Combined Pre- and Posttreatment of Paraoxon Exposure
Abstract
1. Introduction
2. Results
2.1. Mortalities
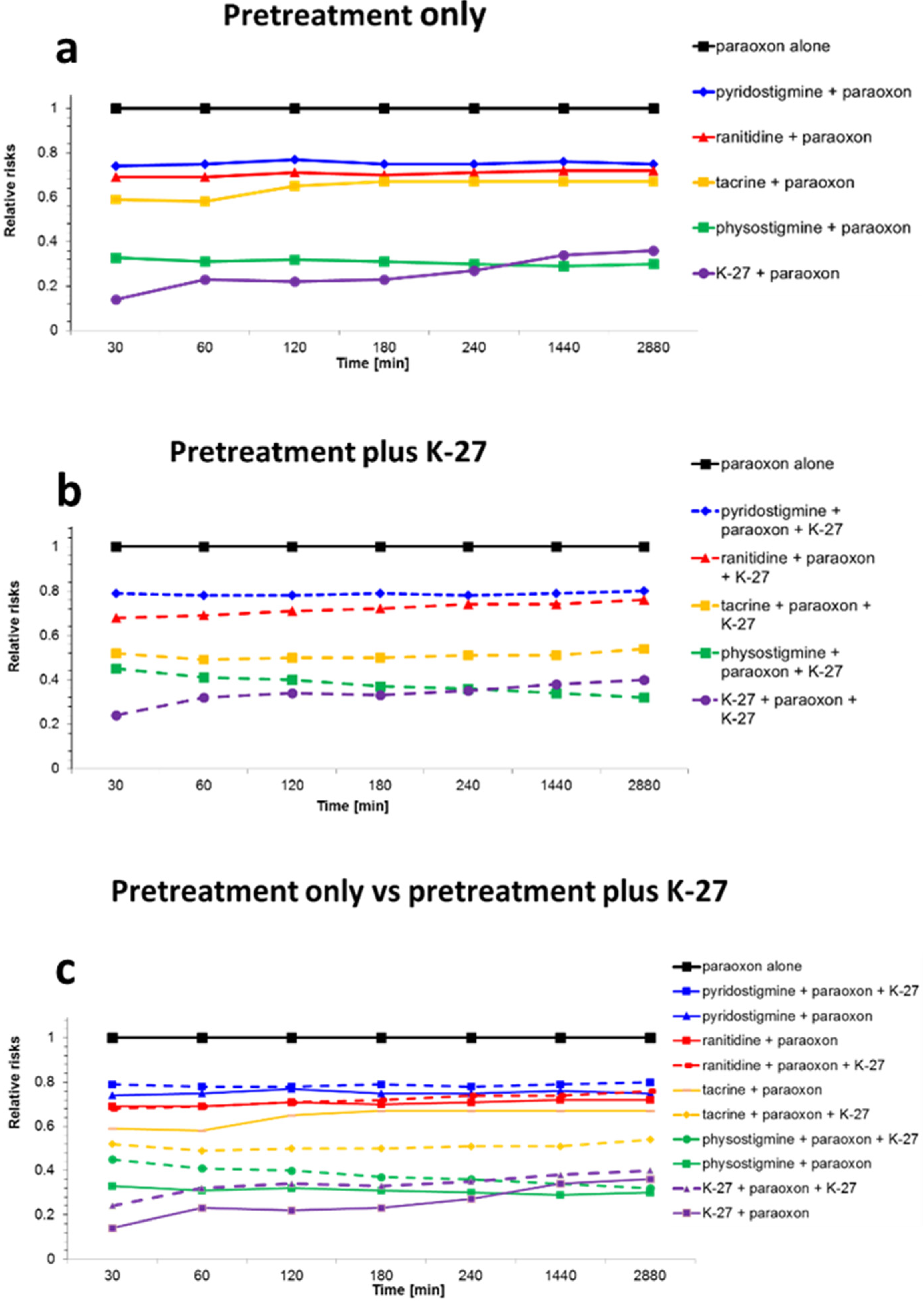
2.2. Survival Analysis
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Animals
4.2.1. Choice of Dosage for Pretreatment
- 2 (pre- and posttreatment) reference groups: only paraoxon exposure.
