In Vitro Bioactivity and Biocompatibility of Bio-Inspired Ti-6Al-4V Alloy Surfaces Modified by Combined Laser Micro/Nano Structuring
Abstract
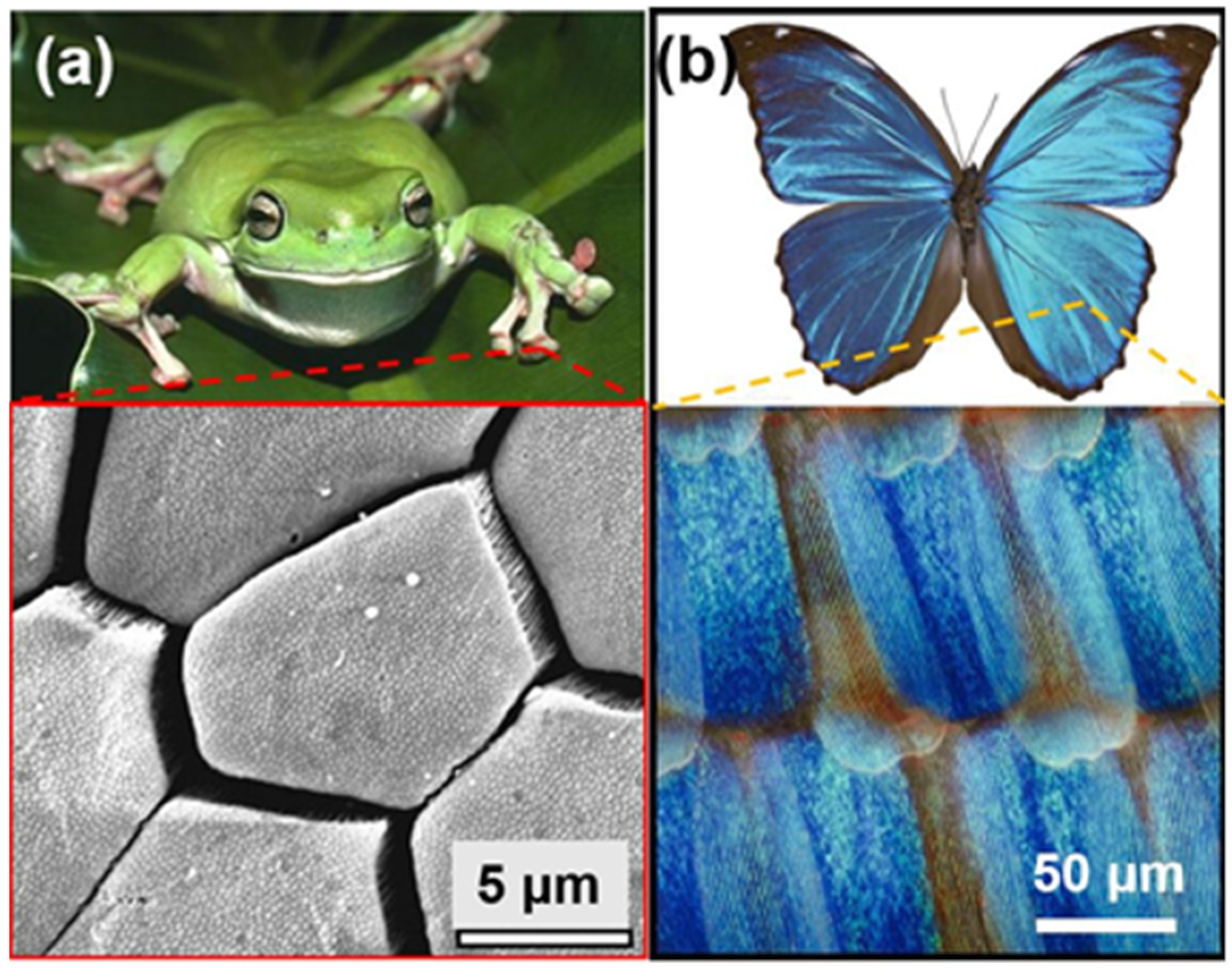
1. Introduction
2. Results and Discussion
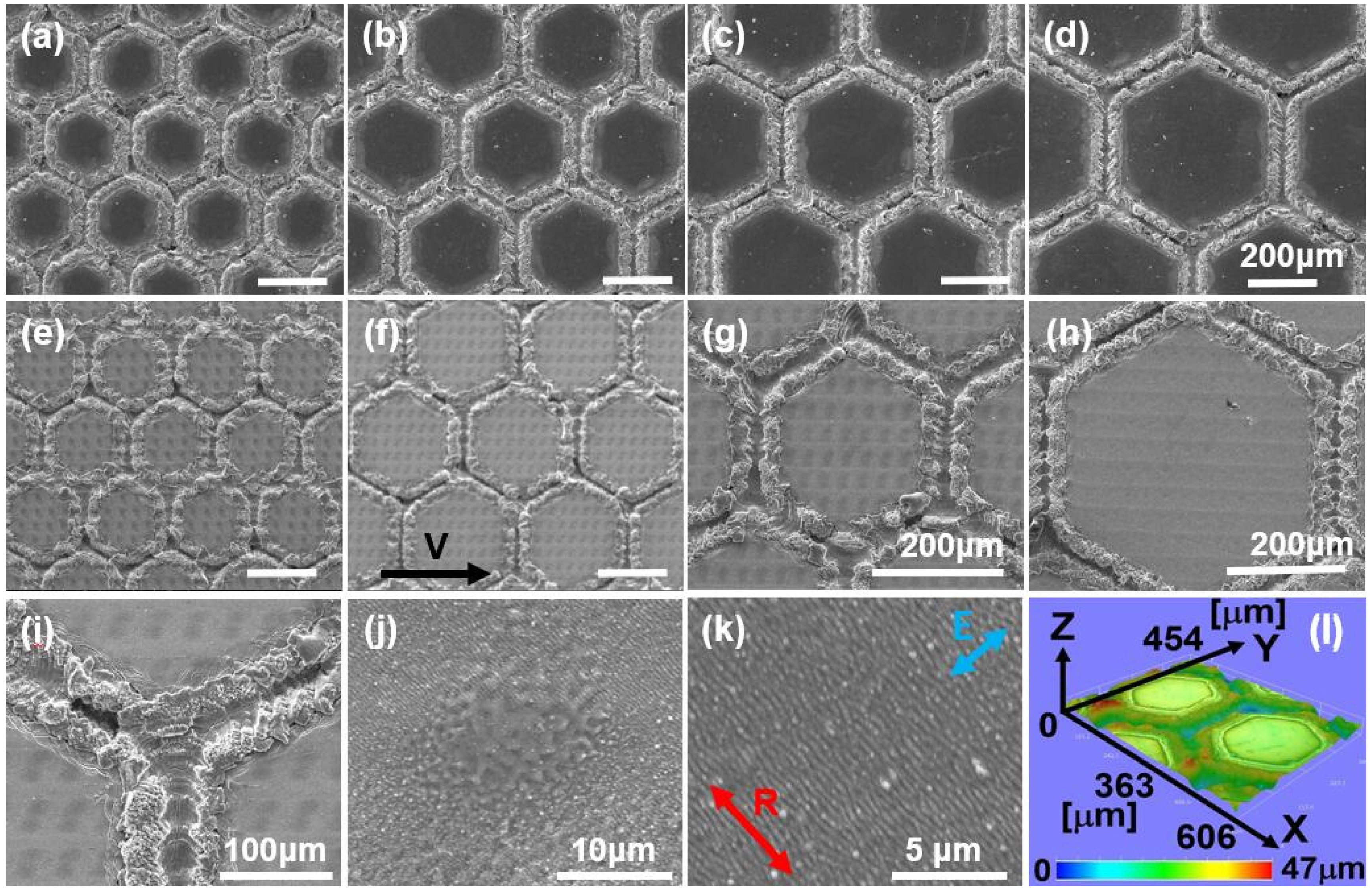
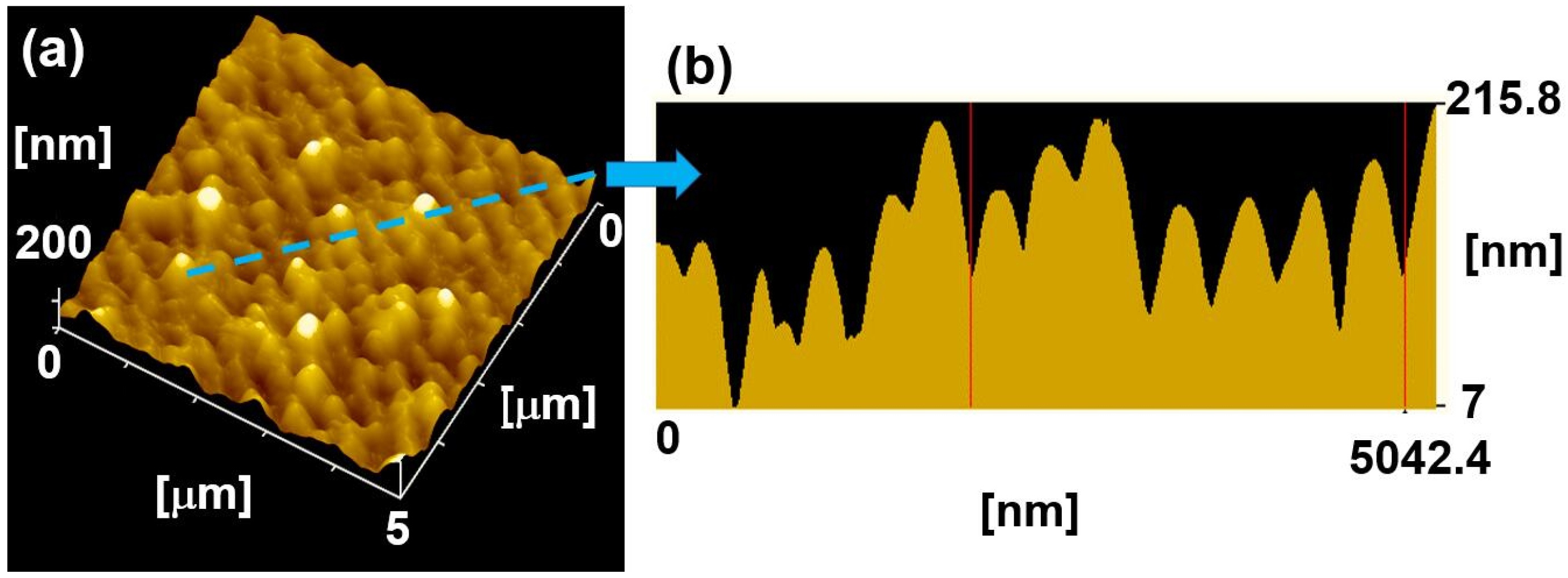
2.1. The Characterization of Bioinspired Surface Micro/Nano-Structures
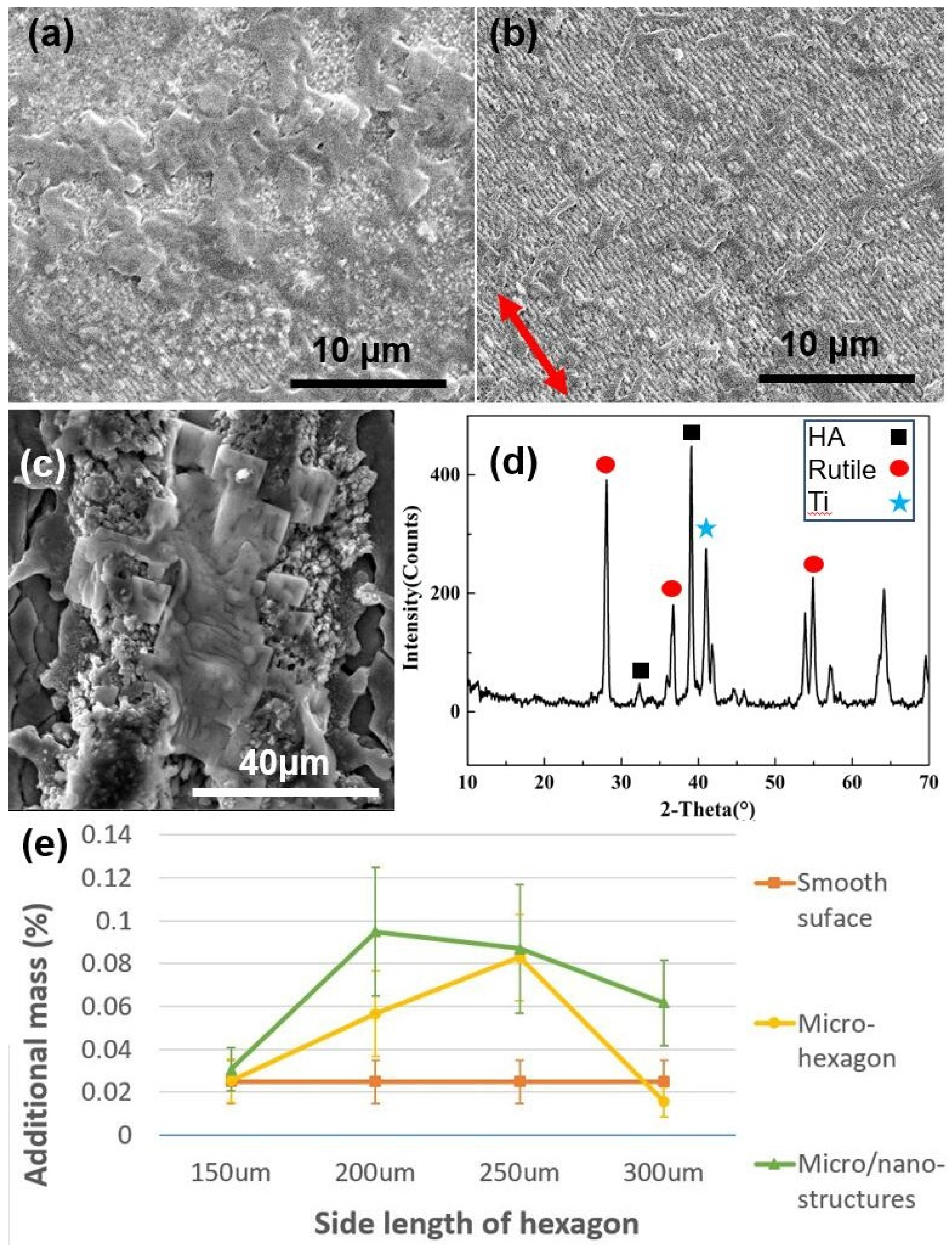
2.2. In Vitro SBF Experimental Results and Analysis
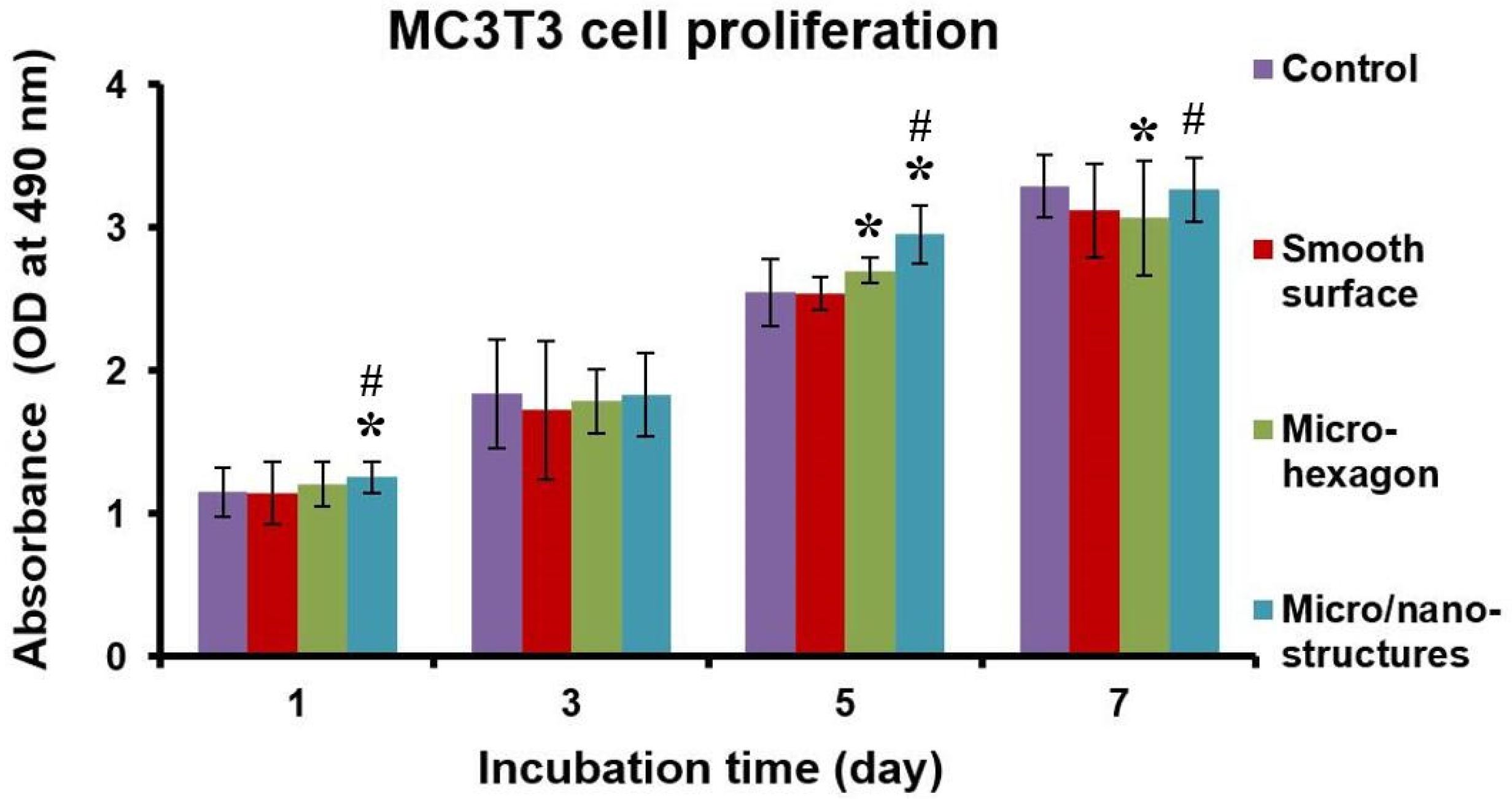
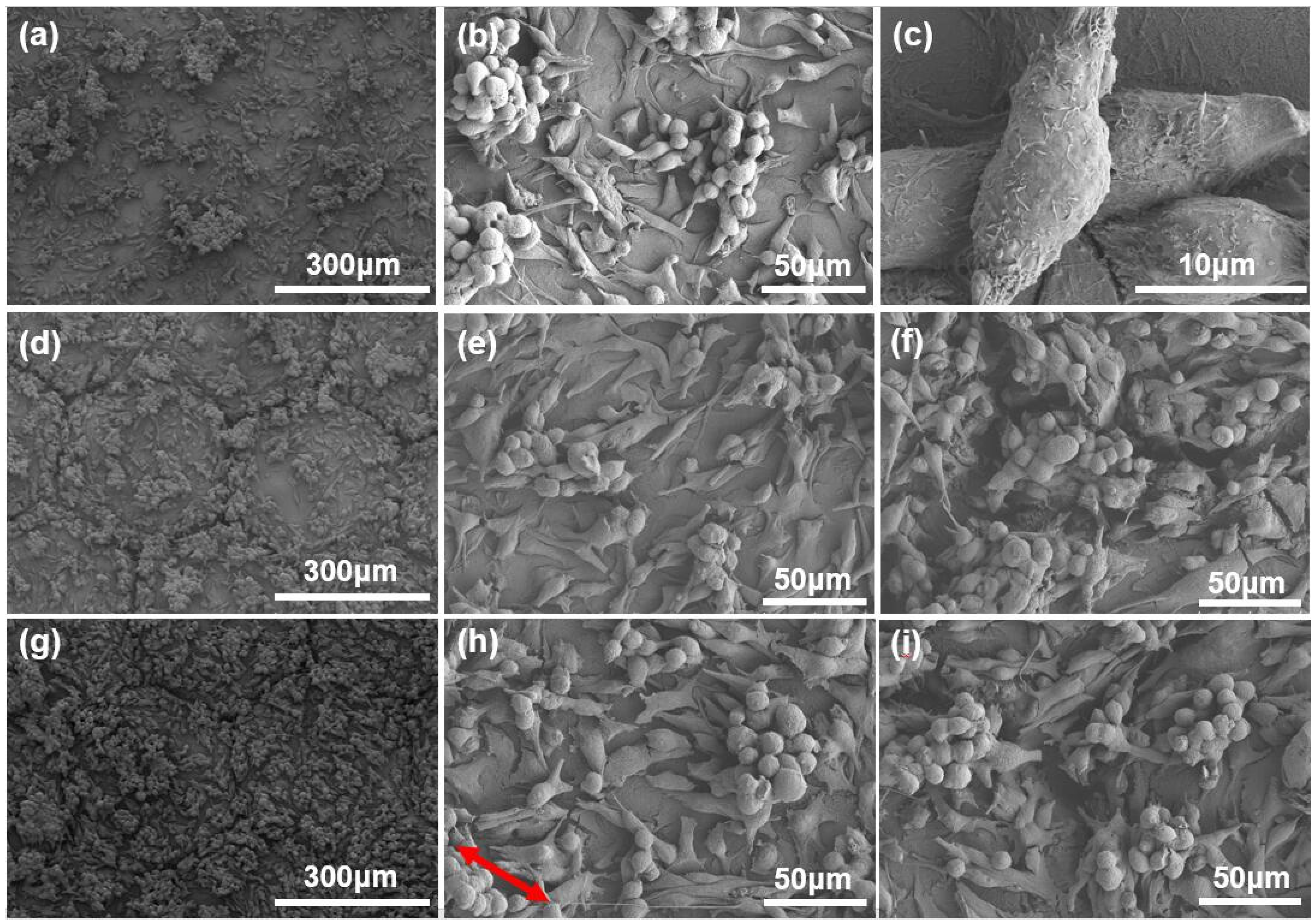
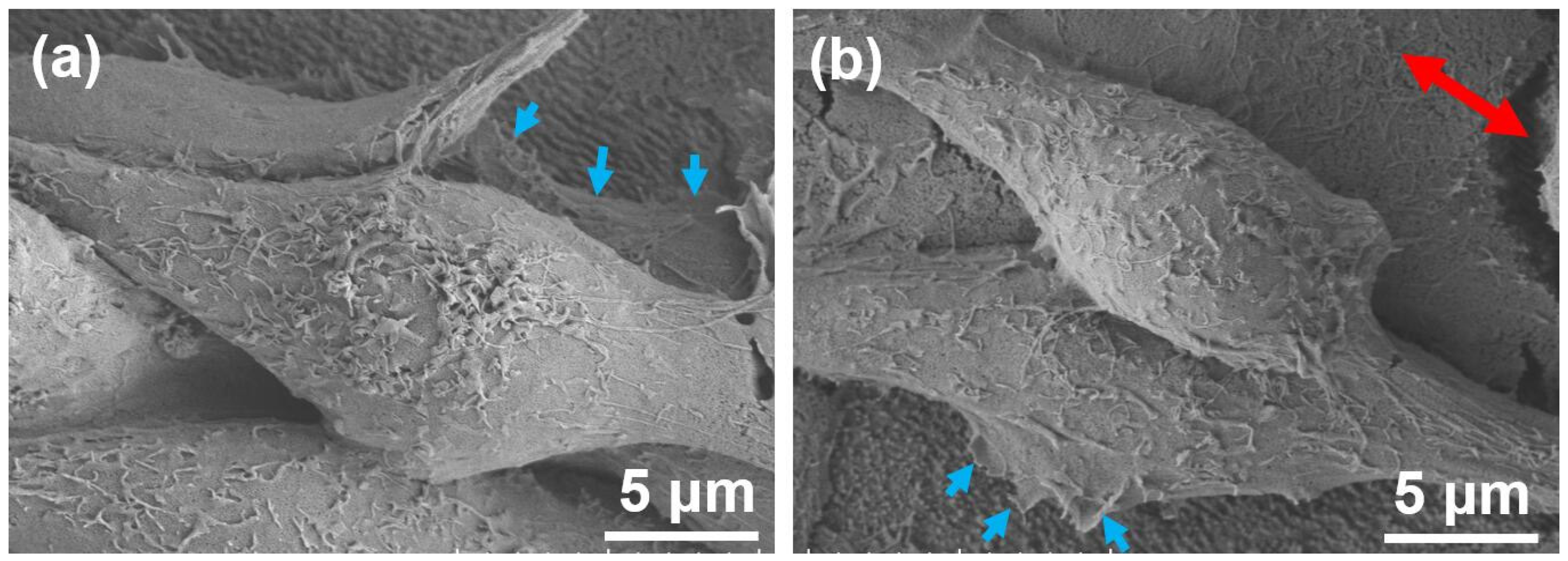
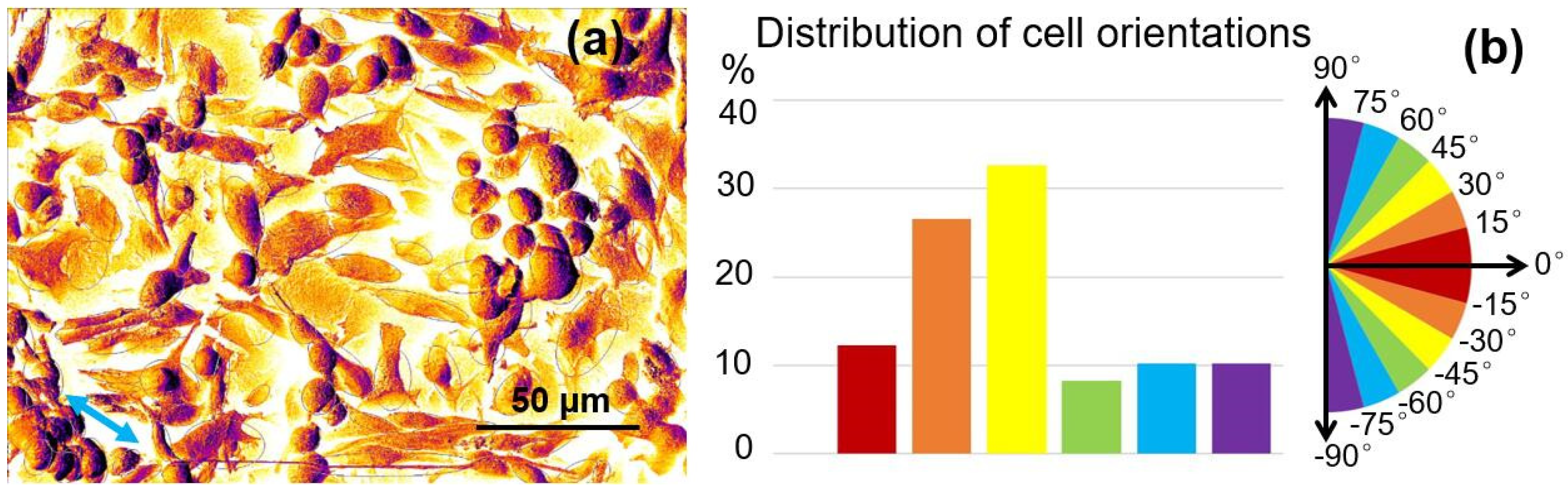
2.3. Cell Culture Results and Discussion
3. Experimental
3.1. Materials
3.2. Laser Surface Structuring
3.3. Surface Morphology and Crystal Analysis
3.4. In Vitro SBF Experiments
3.5. In Vitro Cell Culture Tests
3.6. Image Analysis
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohammadi, M.; Shaegh, S.A.M.; Alibolandi, M.; Ebrahimzadeh, M.H.; Tamayol, A.; Jaafari, M.R.; Ramezani, M. Micro and nanotechnologies for bone regeneration: Recent advances and emerging designs. J. Control. Release 2018, 274, 35–55. [Google Scholar] [CrossRef]
- Wang, R.; Xu, Y.; Yu, X.L.; Zhou, Y.M. Enhanced adhesion of human osteoblast-like cells on femtosecond laser treated Ti-6Al-4V. Adv. Mater. Res. 2013, 739, 101–105. [Google Scholar] [CrossRef]
