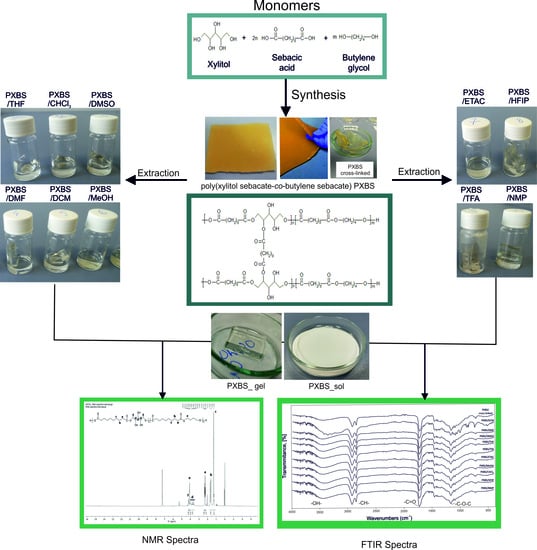
Structure and Properties of Biodegradable Poly (Xylitol Sebacate-Co-Butylene Sebacate) Copolyester
Abstract
:1. Introduction
2. Results and Discussion
2.1. Swelling
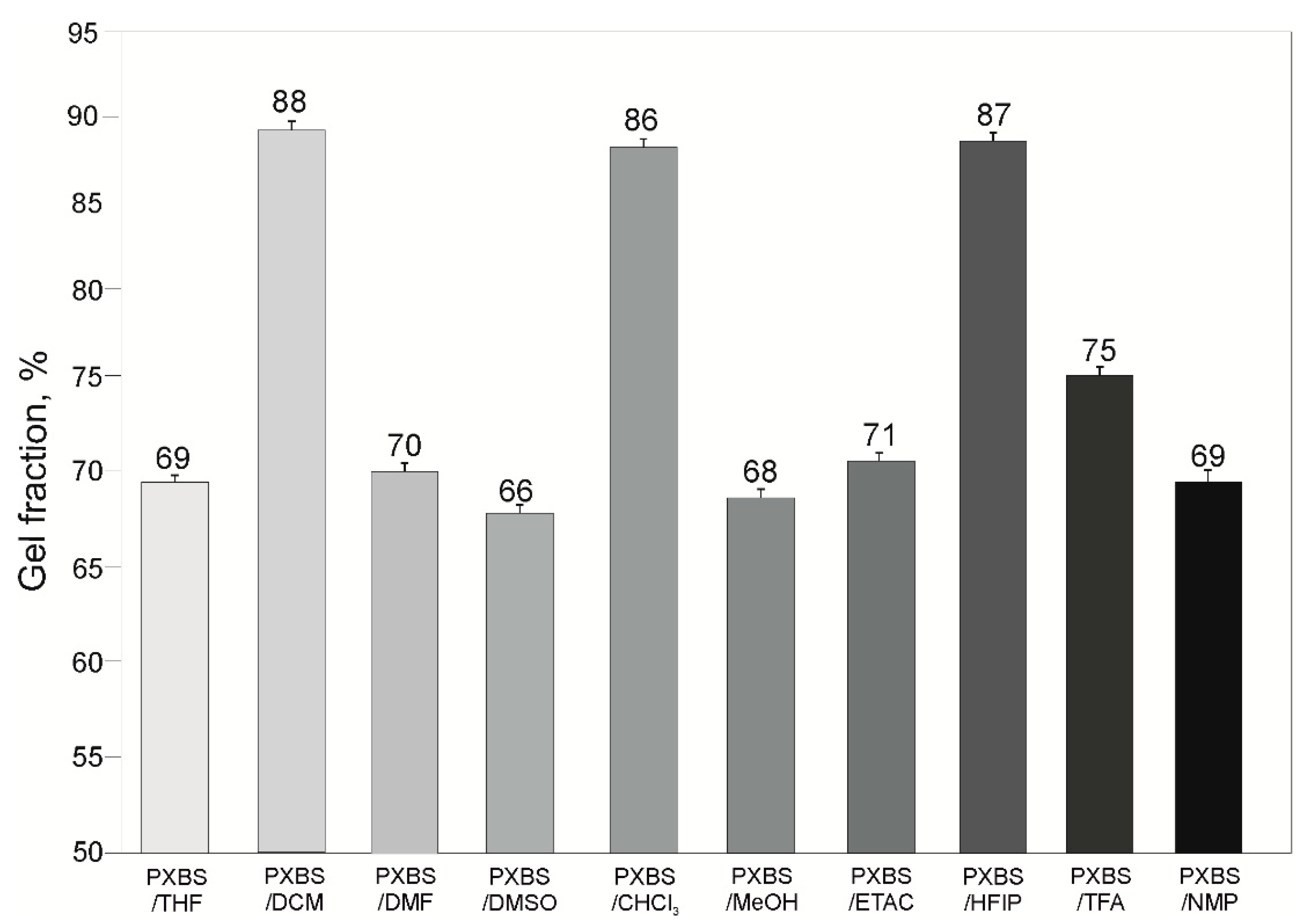
2.2. Gel Fraction
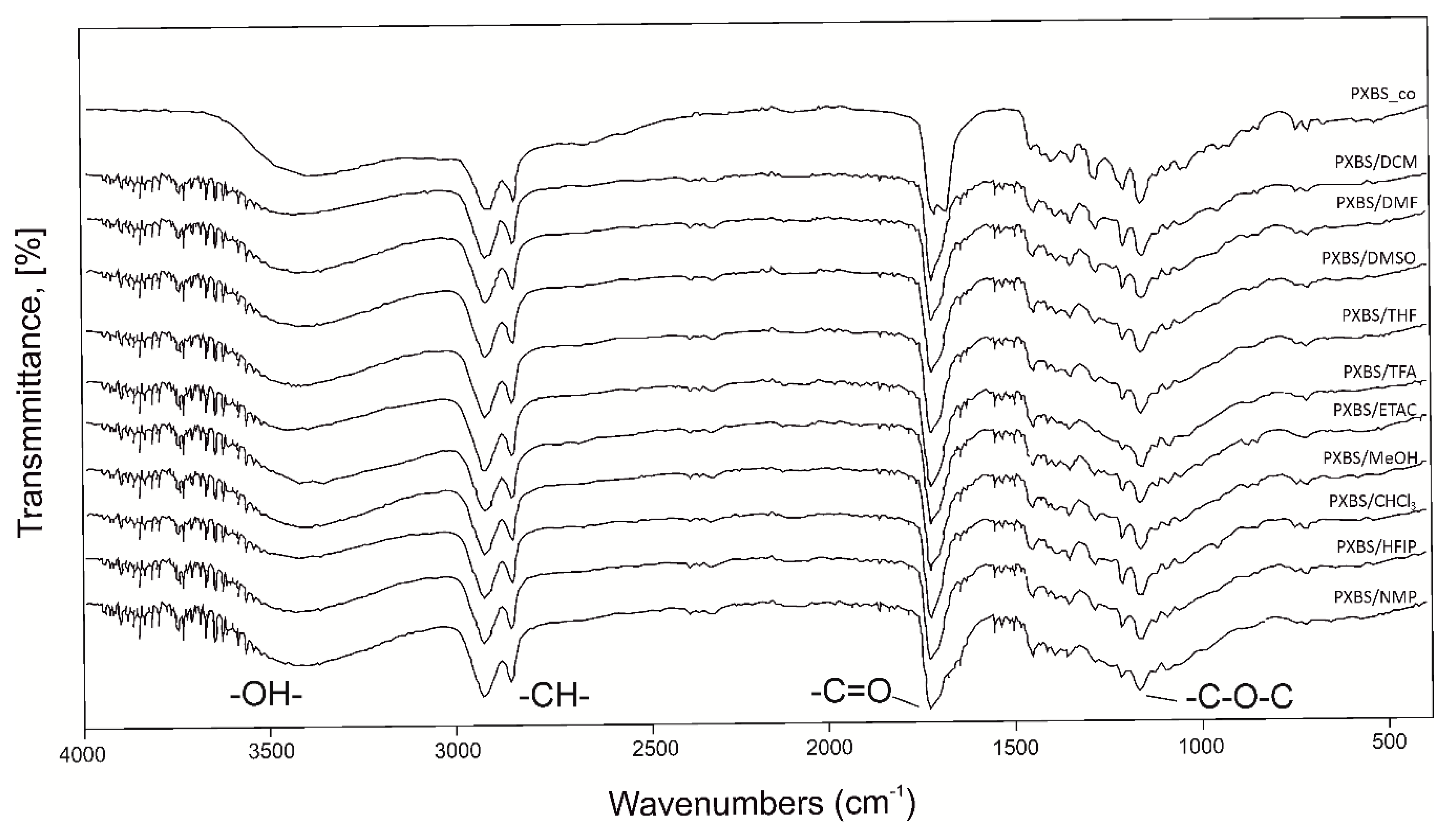
2.3. Fourier Transform Infrared Spectroscopy (FTIR)
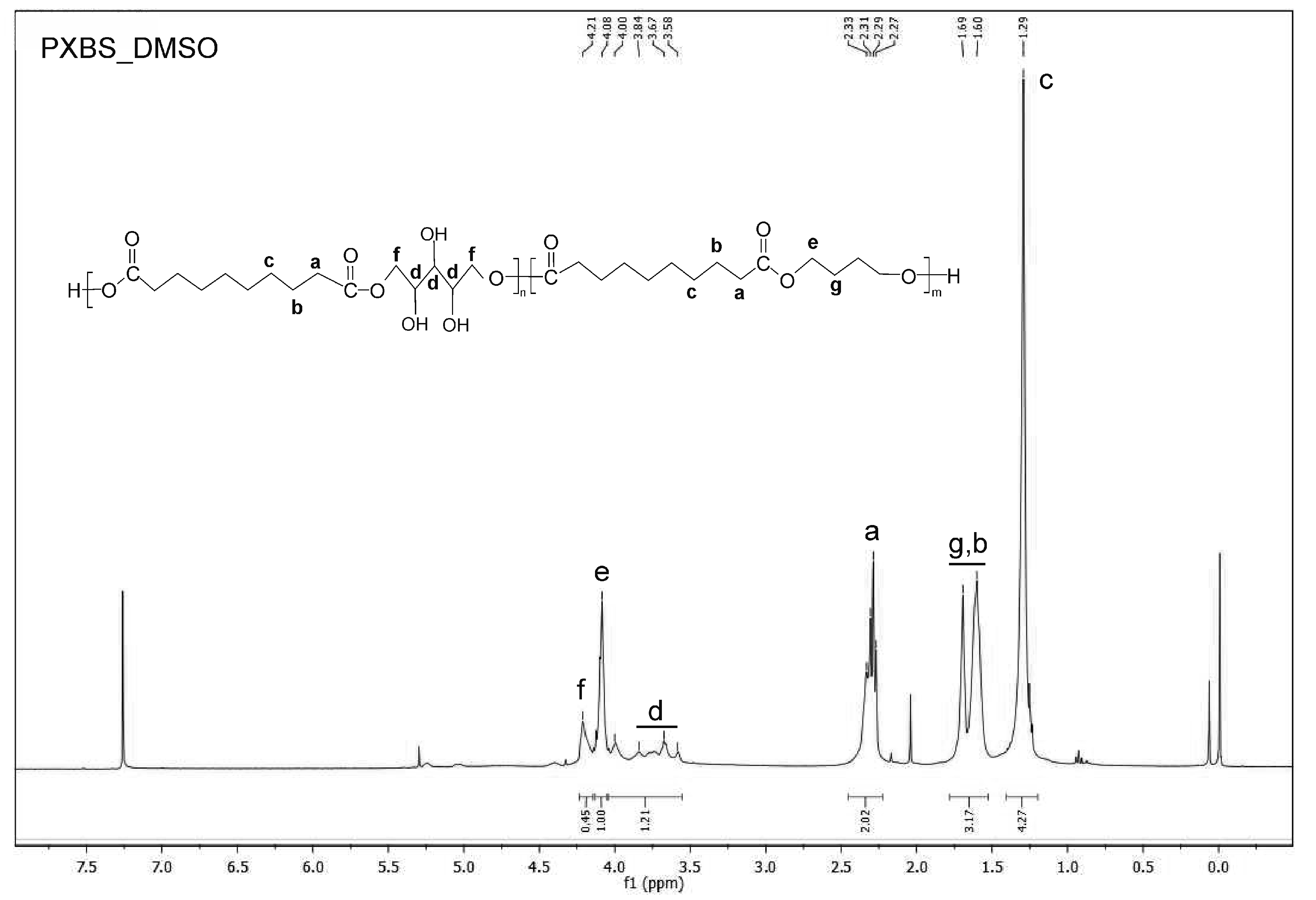
2.4. NMR Spectroscopy
2.5. Differential Scanning Calorimetry (DSC).
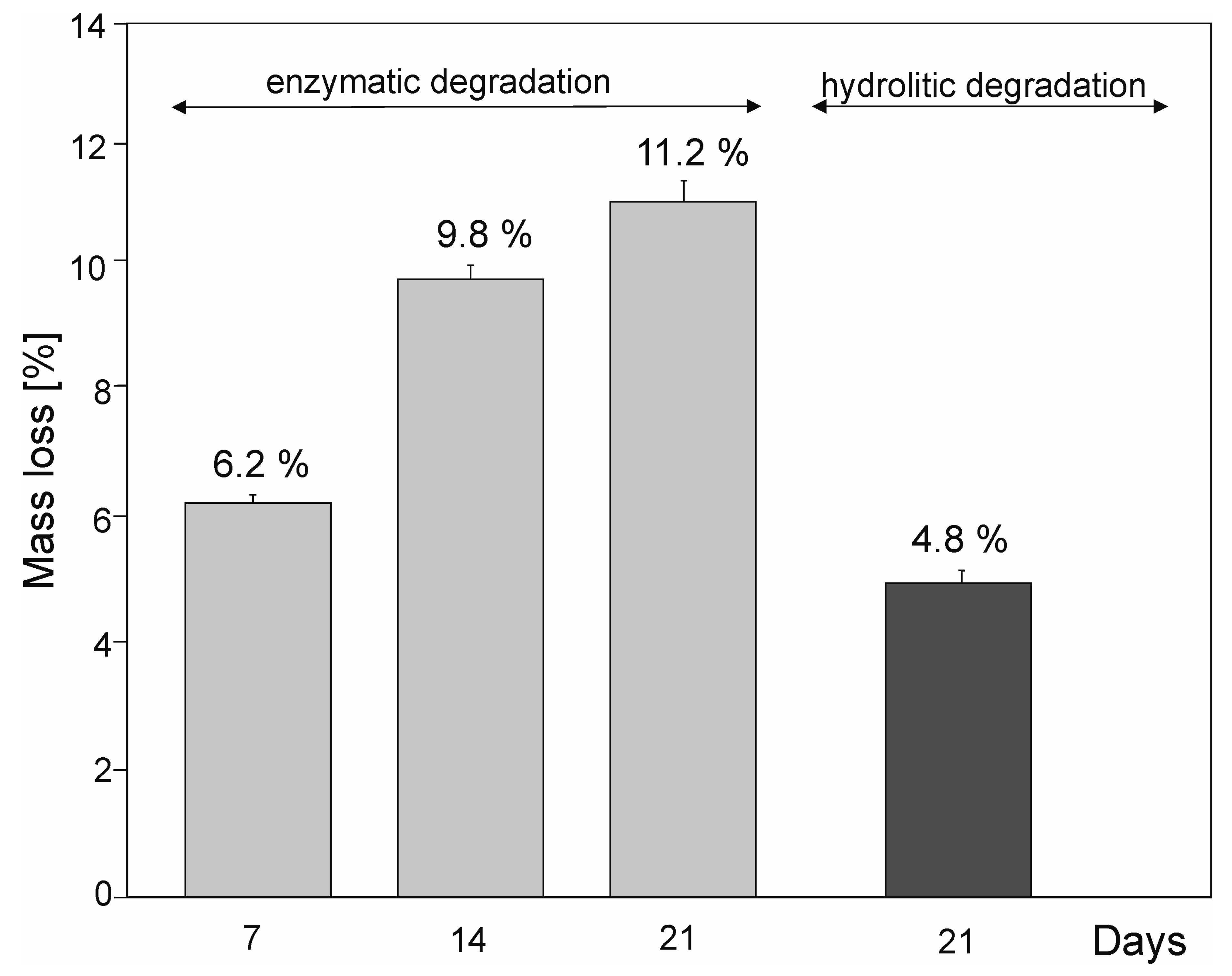
2.6. Hydrolytic and Enzymatic Degradation
3. Materials and Methods
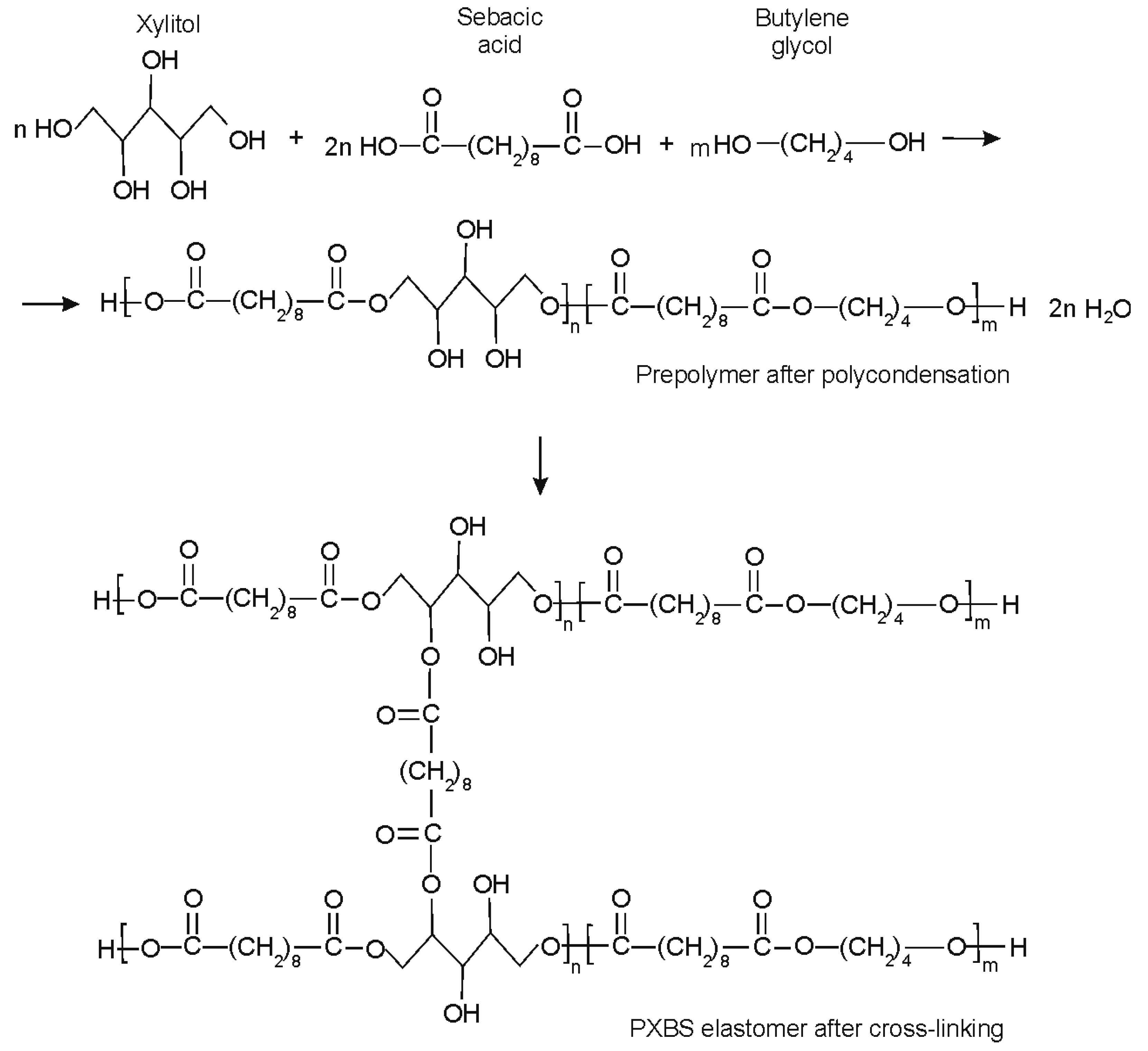
3.1. Synthesis of PXBS
3.2. Extraction
3.3. Experimental Methods
3.3.1. Swelling
3.3.2. Gel Fraction
3.3.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.3.4. NMR Spectroscopy
3.3.5. Differential Scanning Calorimetry (DSC).
3.3.6. Mechanical Properties
3.3.7. Water Contact Angle
3.3.8. Hydrolytic Degradation
3.3.9. Enzymatic Degradation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Akiyama, H.; Yoshida, M. Photochemically reversible liquefaction and solidification of single compounds based on a sugar alcohol scaffold with multi azo-arms. Adv. Mater. 2012, 24, 2353–2356. [Google Scholar] [CrossRef] [PubMed]
