Heterocyclic Amine Formation in Grilled Chicken Depending on Body Parts and Treatment Conditions
Abstract
1. Introduction
2. Results
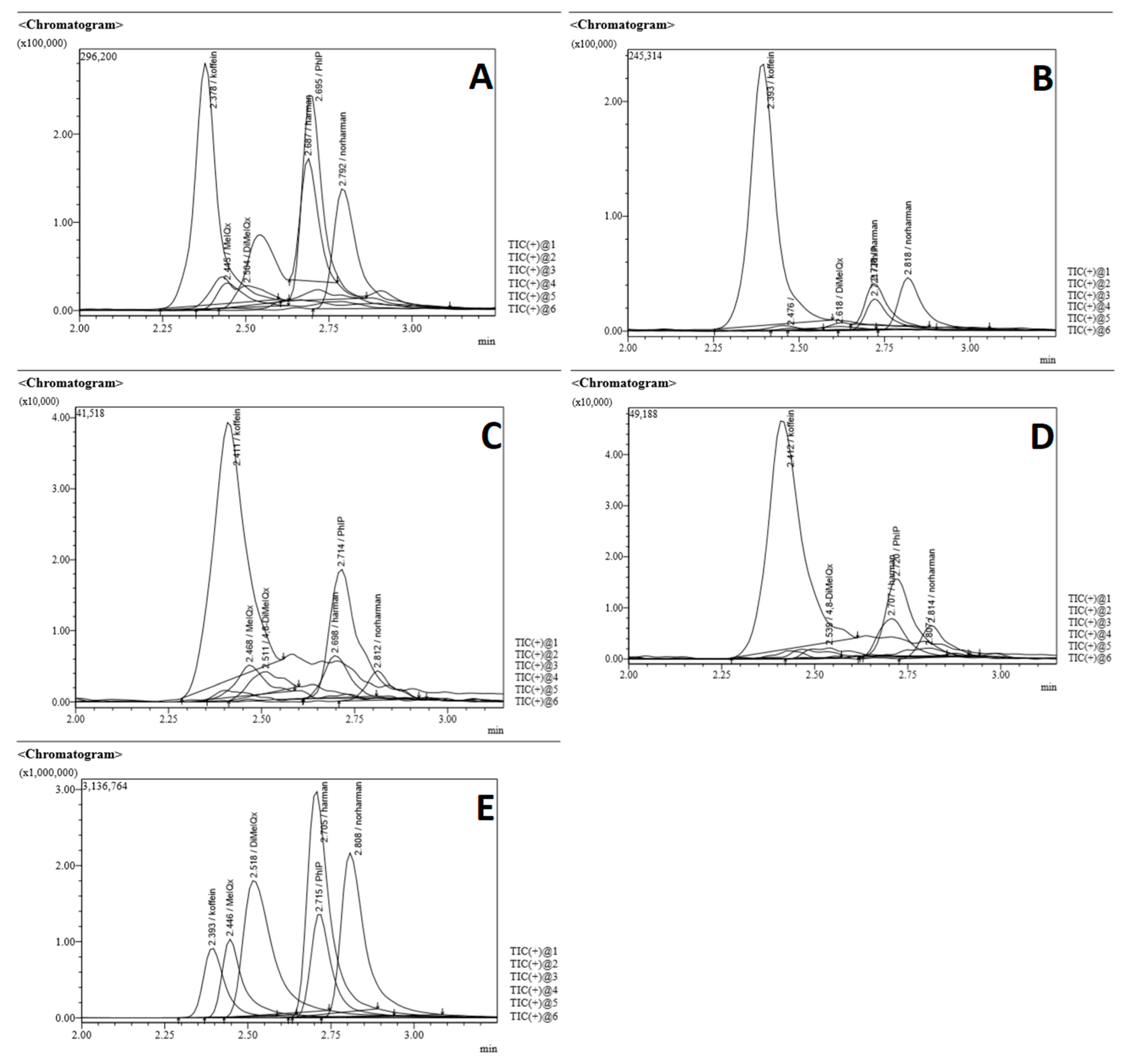
2.1. Validation of the LC-MS/MS Method
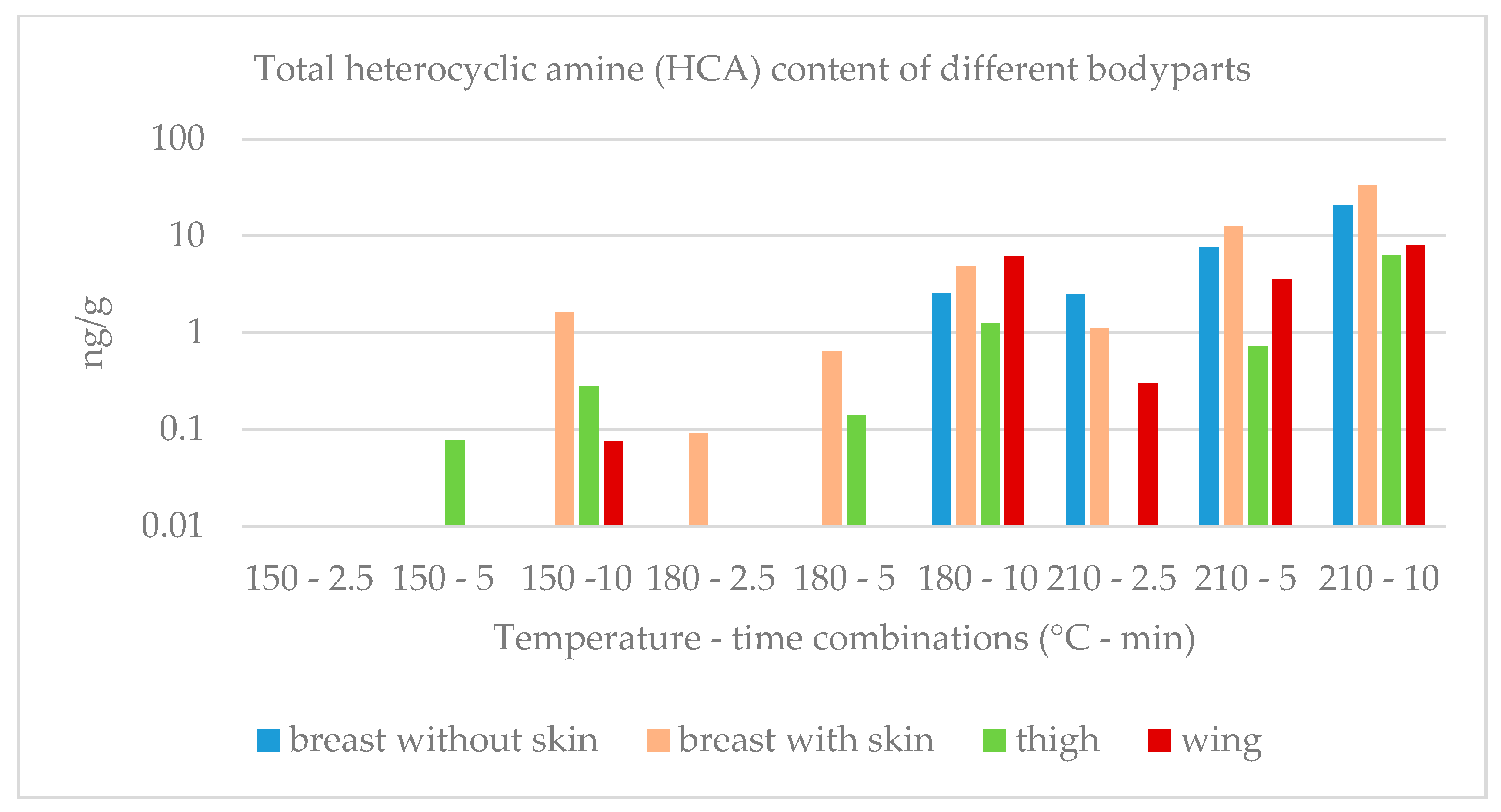
2.2. HCA Content of Chicken Bodyparts
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Instruments and Equipment
4.3. Grilling Trials
4.4. Sample Preparation
4.5. LC-MS/MS Analysis
4.6. Validation
4.7. Data Processing and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kizil, M.; Oz, F.; Besler, H.T. A Review on the Formation of Carcinogenic/Mutagenic Heterocyclic Aromatic Amines. J. Food Process. Technol. 2011, 2, 1–5. [Google Scholar] [CrossRef]
- Martins, S.I.F.S.; Jongen, W.M.F.; van Boekel, M.A.J.S. A review of Maillard reaction in food and implications to kinetic modelling. Trends Food Sci. Technol. 2000, 11, 364–373. [Google Scholar] [CrossRef]
- Buła, M.; Przybylski, W.; Jaworska, D.; Kajak-Siemaszko, K. Formation of heterocyclic aromatic amines in relation to pork quality and heat treatment parameters. Food Chem. 2019, 276, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Zhang, M.; Chen, J.; He, Z.; Qin, F.; Hu, C.; Xu, H.; Tao, G.; Zhang, S.; Chen, J. UPLC-MS/MS and multivariate analysis of inhibition of heterocyclic amine profiles by black pepper and piperine in roast beef patties. Chemom. Intell. Lab. Syst. 2017, 168, 96–106. [Google Scholar] [CrossRef]
- Nagao, M.; Honda, M.; Seino, Y.; Yahagi, T.; Sugimura, T. Mutagenicities of smoke condensates and the charred surface of fish and meat. Cancer Lett. 1977, 2, 221–226. [Google Scholar] [CrossRef]
- Sugimura, T.; Wakabayashi, K.; Nakagama, H.; Nagao, M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004, 95, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Jamin, E.L.; Riu, A.; Douki, T.; Debrauwer, L.; Cravedi, J.-P.; Zalko, D.; Audebert, M. Combined genotoxic effects of a polycyclic aromatic hydrocarbon (B(a)P) and an heterocyclic amine (PhIP) in relation to colorectal carcinogenesis. PLoS ONE 2013, 8, e58591. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, T. Food and cancer. Toxicology 2002, 181–182, 17–21. [Google Scholar] [CrossRef]
- Puangsombat, K.; Gadgil, P.; Houser, T.A.; Hunt, M.C.; Smith, J.S. Occurrence of heterocyclic amines in cooked meat products. Meat Sci. 2012, 90, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Loomis, D.; Guyton, K.Z.; Grosse, Y.; Ghissassi, F.E.; Benbrahim-Tallaa, L.; Guha, N.; Mattock, H.; Straif, K. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015, 16, 1599–1600. [Google Scholar] [CrossRef]
- Dennis, C.; Karim, F.; Smith, J.S. Evaluation of maillard reaction variables and their effect on heterocyclic amine formation in chemical model systems. J. Food Sci. 2015, 80, T472–T478. [Google Scholar] [CrossRef] [PubMed]
- Skog, K.I.; Johansson, M.A.E.; Jägerstad, M.I. Carcinogenic Heterocyclic Amines in Model Systems and Cooked Foods: A Review on Formation, Occurrence and Intake. Food Chem. Toxicol. 1998, 36, 879–896. [Google Scholar] [CrossRef]
- Salmon, C.P.; Knize, M.G.; Felton, J.S. Effects of marinating on heterocyclic amine carcinogen formation in grilled chicken. Food Chem. Toxicol. 1997, 35, 433–441. [Google Scholar] [CrossRef]
- Eurostat—Data Explorer. Available online: https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=apro_mt_pann&lang=en (accessed on 15 November 2019).
