Green Tea Catechins Trigger Immediate-Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening
Abstract
1. Introduction
2. Results
2.1. Effect of GT-Catechin Ingestion on Lifespan
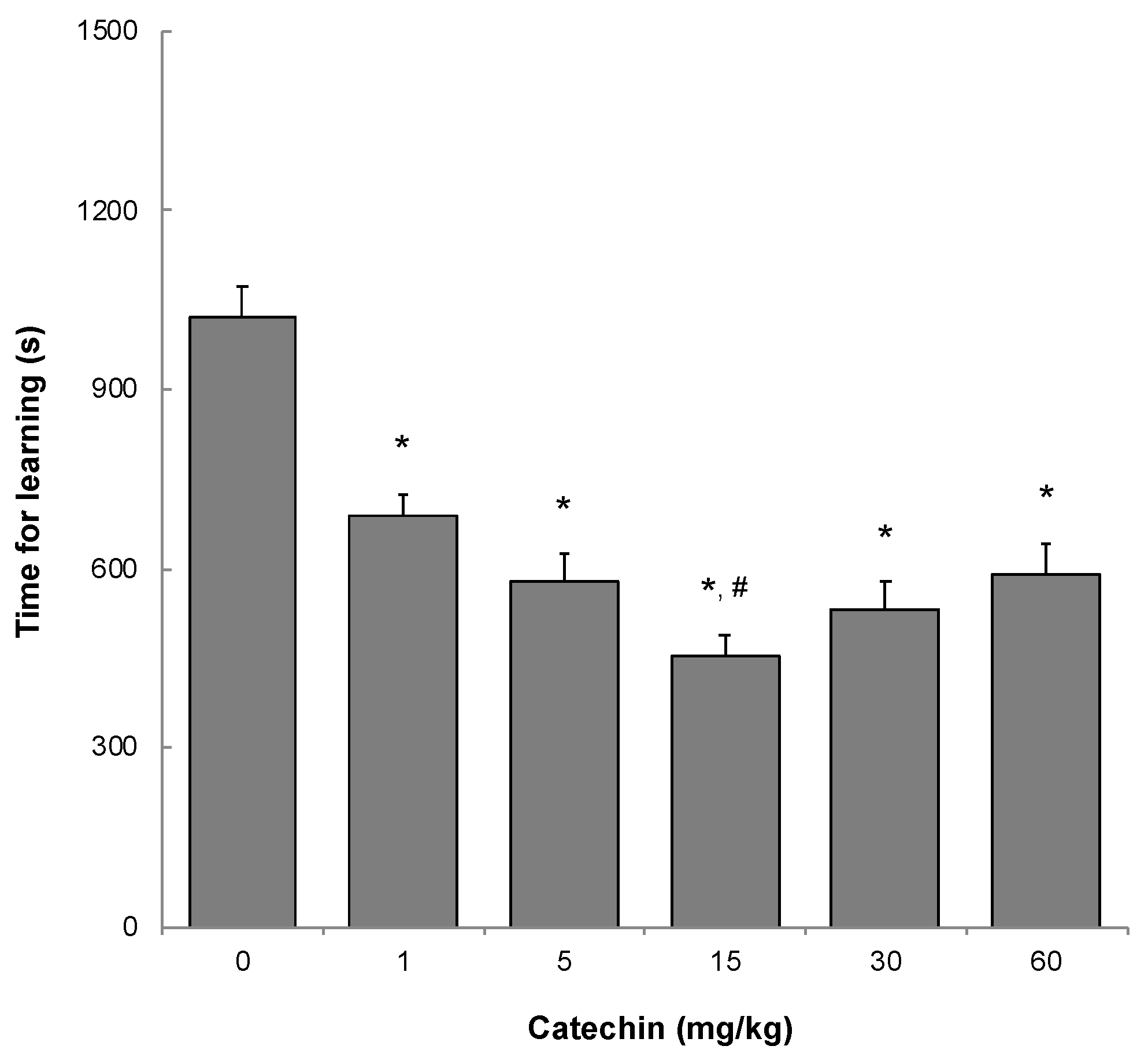
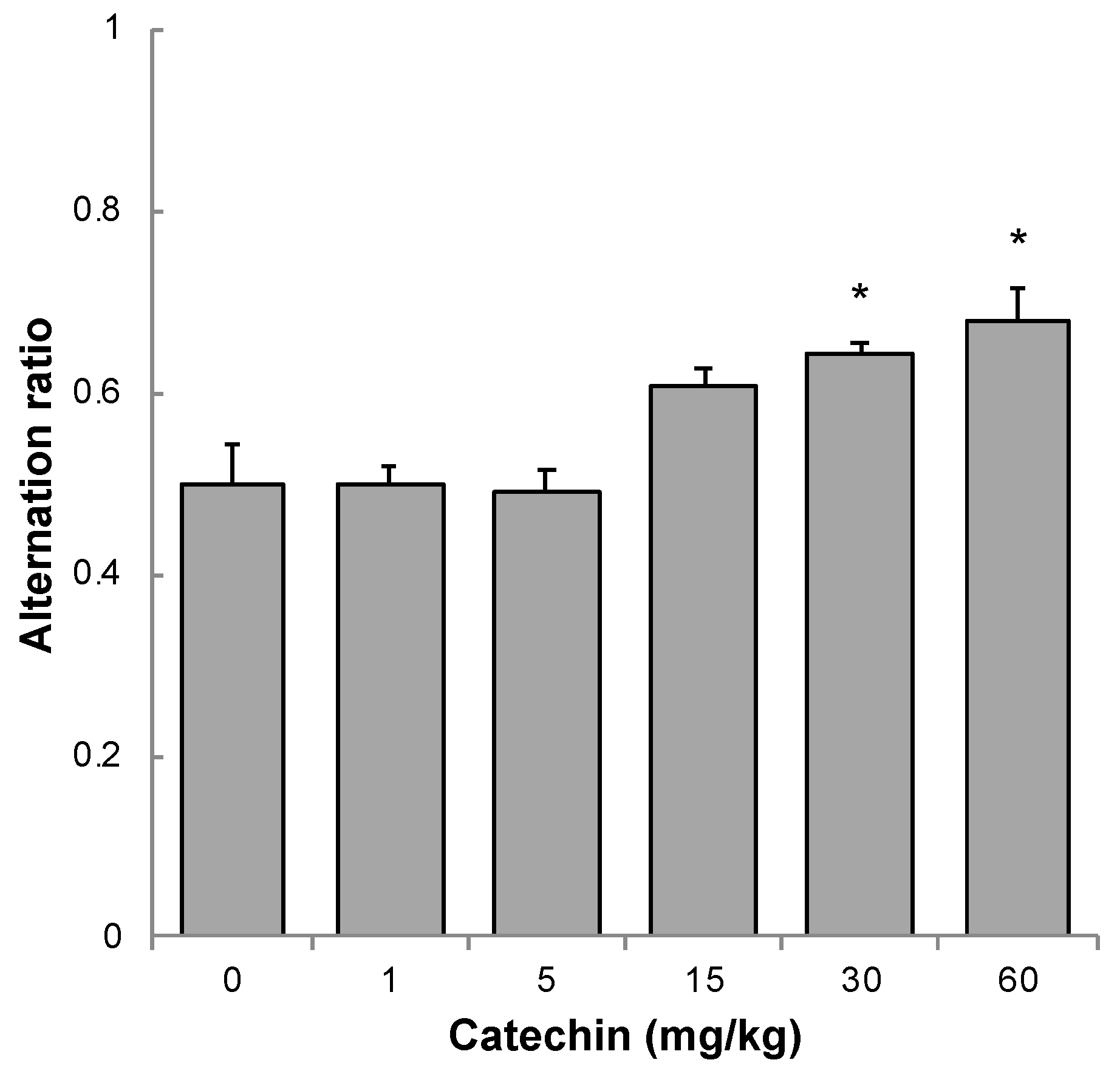
2.2. Memory Acquisition, Memory Retention, and Working Memory
2.3. Transcriptome
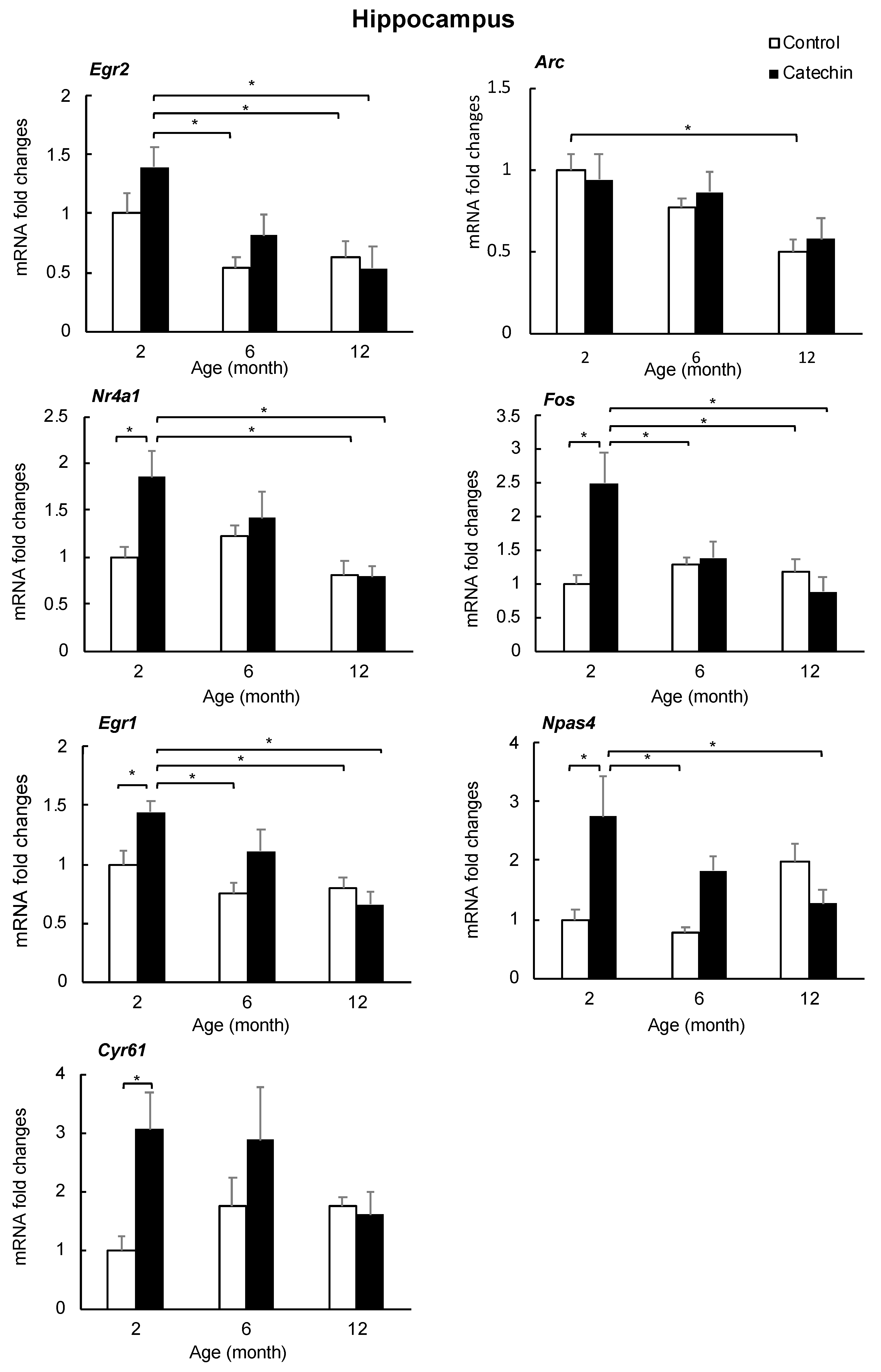
2.4. Effect of GT-Catechin Intake on the IEG Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Design
4.3. Memory Acquisition and Retention Tests
4.4. Working Memory
4.5. Measurement of DNA Microarray and Principal Component Analysis
4.6. Quantitative Real-time Reverse Transcription PCR (qRT-PCR)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Unno, K.; Takabayashi, F.; Kishido, T.; Oku, N.A. Suppressive effect of green tea catechins on morphologic and functional regression of the brain in aged mice with accelerated senescence (SAMP10). Exp. Gerontol. 2004, 39, 1027–1034. [Google Scholar] [CrossRef]
- Unno, K.; Takabayashi, F.; Yoshida, H.; Choba, D.; Fukutomi, R.; Kikunaga, N.; Kishido, T.; Oku, N.; Hoshino, M. Daily consumption of green tea catechin delays memory regression in aged mice. Biogerontology 2007, 8, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Shimada, A.; Tsuzuki, M.; Keino, H.; Satoh, M.; Chiba, Y.; Saitoh, Y.; Hosokawa, M. Apical vulnerability to dendritic retraction in prefrontal neurones of ageing SAMP10 mouse: A model of cerebral degeneration. Neuropathol. Appl. Neurobiol. 2006, 32, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Takeda, T. Senescence-accelerated mouse (SAM) with special references to neurodegeneration models, SAMP8 and SAMP10 mice. Neurochem. Res. 2009, 34, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Unno, K.; Tahara, S.; Shimada, A.; Chiba, Y.; Hoshino, M.; Kaneko, T. Age-related increase of superoxide generation in the brains of mammals and birds. Aging Cell 2008, 7, 459–469. [Google Scholar] [CrossRef]
- Kishido, T.; Unno, K.; Yoshida, H.; Choba, D.; Fukutomi, R.; Asahina, S.; Iguchi, K.; Oku, N.; Hoshino, M. Decline in glutathione peroxidase activity is a reason for brain senescence: Consumption of green tea catechin prevents the decline in its activity and protein oxidative damage in ageing mouse brain. Biogerontology 2007, 8, 423–430. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Nakagawa, A.; Takahashi, Y.; Iguchi, K.; Yamamoto, H.; Hoshino, M.; Hara, A.; Takagaki, A.; Nanjo, F.; et al. Blood brain barrier permeability of (-)-epigallocatechin gallate, its proliferation-enhancing activity of human neuroblastoma SH-SY5Y cells, and its preventive effect on age-related cognitive dysfunction in mice. Biochem. Biophys. Rep. 2017, 9, 180–186. [Google Scholar] [CrossRef]
