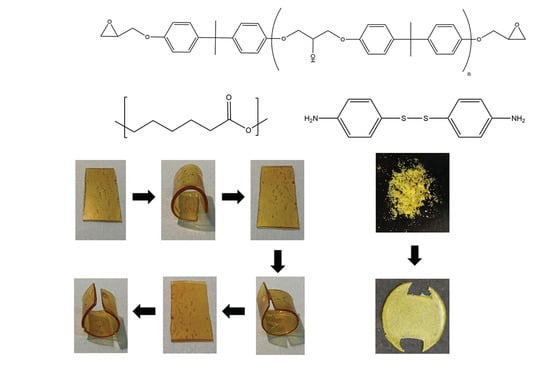
Reprogrammable Permanent Shape Memory Materials Based on Reversibly Crosslinked Epoxy/PCL Blends
Abstract
1. Introduction
2. Results and Discussion
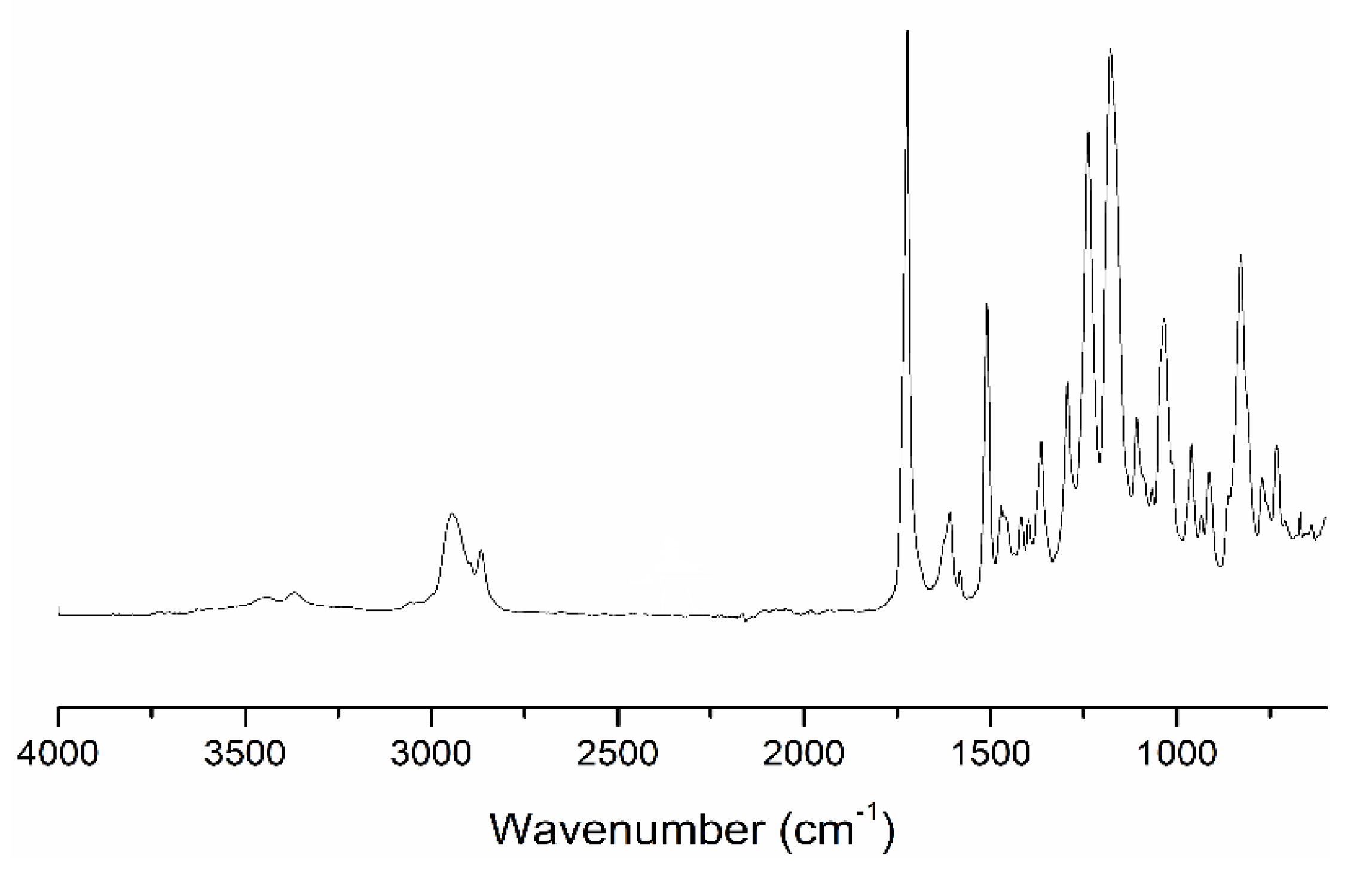
2.1. Characterization of Samples with DDM
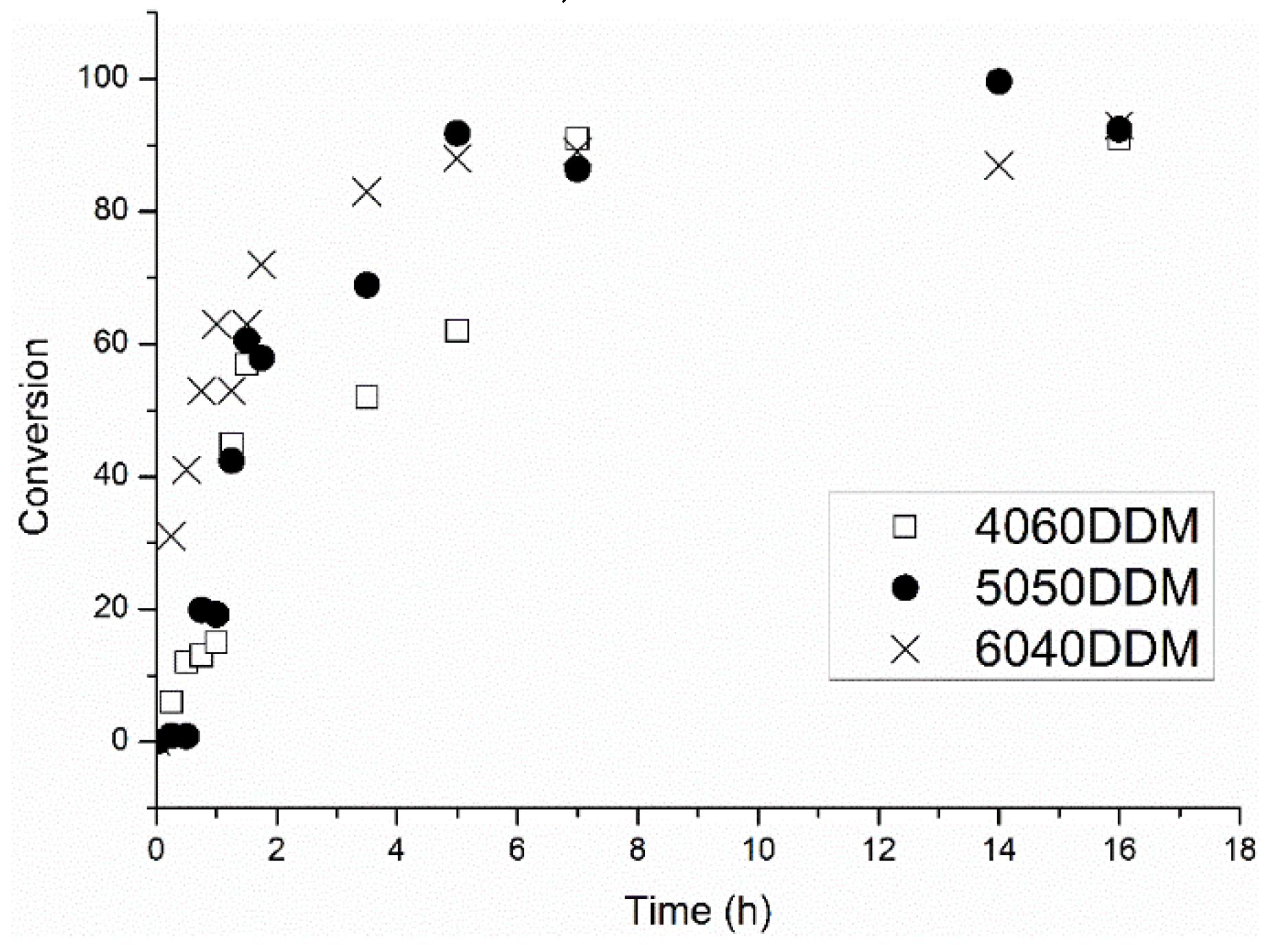
2.2. Characterization of Samples Cured with DDM/DSS Mixtures
2.3. Morphology of the Material
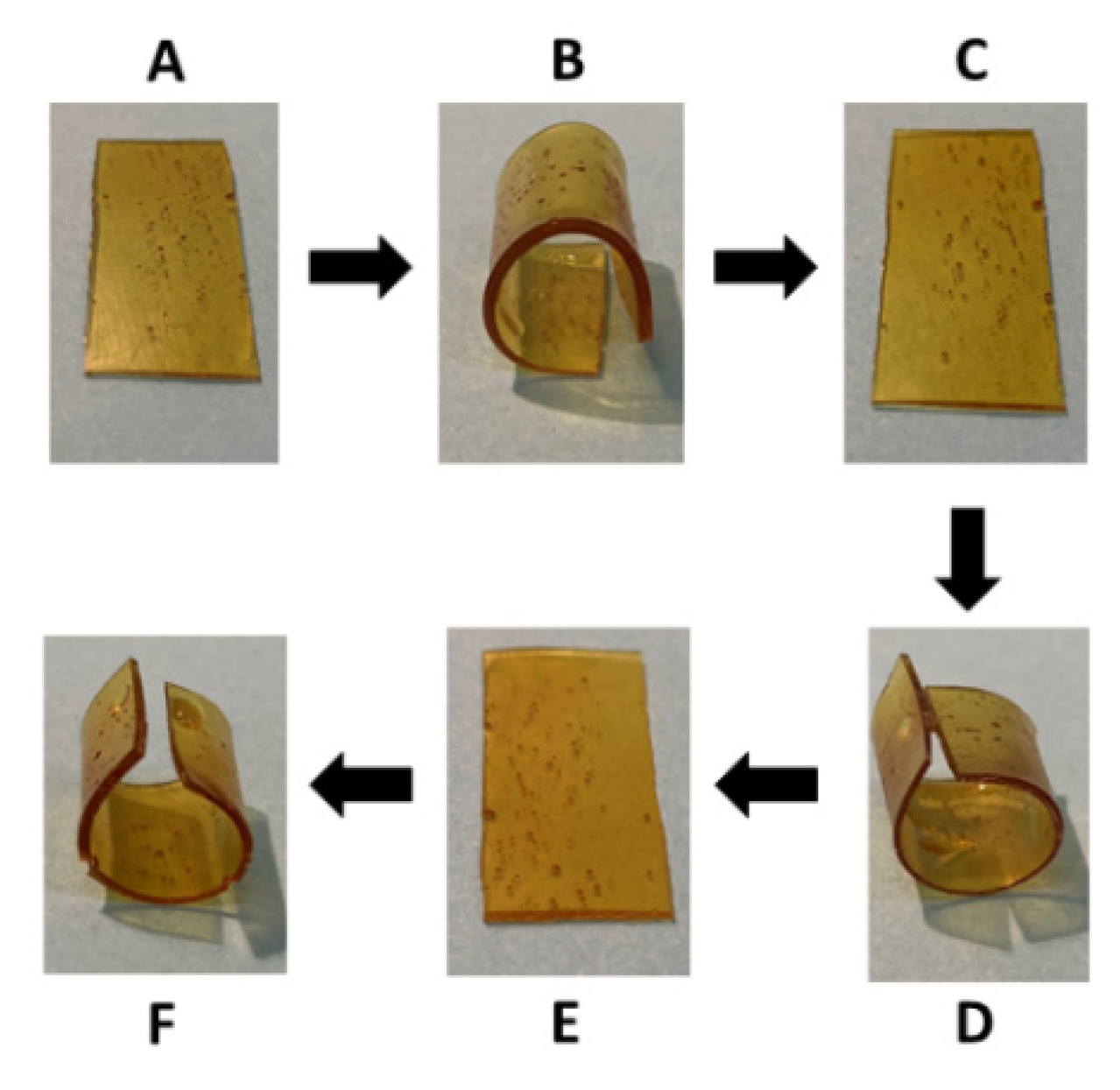
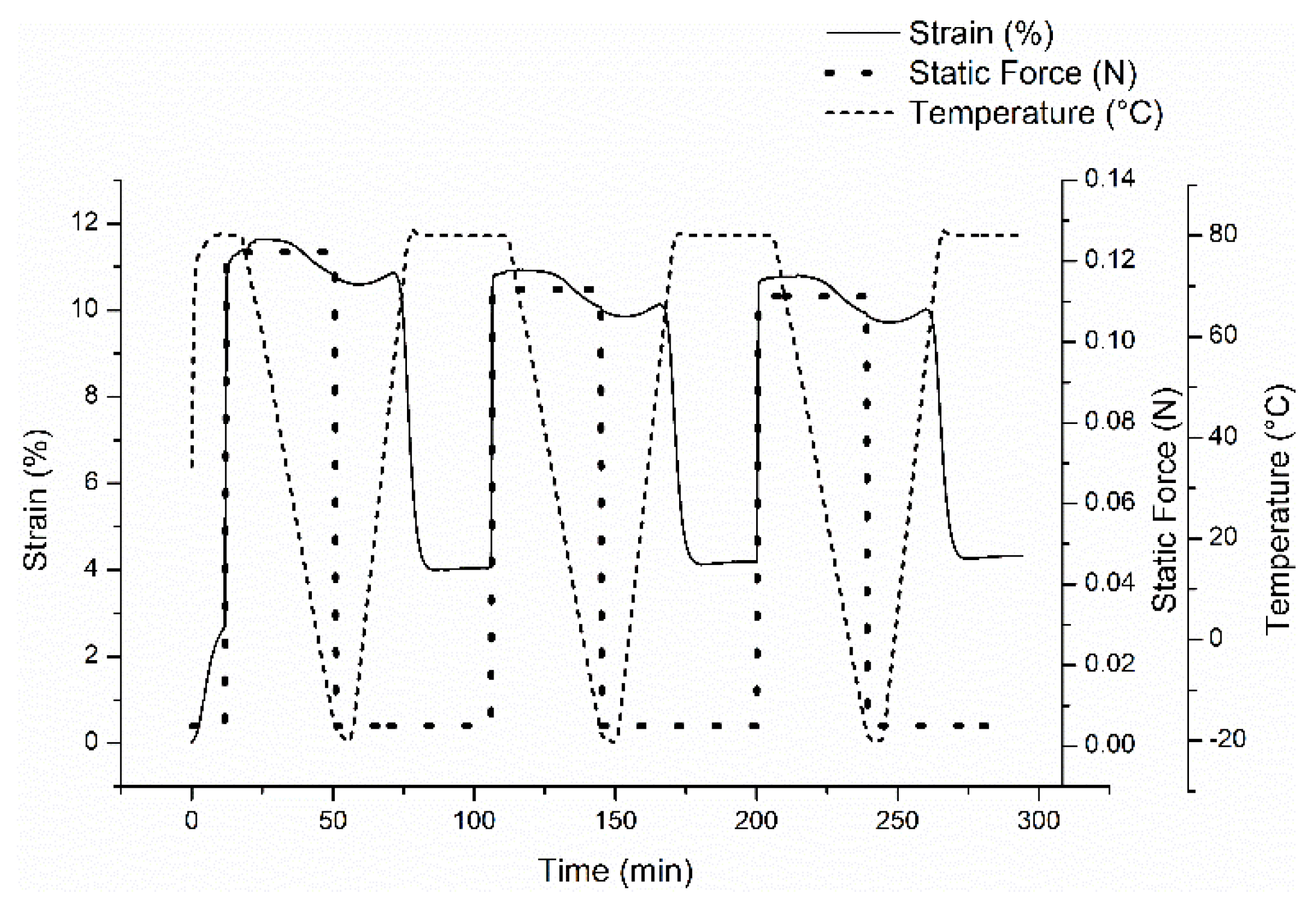
2.4. Shape Memory Properties
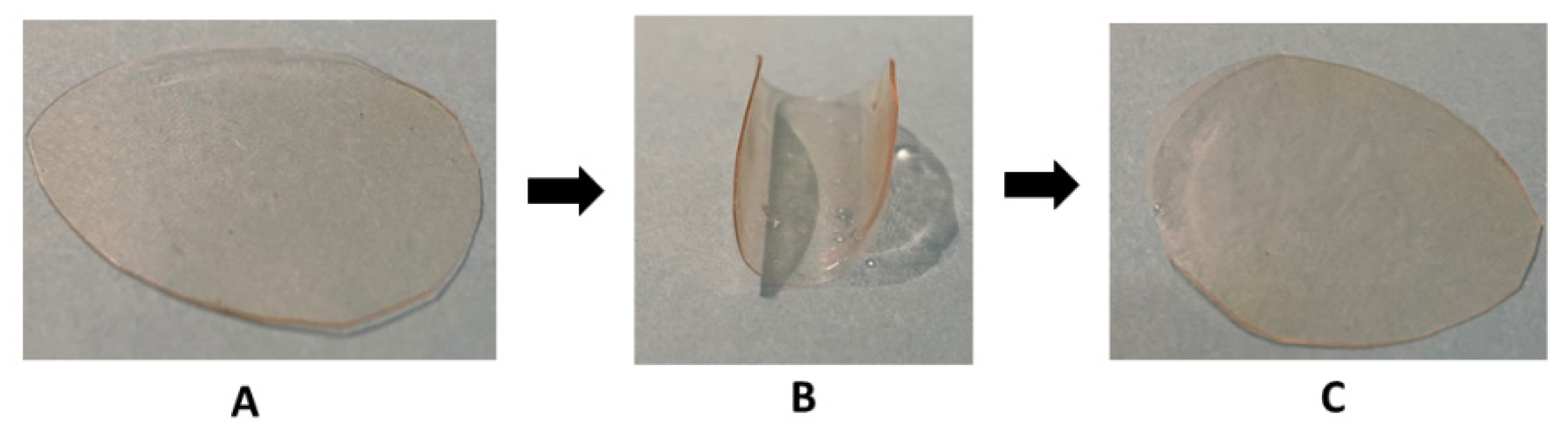
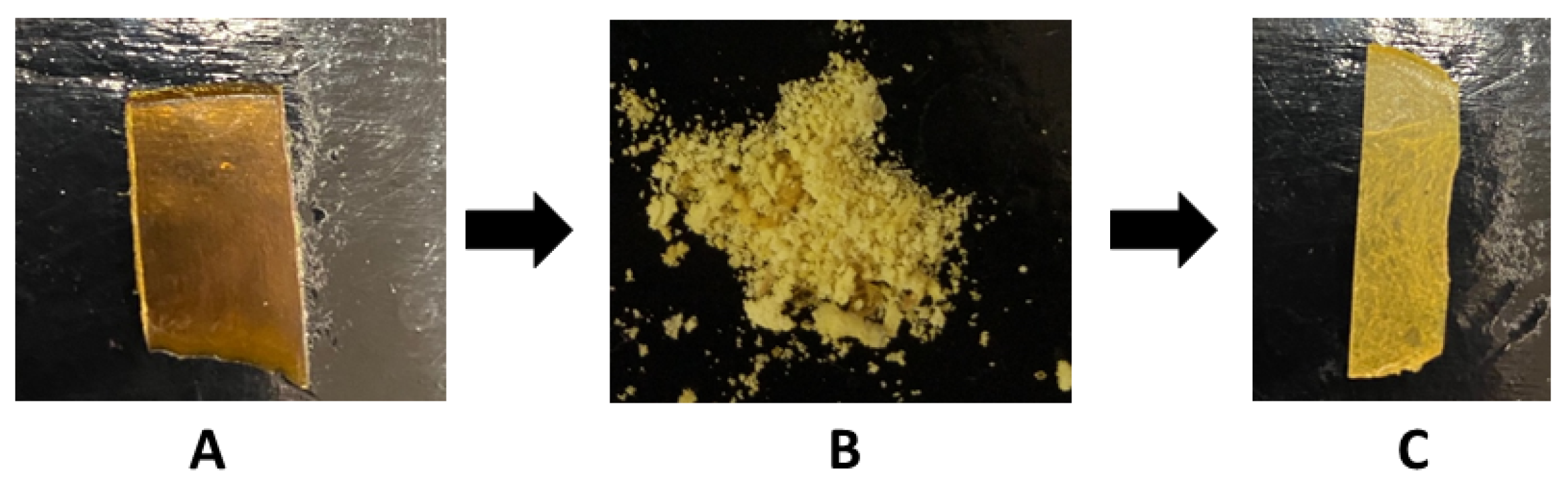
2.5. Recycling Properties
3. Materials and Methods
3.1. Materials
3.2. Synthesis
3.3. Characterization
3.4. Shape Memory Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lester, B.; Baxevanis, T.; Chemisky, Y.; Lagoudas, D.; Lester, B.; Baxevanis, T.; Chemisky, Y.; Dimitris, L. Review and Perspectives: Shape Memory Alloy Composite Systems To cite this version: Science Arts & Métiers (SAM). Acta Mech. 2015, 226, 3907–3960. [Google Scholar]
- Hager, M.D.; Bode, S.; Weber, C.; Schubert, U.S. Shape memory polymers: Past, present and future developments. Prog. Polym. Sci. 2015, 49–50, 3–33. [Google Scholar] [CrossRef]
- Hodgson, D.E.; Wu, M.H.; Biermann, R.J. Shape Memory Alloys. En ASM Handbook Volume 2: Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; ASM International: Russell, OH, USA, 1990. [Google Scholar]