- Pyridostigmine: 1 µmol/rat = 0.26 mg/rat (= 1 mg/kg average body weight).
- Physostigmine: 0.25 µmol/rat = 0.07 mg/rat (= 0.28 mg/kg average body weight).
- Ranitidine hydrochloride: 12 µmol/rat = 4.21 mg/rat (= 17.0 mg/kg average body weight).
- Tacrine: 4 µmol/rat = 1 mg/rat (= 4.0 mg/kg average body weight).
- K-27: 60 µmol/rat = 26.77 mg/rat (= 108.0 mg/kg average body weight).
4.2.2. Pretreatment, Paraoxon, and Oxime Exposure
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, R.C. Classification and uses of organophosphates and carbamates. In Toxicology of Organophosphate and Carbamate Compounds; Gupta, R.C., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2006; pp. 5–24. [Google Scholar]
- Eddleston, M.; Buckley, N.A.; Eyer, P.; Dawson, A.H. Management of acute organophosphorus pesticide poisoning. Lancet 2008, 371, 597–607. [Google Scholar] [CrossRef]
- Gunnell, D.; Eddleston, M.; Phillips, M.R.; Konradsen, F. The global distribution of fatal pesticide self-poisoning: Systematic review. BMC Public Health 2007, 7, 357. [Google Scholar] [CrossRef] [PubMed]
- Delfino, R.T.; Ribeiro, T.S.; Figueroa-Villar, J.D. Organophosphorus Compounds as Chemical Warfare Agents: A Review. J. Braz. Chem. Soc. 2009, 20, 407–428. [Google Scholar] [CrossRef]
- Masson, P.; Nachon, F. Cholinesterase reactivators and bioscavengers for pre- and post-exposure treatments of organophosphorus poisoning. J. Neurochem. 2017, 142, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Balali-Mood, M.; Balali-Mood, K. Neurotoxic disorders of organophosphorus compounds and their managements. Arch. Iran. Med. 2008, 11, 65–89. [Google Scholar] [PubMed]
- Macilwain, C. Study proves Iraq used nerve gas. Nature 1993, 363, 3. [Google Scholar] [CrossRef]
- Wiener, S.W.; Hoffman, R.S. Nerve agents: A comprehensive review. J. Intensive Care Med. 2004, 19, 22–37. [Google Scholar] [CrossRef]
- Yanagisawa, N.; Morita, H.; Nakajima, T. Sarin experiences in Japan: Acute toxicity and long-term effects. J. Neurol. Sci. 2006, 249, 76–85. [Google Scholar] [CrossRef]
- Dolnik, A.; Bhattacharjee, A. Hamas: Suicide bombings, rockets, or WMD? Terror. Polit. Violenc. 2002, 14, 109–128. [Google Scholar] [CrossRef]
- Kostadinov, R.; Kanev, K.; Dimov, D. Chemical Terrorism, History and Threat Assessment. Med. Manag. Chem. Biol. Casualties 2010, 8, 77–84. [Google Scholar]
- Dolgin, E. Syrian gas attack reinforces need for better anti-sarin drugs. Nat. Med. 2013, 19, 1194–1195. [Google Scholar] [CrossRef] [PubMed]
- Pita, R.; Domingo, J. The Use of Chemical Weapons in the Syrian Conflict. Toxics 2014, 2, 391–402. [Google Scholar] [CrossRef]
- Brooks, J.; Erickson, T.B.; Kayden, S.; Ruiz, R.; Wilkinson, S.; Burkle Jr, F.M. Responding to chemical weapons violations in Syria: Legal, health, and humanitarian recommendations. Confl. Health 2018, 12, 12. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Wille, T.; Koller, M.; Thiermann, H. Toxicology of organophosphorus compounds in view of an increasing terrorist threat. Arch. Toxicol. 2016, 90, 2131–2145. [Google Scholar] [CrossRef]