- Gittens, R.A.; Mclachlan, T.; Olivares-Navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Kubo, K.; Tsukimura, N.; Iwasa, F.; Ueno, T.; Saruwatari, L.; Aita, H.; Chiou, W.A.; Ogawa, T. Cellular behavior on TiO2 nanonodular structures in a micro-to-nanoscale hierarchy model. Biomaterials 2009, 30, 5319–5329. [Google Scholar] [CrossRef]
- Hu, G.; Guan, K.; Lu, L.; Zhang, J.; Lu, N.; Guan, Y. Engineered functional surfaces by laser microprocessing for biomedical applications. Engineering 2018, 6, 822–830. [Google Scholar] [CrossRef]
- Han, C.; Li, Y.; Wang, Q.; Wen, S.; Wei, Q.; Yan, C.; Hao, L.; Liu, J.; Shi, Y. Continuous functionally graded porous titanium scaffolds manufactured by selective laser melting for bone implants. J. Mech. Behav. Biomed. Mater. 2018, 80, 119. [Google Scholar] [CrossRef]
- Yang, Y.Z.; Tian, J.M.; Tian, J.T.; Chen, Z.Q.; Deng, X.J.; Zhang, D.H. Preparation of graded porous titanium coatings on titanium implant materials by plasma spraying. J. Biomed. Mater. Res. 2000, 52, 333–337. [Google Scholar] [CrossRef]
- Aparicio, C.; Rodriguez, D.; Gil, F.J. Variation of roughness and adhesion strength of deposited apatite layers on titanium dental implants. Mater. Sci. Eng. C 2011, 31, 320–324. [Google Scholar] [CrossRef]
- den Braber, E.T.; Jansen, H.V.; de Boer, M.J.; Croes, H.J.E.; Elwenspoek, M.; Ginsel, L.A.; Jansen, J.A. Scanning electron microscopic, transmission electron microscopic and confocal laser scanning microscopic observation of fibroblasts cultured on microgrooved surfaces of bulk titanium substrata. J. Biomed. Mater. Res. 1998, 40, 425–433. [Google Scholar] [CrossRef]