- Oláh, B. Baromfihús Elkészítési Szokások a Fogyasztók Rákkeltő Heterociklusos Amin Terhelése Fényében. Master’s Thesis, University of Veterinary Medicine, Budapest, Hungary, 2018. [Google Scholar]
- Pleva, D.; Lanyi, K.J.; Oláh, B.; Laczay, P. Study on the Consumers’ Habits for Meat Preparation in the Light of Cancer Hazards; Crimson Publishers: New York, NY, USA, 2019. [Google Scholar]
- Khan, M.R.; Bertus, L.M.; Busquets, R.; Puignou, L. Mutagenic heterocyclic amine content in thermally processed offal products. Food Chem. 2009, 112, 838–843. [Google Scholar] [CrossRef]
- Jian, S.-H.; Yeh, P.-J.; Wang, C.-H.; Chen, H.-C.; Chen, S.-F. Analysis of heterocyclic amines in meat products by liquid chromatography—Tandem mass spectrometry. J. Food Drug Anal. 2019, 27, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, M.; Kataoka, H.; Ishihara, J.; Takachi, R.; Hamada, G.S.; Sharma, S.; Le Marchand, L.; Tsugane, S. Heterocyclic amines content of meat and fish cooked by Brazilian methods. J. Food Compost. Anal. 2010, 23, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kondjoyan, A.; Chevolleau, S.; Portanguen, S.; Molina, J.; Ikonic, P.; Clerjon, S.; Debrauwer, L. Relation between crust development and heterocyclic aromatic amine formation when air-roasting a meat cylinder. Food Chem. 2016, 213, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef] [PubMed]
- Straková, E.; Suchý, P.; Vitula, F.; Večerek, V. Differences in the amino acid composition of muscles from pheasant and broiler chickens. Arch. Anim. Breed. 2006, 49, 508–514. [Google Scholar] [CrossRef]
- The Nutritional Value of Chicken. Available online: https://www.nationalchickencouncil.org/chicken-the-preferred-protein-for-your-health-and-budget/the-nutritional-value-of-chicken/ (accessed on 22 November 2019).
- Consumers’ Attitude Study about Red Meat Choice and Consumption in the Light of Cancer Hazards. Available online: https://www.researchgate.net/publication/325285904_Consumers’_attitude_study_about_red_meat_choice_and_consumption_in_the_light_of_cancer_hazards (accessed on 25 January 2020).
- The Difference Between Grilling and BBQ|ThermoWorks. Available online: https://blog.thermoworks.com/thermometer/grilling-bbqwhats-difference/ (accessed on 9 March 2020).
- Union, P.O. of the E. CELEX1, 2002/657/EC: Commission Decision of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results (Text with EEA relevance) (notified under document number C(2002) 3044). Available online: https://op.europa.eu:443/en/publication-detail/-/publication/ed928116-a955-4a84-b10a-cf7a82bad858/language-en (accessed on 9 March 2020).
- Bioanalytical Method Validation. Available online: https://www.ema.europa.eu/en/bioanalytical-method-validation (accessed on 9 March 2020).
