. Cancer Ther. 2010, 9, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Bose, P.; Simmons, G.L.; Grant, S. Cyclin-dependent kinase inhibitor therapy for hematologic malignancies. Expert Opin. Investig. Drugs 2013, 22, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Hassan, A.S.; Askar, A.A.; Nossier, E.S.; Naglah, A.M.; Moustafa, G.O.; Al-Omar, M.A. Antibacterial Evaluation, In Silico Characters and Molecular docking of Schiff Bases Derived from 5-aminopyrazoles. Molecules 2019, 24, 3130. [Google Scholar] [CrossRef]
- Magd-El-Din, A.A.; Mousa, H.A.; Labib, A.A.; Hassan, A.S.; Abd El-All, A.S.; Ali, M.M.; El-Rashedy, A.A.; El-Desoky, A.H. Benzimidazole-Schiff bases and their complexes: Synthesis, anticancer activity and molecular modeling as Aurora kinase inhibitor. Z. Nat. C 2018, 73, 465–478. [Google Scholar] [CrossRef]
- Khatab, T.K.; Abdelghany, A.M.; Soliman, H.A. V2O5 based quadruple nano-perovskite as a new catalystfor the synthesis of bis and tetrakis heterocyclic compounds. Appl. Organomet. Chem. 2019, 33, e4783. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hafez, T.S. Antimicrobial Activities of Ferrocenyl Complexes: A Review. J. App. Pharm. Sci. 2018, 8, 156–165. [Google Scholar]
- Hassan, A.S.; Awad, H.M.; Magd-El-Din, A.A.; Hafez, T.S. Synthesis and in vitro antitumor evaluation of novel Schiff bases. Med. Chem. Res. 2018, 27, 915–927. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hafez, T.S.; Ali, M.M.; Khatab, T.K. Design, synthesis and cytotoxic activity of some new pyrazolines bearing benzofuran and pyrazole moieties. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 417–429. [Google Scholar]
- Abd El-All, A.S.; Hassan, A.S.; Osman, S.A.; Yosef, H.A.A.; Abdel-Hady, W.H.; El-Hashash, M.A.; Atta-Allah, S.R.; Ali, M.M.; El Rashedy, A.A. Synthesis, characterization and biological evaluation of new fused triazine derivatives based on 6-methyl-3-thioxo-1,2,4-triazin-5-one. Acta Pol. Pharm. 2016, 73, 79–92. [Google Scholar]
- Hassan, A.S.; Osman, S.A.; Hafez, T.S. 5-Phenyl-2-furaldehyde: Synthesis, Reactions and Biological Activities. Egypt. J. Chem. 2015, 58, 113–139. [Google Scholar]
- Osman, S.A.; Mousa, H.A.; Yosef, H.A.A.; Hafez, T.S.; El-Sawy, A.A.; Abdallah, M.M.; Hassan, A.S. Synthesis, characterization and cytotoxicity of mixed ligand Mn(II), Co(II) and Ni(II) complexes. J. Serb. Chem. Soc. 2014, 79, 953–964. [Google Scholar] [CrossRef]
- Osman, S.A.; Yosef, H.A.A.; Hafez, T.S.; El-Sawy, A.A.; Mousa, H.A.; Hassan, A.S. Synthesis and antibacterial activity of some novel chalcones, pyrazoline and 3-cyanopyridine derivatives based on khellinone as well as Ni(II), Co(II) and Zn(II) complexes. Aust. J. Basic Appl. Sci. 2012, 6, 852–863. [Google Scholar]