- Unno, K.; Yamamoto, H.; Ohtaki, T.; Ishikawa, Y.; Noda, S.; Maeda, K.; Fujitani, K.; Miyazaki, H.; Takabayashi, F.; Sasaki, T.; et al. Active component in green tea catechins and effective intake period for prevention of age-related brain dysfunction. Anti-Aging Med. 2011, 8, 75–81. [Google Scholar] [CrossRef][Green Version]
- Pallauf, K.; Duckstein, N.; Rimbach, G. A literature review of flavonoids and lifespan in model organisms. Proc. Nutr. Soc. 2017, 76, 145–162. [Google Scholar] [CrossRef]
- Xiong, L.G.; Chen, Y.J.; Tong, J.W.; Gong, Y.S.; Huang, J.A.; Liu, Z.H. Epigallocatechin-3-gallate promotes healthy lifespan through mitohormesis during early-to-mid adulthood in Caenorhabditis elegans. Redox Biol. 2018, 14, 305–315. [Google Scholar] [CrossRef]
- Mori, T.; Koyama, N.; Tan, J.; Segawa, T.; Maeda, M.; Town, T. Combined treatment with the phenolics (-)-epigallocatechin-3-gallate and ferulic acid improves cognition and reduces Alzheimer-like pathology in mice. J. Biol. Chem. 2019, 294, 2714–2731. [Google Scholar] [CrossRef] [PubMed]
- Stagni, F.; Giacomini, A.; Emili, M.; Trazzi, S.; Guidi, S.; Sassi, M.; Ciani, E.; Rimondini, R.; Bartesaghi, R. Short- and long-term effects of neonatal pharmacotherapy with epigallocatechin-3-gallate on hippocampal development in the Ts65Dn mouse model of Down syndrome. Neuroscience 2016, 333, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Rong, C.; Chen, Y.; Yang, C.; Hu, Q.; Mo, Y.; Zhang, C.; Gu, X.; Zhang, L.; He, W.; et al. (-)-Epigallocatechin-3-gallate attenuates cognitive deterioration in Alzheimer’s disease model mice by upregulating neprilysin expression. Exp. Cell Res. 2015, 334, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lee, Y.K.; Ban, J.O.; Ha, T.Y.; Yun, Y.P.; Han, S.B.; Oh, K.W.; Hong, J.T. Green tea (-)-epigallocatechin-3-gallate inhibits beta-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-kappaB pathways in mice. J. Nutr. 2009, 139, 1987–1993. [Google Scholar] [CrossRef]