- Ratna, D.; Karger-Kocsis, J. Recent advances in shape memory polymers and composites: A review. J. Mater. Sci. 2008, 43, 254–269. [Google Scholar] [CrossRef]
- Lendlein, A. Shape-Memory Polymers; Springer: New York, NY, USA, 2010. [Google Scholar]
- Leng, J.; Lan, X.; Liu, Y.; Du, S. Shape-memory polymers and their composites: Stimulus methods and applications. Prog. Mater. Sci. 2011, 56, 1077–1135. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, J.; Lu, H.; Xu, B.; Fu, Y.; Liu, Y.; Leng, J. Thermosetting epoxy resin/thermoplastic system with combined shape memory and self-healing properties. Smart Mater. Struct. 2015, 25, 015021. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, Z.; Liu, Y.; Cheng, W.; Huang, Y.; Leng, J. Thermosetting epoxy reinforced shape memory composite microfiber membranes: Fabrication, structure and properties. Compos. Part A Appl. Sci. Manuf. 2015, 76, 54–61. [Google Scholar] [CrossRef]
- Song, W.B.; Wang, L.Y.; Wang, Z.D. Synthesis and thermomechanical research of shape memory epoxy systems. Mater. Sci. Eng. A 2011, 529, 29–34. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Kéki, S. Review of progress in shape memory epoxies and their composites. Polymers 2017, 10, 34. [Google Scholar] [CrossRef]