- Ferri, Y.; Piotrowski, O.; Chauvy, P.F.; Madore, C.; Landolt, D. Two-level electrochemical micromachining of titanium for device fabrication. J. Micromech. Microeng. 2001, 11, 522–527. [Google Scholar] [CrossRef]
- Babaliari, E.; Kavatzikidou, P.; Angelaki, D.; Chaniotaki, L.; Manousaki, A.; Siakouli-Galanopoulou, A.; Ranella, A.; Stratakis, E. Engineering cell adhesion and orientation via ultrafast laser fabricated microstructured substrates. Int. J. Mol. Sci. 2018, 19, 2053. [Google Scholar] [CrossRef]
- Lu, X.; Leng, Y.; Zhang, X.; Xu, J.; Qin, L.; Chan, C.W. Comparative study of osteoconduction on micromachined and alkali-treated titanium alloy surfaces in vitro and in vivo. Biomaterials 2005, 26, 1793–1801. [Google Scholar] [CrossRef]
- Li, C.; Yang, L.; Ren, X.; Yang, Y.; Cheng, G. Femtosecond laser-induced non-centrosymmetric surface microstructures on bulk metallic glass for unidirectional droplet micro-displacement. J. Phys. D Appl. Phys. 2020, 53, 105305. [Google Scholar] [CrossRef]
- Li, C.; Yang, Y.; Yang, L.; Shi, Z. Biomimetic Anti-Adhesive Surface Microstructures on Electrosurgical Blade Fabricated by Long-Pulse Laser Inspired by Pangolin Scales. Micromachines 2019, 10, 816. [Google Scholar] [CrossRef]