- Sonseca, Á.; Camarero-Espinosa, S.; Peponi, L.; Weder, C.; Foster, E.J.; Kenny, J.M.; Giménez, E. Mechanical and shape-memory properties of poly (mannitol sebacate)/cellulose nanocrystal nanocomposites. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3123–3133. [Google Scholar] [CrossRef]
- Tham, W.H.; Wahit, M.U.; Kadir, M.R.A.; Wong, T.W. Mechanical and thermal properties of biodegradable hydroxyapatite/poly (sorbitol sebacate malate) composites. Songklanakarin J. Sci. Technol. 2013, 35, 57–61. [Google Scholar]
- Lee, S.H.; Shin, S.R.; Lee, D.S. Sorbitol as a chain extender of polyurethane prepolymers to prepare self-healable and robust polyhydroxyurethane elastomers. Molecules 2018, 23, 2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, A.; Kulkarni, R.D.; Patil, C.K.; Gite, V.V. Utilization of renewable bio-based resources, viz. sorbitol, diol, and diacid, in the preparation of two pack PU anticorrosive coatings. RSC Adv. 2016, 6, 9843–9850. [Google Scholar] [CrossRef]
- Li, Y.; Thouas, G.A.; Chen, Q. Novel elastomeric fibrous networks produced from poly (xylitol sebacate) 2:5 by core/shell electrospinning: Fabrication and mechanical properties. J. Mech. Behav. Biomed. Mater. 2014, 40, 210–221. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Chen, Q.Z. Fabrication of mechanically tissue-like fibrous poly (xylitol sebacate) using core/shell electrospinning technique. Adv. Eng. Mater. 2015, 17, 324–329. [Google Scholar] [CrossRef]
- Deepa, K.; Jaisankar, V. A Study on Xylitol Based Copolyester for In vitro Degradation Applications. Int. J. ChemTech Res. 2018, 11, 69–76. [Google Scholar] [CrossRef]
- Firoozi, N.; Kang, Y. A Highly Elastic and Autofluorescent Poly (xylitol-dodecanedioic Acid) for Tissue Engineering. ACS Biomater. Sci. Eng. 2019, 5, 1257–1267. [Google Scholar] [CrossRef]
- Selvam, S.; Pithapuram, M.V.; Victor, S.P.; Muthu, J. Injectable in situ forming xylitol-PEG-based hydrogels for cell encapsulation and delivery. Colloids Surf. B Biointerfaces 2015, 126, 35–43. [Google Scholar] [CrossRef]