- Lu, L.; Fan, J.; Li, G. Intrinsic healable and recyclable thermoset epoxy based on shape memory effect and transesterification reaction. Polymer 2016, 105, 10–18. [Google Scholar] [CrossRef]
- Feldkamp, D.M.; Rousseau, I.A. Effect of chemical composition on the deformability of shape-memory epoxies. Macromol. Mater. Eng. 2011, 296, 1128–1141. [Google Scholar] [CrossRef]
- Chang, Y.W.; Eom, J.P.; Kim, J.G.; Kim, H.T.; Kim, D.K. Preparation and characterization of shape memory polymer networks based on carboxylated telechelic poly(ε-caprolactone)/epoxidized natural rubber blends. J. Ind. Eng. Chem. 2010, 16, 256–260. [Google Scholar] [CrossRef]
- Lützen, H.; Gesing, T.M.; Kim, B.K.; Hartwig, A. Novel cationically polymerized epoxy/poly(ε-caprolactone) polymers showing a shape memory effect. Polymer 2012, 53, 6089–6095. [Google Scholar] [CrossRef]
- Arnebold, A.; Hartwig, A. Fast switchable, epoxy based shape-memory polymers with high strength and toughness. Polymer 2016, 83, 40–49. [Google Scholar] [CrossRef]
- Iregui, A.; Irusta, L.; Llorente, O.; Martin, L.; Calvo-Correas, T.; Eceiza, A.; González, A. Electrospinning of cationically polymerized epoxy/polycaprolactone blends to obtain shape memory fibers (SMF). Eur. Polym. J. 2017, 94, 376–383. [Google Scholar] [CrossRef]
- Iregui, A.; Irusta, L.; Martin, L.; González, A. Analysis of the process parameters for obtaining a stable electrospun process in different composition epoxy/poly ε-Caprolactone blends with shape memory properties. Polymers. 2019, 11, 475. [Google Scholar] [CrossRef]