- Li, C.; Yang, L.; Yan, C.; Chen, W.; Cheng, G. Biomimetic anti-adhesive surface micro-structures of electrosurgical knife fabricated by fiber laser. J. Laser Micro Nanoen. 2018, 13, 309–313. [Google Scholar]
- Li, C.; Cheng, G.H.; Colombier, J.P.; Faure, N.; Reynaud, S.; Zhang, H.; Jamon, D.; Stoian, R. Impact of evolving surface nanoscale topologies in femtosecond laser structuring of Ni-based superalloy cmsx-4. J. Opt. 2016, 18, 015402. [Google Scholar] [CrossRef]
- Li, C.; Zhang, H.; Cheng, G.; Faure, N.; Jamon, D.; Colombier, J.P.; Stoian, R. Initial Cumulative Effects in Femtosecond Pulsed Laser-induced Periodic Surface Structures on Bulk Metallic Glasses. J. Laser Micro Nanoen. 2016, 11, 357–365. [Google Scholar]
- Li, C.; Cheng, G.; Sedao, X.; Zhang, W.; Zhang, H.; Faure, N.; Jamon, D.; Colombier, J.P.; Stoian, R. Scattering effects and high-spatial-frequency nanostructures on ultrafast laser irradiated surfaces of zirconium metallic alloys with nano-scaled topographies. Opt. Express 2016, 24, 1558–1568. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Valderrama, P.; Jones, A.A.; Wilson, T.G.; Seibl, R.; Cochran, D.L. Bone apposition around two different sandblasted and acid-etched titanium implant surfaces: A histomorphometric study in canine mandibles. Clin. Oral Implant Res. 2008, 19, 233–241. [Google Scholar] [CrossRef]