- Sugane, K.; Takahashi, H.; Shimasaki, T.; Teramoto, N.; Shibata, M. Stereocomplexation, thermal and mechanical properties of conetworks composed of star-shaped l-lactide, d-lactide and ε-caprolactone oligomers utilizing sugar alcohols as core molecules. Polymers 2017, 9, 582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moorhoff, C.; Li, Y.; Cook, W.D.; Braybrook, C.; Chen, Q.Z. Characterization of the prepolymer and gel of biocompatible poly (xylitol sebacate) in comparison with poly (glycerol sebacate) using a combination of mass spectrometry and nuclear magnetic resonance. Polym. Int. 2015, 64, 668–688. [Google Scholar] [CrossRef]
- Dasgupta, Q.; Chatterjee, K.; Madras, G. Combinatorial approach to develop tailored biodegradable poly (xylitol dicarboxylate) polyesters. Biomacromolecules 2014, 15, 4302–4313. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.G.; Luo, W.; Yousaf, M.N. Aliphatic polyester elastomers derived from erythritol and α,ω-diacids. Polym. Chem. 2010, 1, 296–302. [Google Scholar] [CrossRef]
- Ning, Z.Y.; Zhang, Q.S.; Wu, Q.P.; Li, Y.Z.; Ma, D.X.; Chen, J.Z. Efficient synthesis of hydroxyl functioned polyesters from natural polyols and sebacic acid. Chinese Chem. Lett. 2011, 22, 635–638. [Google Scholar] [CrossRef]
