Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives
Abstract
1. Blood Coagulation: A Process Dependent on Vitamin K Wanted or Dreadful
2. Coagulation Pathways
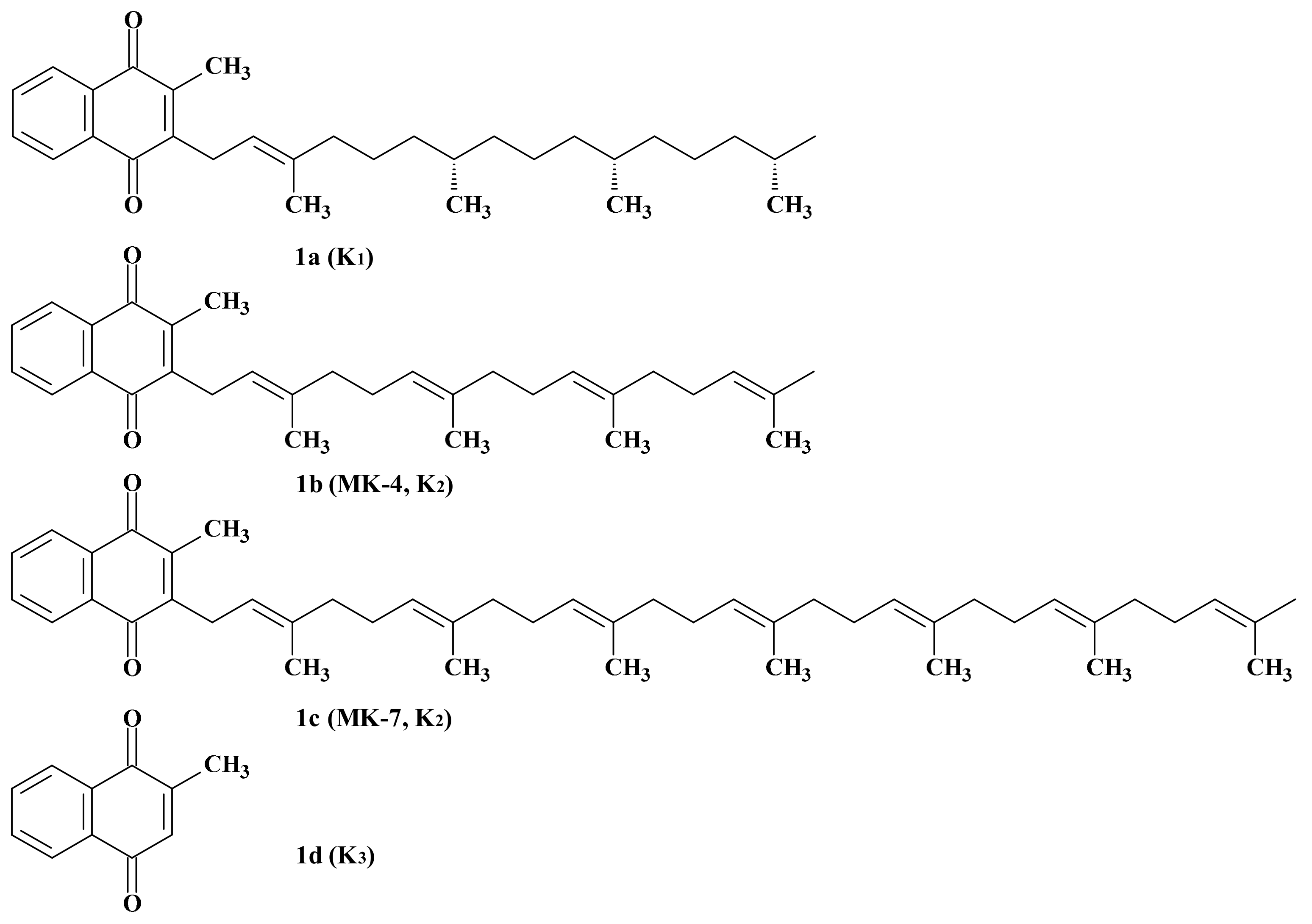
3. Members of the Vitamin K Family
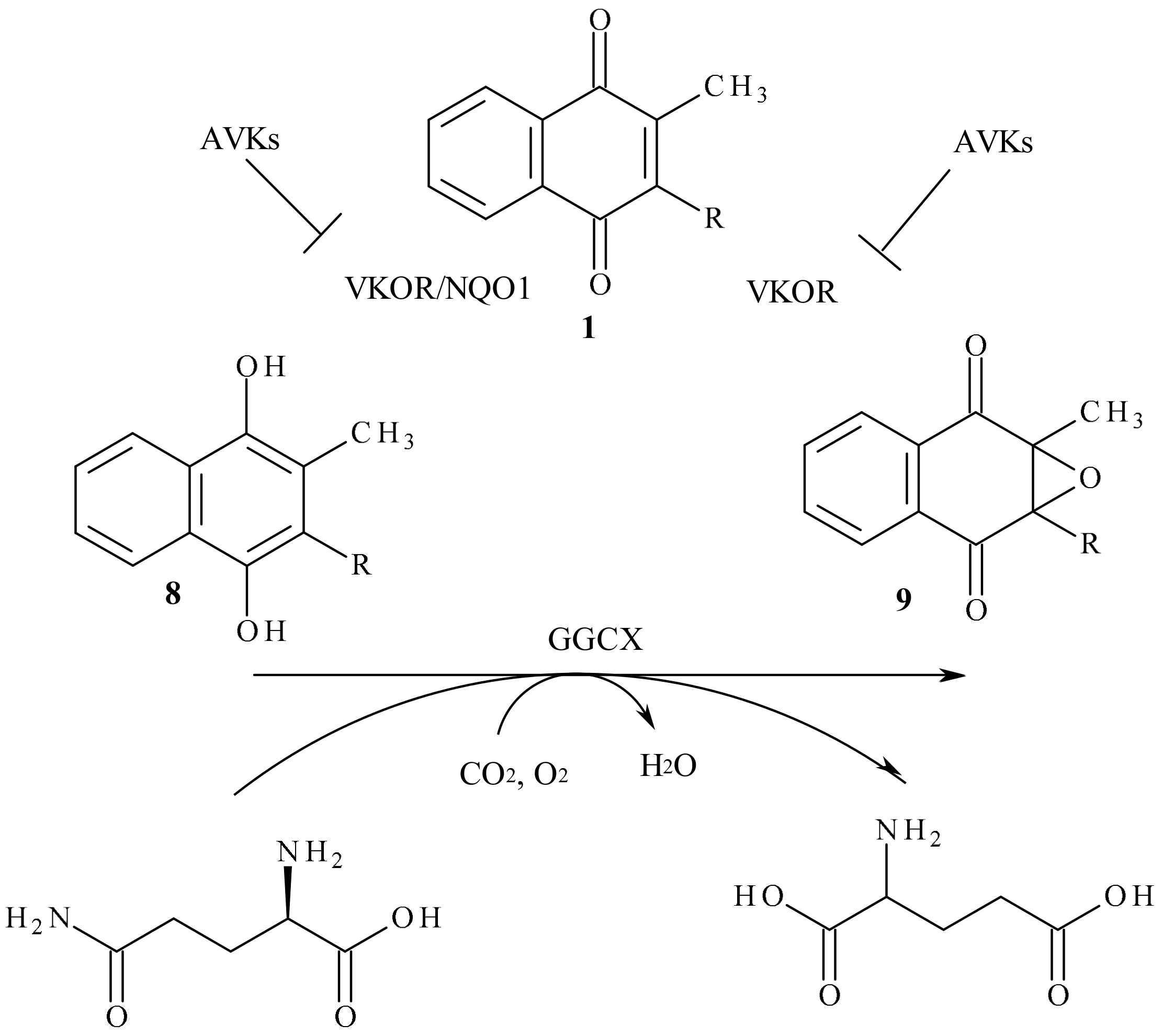