- De la Torre, R.; De Sola, S.; Pons, M.; Duchon, A.; de Lagran, M.M.; Farré, M.; Fitó, M.; Benejam, B.; Langohr, K.; Rodriguez, J.; et al. Epigallocatechin-3-gallate, a DYRK1A inhibitor, rescues cognitive deficits in Down syndrome mouse models and in humans. Mol. Nutr. Food Res. 2014, 58, 278–288. [Google Scholar] [CrossRef]
- Mi, Y.; Qi, G.; Fan, R.; Qiao, Q.; Sun, Y.; Gao, Y.; Liu, X. EGCG ameliorates high-fat- and high-fructose-induced cognitive defects by regulating the IRS/AKT and ERK/CREB/BDNF signaling pathways in the CNS. FASEB J. 2017, 31, 4998–5011. [Google Scholar] [CrossRef]
- Han, J.Y.; Kim, J.K.; Kim, J.H.; Oh, B.S.; Cho, W.J.; Jung, Y.D.; Lee, S.G. Neurorestorative effects of epigallocatechin-3-Gallate on cognitive function in a chronic cerebral hypoperfusion rat model. Restor. Neurol. Neurosci. 2016, 34, 367–377. [Google Scholar] [CrossRef]
- Soung, H.S.; Wang, M.H.; Tseng, H.C.; Fang, H.W.; Chang, K.C. (-)Epigallocatechin-3-gallate decreases the stress-induced impairment of learning and memory in rats. Neurosci. Lett. 2015, 602, 27–32. [Google Scholar] [CrossRef]
- Biasibetti, R.; Tramontina, A.C.; Costa, A.P.; Dutra, M.F.; Quincozes-Santos, A.; Nardin, P.; Bernardi, C.L.; Wartchow, K.M.; Lunardi, P.S.; Gonçalves, C.A. Green tea (-)epigallocatechin-3-gallate reverses oxidative stress and reduces acetylcholinesterase activity in a streptozotocin-induced model of dementia. Behav. Brain Res. 2013, 236, 186–193. [Google Scholar] [CrossRef]
- Itoh, T.; Tabuchi, M.; Mizuguchi, N.; Imano, M.; Tsubaki, M.; Nishida, S.; Hashimoto, S.; Matsuo, K.; Nakayama, T.; Ito, A.; et al. Neuroprotective effect of (-)-epigallocatechin-3-gallate in rats when administered pre- or post-traumatic brain injury. J. Neural Transm. 2013, 120, 767–783. [Google Scholar] [CrossRef]
- Yamanaka, D.; Kawano, T.; Nishigaki, A.; Aoyama, B.; Tateiwa, H.; Shigematsu-Locatelli, M.; Locatelli, F.M.; Yokoyama, M. Effects of epigallocatechin-3-gallate on systemic inflammation-induced cognitive dysfunction in aged rats. J. Anesth. 2017, 31, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Stringer, M.; Abeysekera, I.; Dria, K.J.; Roper, R.J.; Goodlett, C.R. Low dose EGCG treatment beginning in adolescence does not improve cognitive impairment in a Down syndrome mouse model. Pharmacol. Biochem. Behav. 2015, 138, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Pence, B.D.; Bhattacharya, T.K.; Park, P.; Rytych, J.L.; Allen, J.M.; Sun, Y.; McCusker, R.H.; Kelley, K.W.; Johnson, R.W.; Rhodes, J.S.; et al. Long-term supplementation with EGCG and beta-alanine decreases mortality but does not affect cognitive or muscle function in aged mice. Exp. Gerontol. 2017, 98, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, M.; Xu, X.; Song, M.; Tao, H.; Bai, Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol. Nutr. Food Res. 2012, 56, 1292–1303. [Google Scholar] [CrossRef]