- Fejos, M.; Molnár, K.; Karger-Kocsis, J. Epoxy/polycaprolactone systems with triple-shape memory effect: Electrospun nanoweb with and without graphene Versus co-continuous morphology. Materials 2013, 6, 4489–4504. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Takayama, T.; Uyama, H. Biodegradable shape memory polymeric material from epoxidized soybean oil and polycaprolactone. Polymers 2015, 7, 2165–2174. [Google Scholar] [CrossRef]
- Pomponi, F.; Moncaster, A. Circular economy for the built environment: A research framework. J. Clean. Prod. 2017, 143, 710–718. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Bowman, C.N. Covalent adaptable networks: Smart, reconfigurable and responsive network systems. Chem. Soc. Rev. 2013, 42, 7161–7173. [Google Scholar] [CrossRef]
- Kloxin, C.J.; Scott, T.F.; Adzima, B.J.; Bowman, C.N. Covalent adaptable networks (CANs): A unique paradigm in cross-linked polymers. Macromolecules 2010, 43, 2643–2653. [Google Scholar] [CrossRef]
- Denissen, W.; Winne, J.M.; Du Prez, F.E. Vitrimers: Permanent organic networks with glass-like fluidity. Chem. Sci. 2016, 7, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gandini, A. The furan/maleimide Diels-Alder reaction: A versatile click-unclick tool in macromolecular synthesis. Prog. Polym. Sci. 2013, 38, 1–29. [Google Scholar] [CrossRef]
