Chair- and V-Shaped of H-bonded Supramolecular Complexes of Azophenyl Nicotinate Derivatives; Mesomorphic and DFT Molecular Geometry Aspects
Abstract
1. Introduction
2. Experimental
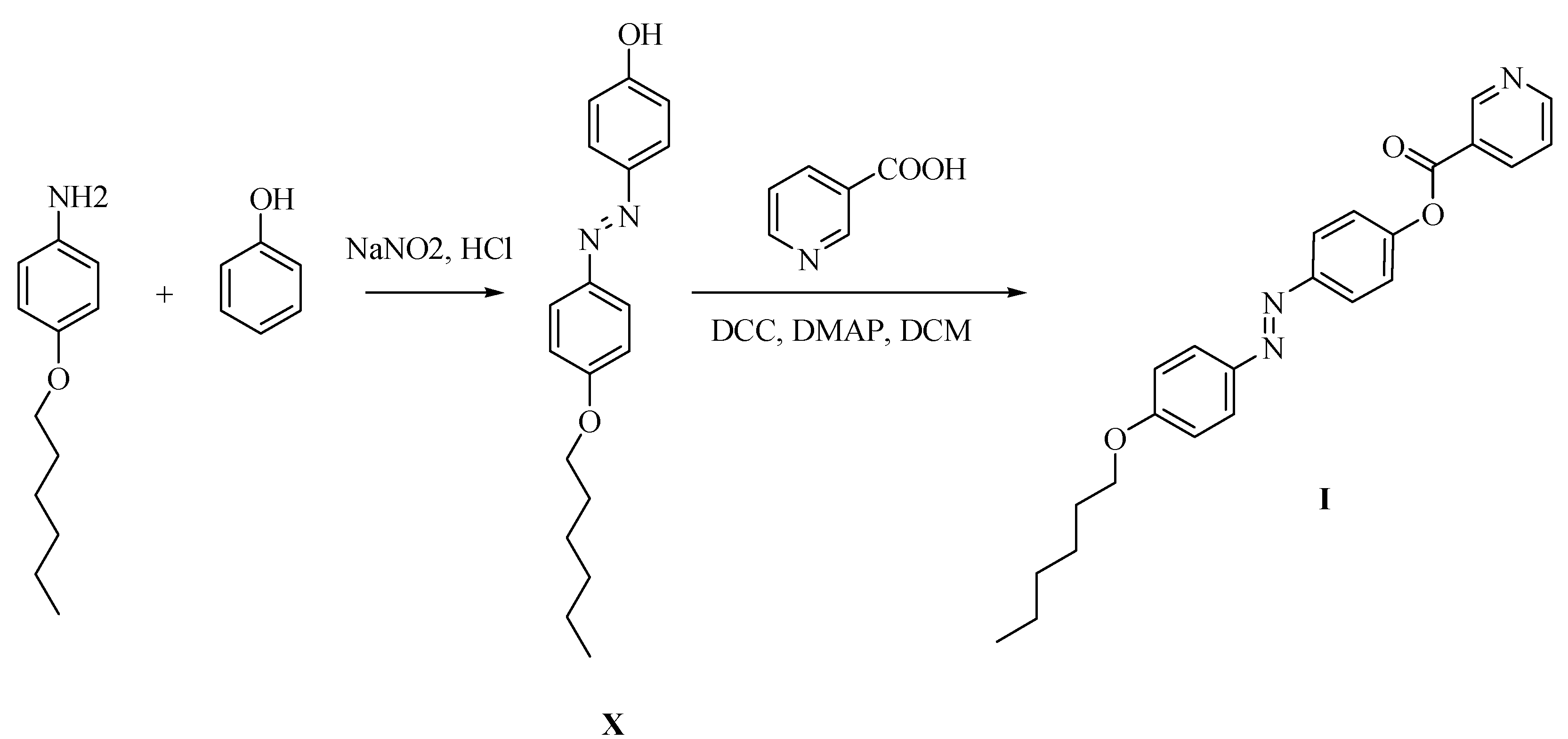
2.1. Preparation of 4-(4-(Hexyloxy)phenylazo)methyl)phenyl Nicotinate
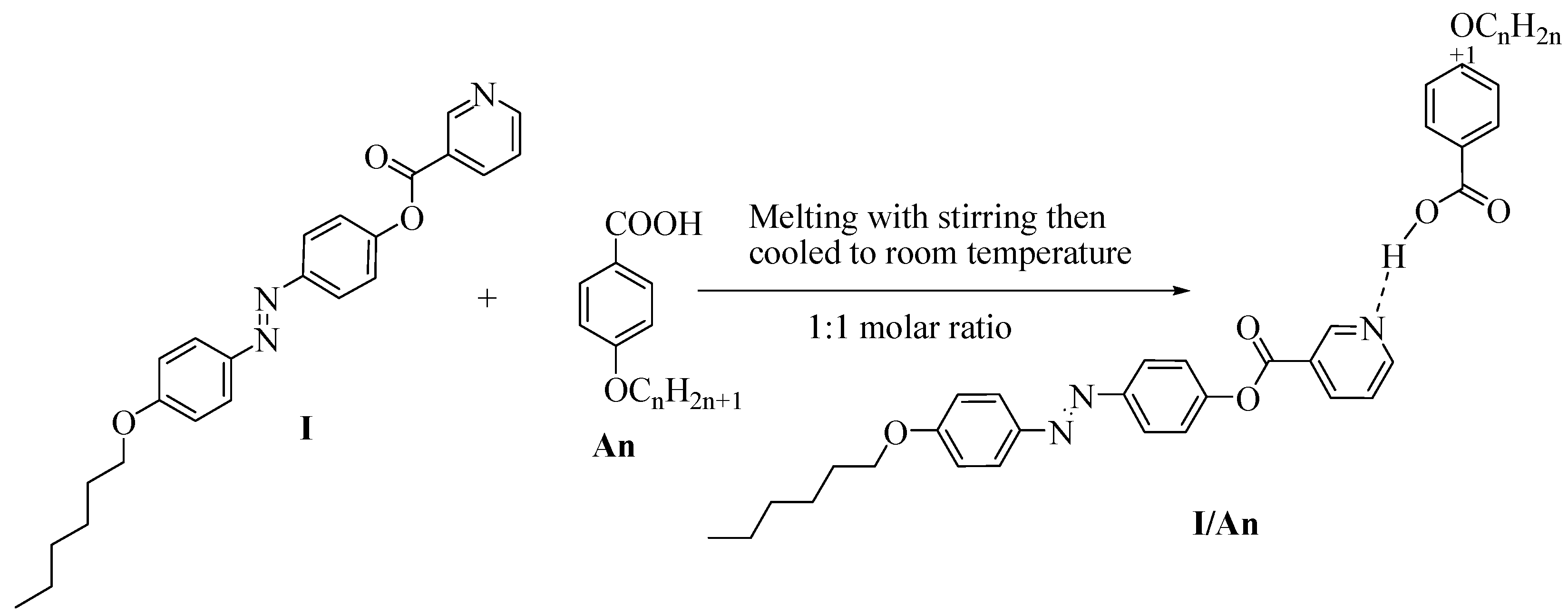
2.2. Preparation of 1:1 Supramolecular H-bonded Complexes
3. Results and Discussion
3.1. FT-IR Conformation
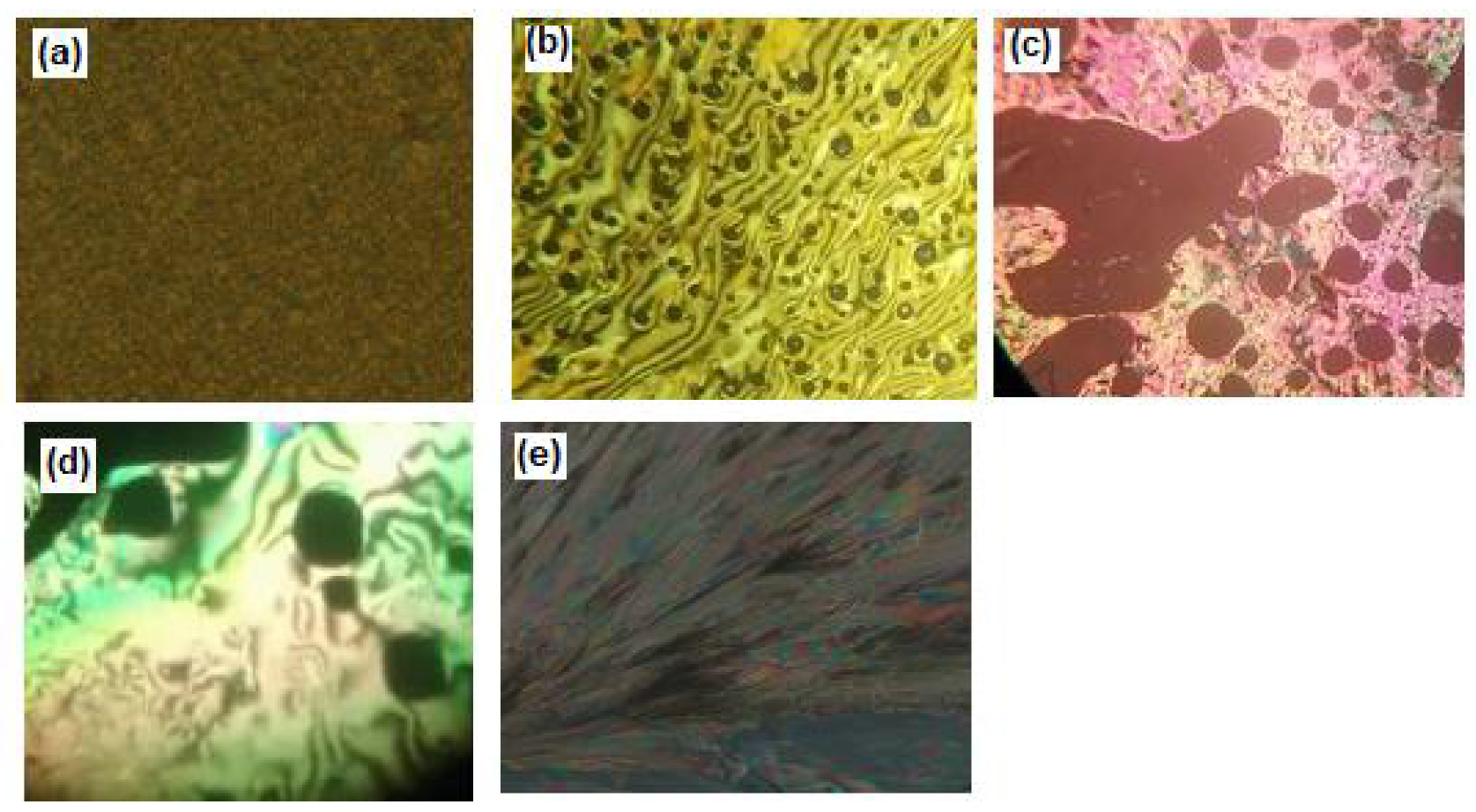
3.2. Mesophase and Optical Study
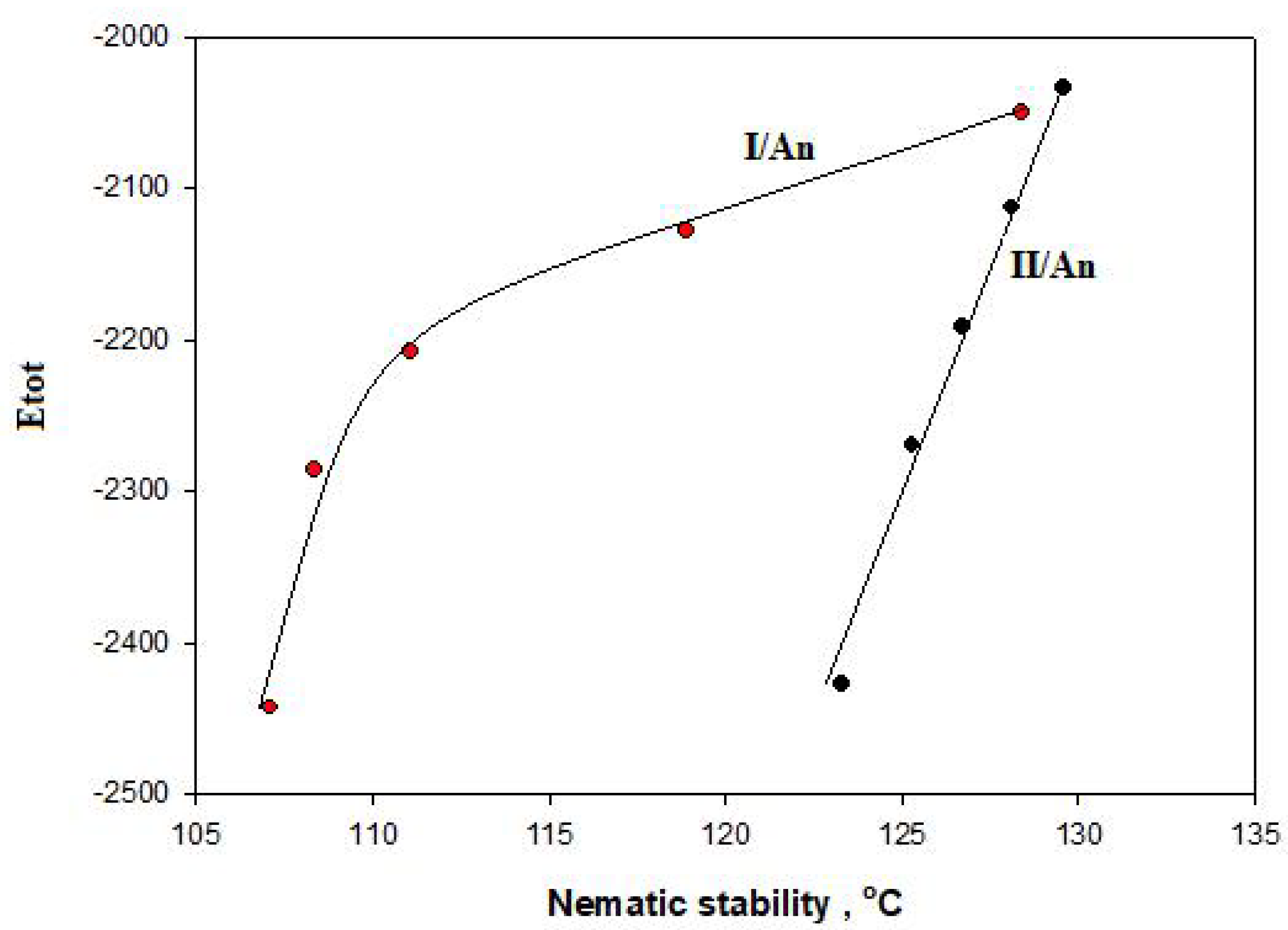
3.3. DFT Theoretical Calculations
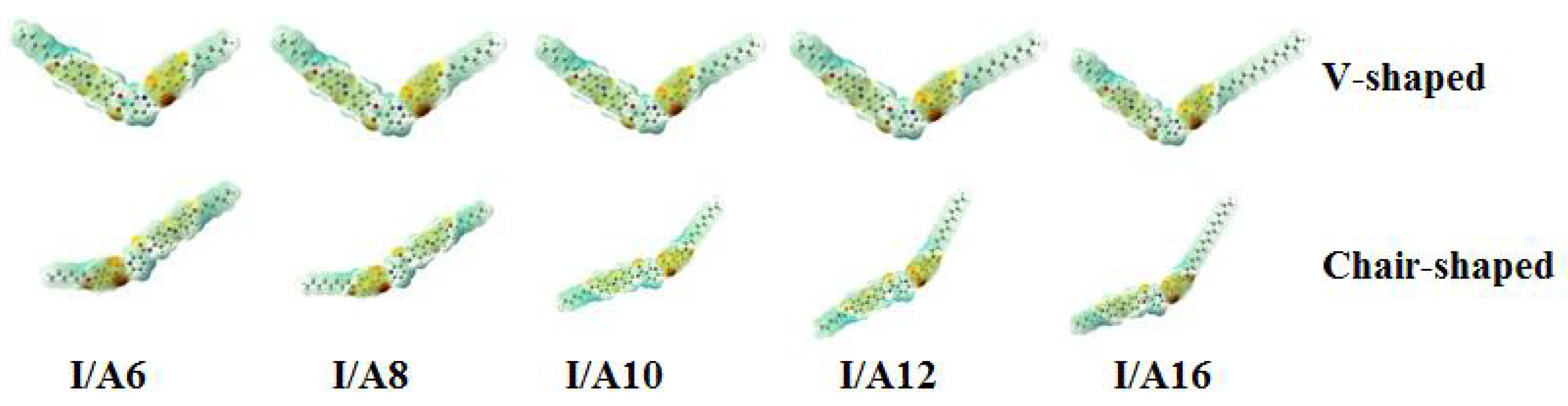
3.3.1. Molecular Geometry of SMHBCs
3.3.2. Thermal Parameters
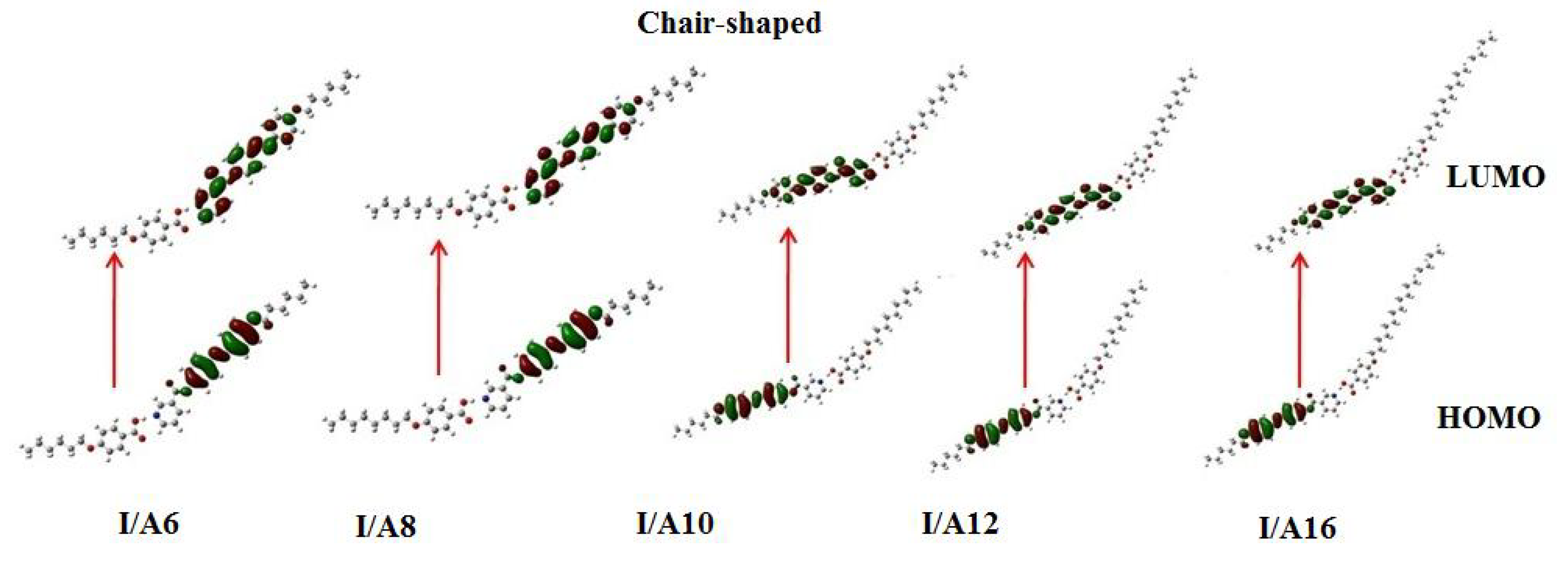
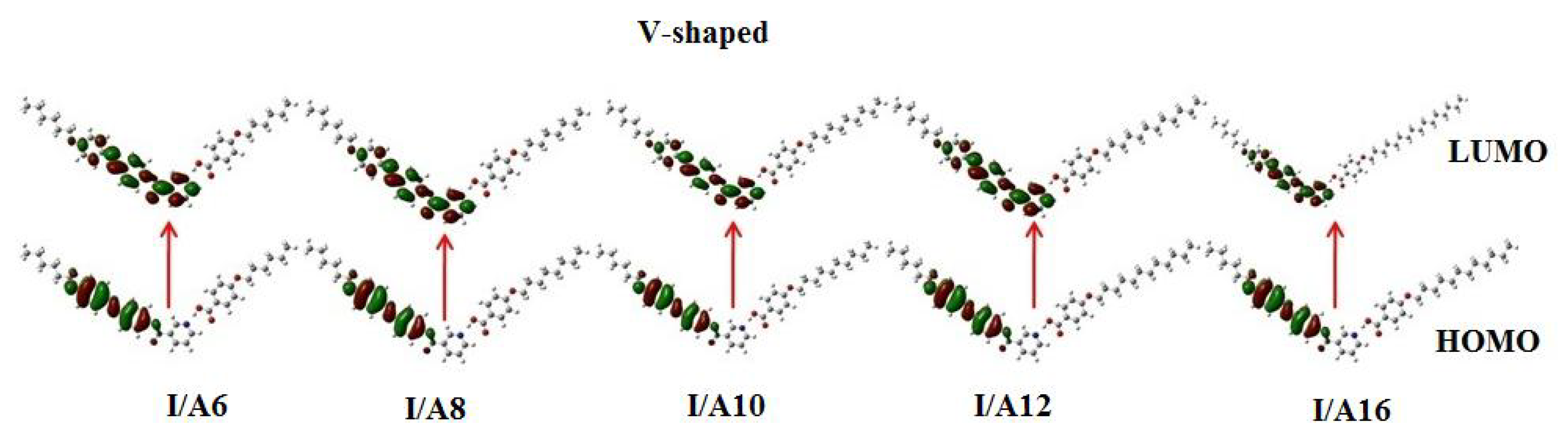
3.3.3. Frontier Molecular Orbitals and Polarizability
3.3.4. Molecular Electrostatic Potential (MEP)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bisoyi, H.K.; Li, Q. Light-driven liquid crystalline materials: From photo-induced phase transitions and property modulations to applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef]
- Beharry, A.A.; Woolley, G.A. Azobenzene photoswitches for biomolecules. Chem. Soc. Rev. 2011, 40, 4422–4437. [Google Scholar] [CrossRef] [PubMed]
- Setia, S.; Sidiq, S.; De, J.; Pani, I.; Pal, S.K. Applications of liquid crystals in biosensing and organic light-emitting devices: Future aspects. Liq. Cryst. 2016, 43, 2009–2050. [Google Scholar] [CrossRef]
- Nayek, P.; Ghosh, S.; Roy, S.; Majumder, T.P.; Dabrowski, R. Electro-optic and dielectric investigations of a perfluorinated compound showing orthoconic antiferroelectric liquid crystal. J. Mol. Liq. 2012, 175, 91–96. [Google Scholar] [CrossRef]
- Imrie, C.T.; Henderson, P.A.; Yeap, G.-Y. Liquid crystal oligomers: Going beyond dimers. Liq. Cryst. 2009, 36, 755–777. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Lee, H.-C.; Mahmood, W.A.K.; Imrie, C.T.; Takeuchi, D.; Osakada, K. Synthesis, thermal and optical behaviour of non-symmetric liquid crystal dimers α-(4-benzylidene-substituted-aniline-4′-oxy)-ω-[pentyl-4-(4′-phenyl) benzoateoxy] hexane. Phase Transit. 2011, 84, 29–37. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Osman, F.; Imrie, C.T. Non-symmetric dimers: Effects of varying the mesogenic linking unit and terminal substituent. Liq. Cryst. 2015, 42, 543–554. [Google Scholar] [CrossRef]