- Elgemeie, G.H.; Elsayed, S.H.; Hassan, A.S. Design and synthesis of the first thiophene thioglycosides. Synth. Commun. 2009, 39, 1781–1792. [Google Scholar] [CrossRef]
- Elgemeie, G.H.; Elsayed, S.H.; Hassan, A.S. Direct route to a new class of acrylamide thioglycosides and their conversions to pyrazole derivatives. Synth. Commun. 2008, 38, 2700–2706. [Google Scholar] [CrossRef]
- Khatab, T.K.; Hassan, A.S.; Hafez, T.S. V2O5/SiO2 as an efficient catalyst in the synthesis of 5-aminopyrazole derivatives under solvent free condition. Bull. Chem. Soc. Ethiop. 2019, 33, 135–142. [Google Scholar] [CrossRef]
- Cappuccino, J.G.; Sherman, N. Microbiology, Laboratory Manual; Pearson Education, Inc.: New Delhi, India, 2004; pp. 282–283. [Google Scholar]
- El-sherbiny, G.M.; El-Batal, A.I.; El-Sherbiny, I.M.; Askar, A.A. Antibacterial Potential with Molecular Docking Study against Multi-Drug Resistant Bacteria and Mycobacterium tuberculosis of Streptomycin Produced by Streptomyces atroverins, strain Askar-SH50. J. Chem. Pharm. Res. 2017, 9, 189–208. [Google Scholar]
- Cooper, K. The theory of antibiotic inhibition zones. In Analytical Microbiology; Academic Press: New York, NY, USA; London, UK, 1963; pp. 1–85. [Google Scholar]
- El-Batal, A.I.; El-Sayed, M.H.; Refaat, B.M.; Askar, A.A. Marine Streptomyces cyaneus Strain Alex-SK121 Mediated Eco-friendly Marine Streptomyces cyaneus Strain Alex-SK121 Mediated Eco-friendly Synthesis of Silver Nanoparticles Using Gamma Radiation. Br. J. Pharm. Res. 2014, 4, 2525–2547. [Google Scholar]
- Ghorab, M.M.; Soliman, A.M.; Alsaid, M.S.; Askar, A.A. Synthesis, antimicrobial activity and docking study of some novel 4-(4,4-dimethyl-2,6 dioxocyclohexylidene) methylamino derivatives carrying biologically active sulfonamide moiety. Arab. J. Chem. 2020, 13, 545–556. [Google Scholar] [CrossRef]
- Sun, N.; Li, M.; Cai, S.; Li, Y.; Chen, C.; Zheng, Y.; Li, X.; Fang, Z.; Lv, H.; Lu, Y.J. Antibacterial evaluation and mode of action study of BIMQ, a novel bacterial cell division inhibitor. Biochem. Biophys. Res. Commun. 2019, 514, 1224–1230. [Google Scholar] [CrossRef]
- Kusakabe, Y.; Mizutani, S.; Kamo, S.; Yoshimoto, T.; Tomoshige, S.; Kawasaki, T.; Takasawa, R.; Tsubaki, K.; Kuramochi, K. Synthesis, antibacterial and cytotoxic evaluation of flavipucine and its derivatives. Bioorg. Med. Chem. Lett. 2019, 29, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Akbay, P.; Basaran, A.A.; Undeger, U.; Basaran, N. In vitro immunomodulatory activity of flavonoid glycosides from Urtica dioica L. Phytother. Res. 2003, 17, 34–37. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef] [PubMed]
- Desai, N.C.; Kotadiya, G.M.; Trivedi, A.R. Studies on molecular properties prediction, antitubercular and antimicrobial activities of novel quinoline based pyrimidine motifs. Bioorg. Med. Chem. Lett. 2014, 24, 3126–3130. [Google Scholar] [CrossRef] [PubMed]