- Atkinson-Leadbeater, K.; Hehr, C.L.; Johnston, J.; Bertolesi, G.; McFarlane, S. EGCG stabilizes growth cone filopodia and impairs retinal ganglion cell axon guidance. Dev. Dyn. 2016, 245, 667–677. [Google Scholar] [CrossRef]
- Arámburo, C.; Alba-Betancourt, C.; Luna, M.; Harvey, S. Expression and function of growth hormone in the nervous system: A brief review. Gen. Comp. Endocrinol. 2014, 203, 35–42. [Google Scholar] [CrossRef]
- Kim, S.; Kim, H.; Um, J.W. Synapse development organized by neuronal activity-regulated immediate-early genes. Exp. Mol. Med. 2018, 50, 11. [Google Scholar] [CrossRef]
- Gallo, F.T.; Katche, C.; Morici, J.F.; Medina, J.H.; Weisstaub, N.V. Immediate early genes, memory and psychiatric disorders: Focus on c-Fos, Egr1 and Arc. Front. Behav. Neurosci. 2018, 12, 79. [Google Scholar] [CrossRef]
- Malik, A.R.; Urbanska, M.; Gozdz, A.; Swiech, L.J.; Nagalski, A.; Perycz, M.; Blazejczyk, M.; Jaworski, J. Cyr61, a matricellular protein, is needed for dendritic arborization of hippocampal neurons. J. Biol. Chem. 2013, 288, 8544–8559. [Google Scholar] [CrossRef]
- Horita, H.; Wada, K.; Rivas, M.V.; Hara, E.; Jarvis, E.D. The dusp1 immediate early gene is regulated by natural stimuli predominantly in sensory input neurons. J. Comp. Neurol. 2010, 518, 2873–2901. [Google Scholar] [CrossRef] [PubMed]
- Leone, A.; Mitsiades, N.; Ward, Y.; Spinelli, B.; Poulaki, V.; Tsokos, M.; Kelly, K. The Gem GTP-binding protein promotes morphological differentiation in neuroblastoma. Oncogene 2001, 20, 3217–3225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zlatković, J.; Bernardi, R.E.; Filipović, D. Protective effect of Hsp70i against chronic social isolation stress in the rat hippocampus. J. Neural Transm. 2014, 121, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Oza, J.; Yang, J.; Chen, K.Y.; Liu, A.Y. Changes in the regulation of heat shock gene expression in neuronal cell differentiation. Cell Stress Chaperones 2008, 13, 73–84. [Google Scholar] [CrossRef]