- Yeap, G.-Y.; Hng, T.-C.; Yeap, S.-Y.; Gorecka, E.; Ito, M.M.; Ueno, K.; Okamoto, M.; Mahmood, W.A.K.; Imrie, C.T. Why do non-symmetric dimers intercalate? The synthesis and characterisation of the α-(4-benzylidene-substituted-aniline-4′-oxy)-ω-(2-methylbutyl-4′-(4 ″-phenyl) benzoateoxy) alkanes. Liq. Cryst. 2009, 36, 1431–1441. [Google Scholar] [CrossRef]
- Yagai, S.; Kitamura, A. Recent advances in photoresponsive supramolecular self-assemblies. Chem. Soc. Rev. 2008, 37, 1520–1529. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; El-Sayed, T.; Alnoman, R. Mesophase behavior and DFT conformational analysis of new symmetrical diester chalcone liquid crystals. J. Mol. Liq. 2019, 285, 96–105. [Google Scholar] [CrossRef]
- Aoki, K.I.; Nakagawa, M.; Ichimura, K. Self-assembly of amphoteric azopyridine carboxylic acids: Organized structures and macroscopic organized morphology influenced by heat, pH change, and light. J. Am. Chem. Soc. 2000, 122, 10997–11004. [Google Scholar] [CrossRef]
- Zaki, A.A.; Ahmed, H.; Hagar, M. Impact of fluorine orientation on the optical properties of difluorophenylazophenyl benzoates liquid crystal. Mater. Chem. Phys. 2018, 216, 316–324. [Google Scholar] [CrossRef]
- Zhou, W.; Yu, H. Different morphologies of self-assembled nanofibers fabricated with amphiphilic low-molecular-weight azopyridinium salts. Rsc Adv. 2013, 3, 22155–22159. [Google Scholar] [CrossRef]
- Garcia-Amorós, J.; Reig, M.; Castro, M.C.R.; Cuadrado, A.; Raposo, M.M.M.; Velasco, D. Molecular photo-oscillators based on highly accelerated heterocyclic azo dyes in nematic liquid crystals. Chem. Commun. 2014, 50, 6704–6706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kobayashi, T.; Zhu, H.; Yu, H. Electrically conductive hybrid nanofibers constructed with two amphiphilic salt components. Chem. Commun. 2011, 47, 12768–12770. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hao, R.; Jackson, J.K.; Chiao, M.; Yu, H. Janus ultrathin film from multi-level self-assembly at air–water interfaces. Chem. Commun. 2014, 50, 14843–14846. [Google Scholar] [CrossRef]
- Mamiya, J.-i.; Yoshitake, A.; Kondo, M.; Yu, Y.; Ikeda, T. Is chemical crosslinking necessary for the photoinduced bending of polymer films? J. Mater. Chem. 2008, 18, 63–65. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The synthesis of new thermal stable schiff base/ester liquid crystals: A computational, mesomorphic, and optical study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, H.; Zhang, L.; Yang, H.; Lu, Y. Photoresponsive liquid crystals based on halogen bonding of azopyridines. Chem. Commun. 2014, 50, 9647–9649. [Google Scholar] [CrossRef]