- Panchal, M.; Trivedi, D. Clinical profile in patients of organophosphorus poisoning. Int. J. Sci. Res. 2016, 5, 97–99. [Google Scholar]
- Petroianu, G.; Toomes, L.M.; Petroianu, A.; Bergler, W.; Rufer, R. Control of blood pressure, heart rate and haematocrit during high-dose intravenous paraoxon exposure in mini pigs. J. Appl. Toxicol. 1998, 18, 293–298. [Google Scholar] [CrossRef]
- Petroianu, G.A. Organophosphate poisoning: The lesser-known face of a toxidrome. Eur. J. Emerg. Med. 2005, 12, 102–103. [Google Scholar] [CrossRef]
- Antonijevic, B.; Stojiljkovic, M.P. Unequal efficacy of pyridinium oximes in acute organophosphate poisoning. Clin. Med. Res. 2007, 5, 71–82. [Google Scholar] [CrossRef]
- Hrabetz, H.; Thiermann, H.; Felgenhauer, N.; Zilker, T.; Haller, B.; Nahrig, J.; Saugel, B.; Eyer, F. Organophosphate poisoning in the developed world-a single centre experience from here to the millennium. Chem. Biol. Interact. 2013, 206, 561–568. [Google Scholar] [CrossRef]
- Becker, G.; Kawan, A.; Gutzeit, D.; Worek, F.; Szinicz, L. Direct reaction of oximes with crotylsarin, cyclosarin, or VX in vitro. Arch. Toxicol. 2007, 81, 415–420. [Google Scholar] [CrossRef]
- Masson, P. Evolution of and perspectives on therapeutic approaches to nerve agent poisoning. Toxicol. Lett. 2011, 206, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Worek, F.; Thiermann, H.; Wille, T. Oximes in organophosphate poisoning: 60 years of hope and despair. Chem.-Biol. Interact. 2016, 259, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Eddleston, M.; Eyer, P.; Worek, F.; Juszczak, E.; Alder, N.; Mohamed, F.; Senarathna, L.; Hittarage, A.; Azher, S.; Jeganathan, K.; et al. Pralidoxime in acute organophosphorus insecticide poisoning--a randomised controlled trial. PLoS Med. 2009, 6, e1000104. [Google Scholar] [CrossRef] [PubMed]
- Eyer, F.; Meischner, V.; Kiderlen, D.; Thiermann, H.; Worek, F.; Haberkorn, M.; Felgenhauer, N.; Zilker, T.; Eyer, P. Human parathion poisoning. A toxicokinetic analysis. Toxicol. Rev. 2003, 22, 143–163. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Petroianu, G.A. Reversible cholinesterase inhibitors as pretreatment for exposure to organophosphates. A review. J. Appl. Toxicol. 2019, 39, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Keeler, J.R.; Hurst, C.G.; Dunn, M.A. Pyridostigmine used as a nerve agent pretreatment under wartime conditions. J. Am. Med Assoc. (JAMA) 1991, 266, 693–695. [Google Scholar] [CrossRef]
- McCauley, L.A. Organophosphates and the gulf war syndrome. In Toxicology of Organophosphate and Carbamate Compounds; Gupta, R.C., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2006; pp. 69–78. [Google Scholar]
- Pope, C.N. Central nervous system effects and neurotoxicity. In Toxicology of Organophosphate and Carbamate Compounds; Gupta, R.C., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Boston, MA, USA; Heidelberg, Germany, 2006; pp. 271–291. [Google Scholar]
- US Food and Drug Administration. FDA Approves Pyridostigmine Bromide as Pretreatment against Nerve Gas. Available online: http://www.fda.gov/Drugs/EmergencyPreparedness/BioterrorismandDrugPreparedness/ucm130342.htm (accessed on 20 January 2020).