- Laita, H.; Boufi, S.; Gandini, A. The application of the Diels-Alder reaction to polymers bearing furan moieties. 1. Reactions with maleimides. Eur. Polym. J. 1997, 33, 1203–1211. [Google Scholar] [CrossRef]
- Turkenburg, D.H.; Fischer, H.R. Diels-Alder based, thermo-reversible cross-linked epoxies for use in self-healing composites. Polymer 2015, 79, 187–194. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Shape memory epoxy vitrimers based on DGEBA crosslinked with dicarboxylic acids and their blends with citric acid. RSC Adv. 2016, 6, 88647–88655. [Google Scholar] [CrossRef]
- Gyarmati, B.; Némethy, Á.; Szilágyi, A. Reversible disulphide formation in polymer networks: A versatile functional group from synthesis to applications. Eur. Polym. J. 2013, 49, 1268–1286. [Google Scholar] [CrossRef]
- Li, K.; Xu, Z.; Zhao, S.; Meng, X.; Zhang, R.; Li, J.; Leng, J.; Zhang, G.; Cao, D.; Sun, R. Biomimetic, recyclable, highly stretchable and self-healing conductors enabled by dual reversible bonds. Chem. Eng. J. 2019, 371, 203–212. [Google Scholar] [CrossRef]
- Zhang, L.; Qiu, T.; Zhu, Z.; Guo, L.; Li, X. Self-Healing Polycaprolactone Networks through Thermo-Induced Reversible Disulfide Bond Formation. Macromol. Rapid Commun. 2018, 39, 1–5. [Google Scholar] [CrossRef]