- Yu, H. Recent advances in photoresponsive liquid-crystalline polymers containing azobenzene chromophores. J. Mater. Chem. C 2014, 2, 3047–3054. [Google Scholar] [CrossRef]
- Yu, H. Photoresponsive liquid crystalline block copolymers: From photonics to nanotechnology. Prog. Polym. Sci. 2014, 39, 781–815. [Google Scholar] [CrossRef]
- Cui, L.; Zhao, Y. Azopyridine side chain polymers: An efficient way to prepare photoactive liquid crystalline materials through self-assembly. Chem. Mater. 2004, 16, 2076–2082. [Google Scholar] [CrossRef]
- Zhou, H.; Xue, C.; Weis, P.; Suzuki, Y.; Huang, S.; Koynov, K.; Auernhammer, G.K.; Berger, R.; Butt, H.-J.; Wu, S. Photoswitching of glass transition temperatures of azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat. Chem. 2017, 9, 145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Sun, Y.; Yang, H.; Yu, H. Erasable thin-film optical diode based on a photoresponsive liquid crystal polymer. Nanoscale 2014, 6, 3854–3860. [Google Scholar] [CrossRef]
- Tan, X.; Li, Z.; Xia, M.; Cheng, X. Reversible photoresponsive chiral liquid crystal and multistimuli responsive organogels based on a cholesterol-azobenzene dimesogen. Rsc Adv. 2016, 6, 20021–20026. [Google Scholar] [CrossRef]
- Shen, P.; Zhang, X.; Lu, H.; Su, Z.; Zhou, Y.; Song, B.; Li, X.; Yang, X.; Tu, Y.; Li, C.Y. Effect of Fullerene Volume Fraction on Two-Dimensional Crystal-Constructed Supramolecular Liquid Crystals. Chem. Asian J. 2019, 14, 125–129. [Google Scholar] [CrossRef]
- Lehmann, M.; Dechant, M.; Gerbig, L.; Baumann, M. Supramolecular click procedures in liquid crystals. Liq. Cryst. 2019, 46, 1985–1994. [Google Scholar] [CrossRef]
- Saccone, M.; Pfletscher, M.; Kather, S.; Wölper, C.; Daniliuc, C.; Mezger, M.; Giese, M. Improving the mesomorphic behaviour of supramolecular liquid crystals by resonance-assisted hydrogen bonding. J. Mater. Chem. C 2019, 7, 8643–8648. [Google Scholar] [CrossRef]
- Sharma, V.S.; Shah, A.P.; Sharma, A.S. A new class of supramolecular liquid crystals derived from azo calix [4] arene functionalized 1, 3, 4-thiadiazole derivatives. New J. Chem. 2019, 43, 3556–3564. [Google Scholar] [CrossRef]