Sample Availability: Samples are not available from the authors. |
| Calibration Curve Parameters | LOD (ng·g−1) | LOQ (ng·g−1) | Precision | Recovery | ||||
|---|---|---|---|---|---|---|---|---|
| Compound | Equation (y = a·x + b)* | Intra-Day | Inter-Day | |||||
| a | b | r** | % | % | % | |||
| MeIQx | 8 ± 1 | 0 ± 1E−3 | 0.9974 ± 0.0007 | 0.04 | 0.12 | 5 ± 1 | 7 ± 2 | 110 ± 10 |
| 4,8-DiMeIQx | 11 ± 2 | 0 ± 2E−3 | 0.9965 ± 0.0002 | 0.02 | 0.08 | 5 ± 1 | 5.8 ± 0.9 | 110 ± 10 |
| PhIP | 7 ± 1 | 0 ± 3E−3 | 0.9982 ± 0.0005 | 0.03 | 0.12 | 4 ± 1 | 10 ± 2 | 112 ± 9 |
| HAR | 8 ± 1 | 0 ± 2.5E−3 | 0.9974 ± 0.0007 | 0.04 | 0.11 | 2.9 ± 0.8 | 6 ± 1 | 98 ± 8 |
| NOR | 8 ± 1 | 0 ± 2E−3 | 0.9973 ± 0.0003 | 0.05 | 0.17 | 4 ± 1 | 8 ± 2 | 100 ± 10 |
| Breast Without Skin | HAR | NOR | MeIQx | 4,8-DiMeIQx | PhIP | Total | |
|---|---|---|---|---|---|---|---|
| T (°C) | t (min) | Amount (ng/g) | |||||
| 150 | 2.5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 |
| 5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | |
| 10 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | |
| 180 | 2.5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 |
| 5 | <0.08 | 0.13 ± 0.03 a | 0.09 ± 0.01 a | <0.08 | 0.42 ± 0.06 a | 0.64 ± 0.06 a | |
| 10 | <0.08 | 0.18 ± 0.02 b | 0.33 ± 0.05 b | 0.17 ± 0.02 a | 1.9 ± 0.3 b | 2.5 ± 0.3 b | |
| 210 | 2.5 | 0.08 ± 0.02 a | 0.20 ± 0.03 b | 0.35 ± 0.06 b | 0.14 ± 0.02 b | 1.7 ± 0.2 b | 2.5 ± 0.2 b |
| 5 | 0.20 ± 0.03 b | 0.47 ± 0.04 c | 1.0 ± 0.2 c | 0.49 ± 0.07 c | 5.5 ± 0.6 c | 7.6 ± 0.8 c | |
| 10 | 0.48 ± 0.05 c | 1.0 ± 0.1 d | 2.3 ± 0.2 d | 1.5 ± 0.2 d | 16 ± 2 d | 21 ± 2 d | |
| Breast With Skin | HAR | NOR | MeIQx | 4,8-DiMeIQx | PhIP | Total HCA | |
|---|---|---|---|---|---|---|---|
| T (°C) | t (min) | Amount (ng/g) | |||||
| 150 | 2.5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 |
| 5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | |
| 10 | 0.19 ± 0.3 a | 0.32 ± 0.06 a | <0.08 | 0.09 ± 0.01 a | 1.0 ± 0.1 a | 1.6 ± 0.2 a | |
| 180 | 2.5 | <0.08 | 0.09 ± 0.01 b | <0.08 | <0.08 | <0.08 | 0.09 ± 0.01 b |
| 5 | 0.30 ± 0.04 b | 0.15 ± 0.02 c | <0.08 | <0.08 | 0.20 ± 0.02 b | 0.64 ± 0.04 c | |
| 10 | 0.7 ± 0.1 c | 0.33 ± 0.04 a | 0.57 ± 0.07 a | 0.35 ± 0.04 b | 2.9 ± 0.3 c | 4.9 ± 0.3 d | |
| 210 | 2.5 | 0.09 ± 0.01 d | 0.16 ± 0.02 d | <0.08 | <0.08 | 0.9 ± 0.1 d | 1.1 ± 0.1 e |
| 5 | 0.39 ± 0.05 e | 0.51 ± 0.07 e | 0.7 ± 0.1 b | 0.49 ± 0.07 c | 10 ± 1 e | 12.6 ± 1.5 f | |
| 10 | 1.1 ± 0.2 f | 1.4 ± 0.2 f | 8.0 ± 0.9 c | 2.3 ± 0.3 d | 20.5 ± 2.5 f | 33 ± 4 g | |
| Thigh Without Skin | HAR | NOR | MeIQx | 4,8-DiMeIQx | PhIP | Total HCA | |