- Zhou, F.; Guo, Z.; Wang, W.; Lei, X.; Zhang, B.; Zhang, H.; Zhang, Q. Preparation of self-healing, recyclable epoxy resins and low-electrical resistance composites based on double-disulfide bond exchange. Compos. Sci. Technol. 2018, 167, 79–85. [Google Scholar] [CrossRef]
- Ruiz De Luzuriaga, A.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horizons 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Chen, T.; Fang, L.; Lu, C.; Xu, Z. Effects of Blended Reversible Epoxy Domains on Structures and Properties of Self-Healing/Shape-Memory Thermoplastic Polyurethane. Macromol. Mater. Eng. 2020, 305, 1–13. [Google Scholar] [CrossRef]
- Rigail-Cedeño, A.; Sung, C.S.P. Fluorescence and IR characterization of epoxy cured with aliphatic amines. Polymer 2005, 46, 9378–9384. [Google Scholar] [CrossRef]
- Parameswaranpillai, J.; Sidhardhan, S.K.; Jose, S.; Hameed, N.; Salim, N.V.; Siengchin, S.; Pionteck, J.; Magueresse, A.; Grohens, Y. Miscibility, Phase Morphology, Thermomechanical, Viscoelastic and Surface Properties of Poly(ϵ-caprolactone) Modified Epoxy Systems: Effect of Curing Agents. Ind. Eng. Chem. Res. 2016, 55, 10055–10064. [Google Scholar] [CrossRef]
- Chen, J.; Chang, F. Phase Separation and Melting Behavior in Poly (e- caprolactone) -Epoxy Blends Cured by 3, 3-dimethylmethylene-di (cyclohexylamine). J. Appl. Polym. Sci. 2003, 89, 3107–3114. [Google Scholar] [CrossRef]