- Wang, X.; Bai, L.; Kong, S.; Song, Y.; Meng, F. Star-shaped supramolecular ionic liquid crystals based on pyridinium salts. Liq. Cryst. 2019, 46, 512–522. [Google Scholar] [CrossRef]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Phase behavior and DFT calculations of laterally methyl supramolecular hydrogen-bonding complexes. Crystals 2019, 9, 133. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Refaie, A.A.; Alaasar, M.A. Novel hydrogen-bonded angular supramolecular liquid crystals. Liq. Cryst. 2012, 39, 47–61. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular Hydrogen-Bonded Liquid Crystals Formed from 4-(4′-Pyridylazophenyl)-4 ″–Substituted Benzoates and 4-Alkoxybenzoic Acids. Mol. Cryst. Liq. Cryst. 2008, 482, 57–70. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular hydrogen-bonded liquid crystals formed from 4-(4′-pyridylazophenyl)-4 ″-alkoxy benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2008, 487, 74–91. [Google Scholar] [CrossRef]
- Naoum, M.; Fahmi, A.; Alaasar, M. Supramolecular liquid crystals induced by hydrogen-bonding interactions between non-mesomorphic compounds. I. 4-(4′-Pyridylazophenyl)-4 ″-substituted benzoates and 4-substituted benzoic acids. Mol. Cryst. Liq. Cryst. 2009, 506, 22–23. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.G.A.; Almllal, W.A. Supramolecular Liquid Crystals Induced by Hydrogen-Bonding Interactions between Non-Mesomorphic Compounds. II. Effect of Lateral Substitution. Mol. Cryst. Liq. Cryst. 2010, 518, 109–128. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddadd, O. DFT Calculations and Mesophase Study of Coumarin Esters and Its Azoesters. Crystals 2018, 8, 359. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Parveen, S.; Hagar, M.; Ahmed, H.A.; Knight, J.G. A new chiral Boron–dipyrromethene (BODIPY)–based fluorescent probe: Molecular docking, DFT, antibacterial and antioxidant approaches. J. Biomol. Struct. Dyn. 2019. [Google Scholar] [CrossRef]
- Chen, R.; An, Z.; Wang, W.; Chen, X.; Chen, P. Lateral substituent effects on UV stability of high-birefringence liquid crystals with the diaryl-diacetylene core: DFT/TD-DFT study. Liq. Cryst. 2017, 44, 1515–1524. [Google Scholar] [CrossRef]
- Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; Hagar, M.; Al-Ola, K.A.A. New Symmetrical U- and Wavy-Shaped Supramolecular H-Bonded Systems; Geometrical and Mesomorphic Approaches. Molecules 2020, 25, 1420. [Google Scholar] [CrossRef]