- Petroianu, G.A.; Nurulain, S.M.; Shafiullah, M.; Hasan, M.Y.; Kuca, K.; Lorke, D.E. Usefulness of administration of non-organophosphate cholinesterase inhibitors before acute exposure to organophosphates: Assessment using paraoxon. J. Appl. Toxicol. 2013, 33, 894–900. [Google Scholar] [CrossRef]
- Lorke, D.E.; Hasan, M.Y.; Nurulain, S.M.; Shafiullah, M.; Kuca, K.; Petroianu, G.A. Acetylcholinesterase inhibitors as pretreatment before acute exposure to organophosphates: Assessment using methyl-paraoxon. CNS Neurol. Disord. Drug Targets 2012, 11, 1052–1060. [Google Scholar] [CrossRef]
- Lorke, D.E.; Hasan, M.Y.; Nurulain, S.M.; Shafiullah, M.; Kuca, K.; Petroianu, G.A. Pretreatment for acute exposure to diisopropylfluorophosphate: In vivo efficacy of various acetylcholinesterase inhibitors. J. Appl. Toxicol. 2011, 31, 515–523. [Google Scholar] [CrossRef]
- Lorke, D.E.; Nurulain, S.M.; Hasan, M.Y.; Kuca, K.; Petroianu, G.A. Prophylactic administration of non-organophosphate cholinesterase inhibitors before acute exposure to organophosphates: Assessment using terbufos sulfone. J. Appl. Toxicol. 2014, 34, 1096–1103. [Google Scholar] [CrossRef]
- Petroianu, G.A.; Nurulain, S.M.; Hasan, M.Y.; Kuca, K.; Lorke, D.E. Reversible cholinesterase inhibitors as pre-treatment for exposure to organophosphates: Assessment using azinphos-methyl. J. Appl. Toxicol. 2015, 35, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Nurulain, S.M.; Hasan, M.Y.; Kuca, K.; Petroianu, G.A. Optimal Pre-treatment for Acute Exposure to the Organophosphate Dicrotophos. Curr. Pharm. Des. 2017, 23, 3432–3439. [Google Scholar] [CrossRef] [PubMed]
- Kuca, K.; Bielavsky, J.; Cabal, J.; Bielavska, M. Synthesis of a potential reactivator of acetylcholinesterase—1-(4-hydroxyiminomethylpyridinium)-3-(carbamoylpyridinium) propane dibromide. Tetrahedron Lett. 2003, 44, 3123–3125. [Google Scholar] [CrossRef]
- Kuca, K.; Cabal, J. In vitro reactivation of tabun-inhibited acetylcholinesterase using new oximes--K027, K005, K033 and K048. Cent. Eur. J. Public Health 2004, 12, S59–S61. [Google Scholar]
- Kuča, K.; Cabal, J.; Kassa, J. A comparison of the potency of newly developed oximes (K005, K027, K033, K048) and currently used oximes (pralidoxime, obidoxime, HI-6) to reactivate sarin-inhibited rat brain acetylcholinesterase by in vitro methods. J. Toxicol. Environ. Health. Part A 2005, 68, 677–686. [Google Scholar] [CrossRef]
- Kuca, K.; Kassa, J. In vitro reactivation of acetylcholinesterase using the oxime K027. Vet. Hum. Toxicol. 2004, 46, 15–18. [Google Scholar]
- Kuca, K.; Musilek, K.; Jun, D.; Pohanka, M.; Ghosh, K.K.; Hrabinova, M. Oxime K027: Novel low-toxic candidate for the universal reactivator of nerve agent- and pesticide-inhibited acetylcholinesterase. J. Enzym. Inhib. Med. Chem. 2010, 25, 509–512. [Google Scholar] [CrossRef]