- Holtz, K.M.; Stec, B.; Myers, J.K.; Antonelli, S.M.; Widlanski, T.S.; Kantrowitz, E.R. Alternate modes of binding in two crystal structures of alkaline phosphatase-inhibitor complexes. Protein Sci. 2000, 9, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Soliman, H.A.; Khatab, T.K. New approach for tetrachlorosilane promoted one pot, condensation reaction for tetrahydrobenzo[a]xanthene-11-ones with docking validation as aurora kinase inhibitor. Silicon 2018, 10, 229–233. [Google Scholar] [CrossRef]
- Khatab, T.K.; Mubarak, A.Y.; Soliman, H.A. Design and Synthesis Pairing Between Xanthene and Tetrazole in Pentacyclic System Using Tetrachlorosilane with Aurora Kinase Inhibitor Validation. J. Heterocycl. Chem. 2017, 54, 2463–2470. [Google Scholar] [CrossRef]
- Soliman, H.A.; Khatab, T.K.; Abdel-Megeid, F.M.E. Utilization of bromine azide access to vicinal-azidobromides from arylidene malononitrile. Chin. Chem. Lett. 2016, 27, 1515–1518. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hafez, T.S.; Osman, S.A. Synthesis, characterization, and cytotoxicity of some new 5-aminopyrazole and pyrazolo[1,5-a]pyrimidine derivatives. Sci. Pharm. 2015, 83, 27–39. [Google Scholar] [CrossRef]
- Hassan, A.S.; Mady, M.F.; Awad, H.M.; Hafez, T.S. Synthesis and antitumor activity of some new pyrazolo[1,5-a]pyrimidines. Chin. Chem. Lett. 2017, 28, 388–393. [Google Scholar] [CrossRef]
- El-Naggar, M.; Hassan, A.S.; Awad, H.M.; Mady, M.F. Design, synthesis and antitumor evaluation of novel pyrazolopyrimidines and pyrazoloquinazolines. Molecules 2018, 23, 1249. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.; Thong, Y.H. Optimal conditions for simultaneous purification of mononuclear and polymorphonuclear leucocytes from human blood by the Hypaque-Ficoll method. J. Immunol. Methods 1980, 36, 109–117. [Google Scholar] [CrossRef]
- Baehner, R.L.; Nathan, D.G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N. Engl. J. Med. 1968, 278, 971–976. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Comp. | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Bc | Sa | Ef | Ec | Pa | St | Ca | Fo | Ab | |
| 5a | 11 ± 0.61 | 20 ± 0.25 | 13 ± 0.23 | 13 ± 0.34 | NA | NA | NA | NA | NA |
| 5b | 14 ± 0.54 | 11 ± 0.84 | 13 ± 0.23 | 10 ± 0.62 | NA | NA | 12 ± 0.63 | 7 ± 0.34 | NA |
| 5c | 19 ± 0.14 | 21 ± 0.49 | 22 ± 0.68 | 18 ± 0.24 | 19 ± 0.43 | 18 ± 0.39 | 17 ± 0.33 | 12 ± 0.95 | 13 ± 0.95 |
| 9a | 23 ± 0.18 | 19 ± 0.37 | 16 ± 0.83 | 14 ± 0.21 | 16 ± 0.33 | 16 ± 0.77 | 15 ± 0.25 | 13 ± 0.39 | 16 ± 0.79 |
| 9b | 10 ± 0.24 | 15 ± 0.15 | NA | 18 ± 0.18 | NA | 14 ± 0.48 | NA | 10 ± 0.28 | NA |
| 9c | 16 ± 0.78 | 21 ± 0.24 | 25 ± 0.23 | 17 ± 0.84 | 15 ± 0.34 | 18 ± 0.18 | 19 ± 0.24 | 16 ± 0.78 | 13 ± 0.43 |