|---|---|---|---|---|---|---|---|
| T (°C) | t (min) | Amount (ng/g) | |||||
| 150 | 2.5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 |
| 5 | 0.08 ± 0.01 a | <0.08 | <0.08 | <0.08 | <0.08 | 0.08 ± 0.01 a | |
| 10 | 0.17 ± 0.02 b | 0.11 ± 0.02 a | <0.08 | <0.08 | <0.08 | 0.28 ± 0.02 b | |
| 180 | 2.5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 |
| 5 | 0.14 ± 0.01 c | <0.08 | <0.08 | <0.08 | <0.08 | 0.14 ± 0.01 c | |
| 10 | 0.19 ± 0.03 d | 0.17 ± 0.02 b | 0.38 ± 0.05 a | 0.11 ± 0.02 a | 0.41 ± 0.05 a | 1.3 ± 0.1 d | |
| 210 | 2.5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 |
| 5 | 0.17 ± 0.03 b,d | 0.13 ± 0.02 c | <0.08 | <0.08 | 0.41 ± 0.05 a | 0.71 ± 0.06 e | |
| 10 | 0.57 ± 0.06 e | 0.54 ± 0.07 d | 1.2 ± 0.2 b | 0.66 ± 0.07 b | 3.4 ± 0.4 b | 6.3 ± 0.6 f | |
| Wing With Skin | HAR | NOR | MeIQx | 4,8-DiMeIQx | PhIP | Total HCA | |
|---|---|---|---|---|---|---|---|
| T (°C) | t (min) | Amount (ng/g) | |||||
| 150 | 2.5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 |
| 5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | |
| 10 | 0.08 ± 0.01 a | <0.08 | <0.08 | <0.08 | <0.08 | 0.08 ± 0.01 a | |
| 180 | 2.5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 |
| 5 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | <0.08 | |
| 10 | 0.38 ± 0.05 b | 0.34 ± 0.05 a | 0.8 ± 0.1 a | 0.27 ± 0.04 a | 3.9 ± 0.5 a | 6.2 ± 0.7 b | |
| 210 | 2.5 | 0.08 ± 0.01 a | <0.08 | <0.08 | <0.08 | 0.23 ± 0.03 b | 0.30 ± 0.04 c |
| 5 | 0.81 ± 0.05 c | 0.36 ± 0.04 a | 0.35 ± 0.05 b | <0.08 | 2.0 ± 0.3 c | 3.6 ± 0.4 d | |
| 10 | 0.9 ± 0.1 d | 0.8 ± 0.1 b | 0.91 ± 0.09 c | 0.36 ± 0.04 b | 4.5 ± 0.4 d | 8.1 ± 0.6 e | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pleva, D.; Lányi, K.; Monori, K.D.; Laczay, P. Heterocyclic Amine Formation in Grilled Chicken Depending on Body Parts and Treatment Conditions. Molecules 2020, 25, 1547. https://doi.org/10.3390/molecules25071547
Pleva D, Lányi K, Monori KD, Laczay P. Heterocyclic Amine Formation in Grilled Chicken Depending on Body Parts and Treatment Conditions. Molecules. 2020; 25(7):1547. https://doi.org/10.3390/molecules25071547
Chicago/Turabian StylePleva, Dániel, Katalin Lányi, Kitti Dóra Monori, and Péter Laczay. 2020. "Heterocyclic Amine Formation in Grilled Chicken Depending on Body Parts and Treatment Conditions" Molecules 25, no. 7: 1547. https://doi.org/10.3390/molecules25071547
APA StylePleva, D., Lányi, K., Monori, K. D., & Laczay, P. (2020). Heterocyclic Amine Formation in Grilled Chicken Depending on Body Parts and Treatment Conditions. Molecules, 25(7), 1547. https://doi.org/10.3390/molecules25071547