- Kishor, M.H.; Mohan, M.M. Investigations on smectic X* and re-entrant smectic C* orderings in hydrogen bonded ferroelectric liquid crystals. J. Mol. Liq. 2019, 273, 504–524. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; El-Sayed, T.H.; Alnoman, R.B. Schiff base/ester liquid crystals with different lateral substituents: Mesophase behaviour and DFT calculations. Liq. Cryst. 2019, 46, 1–11. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Saad, G.R. Synthesis and mesophase behaviour of Schiff base/ester 4-(arylideneamino)phenyl-4″-alkoxy benzoates and their binary mixtures. J. Mol. Liq. 2019, 273, 266–273. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Saad, G.R. Mesophase stability of new Schiff base ester liquid crystals with different polar substituents. Liq. Cryst. 2018, 45, 1324–1332. [Google Scholar] [CrossRef]
- Arakawa, Y.; Sasaki, Y.; Tsuji, H. Supramolecular hydrogen-bonded liquid crystals based on 4-n-alkylthiobenzoic acids and 4, 4′-bipyridine: Their mesomorphic behavior with comparative study including alkyl and alkoxy counterparts. J. Mol. Liq. 2019, 280, 153–159. [Google Scholar] [CrossRef]
- Vasanthi, T.; Subhasri, P.; Jayaprakasam, R.; Vijayakumar, V. Experimental and computational studies on induced thermochromic effect and re-entrant smectic phase in linear double hydrogen-bonded binary liquid crystal mixtures. Phase Transit. 2019, 92, 229–248. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Alaasar, M.A.; Salem, R.A. Supramolecular liquid crystals in binary and ternary systems. Thermochim. Acta 2011, 517, 63–73. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Mohammady, S.Z.; Abaza, A.H. Effect of lateral substitution on supramolecular liquid crystal associates induced by hydrogen-bonding interactions between 4-(4′-pyridylazo-3-methylphenyl)-4′′-alkoxy benzoates and 4-substituted benzoic acids. Liq. Cryst. 2010, 37, 475–486. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2019, 46, 550–559. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. Rsc Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Naoum, M.M. Mesophase behavior of binary and ternary mixtures of benzoic acids bearing terminal substituents of different polarity and chain-lengths. Thermochim. Acta 2014, 575, 122–128. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Naoum, M.M.; Saad, G.R. Mesophase behaviour of 1:1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Naoum, M.M. Mesophase behaviour of azobenzene-based angular supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2016, 43, 222–234. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Alhaddad, O.A. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1440–1451. [Google Scholar] [CrossRef]
- Ahmed, H.; Hagar, M.; Alhaddad, O. New chair shaped supramolecular complexes-based aryl nicotinate derivative; mesomorphic properties and DFT molecular geometry. Rsc. Adv. 2019, 9, 16366–16374. [Google Scholar] [CrossRef]
- Dave, J.S.; Menon, M. Azomesogens with a heterocyclic moiety. Bull. Mater. Sci. 2000, 23, 237–238. [Google Scholar] [CrossRef]
- Chen, H.; Liu, Y.; Gong, T.; Wang, L.; Zhao, K.; Zhou, S. Use of intermolecular hydrogen bonding to synthesize triple-shape memory supermolecular composites. Rsc. Adv. 2013, 3, 7048–7056. [Google Scholar] [CrossRef]
- Weinhold, F. Resonance character of hydrogen-bonding interactions in water and other H-bonded species. Adv. Protein Chem. 2005, 72, 121–155. [Google Scholar]
- Nafee, S.S.; Ahmed, H.A.; Hagar, M. New architectures of supramolecular H-bonded liquid crystal complexes based on dipyridine derivatives. Liq. Cryst. 2020, 1–14. [Google Scholar] [CrossRef]
- Alhaddad, O.; Ahmed, H.; Hagar, M. Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals. Molecules 2020, 25, 365. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Saad, G. New calamitic thermotropic liquid crystals of 2-hydroxypyridine ester mesogenic core: Mesophase behaviour and DFT calculations. Liq. Cryst. 2019, 47, 114–124. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.; Alhaddad, O. New azobenzene-based natural fatty acid liquid crystals with low melting point: Synthesis, DFT calculations and binary mixtures. Liq. Cryst. 2019, 46, 2223–2234. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Martinez-Felipe, A.; Storey, J.; Gorecka, E.; Imrie, C.T. Twist-Bend Nematogenic Supramolecular Dimers and Trimers Formed by Hydrogen Bonding. Crystals 2020, 10, 175. [Google Scholar] [CrossRef]
- Cleland, W.; Kreevoy, M.M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef]