- Bruggeman, J.P.; de Bruin, B.J.; Bettinger, C.J.; Langer, R. Biodegradable poly (polyol sebacate) polymers. Biomaterials 2008, 29, 4726–4735. [Google Scholar] [CrossRef] [Green Version]
- Bruggeman, J.P.; Bettinger, C.J.; Nijst, C.L.E.; Kohane, D.S.; Langer, R. Biodegradable xylitol-based polymers. Adv. Mater. 2008, 20, 1922–1927. [Google Scholar] [CrossRef]
- Bruggeman, J.P.; Bettinger, C.J.; Langer, R. Biodegradable xylitol-based elastomers: In vivo behavior and biocompatibility. J. Biomed. Mater. Res. Part A 2010, 95, 92–104. [Google Scholar] [CrossRef] [Green Version]
- Kavimani, V.; Jaisankar, V. Synthesis and Characterisation of Sorbitol Based Copolyesters for Biomedical Applications. J. Phys. Sci. Appl. 2014, 4, 507–515. [Google Scholar]
- Hu, J.; Gao, W.; Kulshrestha, A.; Gross, R.A. “Sweet polyesters”: Lipase-catalyzed condensation-polymerizations of alditols. ACS Symp. Ser. 2008, 999, 275–284. [Google Scholar]