4. Vitamin K Oxide Reductase, VKOR
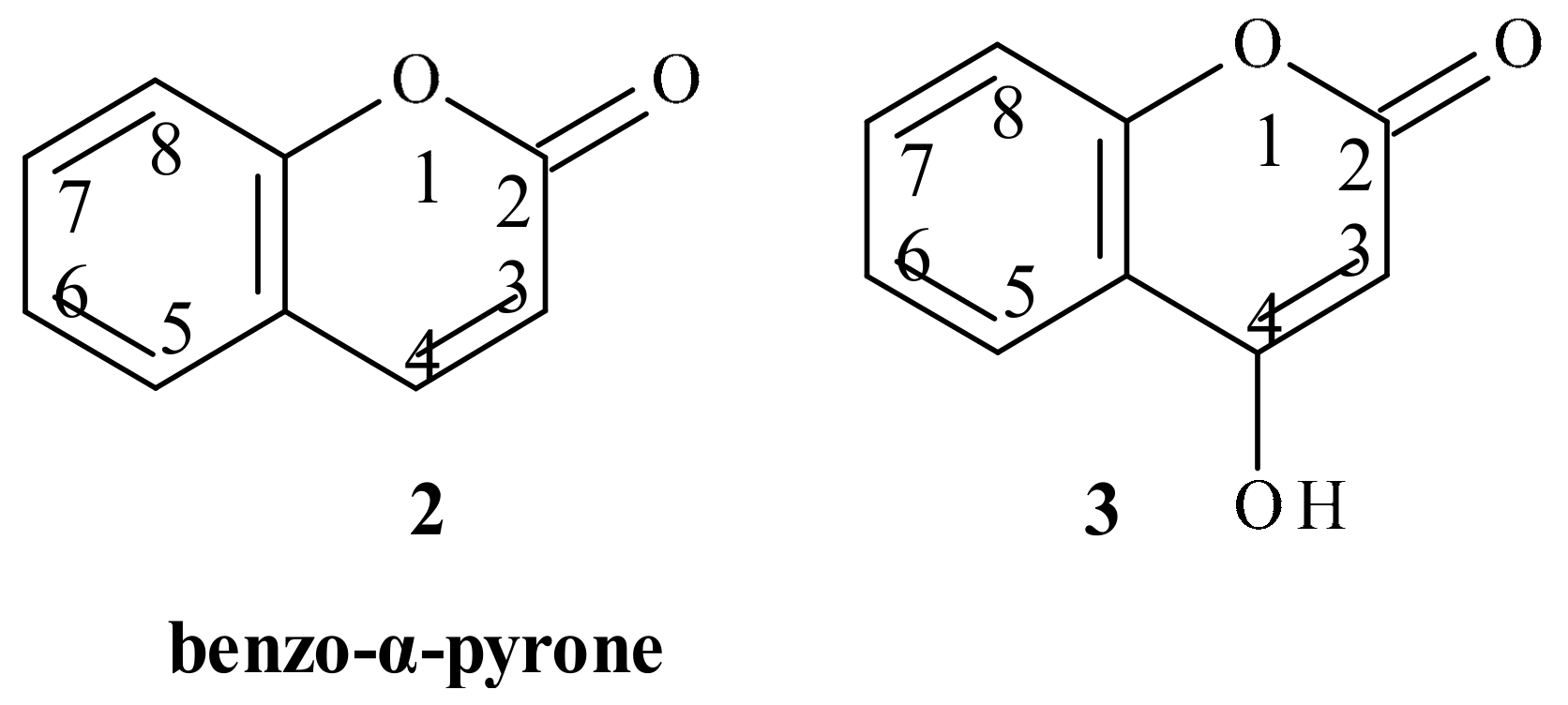
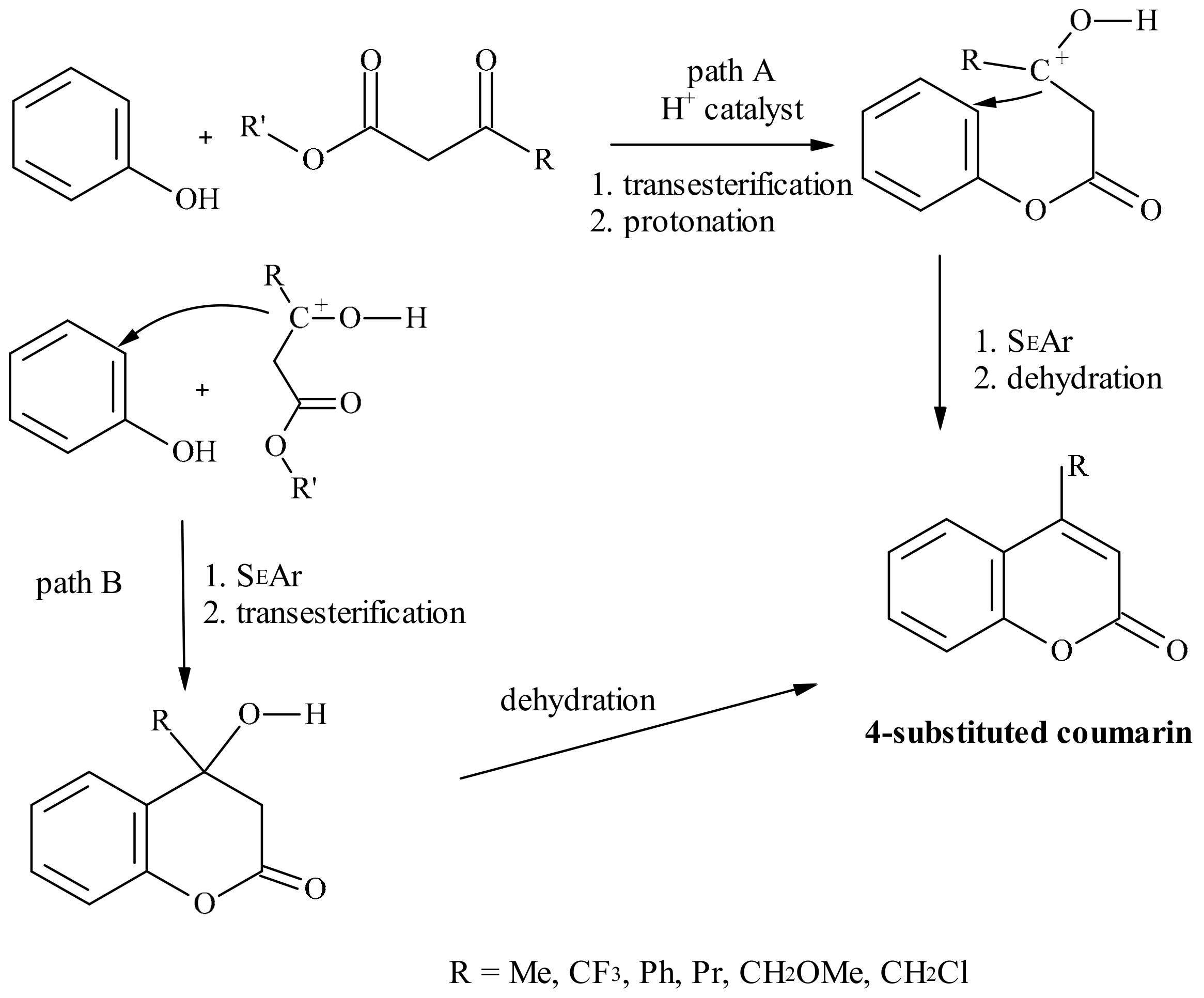