- Lizu, M.; Lutfor, M.; Surugau, N.; How, S.; Arshad, S.E. Synthesis and characterization of ethyl cellulose–based liquid crystals containing azobenzene chromophores. Mol. Cryst. Liq. Cryst. 2010, 528, 64–73. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.; Imrie, C.T. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentoxybenzoic acid. Rsc Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef]
- Martínez-Felipe, A.; Imrie, C.T. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Ghanem, A.; Noel, C. FTIR investigation of two alkyl-p-terphenyl-4, 4 ″-dicarboxylates in their crystalline, smectic and isotropic phases. Mol. Cryst. Liq. Cryst. 1987, 150, 447–472. [Google Scholar] [CrossRef]
- Paterson, D.A.; Martínez-Felipe, A.; Jansze, S.M.; TM Marcelis, A.; MD Storey, J.; Imrie, C.T. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 928–939. [Google Scholar] [CrossRef]
- Walker, R.; Pociecha, D.; Abberley, J.; Martinez-Felipe, A.; Paterson, D.; Forsyth, E.; Lawrence, G.; Henderson, P.; Storey, J.; Gorecka, E. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef] [PubMed]
- Gray, G.W. Molecular Structure and the Properties of Liquid Crystals; Academic press: London, UK, 1962. [Google Scholar]
- Imrie, C.; Taylor, L. The preparation and properties of low molar mass liquid crystals possessing lateral alkyl chains. Liq. Cryst. 1989, 6, 1–10. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds I/An are available from the authors. |


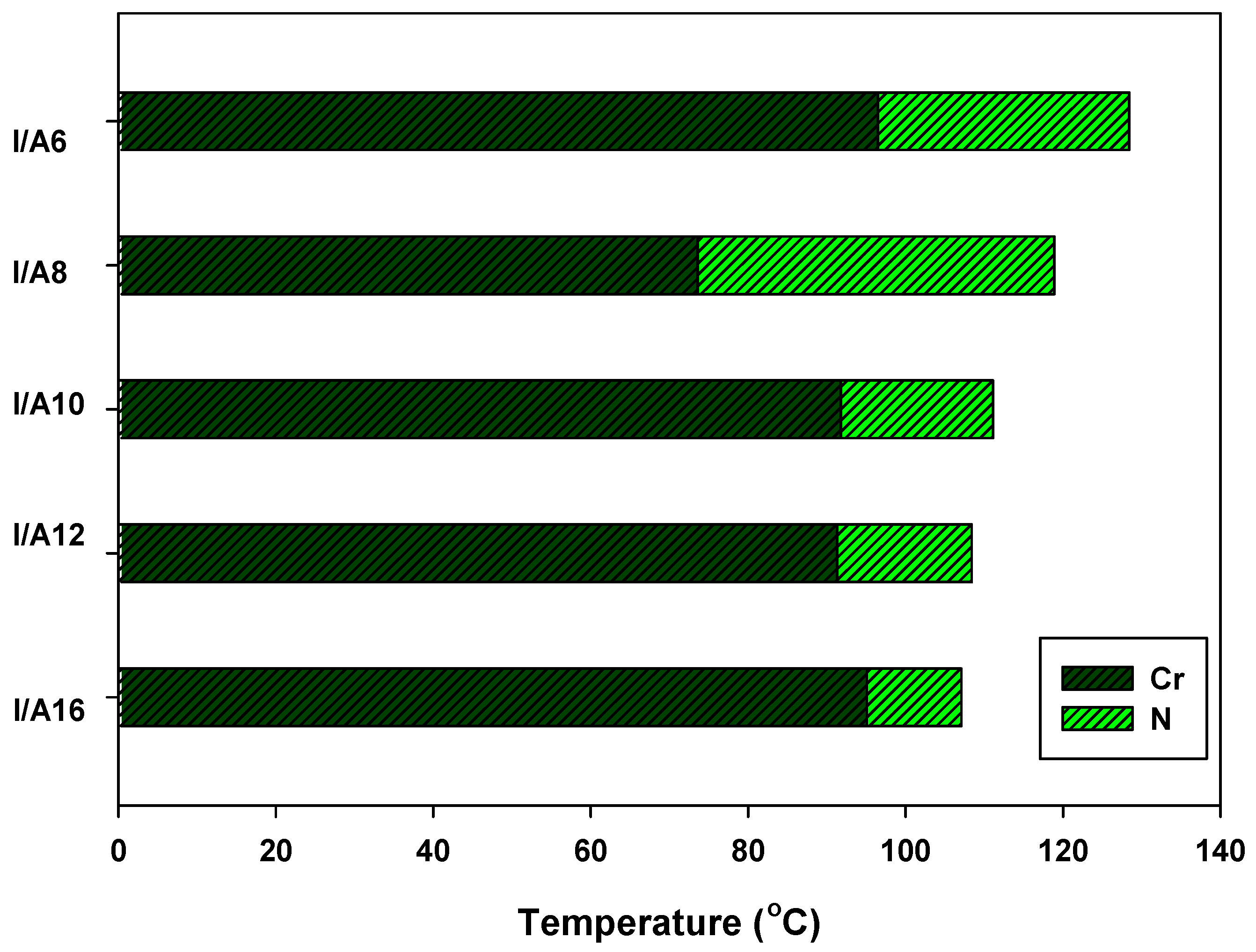
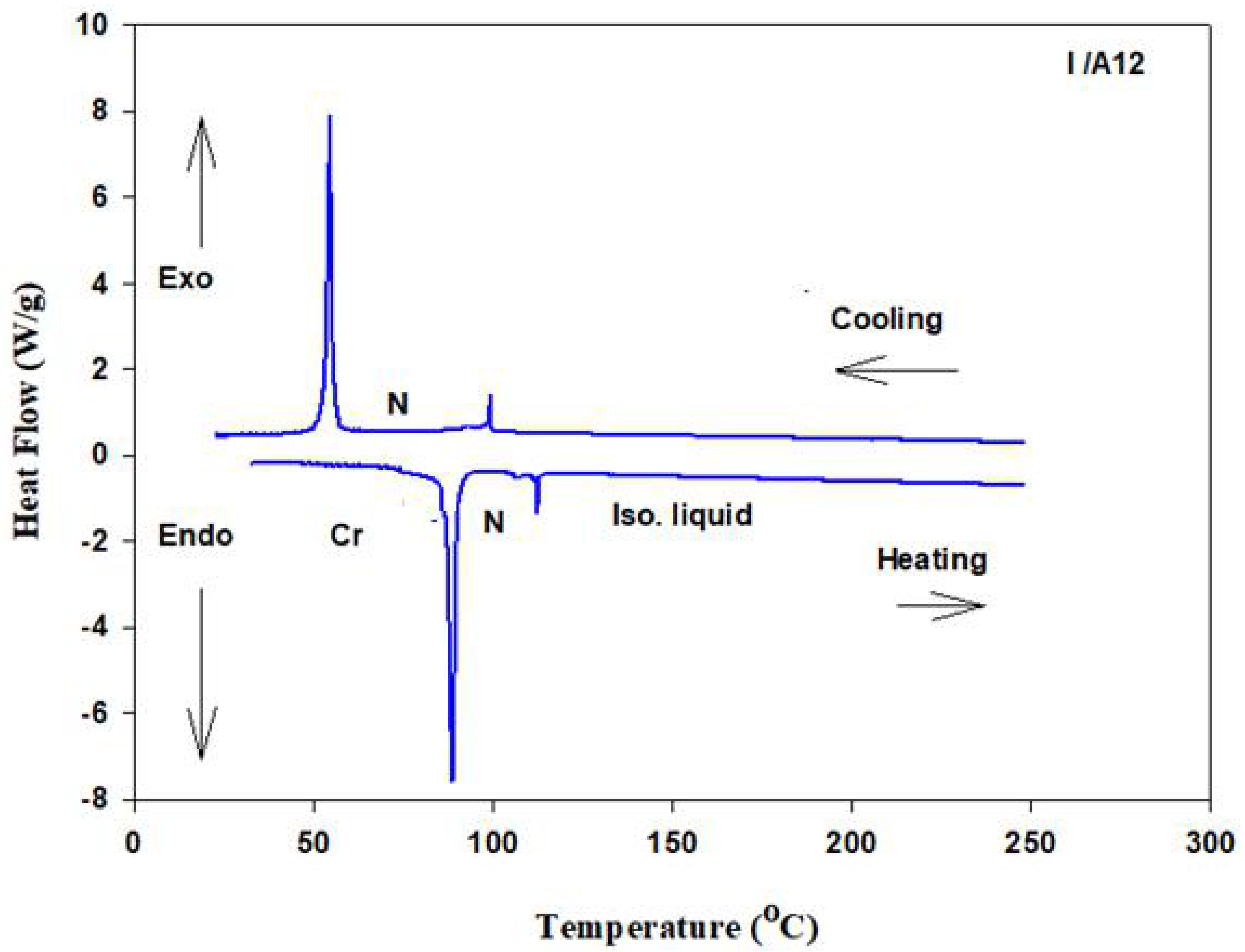
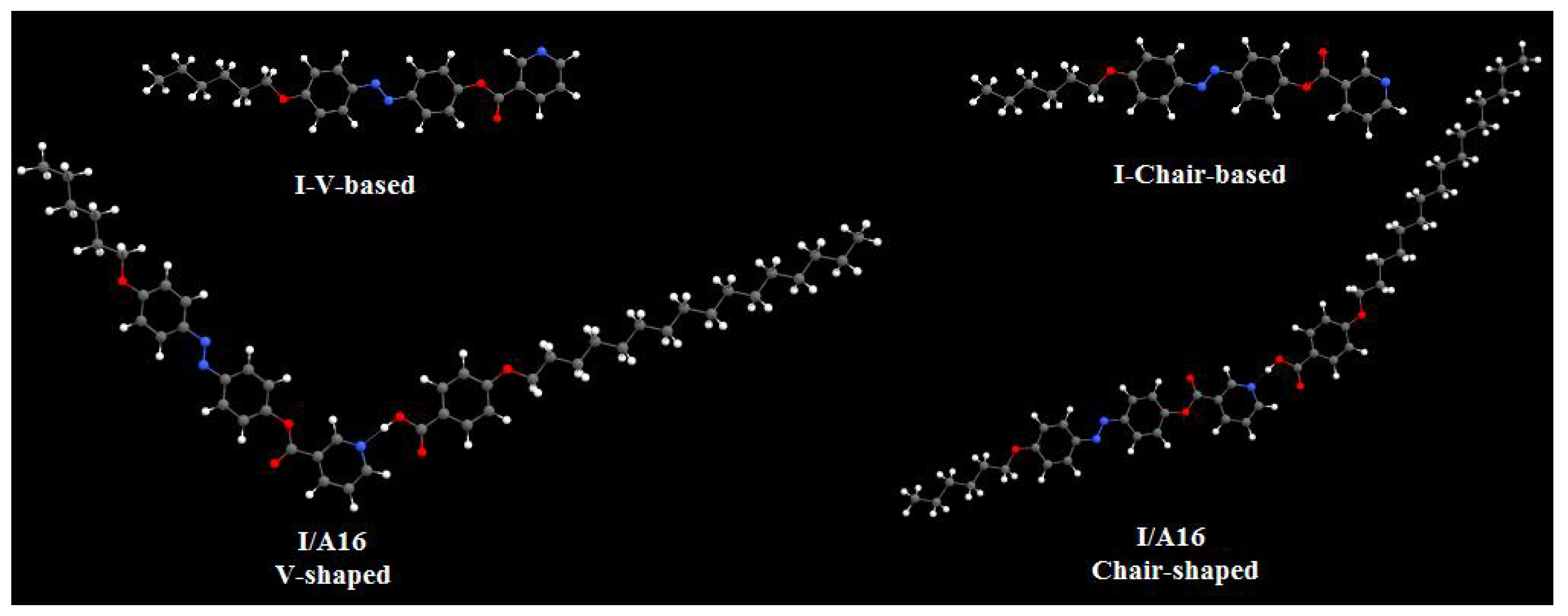
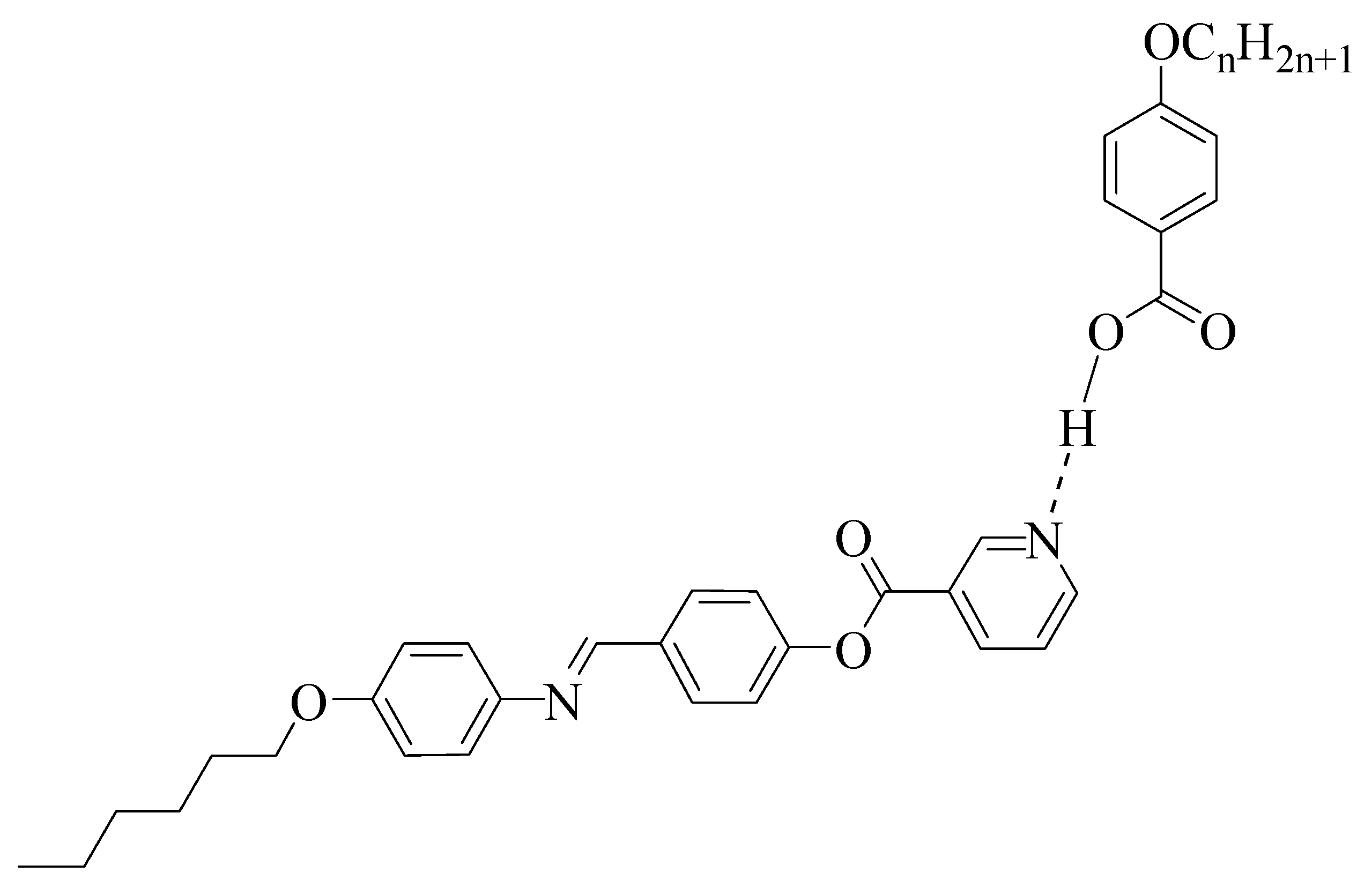
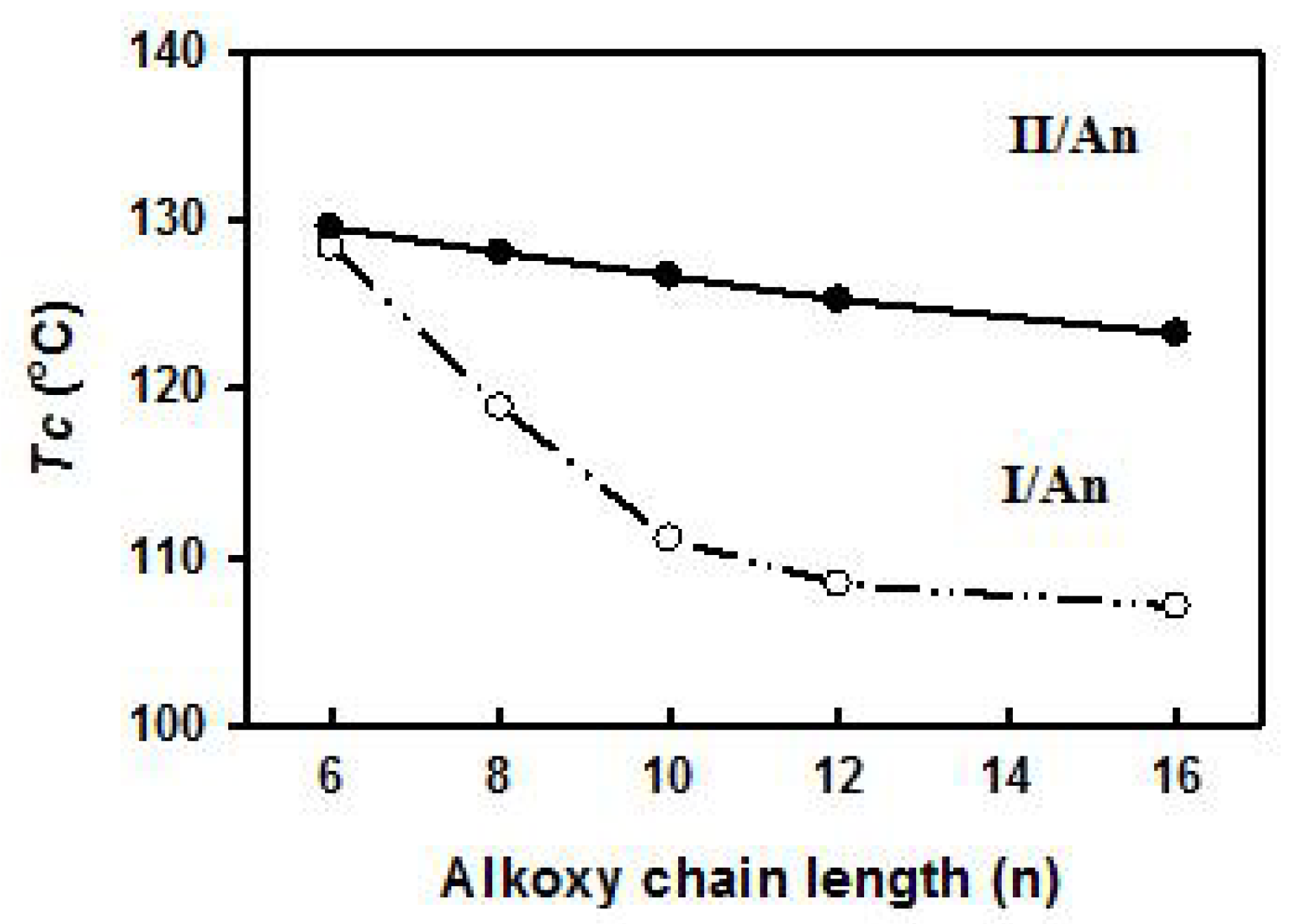
| Complex | TCr-N | ∆HCr-N | TN-I | ∆HN-I | ∆S/RN-I |
|---|---|---|---|---|---|
| I/A6 | 96.5 | 58.13 | 128.4 | 3.86 | 1.16 |
| I/A8 | 73.6 | 58.37 | 118.9 | 2.55 | 0.78 |
| I/A10 | 91.8 | 53.39 | 111.1 | 1.86 | 0.58 |
| I/A12 | 91.3 | 53.31 | 108.4 | 1.22 | 0.38 |
| I/A16 | 95.1 | 52.56 | 107.1 | 1.19 | 0.38 |
| Conformer | Chair-Shaped | V-Shaped | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | I/A6 | I/A8 | I/A10 | I/A12 | I/A16 | I/A6 | I/A8 | I/A10 | I/A12 | I/A16 |
| Ecorr | 0.741 | 0.798 | 0.855 | 0.912 | 1.026 | 0.741 | 0.798 | 0.855 | 0.912 | 1.026 |
| ZPVE | −2050.616 | −2129.183 | −2207.750 | −2286.317 | −2443.451 | −2050.615 | −2129.182 | −2207.749 | −2286.316 | −2443.451 |
| Etot | −2050.570 | −2129.134 | −2207.698 | −2286.263 | −2443.391 | −2050.569 | −2129.133 | −2207.698 | −2286.262 | −2443.391 |
| H | −2050.569 | −2129.133 | −2207.697 | −2286.262 | −2443.391 | −2050.568 | −2129.132 | −2207.697 | −2286.261 | −2443.390 |