- Zheng, L.; Wang, Z.; Wu, S.; Li, C.; Zhang, D.; Xiao, Y. Novel poly (butylene fumarate) and poly(butylene succinate) multiblock copolymers bearing reactive carbon-carbon double bonds: Synthesis, characterization, cocrystallization, and properties. Ind. Eng. Chem. Res. 2013, 52, 6147–6155. [Google Scholar] [CrossRef]
- Zheng, L.; Li, C.; Wang, Z.; Wang, J.; Xiao, Y.; Zhang, D.; Guan, G. Novel biodegradable and double crystalline multiblock copolymers comprising of poly (butylene succinate) and poly(ε-caprolactone): Synthesis, characterization, and properties. Ind. Eng. Chem. Res. 2012, 51, 7264–7272. [Google Scholar] [CrossRef]
- Zheng, L.; Li, C.; Huang, W.; Huang, X.; Zhang, D.; Guan, G.; Xiao, Y.; Wang, D. Synthesis of high-impact biodegradable multiblock copolymers comprising of poly (butylene succinate) and poly (1,2-propylene succinate) with hexamethylene diisocyanate as chain extender. Polym. Adv. Technol. 2011, 22, 279–285. [Google Scholar] [CrossRef]
- Piątek-Hnat, M.; Bomba, K. The influence of of cross-linking process on the physicochemical properties of new copolyesters containing xylitol. Mater. Today Commun. 2020, 20, 100734. [Google Scholar] [CrossRef]