| 13a | 13 ± 0.11 | 16 ± 0.34 | 13 ± 0.23 | 15 ± 0.23 | 14 ± 0.78 | 16 ± 0.33 | 13 ± 0.65 | 14 ± 0.11 | 11 ± 0.42 |
| 13b | 9 ± 0.37 | NA | NA | 12 ± 0.62 | NA | NA | 12 ± 0.46 | 14 ± 0.22 | NA |
| 13c | 17 ± 0.23 | 10 ± 0.47 | 13 ± 0.23 | 15 ± 0.45 | 12 ± 0.61 | 14 ± 0.47 | 14 ± 0.23 | 11 ± 0.22 | 10 ± 0.32 |
| 13d | 14 ± 0.23 | 18 ± 0.87 | 16 ± 0.23 | 19 ± 0.14 | 17 ± 0.46 | 17 ± 0.76 | 16 ± 0.81 | 15 ± 0.66 | 13 ± 0.33 |
| 13e | 17 ± 0.32 | 17 ± 0.74 | 23 ± 0.21 | 16 ± 0.96 | 15 ± 0.77 | 13 ± 0.98 | 18 ± 0.11 | 13 ± 0.44 | 12 ± 0.32 |
| 13f | 10 ± 0.14 | 15 ± 0.64 | NA | 11 ± 0.12 | NA | 13 ± 0.32 | 12 ± 0.44 | 10 ± 0.48 | NA |
| 13g | 12 ± 0.47 | 8 ± 0.2 | 13 ± 0.23 | 11 ± 0.27 | NA | NA | NA | NA | NA |
| 13h | 20 ± 0.34 | 18 ± 0.16 | 20 ± 0.13 | 17 ± 0.15 | 18 ± 0.12 | 16 ± 0.85 | 20 ± 0.26 | 15 ± 0.76 | 14 ± 0.12 |
| 13i | NA | NA | NA | 13 ± 0.85 | NA | 11 ± 0.12 | 15 ± 0.56 | 12 ± 0.15 | NA |
| ST1 | 22 ± 0.42 | 24 ± 0.61 | 25 ± 0.45 | 23 ± 0.33 | 20 ± 0.55 | 22 ± 0.18 | NA | NA | NA |
| ST2 | NA | NA | NA | NA | NA | NA | 15 ± 0.2 | 20 ± 0.32 | 18 ± 0.2 |
| Comp. | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bc | Sa | Ef | Ec | Pa | St | Ca | Fo | Ab | ||||||||||
| MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MFC | MIC | MFC | MIC | MFC | |
| 5c | 31.25 | 62.5 | 3.9 | 7.81 | 3.9 | 7.81 | 6.79 | 15.62 | 3.9 | 7.81 | 3.39 | 7.81 | 7.14 | 16.44 | 52.08 | 62.5 | 29.46 | 41.25 |
| 9a | 3.55 | 7.81 | 6.28 | 13.2 | 7.1 | 15.62 | 7.14 | 16.44 | 14.16 | 29.75 | 7.1 | 15.62 | 7.43 | 15.62 | 27.9 | 44.65 | 21.25 | 29.76 |
| 9c | 5.73 | 13.2 | 2.95 | 6.51 | 1.85 | 3.9 | 6.6 | 13.2 | 6.5 | 15.62 | 2.83 | 6.51 | 3.39 | 7.81 | 27.77 | 41.66 | 24.03 | 31.25 |
| 13a | 18.93 | 41.66 | 29.76 | 62.5 | 19.83 | 41.66 | 7.47 | 16.44 | 12.93 | 29.75 | 7.47 | 16.44 | 15.62 | 31.25 | 32.04 | 41.66 | 41.66 | 62.5 |
| 13c | 29.76 | 62.5 | 56.81 | 125 | 31.25 | 62.5 | 6.79 | 15.62 | 13.02 | 31.25 | 13.52 | 29.75 | 11.27 | 24.8 | 52.08 | 62.5 | 96.15 | 125 |
| 13d | 14.87 | 29.75 | 14.2 | 31.25 | 14.2 | 31.25 | 3.39 | 7.81 | 6.6 | 13.2 | 6.79 | 15.62 | 7.1 | 15.62 | 20.83 | 31.25 | 26.04 | 41.66 |
| 13e | 7.43 | 15.62 | 18.09 | 41.66 | 12.93 | 29.75 | 14.87 | 29.75 | 7.14 | 16.44 | 15.62 | 31.25 | 3.39 | 7.81 | 24.79 | 34.71 | 31.89 | 44.65 |
| 13h | 7.47 | 16.44 | 7.1 | 15.62 | 13.58 | 31.25 | 3.71 | 7.81 | 6.28 | 13.2 | 6.28 | 13.2 | 2.95 | 6.51 | 22.88 | 29.75 | 24.03 | 31.25 |
| ST1 | 7.81 | 15.62 | 3.12 | 6.24 | 3.9 | 7.81 | 5.68 | 12.5 | 9.46 | 20.83 | 6.79 | 15.62 | - | - | - | - | - | - |
| ST2 | - | - | - | - | - | - | - | - | - | - | - | - | 7.81 | 15.62 | 26.4 | 31.25 | 20.2 | 24.13 |
| Compounds | Intracellular Killing Activity % |
|---|---|
| 5c | 76.2 ± 0.23 |
| 9a | 125.6 ± 0.44 |
| 9c | 122.9 ± 0.79 |
| 13a | 98.7 ± 0.61 |