- Lorke, D.E.; Nurulain, S.M.; Hasan, M.Y.; Kuca, K.; Musilek, K.; Petroianu, G.A. Eight new bispyridinium oximes in comparison with the conventional oximes pralidoxime and obidoxime: In vivo efficacy to protect from diisopropylfluorophosphate toxicity. J. Appl. Toxicol. 2008, 28, 920–928. [Google Scholar] [CrossRef]
- Petroianu, G.A.; Lorke, D.E.; Kalasz, H. Comparison of the Ability of Pyridinium Aldoximes to Reactivate Human Red Blood Cell Acetylcholinesterases Inhibited by ethyl- and methyl-paraoxon. Curr. Org. Chem. 2012, 16, 1359–1369. [Google Scholar] [CrossRef]
- Kassa, J.; Kuca, K.; Cabal, J.; Paar, M. A comparison of the efficacy of new asymmetric bispyridinium oximes (K027, K048) with currently available oximes against tabun by in vivo methods. J. Toxicol. Environ. Health. Part A 2006, 69, 1875–1882. [Google Scholar] [CrossRef]
- Nurulain, S.M.; Lorke, D.E.; Hasan, M.Y.; Shafiullah, M.; Kuca, K.; Musilek, K.; Petroianu, G.A. Efficacy of eight experimental bispyridinium oximes against paraoxon-induced mortality: Comparison with the conventional oximes pralidoxime and obidoxime. Neurotox. Res. 2009, 16, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Petroianu, G.A.; Lorke, D.E. Pyridinium oxime reactivators of cholinesterase inhibited by diisopropyl-fluorophosphate (DFP): Predictive value of in-vitro testing for in-vivo efficacy. Mini Rev. Med. Chem. 2008, 8, 1328–1342. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Petroianu, G.A. The Experimental Oxime K027-A Promising Protector from Organophosphate Pesticide Poisoning. A Review Comparing K027, K048, Pralidoxime, and Obidoxime. Front. Neurosci. 2019, 13, 427. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Petroianu, G.A. Treatment of Organophosphate Poisoning with Experimental Oximes: A Review. Curr. Org. Chem. 2019, 23, 628–639. [Google Scholar] [CrossRef]
- Lorke, D.E.; Hasan, M.Y.; Arafat, K.; Kuca, K.; Musilek, K.; Schmitt, A.; Petroianu, G.A. In vitro oxime protection of human red blood cell acetylcholinesterase inhibited by diisopropyl-fluorophosphate. J. Appl. Toxicol. 2008, 28, 422–429. [Google Scholar] [CrossRef]
- Dawson, R.M. Review of oximes available for treatment of nerve agent poisoning. J. Appl. Toxicol. 1994, 14, 317–331. [Google Scholar] [CrossRef]
- Dunn, M.A.; Hackley, B.E.; Sidell, F.R. Pretreatment for nerve agent exposure. In Textbook of Military Medicine: Medical Aspects of Chemical & Biological Warfare; Sidell, F.R., Takafuji, E.T., Franz, D.R., Eds.; Borden Institute, Walter Reed Army Medical Center: Washington, DC, USA, 1997; pp. 181–196. [Google Scholar]
- Inns, R.H.; Leadbeater, L. The efficacy of bispyridinium derivatives in the treatment of organophosphonate poisoning in the guinea-pig. J. Pharm. Pharmacol. 1983, 35, 427–433. [Google Scholar] [CrossRef]
- Myhrer, T.; Aas, P. Pretreatment and prophylaxis against nerve agent poisoning: Are undesirable behavioral side effects unavoidable? Neurosci. Biobehav. Rev. 2016, 71, 657–670. [Google Scholar] [CrossRef]