- Piątek-Hnat, M.; Bomba, K.; Pęksiński, J. Synthesis and selected properties of ester elastomer containing sorbitol. Appl. Sci. 2020, 10, 1628. [Google Scholar] [CrossRef] [Green Version]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds, 4th ed.; Springer-Verlag: Berlin/ Heidelberg, Germany, 2009. [Google Scholar]
- Amass, W.; Amass, A.; Tighe, B. A review of biodegradable polymers: Uses, current developments in the synthesis and characterization of biodegradable polyesters, blends of biodegradable polymers and recent advances in biodegradation studies. Polym. Int. 1998, 47, 89–144. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds poly (xylitol-sebacate co butylene sebacate) are available from the authors. |







| Sample/Material | PXBS_GEL | Sample/Material | PXBS_SOL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Tg1 [°C] | ∆cp [J/g°C] | Tm1 [°C] | ∆Hm1 [J/g] | Tm2 [°C] | ∆Hm2 [J/g] | Tm3 [°C] | ∆Hm3 [J/g] | ||
| PXBS_Cross-Linked | −29.9 | 0.412 | 16.8 | 26.3 | PXBS_co | 19.1 | 26.4 | 44.8 | 50.9 |
| PXBS_THF | −28.3 | 0.550 | 11.1 | 9.1 | PXBS_THF | 16.5 | 44.2 | n.o | n.o |
| PXBS_DMSO | −31.7 | 0.439 | 14.7 | 28.2 | PXBS_DMSO | 20.4 | 33.3 | 47.9 | 18.6 |