- Oh-Hashi, K.; Fujimura, K.; Norisada, J.; Hirata, Y. Expression analysis and functional characterization of the mouse cysteine-rich with EGF-like domains 2. Sci. Rep. 2018, 8, 12236. [Google Scholar] [CrossRef]
- Fukuda, S.; Sumii, M.; Masuda, Y.; Takahashi, M.; Koike, N.; Teishima, J.; Yasumoto, H.; Itamoto, T.; Asahara, T.; Dohi, K.; et al. Murine and human SDF2L1 is an endoplasmic reticulum stress-inducible gene and encodes a new member of the Pmt/rt protein family. Biochem. Biophys. Res. Commun. 2001, 280, 407–414. [Google Scholar] [CrossRef]
- Kwapis, J.L.; Alaghband, Y.; Kramár, E.A.; López, A.J.; Vogel Ciernia, A.; White, A.O.; Shu, G.; Rhee, D.; Michael, C.M.; Montellier, E.; et al. Epigenetic regulation of the circadian gene Per1 contributes to age-related changes in hippocampal memory. Nat. Commun. 2018, 9, 3323. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and oxidative stress: A mutual interplay in age-related diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Caro, A.A.; Davis, A.; Fobare, S.; Horan, N.; Ryan, C.; Schwab, C. Antioxidant and pro-oxidant mechanisms of (+) catechin in microsomal CYP2E1-dependent oxidative stress. Toxicol. In Vitro 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Pervin, M.; Unno, K.; Takagaki, A.; Isemura, M.; Nakamura, Y. Function of green tea catechins in the brain: Epigallocatechin gallate and its metabolites. Int. J. Mol. Sci. 2019, 20, 3630. [Google Scholar] [CrossRef]
- Noguchi-Shinohara, M.; Yuki, S.; Dohmoto, C.; Ikeda, Y.; Samuraki, M.; Iwasa, K.; Yokogawa, M.; Asai, K.; Komai, K.; Nakamura, H.; et al. Consumption of green tea, but not black tea or coffee, is associated with reduced risk of cognitive decline. PLoS ONE 2014, 9, e96013. [Google Scholar] [CrossRef] [PubMed]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki Cohort 2006 Study. Am. J. Geriatr. Psychiatry 2016, 24, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Saito, E.; Inoue, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Iwasaki, M.; Sasazuki, S.; Noda, M.; Iso, H.; Tsugane, S.; et al. Association of green tea consumption with mortality due to all causes and major causes of death in a Japanese population: The Japan Public Health Center-based Prospective Study (JPHC Study). Ann. Epidemiol. 2015, 25, 512–518.e3. [Google Scholar] [CrossRef] [PubMed]