- Jiang, S.; Ji, X.; An, L.; Jiang, B. Crystallization behavior of PCL in hybrid confined environment. Polymer 2001, 42, 3901–3907. [Google Scholar] [CrossRef]
- Alvarado-Tenorio, B.; Romo-Uribe, A.; Mather, P.T. Nanoscale Order and Crystallization in POSS-PCL Shape Memory Molecular Networks. Macromolecules 2015, 48, 5770–5779. [Google Scholar] [CrossRef]
- Liu, Y.; Gall, K.; Dunn, M.L.; McCluskey, P. Thermomechanical recovery couplings of shape memory polymers in flexure. Smart Mater. Struct. 2003, 12, 947–954. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

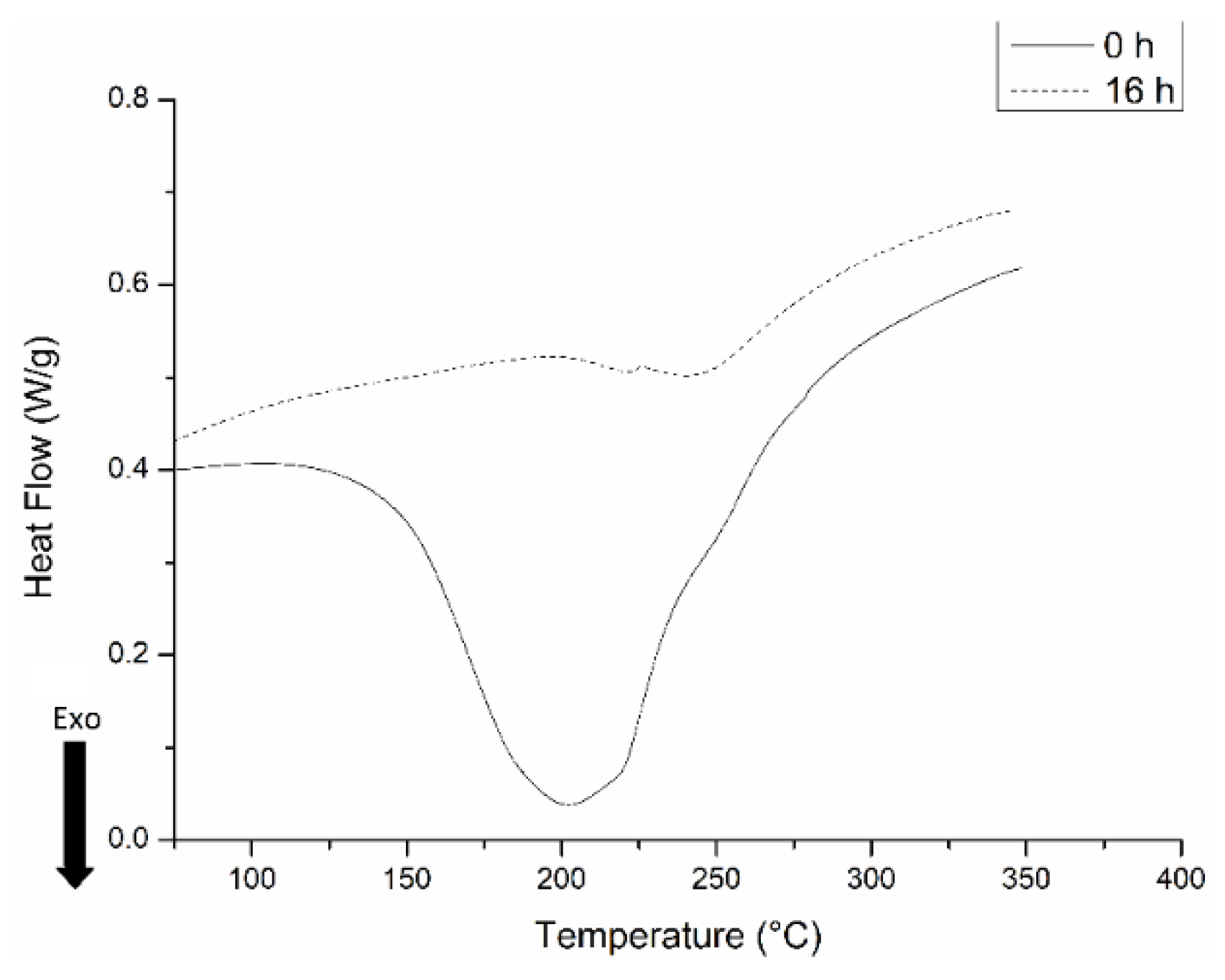
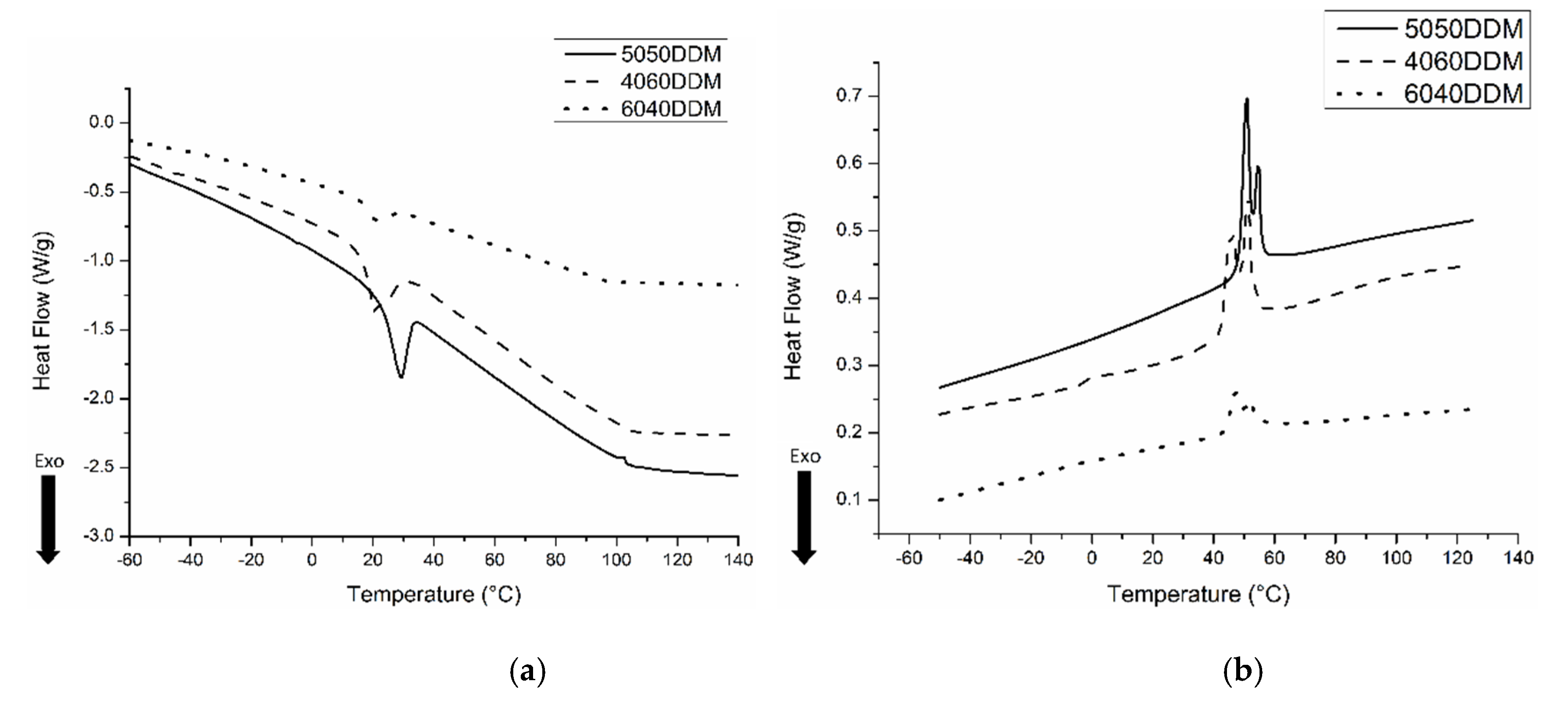
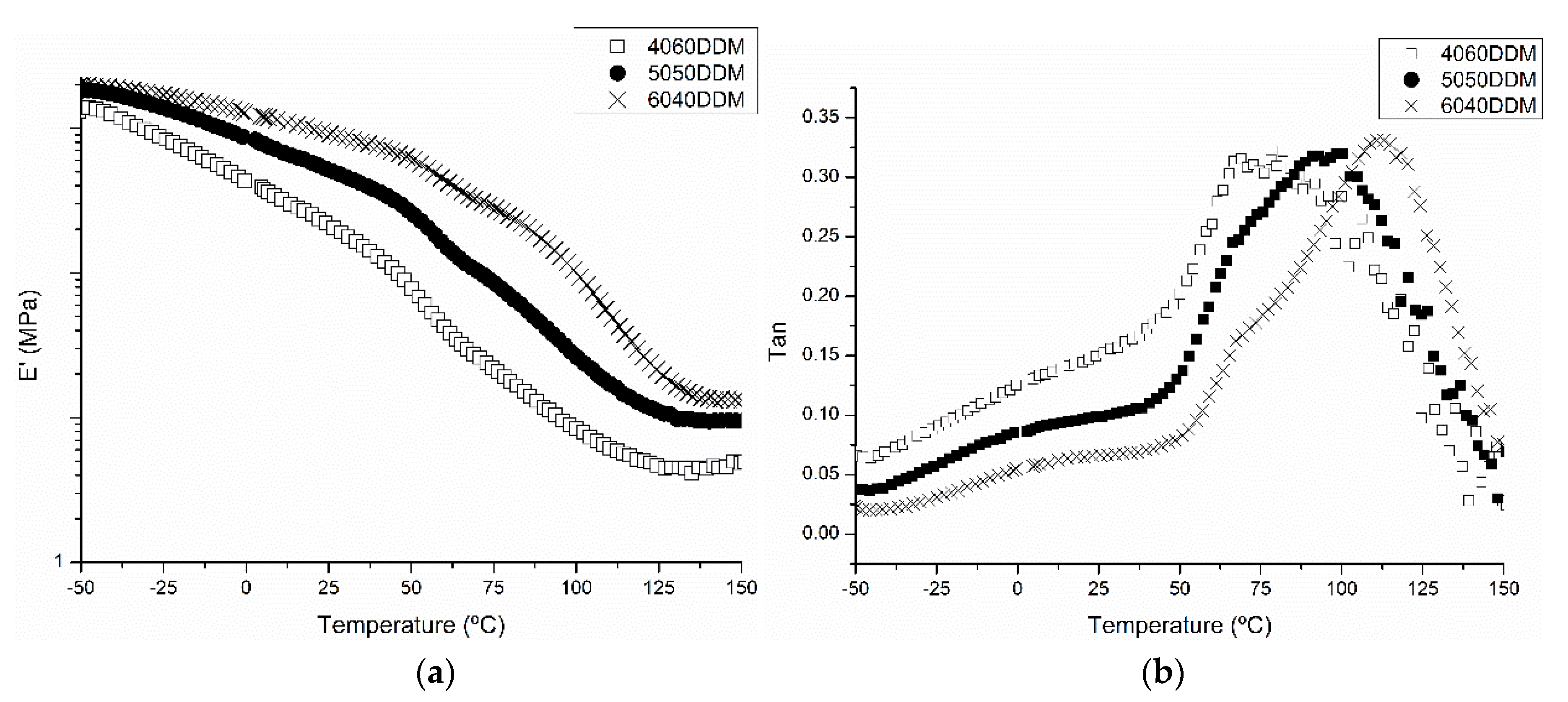


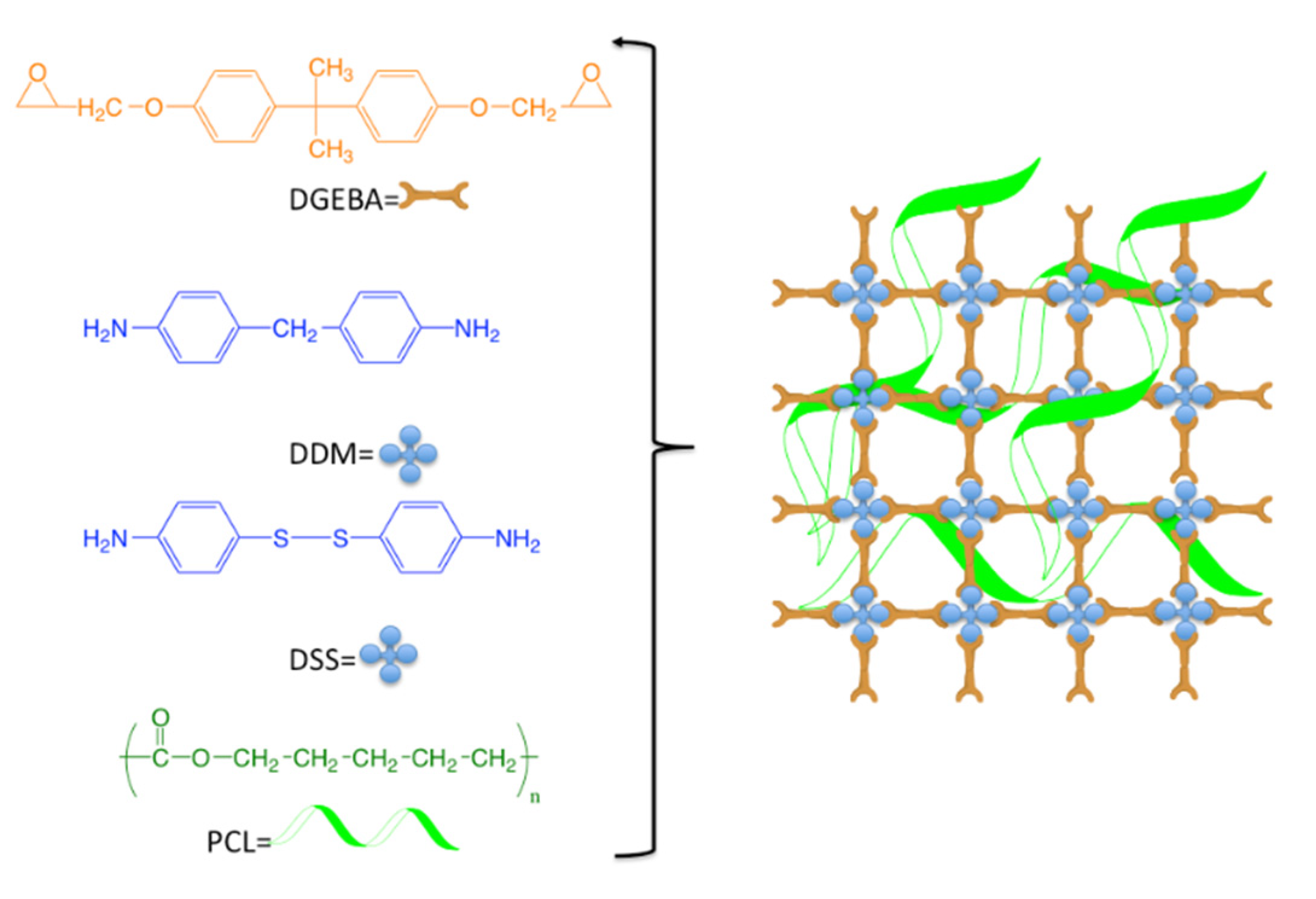
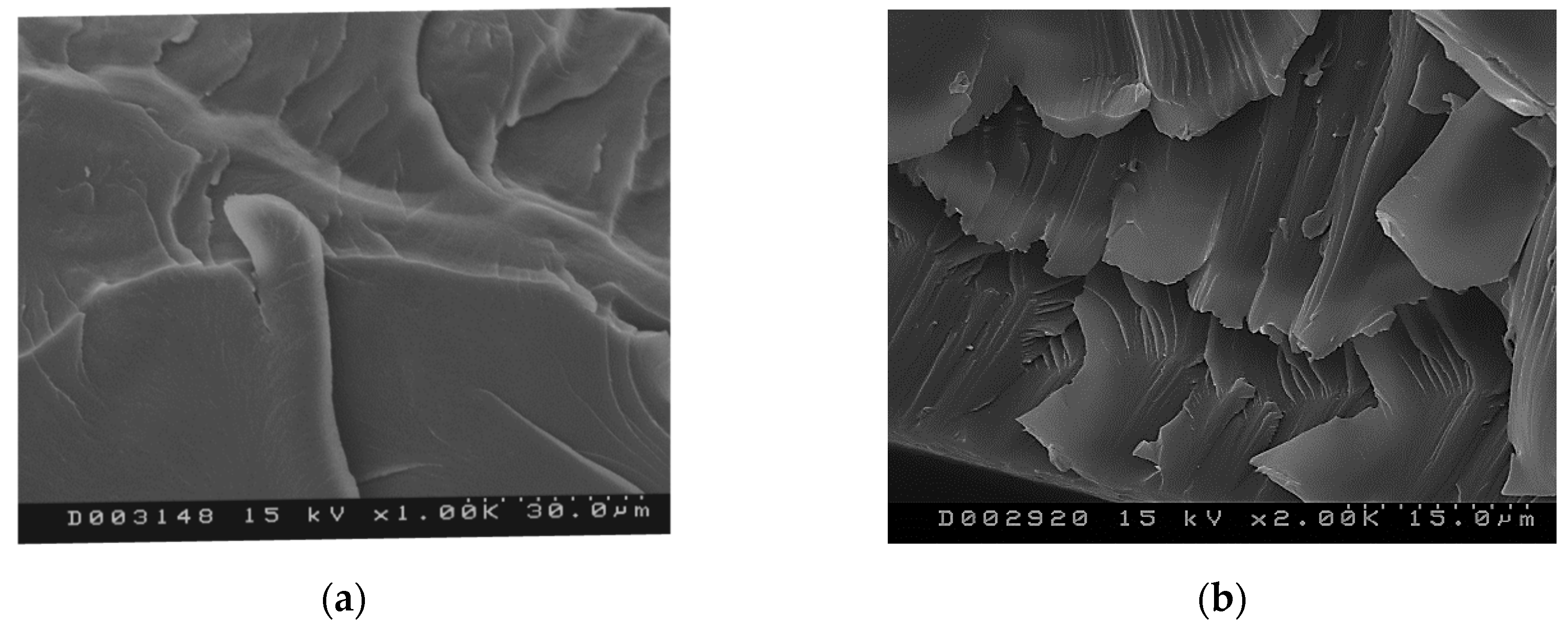

| Sample | Melting Enthalpy (J/g) Second Scan | Crystallinity (%) |
|---|---|---|
| 6040DDM | 1 | 0.7 |
| 5050DDM | 3.4 | 2.5 |
| 4060DDM | 4.6 | 3.4 |
| PCL | 53.7 | 40.9 |
| Sample | Fixity Ratio (%) | Recovery Ratio (%) | ||||
|---|---|---|---|---|---|---|
| Cycle 1 | Cycle 2 | Cycle 3 | Cycle 1 | Cycle 2 | Cycle 3 | |
| 5050DDM | 80 | 68 | 65 | 57 | 90 | 95 |
| 5050DDMDSS | 92 | 80 | 79 | 53 | 96 | 97 |
| 5050DSS | 91 | 88 | 88 | 85 | 98 | 98 |
| 5050DSS Reprocessed | 86 | 80 | 79 | 62 | 89 | 91 |
| Sample | DGEBA (g) | DGEBA (mol) | PCL (g) | DSS (g) | DSS (mol) | DDM (g) | DDM (mol) |