- Buser, D.; Broggini, N.; Wieland, M.; Schenk, R.K.; Denzer, A.J.; Cochran, D.L.; Hoffmann, B.; Lussi, A.; Steinemann, S.G. Enhanced bone apposition to a chemically modified SLA titanium surface. J. Dent. Res. 2004, 83, 529–533. [Google Scholar] [CrossRef]
- Zinger, O.; Zhao, G.; Schwartz, Z.; Simpson, J.; Wieland, M.; Landolt, D.; Boyan, B. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials 2005, 26, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Jeon, H.J.; Jin, G.; Kim, G.H. The effect of microsized roughness in nano/microsized hierarchical surfaces replicated from a lotus leaf on the activities of osteoblast-like cells (MG63). J. Mater. Chem. 2012, 22, 7584–7591. [Google Scholar] [CrossRef]
- Cha, K.J.; Park, K.S.; Kang, S.W.; Cha, B.H.; Lee, B.K.; Han, I.B.; Shin, D.A.; Kim, D.S.; Lee, S.H. Effect of replicated polymeric substrate with lotus surface structure on adipose-derived stem cell behaviors. Macromol. Biosci. 2011, 11, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Federle, W.; Barnes, W.J.P.; Baumgartner, W.; Drechsler, P.; Smith, J.M. Wet but not slippery: Boundary friction in tree frog adhesive toe pads. J. R. Soc. Interface 2006, 3, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Welsch, U.; Storch, V.; Fuchs, W. The fine structure of the digital pads of rhacophorid tree frogs. Cell Tissue Res. 1974, 148, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Berthier, S.; Charron, E.; Boulenguez, J. Morphological structure and optical properties of the wings of Morphidae. Insect Sci. 2006, 13, 145–158. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, X.; Jiang, L. Directional adhesion of superhydrophobic butterfly wings. Soft Matter 2007, 3, 178–182. [Google Scholar] [CrossRef]