- Wetherell, J.; Price, M.; Mumford, H.; Armstrong, S.; Scott, L. Development of next generation medical countermeasures to nerve agent poisoning. Toxicology 2007, 233, 120–127. [Google Scholar] [CrossRef]
- Cox, D.R. Regression models and life tables. J. R. Stat. Soc. 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Lorke, D.E.; Hasan, M.Y.; Nurulain, S.M.; Sheen, R.; Kuca, K.; Petroianu, G.A. Entry of two new asymmetric bispyridinium oximes (K-27 and K-48) into the rat brain: Comparison with obidoxime. J. Appl. Toxicol. 2007, 27, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Lorke, D.E.; Kalasz, H.; Petroianu, G.A.; Tekes, K. Entry of oximes into the brain: A review. Curr. Med. Chem. 2008, 15, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Stojiljkovic, M.P.; Jokanovic, M. Pyridinium oximes: Rationale for their selection as causal antidotes against organophosphate poisonings and current solutions for auto-injectors. Arh. Hig. Rada Toksikol. 2006, 57, 435–443. [Google Scholar] [PubMed]
- Kassa, J.; Fusek, J. Effect of Panpal pretreatment and antidotal treatment (HI-6 plus benactyzine) on respiratory and circulatory function in soman-poisoned rats. Hum. Exp. Toxicol. 1997, 16, 563–569. [Google Scholar] [CrossRef]
- Kassa, J.; Fusek, J. The positive influence of a cholinergic-anticholinergic pretreatment and antidotal treatment on rats poisoned with supralethal doses of soman. Toxicology 1998, 128, 1–7. [Google Scholar] [CrossRef]
- Jun, D.; Kuca, K.; Stodulka, P.; Koleckar, V.; Dolezal, B.; Simon, P.; Veverka, M. HPLC Analysis of HI-6 Dichloride and Dimethanesulfonate—Antidotes against Nerve Agents and Organophosphorus Pesticides. Anal. Lett. 2007, 40, 2783–2787. [Google Scholar] [CrossRef]
- Jun, D.; Stodulka, P.; Kuca, K.; Koleckar, V.; Dolezal, B.; Simon, P.; Veverka, M. TLC analysis of intermediates arising during the preparation of oxime HI-6 dimethanesulfonate. J. Chromatogr. Sci. 2008, 46, 316–319. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds K-27 are available from the authors. |

| Groups (G) | 30 min | 1 h | 2 h | 3 h | 4 h | 24 h | 48 h |
|---|---|---|---|---|---|---|---|
| Paraoxon only (pretreatment group) | 63/88/96 | 63/88/96 | 63/88/96 | 67/88/96 | 67/88/96 | 67/88/96 | 67/88/96 |
| Paraoxon only (pre- and posttreatment group) | 75/88/92 | 79/88/92 | 79/88/92 | 79/92/92 | 83/92/92 | 83/92/96 | 83/92/96 |
| Pyridostigmine pretreatment | 13/88/83 | 21/88/83 | 25/88/88 | 25/88/88 | 25/88/88 | 29/88/88 | 29/88/88 |
| Pyridostigmine pretreatment + K-27 posttreatment | 13/100/96 | 13/100/100 | 13/100/100 | 21/100/100 | 21/100/100 | 29/100/100 | 29/100/100 |
| Physostigmine pretreatment | 4/29/46 | 4/29/46 | 13/33/46 | 13/33/46 | 13/33/46 | 13/33/46 | 13/33/50 |
| Physostigmine pretreatment + K-27 posttreatment | 8/42/54 | 8/42/54 | 8/42/54 | 8/42/54 | 8/42/54 | 8/42/54 | 8/42/54 |