| PXBS_HFIP | −25.8 | 0.342 | 18.4; 41.8 | 26.4; 1.6 | PXBS_HFIP | 20.1 | 29.8 | 41.2 | 14.8 |
| PXBS_TFA | −32.3 | 0.143 | 9.1; 20.8 | 40.3 | PXBS_TFA | 11.1; 25.2 | 19.8 | n.o | n.o. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piątek-Hnat, M.; Bomba, K.; Pęksiński, J. Structure and Properties of Biodegradable Poly (Xylitol Sebacate-Co-Butylene Sebacate) Copolyester. Molecules 2020, 25, 1541. https://doi.org/10.3390/molecules25071541
Piątek-Hnat M, Bomba K, Pęksiński J. Structure and Properties of Biodegradable Poly (Xylitol Sebacate-Co-Butylene Sebacate) Copolyester. Molecules. 2020; 25(7):1541. https://doi.org/10.3390/molecules25071541
Chicago/Turabian StylePiątek-Hnat, Marta, Kuba Bomba, and Jakub Pęksiński. 2020. "Structure and Properties of Biodegradable Poly (Xylitol Sebacate-Co-Butylene Sebacate) Copolyester" Molecules 25, no. 7: 1541. https://doi.org/10.3390/molecules25071541
APA StylePiątek-Hnat, M., Bomba, K., & Pęksiński, J. (2020). Structure and Properties of Biodegradable Poly (Xylitol Sebacate-Co-Butylene Sebacate) Copolyester. Molecules, 25(7), 1541. https://doi.org/10.3390/molecules25071541