- Heroux, N.A.; Osborne, B.F.; Miller, L.A.; Kawan, M.; Buban, K.N.; Rosen, J.B.; Stanton, M.E. Differential expression of the immediate early genes c-Fos, Arc, Egr-1, and Npas4 during long-term memory formation in the context preexposure facilitation effect (CPFE). Neurobiol. Learn. Mem. 2018, 147, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Y.; Ertürk, A.; Kallop, D.; Jiang, Z.; Weimer, R.M.; Kaminker, J.; Sheng, M. Activity-induced Nr4a1 regulates spine density and distribution pattern of excitatory synapses in pyramidal neurons. Neuron 2014, 83, 431–443. [Google Scholar] [CrossRef]
- Kwapis, J.L.; Alaghband, Y.; López, A.J.; Long, J.M.; Li, X.; Shu, G.; Bodinayake, K.K.; Matheos, D.P.; Rapp, P.R.; Wood, M.A. HDAC3-mediated repression of the Nr4a family contributes to age-related impairments in long-term memory. J. Neurosci. 2019, 39, 4999–5009. [Google Scholar] [CrossRef]
- Miyashita, T.; Kubik, S.; Lewandowski, G.; Guzowski, J.F. Networks of neurons, networks of genes: An integrated view of memory consolidation. Neurobiol. Learn. Mem. 2008, 89, 269–284. [Google Scholar] [CrossRef]
- Wang, J.H.; Cheng, J.; Li, C.R.; Ye, M.; Ma, Z.; Cai, F. Modulation of Ca2+ signals by epigallocatechin-3-gallate (EGCG) in cultured rat hippocampal neurons. Int. J. Mol. Sci. 2011, 12, 742–754. [Google Scholar] [CrossRef]
- Bae, J.H.; Mun, K.C.; Park, W.K.; Lee, S.R.; Suh, S.I.; Baek, W.K.; Yim, M.B.; Kwon, T.K.; Song, D.K. EGCG attenuates AMPA-induced intracellular calcium increase in hippocampal neurons. Biochem. Biophys. Res. Commun. 2002, 290, 1506–1512. [Google Scholar]
- Konishi, T. Principal component analysis for designed experiments. BMC Bioinform. 2015, 16, S7. [Google Scholar] [CrossRef]
- Konishi, T. Three-parameter lognormal distribution ubiquitously found in cDNA microarray data and its application to parametric data treatment. BMC Bioinform. 2004, 5, 5. [Google Scholar] [CrossRef]
- Konishi, T. Microarray test results should not be compensated for multiplicity of gene contents. BMC Syst. Biol. 2011, 5, S6. [Google Scholar] [CrossRef] [PubMed]
- Konishi, T. Data distribution of short oligonucleotide expression arrays and its application to the construction of a generalized intellectual framework. Stat. Appl. Genet. Mol. Biol. 2008, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zha, Y.; Driessens, G.; Locke, F.; Gajewski, T.F. Transcriptional regulator early growth response gene 2 (Egr2) is required for T cell anergy in vitro and in vivo. J. Exp. Med. 2012, 209, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Shandilya, M.C.V.; Gautam, A. The temporal effect of hippocampal Arc in the working memory paradigm during novelty exploration. Brain Res. Bull. 2020, 158, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Helbling, J.C.; Minni, A.M.; Pallet, V.; Moisan, M.P. Stress and glucocorticoid regulation of NR4A genes in mice. J. Neurosci. Res. 2014, 92, 825–834. [Google Scholar] [CrossRef]