| Ranitidine pretreatment | 0/79/92 | 0/83/92 | 4/92/ 92 | 4/92/92 | 8/92/92 | 13/92/92 | 13/92/92 |
| Ranitidine pretreatment + K-27 posttreatment | 25/58/83 | 29/67/83 | 33/67/83 | 38/75/83 | 42/83/83 | 46/83/83 | 46/96/83 |
| Tacrine pretreatment | 0/54/92 | 0/58/92 | 13/71/92 | 13/79/92 | 13/79/92 | 13/79/92 | 13/79/92 |
| Tacrine pretreatment + K-27 posttreatment | 0/63/83 | 0/63/83 | 0/67/83 | 0/71/88 | 0/71/92 | 0/75/92 | 0/79/92 |
| K-27 pretreatment | 13/8/13 | 25/17/21 | 25/21/25 | 25/21/29 | 33/29/33 | 46/42/38 | 46/42/42 |
| K-27 pretreatment + K-27 posttreatment | 0/25/33 | 17/25/46 | 21/25/46 | 21/29/46 | 21/42/46 | 25/54/50 | 25/58/50 |
| Groups | Relative Risk (RR) | 95% CI | p-Value |
|---|---|---|---|
| Paraoxon-ethyl only | 1 | 1 --- 1 | --- |
| Pyridostigmine + paraoxon-ethyl | 0.76 ± 0.13 | 0.54–0.97 | ≤0.05 a |
| Pyridostigmine + paraoxon-ethyl+K-27 | 0.91 ± 0.14 | 0.69–1.13 | ≤0.05 |
| Physostigmine + paraoxon-ethyl | 0.30 ± 0.15 | 0.06–0.53 | ≤0.05 a,b |
| Physostigmine + paraoxon-ethyl+K-27 | 0.32 ± 0.13 | 0.11–0.54 | ≤0.05 a |
| Ranitidine + paraoxon-ethyl | 0.72 ± 0.16 | 0.46–0.98 | ≤0.05 a |
| Ranitidine + paraoxon-ethyl+K-27 | 0.77 ± 0.10 | 0.61–0.93 | ≤0.05 a |
| Tacrine + paraoxon-ethyl | 0.67 ± 0.21 | 0.33–1.00 | ≤0.05 a |
| Tacrine + paraoxon-ethyl+K-27 | 0.59 ± 0.12 | 0.41–0.78 | ≤0.05 a |
| K-27+ paraoxon-ethyl | 0.34 ± 0.09 | 0.20–0.48 | ≤0.05 a,b |
| K-27+ paraoxon-ethyl+K-27 | 0.37 ± 0.08 | 0.24–0.49 | ≤0.05 a |
| Molecular Weight | IC50 * [µM] | LD50/LD01 * (µmol/rat) | Injected Dose (≈ ¼ of LD01) (µmol/rat) | Injected Dose (mg/rat) | Injected Dose (mg/kg Average Body Weight ) | |
|---|---|---|---|---|---|---|
| Pyridostigmine | 261.12 | 0.330 | 7.2/3.7 | 1 | 0.26 | 1 |
| Physostigmine (Eserine) | 275.35 | 0.012 | 3.0/0.9 | 0.25 | 0.07 | 0.28 |
| Ranitidine | 350.86 | 2.5 | 59/46 | 12 | 4.21 | 17.0 |
| Tacrine | 250 | 0.200 | 21.5/16 | 4 | 1 | 4.0 |
| K-27 | 446.16 | 414 | 350/250 | 60 | 26.77 | 108.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorke, D.E.; Nurulain, S.M.; Hasan, M.Y.; Kuča, K.; Petroianu, G.A. Combined Pre- and Posttreatment of Paraoxon Exposure. Molecules 2020, 25, 1521. https://doi.org/10.3390/molecules25071521
Lorke DE, Nurulain SM, Hasan MY, Kuča K, Petroianu GA. Combined Pre- and Posttreatment of Paraoxon Exposure. Molecules. 2020; 25(7):1521. https://doi.org/10.3390/molecules25071521
Chicago/Turabian StyleLorke, Dietrich E, Syed M Nurulain, Mohamed Y Hasan, Kamil Kuča, and Georg A Petroianu. 2020. "Combined Pre- and Posttreatment of Paraoxon Exposure" Molecules 25, no. 7: 1521. https://doi.org/10.3390/molecules25071521
APA StyleLorke, D. E., Nurulain, S. M., Hasan, M. Y., Kuča, K., & Petroianu, G. A. (2020). Combined Pre- and Posttreatment of Paraoxon Exposure. Molecules, 25(7), 1521. https://doi.org/10.3390/molecules25071521