|---|---|---|---|---|---|---|---|
| 4060DDM | 4 | 11.7 | 6 | 0 | 0 | 1.16 | 5.85 |
| 5050DDM | 4 | 11.7 | 4 | 0 | 0 | 1.16 | 5.85 |
| 5050DDMDSS | 4 | 11.7 | 4 | 0.72 | 2.92 | 0.58 | 2.92 |
| 5050DSS | 4 | 11.7 | 4 | 1.45 | 5.85 | 0 | 0 |
| 6040DDM | 4 | 11.7 | 2.67 | 0 | 0 | 1.16 | 5.85 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razquin, I.; Iregui, A.; Orduna, L.; Martin, L.; González, A.; Irusta, L. Reprogrammable Permanent Shape Memory Materials Based on Reversibly Crosslinked Epoxy/PCL Blends. Molecules 2020, 25, 1568. https://doi.org/10.3390/molecules25071568
Razquin I, Iregui A, Orduna L, Martin L, González A, Irusta L. Reprogrammable Permanent Shape Memory Materials Based on Reversibly Crosslinked Epoxy/PCL Blends. Molecules. 2020; 25(7):1568. https://doi.org/10.3390/molecules25071568
Chicago/Turabian StyleRazquin, Iker, Alvaro Iregui, Lidia Orduna, Loli Martin, Alba González, and Lourdes Irusta. 2020. "Reprogrammable Permanent Shape Memory Materials Based on Reversibly Crosslinked Epoxy/PCL Blends" Molecules 25, no. 7: 1568. https://doi.org/10.3390/molecules25071568
APA StyleRazquin, I., Iregui, A., Orduna, L., Martin, L., González, A., & Irusta, L. (2020). Reprogrammable Permanent Shape Memory Materials Based on Reversibly Crosslinked Epoxy/PCL Blends. Molecules, 25(7), 1568. https://doi.org/10.3390/molecules25071568