- Simjee, S.U.; Shaheen, F.; Choudhary, M.I.; Rahman, A.U.; Jamall, S.; Shah, S.U.; Khan, N.; Kabir, N.; Ashraf, N. Suppression of c-Fos protein and mRNA expression in pentylenetetrazole-induced kindled mouse brain by isoxylitones. J. Mol. Neurosci. 2012, 47, 559–570. [Google Scholar] [CrossRef]
- Chandra, A.; Lan, S.; Zhu, J.; Siclari, V.A.; Qin, L. Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. J. Biol. Chem. 2013, 288, 20488–20498. [Google Scholar] [CrossRef]
- Ibi, D.; Takuma, K.; Koike, H.; Mizoguchi, H.; Tsuritani, K.; Kuwahara, Y.; Kamei, H.; Nagai, T.; Yoneda, Y.; Nabeshima, T.; et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J. Neurochem. 2008, 105, 921–932. [Google Scholar] [CrossRef]
- Delépine, C.; Nectoux, J.; Letourneur, F.; Baud, V.; Chelly, J.; Billuart, P.; Bienvenu, T. Astrocyte transcriptome from the Mecp2(308)-truncated mouse model of Rett Syndrome. Neuromolecular Med. 2015, 17, 353–363. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds ...... are available from the authors. |
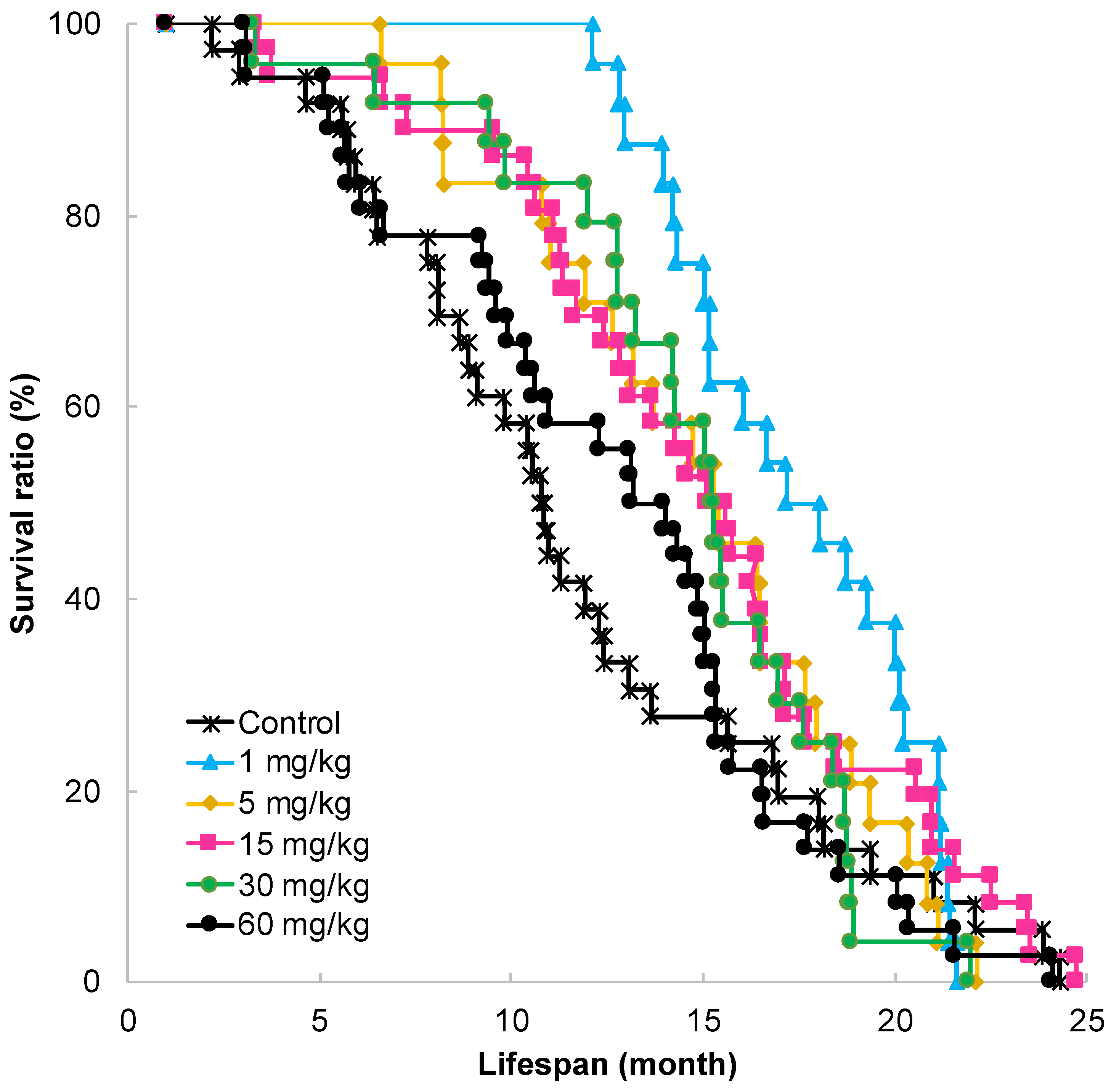
| GT-Catechin | MST (Months) | p-Value | |
|---|---|---|---|
| (mg/kg) | Month | Ratio | |
| 0 | 10.8 | 1.00 | - |
| 1 | 17.2 | 1.59 | 0.027 * |
| 5 | 15.3 | 1.42 | 0.272 |
| 15 | 15.3 | 1.42 | 0.082 |
| 30 | 15.3 | 1.42 | 0.364 |
| 60 | 13.6 | 1.26 | 0.880 |
| Ratio | p-Value | |
|---|---|---|
| 0 | 0.567 | — |
| 1 | 0.583 | 0.533 |
| 5 | 0.720 | 0.186 |
| 15 | 0.737 | 0.185 |
| 30 | 0.800 | 0.060 |
| 60 | 0.833 | 0.024 * |
| Symbol. | Full Name | ΔZ | p |
|---|---|---|---|
| Gh | growth hormone | 0.5621 | 4.55 × 10−7 |
| Egr2 | early growth response 2 | 0.3793 | 1.32 × 10−24 |
| Arc | activity regulated cytoskeletal-associated protein | 0.2996 | 2.49 × 10−35 |
| Nr4a1 | nuclear receptor subfamily 4, group A, member 1 | 0.2858 | 1.79 × 10−37 |
| Fos | FBJ osteosarcoma oncogene | 0.2497 | 4.31 × 10−20 |
| Egr1 | early growth response 1 | 0.2216 | 1.56 × 10−28 |
| Dusp1 | dual specificity phosphatase 1 | 0.2123 | 1.07 × 10−23 |
| Gem | GTP binding protein (gene overexpressed in skeletal muscle) | 0.1948 | 1.21 × 10−12 |