- Stangl, R.; Pries, A.; Loos, B.; Muller, M.; Erben, R.G. Influence of pores created by laser superfinishing on osseointegration of titanium alloy implants. J. Biomed. Mater. Res. A 2004, 69, 444–453. [Google Scholar] [CrossRef]
- McClay, D.R. The role of thin filopodia in motility and morphogenesis. Exp. Cell Res. 1999, 253, 296–301. [Google Scholar] [CrossRef]
- Santana, M.; Estevez, J.O.; Agarwal, V.; Herrera-Becerra, R. Room temperature crystallization of hydroxyapatite in porous silicon structures. Nanoscale Res. Lett. 2016, 11, 497. [Google Scholar] [CrossRef]
- Uchida, M.; Kim, H.M.; Kokubo, T.; Fujibayashi, S.; Nakamura, T. Structural dependence of apatite formation on titania gels in a simulated body fluid. J. Biomed. Mater. Res. A 2002, 64, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Ohshima, M.; Iwata, H. Time-lapse observation of cell alignment on nanogrooved patterns. J. R. Soc. Interface 2009, 6, S269–S277. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Davidson, P.; Popa, A.M.; Giazzon, M.; Liley, M.; Ploux, L. The interaction of cells and bacteria with surfaces structured at the nanometre scale. Acta Biomater. 2010, 6, 3824–3846. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.S.; Olsson, P.; Lidberg, U.; Sutherland, D. The effects of continuous and discontinuous groove edges on cell shape and alignment. Exp. Cell Res. 2003, 288, 177–188. [Google Scholar] [CrossRef]