| G | −2050.710 | −2129.281 | −2207.851 | −2286.422 | −2443.566 | −2050.708 | −2129.278 | −2207.850 | −2286.423 | −2443.563 |
| Parameters | Chair-Shaped | V-Shaped | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I/A6 | I/A8 | I/A10 | I/A12 | I/A16 | I/A6 | I/A8 | I/A10 | I/A12 | I/A16 | |
| ELUMO | −0.098 | −0.098 | −0.098 | −0.098 | −0.098 | −0.098 | −0.098 | −0.098 | −0.098 | −0.098 |
| EHOMO | −0.225 | −0.225 | −0.225 | −0.225 | −0.225 | −0.224 | −0.224 | −0.224 | −0.224 | −0.224 |
| ΔEHOMO-LUMO | 0.127 | 0.127 | 0.127 | 0.127 | 0.127 | 0.126 | 0.126 | 0.127 | 0.126 | 0.126 |
| μ Total | 3.123 | 3.116 | 3.113 | 3.112 | 3.112 | 5.669 | 5.500 | 5.655 | 5.549 | 5.661 |
| Polarizability α | 538.40 | 561.72 | 585.85 | 609.65 | 656.39 | 542.34 | 566.53 | 590.29 | 613.87 | 660.77 |
| Parameters | N=N Derivatives Chair-Shaped | CH=N Derivatives Chair-Shaped | ||
|---|---|---|---|---|
| I/A6 | I/A8 | II/A6 | II/A8 | |
| ELUMO | −0.098 | −0.098 | −0.092 | −0.092 |
| EHOMO | −0.225 | −0.225 | −0.213 | −0.213 |
| ΔEHOMO-LUMO | 0.127 | 0.127 | 0.121 | 0.121 |
| μ Total | 3.123 | 3.116 | 4.982 | 4.808 |
| Polarizability α | 538.40 | 561.72 | 534.36 | 558.54 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhaddad, O.A.; Abu Al-Ola, K.A.; Hagar, M.; Ahmed, H.A. Chair- and V-Shaped of H-bonded Supramolecular Complexes of Azophenyl Nicotinate Derivatives; Mesomorphic and DFT Molecular Geometry Aspects. Molecules 2020, 25, 1510. https://doi.org/10.3390/molecules25071510
Alhaddad OA, Abu Al-Ola KA, Hagar M, Ahmed HA. Chair- and V-Shaped of H-bonded Supramolecular Complexes of Azophenyl Nicotinate Derivatives; Mesomorphic and DFT Molecular Geometry Aspects. Molecules. 2020; 25(7):1510. https://doi.org/10.3390/molecules25071510
Chicago/Turabian StyleAlhaddad, Omaima A., Khulood A. Abu Al-Ola, Mohamed Hagar, and Hoda A. Ahmed. 2020. "Chair- and V-Shaped of H-bonded Supramolecular Complexes of Azophenyl Nicotinate Derivatives; Mesomorphic and DFT Molecular Geometry Aspects" Molecules 25, no. 7: 1510. https://doi.org/10.3390/molecules25071510
APA StyleAlhaddad, O. A., Abu Al-Ola, K. A., Hagar, M., & Ahmed, H. A. (2020). Chair- and V-Shaped of H-bonded Supramolecular Complexes of Azophenyl Nicotinate Derivatives; Mesomorphic and DFT Molecular Geometry Aspects. Molecules, 25(7), 1510. https://doi.org/10.3390/molecules25071510