| 13c | 87.5 ± 0.33 |
| 13d | 136.3 ± 0.16 |
| 13e | 129.8 ± 0.47 |
| 13h | 117.4 ± 0.98 |
| Compounds | MW a | MLogP b | nHBA c | nHBD d | nviolations f |
|---|---|---|---|---|---|
| Rule | <500 | ≤4.15 | ≤10 | ≤5 | 0 |
| 5a | 387.43 | 3.19 | 4 | 2 | 0 |
| 5b | 401.46 | 3.41 | 4 | 2 | 0 |
| 5c | 421.88 | 3.67 | 4 | 2 | 0 |
| 9a | 391.38 | 2.13 | 6 | 4 | 0 |
| 9b | 405.41 | 2.35 | 6 | 4 | 0 |
| 9c | 425.83 | 2.62 | 6 | 4 | 0 |
| 13a | 435.48 | 3.82 | 4 | 2 | 0 |
| 13b | 449.50 | 4.02 | 4 | 2 | 0 |
| 13c | 469.92 | 4.29 | 4 | 2 | 1 |
| 13d | 449.50 | 4.02 | 4 | 2 | 0 |
| 13e | 463.53 | 4.22 | 4 | 2 | 1 |
| 13f | 483.95 | 4.49 | 4 | 2 | 1 |
| 13g | 469.92 | 4.29 | 4 | 2 | 1 |
| 13h | 483.95 | 4.49 | 4 | 2 | 1 |
| 13i | 504.37 | 4.76 | 4 | 2 | 2 |
| Compounds | TPSA a (A2) | Volume b (A3) | %ABS c = 109 − (0.345 × TPSA) | Drug-Likeness Model Score (DLS) |
|---|---|---|---|---|
| 5a | 60.18 | 379.18 | 88.24 | 0.44 |
| 5b | 60.18 | 400.12 | 88.24 | 0.40 |
| 5c | 60.18 | 396.37 | 88.24 | 1.00 |
| 9a | 94.13 | 358.28 | 76.53 | 0.61 |
| 9b | 94.13 | 379.22 | 76.53 | 0.59 |
| 9c | 94.13 | 375.47 | 76.53 | 1.15 |
| 13a | 59.56 | 410.76 | 88.45 | 0.77 |
| 13b | 59.56 | 431.70 | 88.45 | 0.73 |
| 13c | 59.56 | 427.95 | 88.45 | 1.31 |
| 13d | 59.56 | 431.70 | 88.45 | 0.62 |
| 13e | 59.56 | 452.64 | 88.45 | 0.88 |
| 13f | 59.56 | 448.89 | 88.45 | 1.25 |
| 13g | 59.56 | 427.95 | 88.45 | 1.20 |
| 13h | 59.56 | 448.89 | 88.45 | 1.25 |
| 13i | 59.56 | 445.14 | 88.45 | 1.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naglah, A.M.; Askar, A.A.; Hassan, A.S.; Khatab, T.K.; Al-Omar, M.A.; Bhat, M.A. Biological Evaluation and Molecular Docking with In Silico Physicochemical, Pharmacokinetic and Toxicity Prediction of Pyrazolo[1,5-a]pyrimidines. Molecules 2020, 25, 1431. https://doi.org/10.3390/molecules25061431
Naglah AM, Askar AA, Hassan AS, Khatab TK, Al-Omar MA, Bhat MA. Biological Evaluation and Molecular Docking with In Silico Physicochemical, Pharmacokinetic and Toxicity Prediction of Pyrazolo[1,5-a]pyrimidines. Molecules. 2020; 25(6):1431. https://doi.org/10.3390/molecules25061431
Chicago/Turabian StyleNaglah, Ahmed M., Ahmed A. Askar, Ashraf S. Hassan, Tamer K. Khatab, Mohamed A. Al-Omar, and Mashooq A. Bhat. 2020. "Biological Evaluation and Molecular Docking with In Silico Physicochemical, Pharmacokinetic and Toxicity Prediction of Pyrazolo[1,5-a]pyrimidines" Molecules 25, no. 6: 1431. https://doi.org/10.3390/molecules25061431
APA StyleNaglah, A. M., Askar, A. A., Hassan, A. S., Khatab, T. K., Al-Omar, M. A., & Bhat, M. A. (2020). Biological Evaluation and Molecular Docking with In Silico Physicochemical, Pharmacokinetic and Toxicity Prediction of Pyrazolo[1,5-a]pyrimidines. Molecules, 25(6), 1431. https://doi.org/10.3390/molecules25061431