- Walboomers, X.F.; Croes, H.J.; Ginsel, L.A.; Jansen, J.A. Contact guidance of rat fibroblasts on various implant materials. J. Biomed. Mater. Res. 1999, 47, 204–212. [Google Scholar] [CrossRef]
- McBeath, R.; Pirone, D.M.; Nelson, C.M.; Bhadriraju, K.; Chen, C.S. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Dev. Cell 2004, 6, 483–495. [Google Scholar] [CrossRef]
- Velasco, E.; Monsalve-Guil, L.; Jimenez, A.; Ortiz, I.; Moreno-Muñoz, J.; Nuñez-Marquez, E.; Pegueroles, M.; Pérez, R.A.; Gil, F.J. Importance of the roughness and residual stresses of dental implants on fatigue and osseointegration behavior in vivo study in rabbits. J. Oral Implantol. 2016, 42, 469–479. [Google Scholar] [CrossRef]
- Fang, Y.W.; Li, Y.H.; He, W.F.; Li, P.Y. Effects of laser shock processing with different parameters and ways on residual stresses fields of a TC4 alloy blade. Mater. Sci. Eng. A 2013, 559, 683–692. [Google Scholar] [CrossRef]
- Kim, H.M.; Miyazaki, T.; Kukubo, T.; Nakamura, T. Revised simulated body fluid. Key Eng. Mater. 2001, 192, 47–50. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W3. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Dumas, V.; Rattner, A.; Vico, L.; Audouard, E.; Dumas, J.C.; Naisson, P.; Bertrand, P. Multiscale grooved titanium processed with femtosecond laser influences mesenchymal stem cell morphology, adhesion, and matrix organization. J. Biomed. Mater. Res. A 2012, 100, 3108–3116. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the Ti-6Al-4V are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Yang, Y.; Yang, L.; Shi, Z.; Yang, P.; Cheng, G. In Vitro Bioactivity and Biocompatibility of Bio-Inspired Ti-6Al-4V Alloy Surfaces Modified by Combined Laser Micro/Nano Structuring. Molecules 2020, 25, 1494. https://doi.org/10.3390/molecules25071494
Li C, Yang Y, Yang L, Shi Z, Yang P, Cheng G. In Vitro Bioactivity and Biocompatibility of Bio-Inspired Ti-6Al-4V Alloy Surfaces Modified by Combined Laser Micro/Nano Structuring. Molecules. 2020; 25(7):1494. https://doi.org/10.3390/molecules25071494
Chicago/Turabian StyleLi, Chen, Yong Yang, Lijun Yang, Zhen Shi, Pengfei Yang, and Guanghua Cheng. 2020. "In Vitro Bioactivity and Biocompatibility of Bio-Inspired Ti-6Al-4V Alloy Surfaces Modified by Combined Laser Micro/Nano Structuring" Molecules 25, no. 7: 1494. https://doi.org/10.3390/molecules25071494
APA StyleLi, C., Yang, Y., Yang, L., Shi, Z., Yang, P., & Cheng, G. (2020). In Vitro Bioactivity and Biocompatibility of Bio-Inspired Ti-6Al-4V Alloy Surfaces Modified by Combined Laser Micro/Nano Structuring. Molecules, 25(7), 1494. https://doi.org/10.3390/molecules25071494