| Hspa1a | heat shock protein 1A | 0.1916 | 1.28 × 10−11 |
| Rtl1 | retrotransposon-like 1 | 0.1847 | 8.34 × 10−8 |
| Hspa1a | heat shock protein 1A | 0.1827 | 8.76 × 10−22 |
| Hspb1 | heat shock protein 1 | 0.1795 | 3.10 × 10−9 |
| Npas4 | neuronal PAS domain protein 4 | 0.1735 | 7.10 × 10−12 |
| Cyr61 | cysteine rich protein 61 | 0.1687 | 1.93 × 10−10 |
| Creld2 | cysteine-rich with EGF-like domains 2 | 0.1674 | 1.34 × 10−14 |
| Per1 | period homolog 1 (Drosophila) | 0.1671 | 5.43 × 10−15 |
| Unc13c | unc-13 homolog C (C. elegans) | 0.1559 | 4.13 × 10−5 |
| Hey2 | hairy/enhancer-of-split related with YRPW motif 2 | 0.1551 | 1.21 × 10−9 |
| Agxt2l | alanine-glyoxylate aminotransferase 2-like 1 | 0.1526 | 0.000375 |
| Sdf2l1 | stromal cell-derived factor 2-like 1 | 0.1503 | 1.09 × 10−8 |
| ΔZ = expression level (catechin–control) |
| Gene | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) | Ref. |
|---|---|---|---|
| Egr2 | CTACCCGGTGGAAGACCTC | AATGTTGATCATGCCATCTCC | [54] |
| Arc | ACGATCTGGCTTCCTCATTCTGCT | AGGTTCCCTCAGCATCTCTGCTTT | [55] |
| Nr4a1 | CTGCCTTCCTGGAACTCTTCA | CGGGTTTAGATCGGTATGCC | [56] |
| Fos | AAGTAGTGCAGCCCGGAGTA | CCAGTCAAGAGCATCAGCAA | [57] |
| Egr1 | CCTTCCAGTGTCGAATCTGCAT | ACAAATGTCACAGGCAAAAGGC | [58] |
| Npas4 | AGCATTCCAGGCTCATCTGAA | GGCGAAGTAAGTCTTGGTAGGATT | [59] |
| Cyr61 | CCCCCGGCTGGTGAAAGTC | ATGGGCGTGCAGAGGGTTGAAAAG | [60] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Unno, K.; Pervin, M.; Taguchi, K.; Konishi, T.; Nakamura, Y. Green Tea Catechins Trigger Immediate-Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening. Molecules 2020, 25, 1484. https://doi.org/10.3390/molecules25071484
Unno K, Pervin M, Taguchi K, Konishi T, Nakamura Y. Green Tea Catechins Trigger Immediate-Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening. Molecules. 2020; 25(7):1484. https://doi.org/10.3390/molecules25071484
Chicago/Turabian StyleUnno, Keiko, Monira Pervin, Kyoko Taguchi, Tomokazu Konishi, and Yoriyuki Nakamura. 2020. "Green Tea Catechins Trigger Immediate-Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening" Molecules 25, no. 7: 1484. https://doi.org/10.3390/molecules25071484
APA StyleUnno, K., Pervin, M., Taguchi, K., Konishi, T., & Nakamura, Y. (2020). Green Tea Catechins Trigger Immediate-Early Genes in the Hippocampus and Prevent Cognitive Decline and Lifespan Shortening. Molecules, 25(7), 1484. https://doi.org/10.3390/molecules25071484