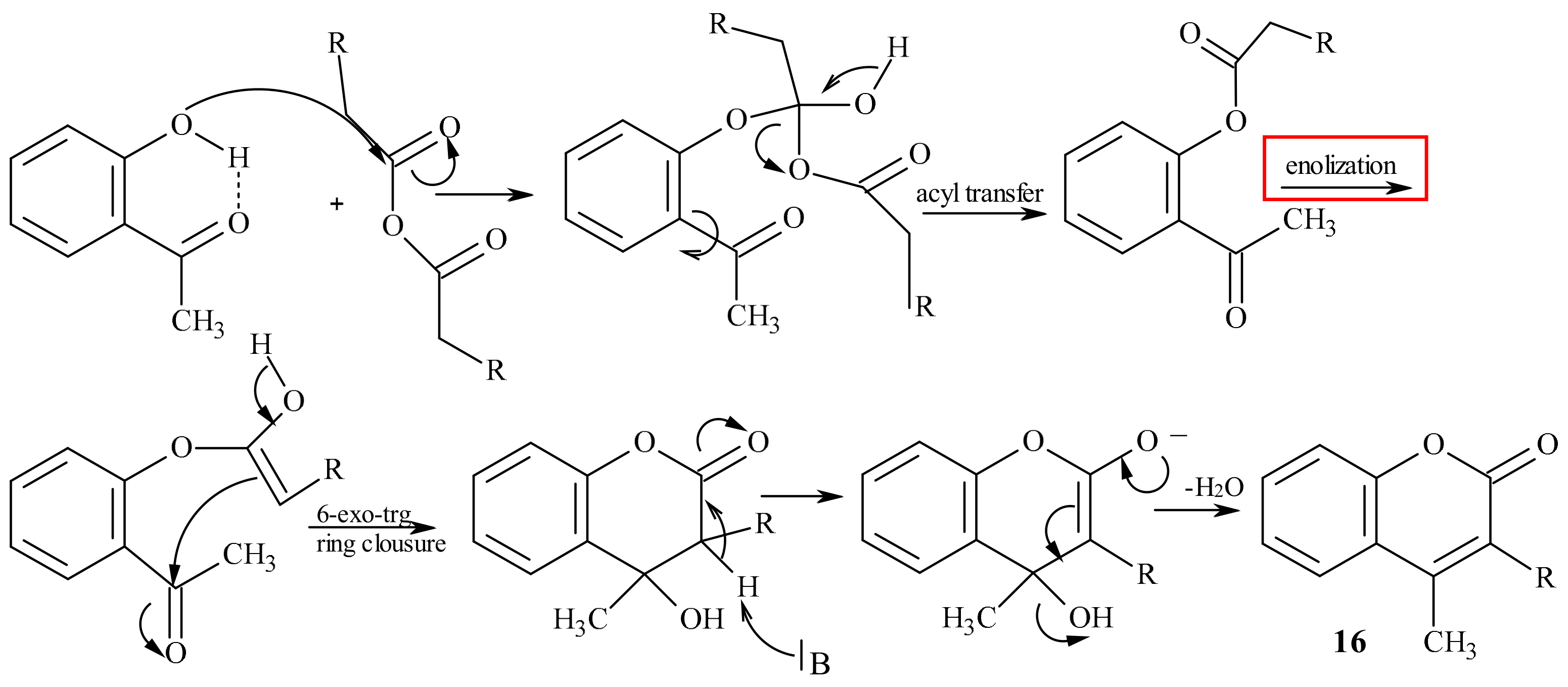
5. Coumarins: Synthesis, Structures, and Properties
5.1. Warfarin: Stereochemistry and Metabolism
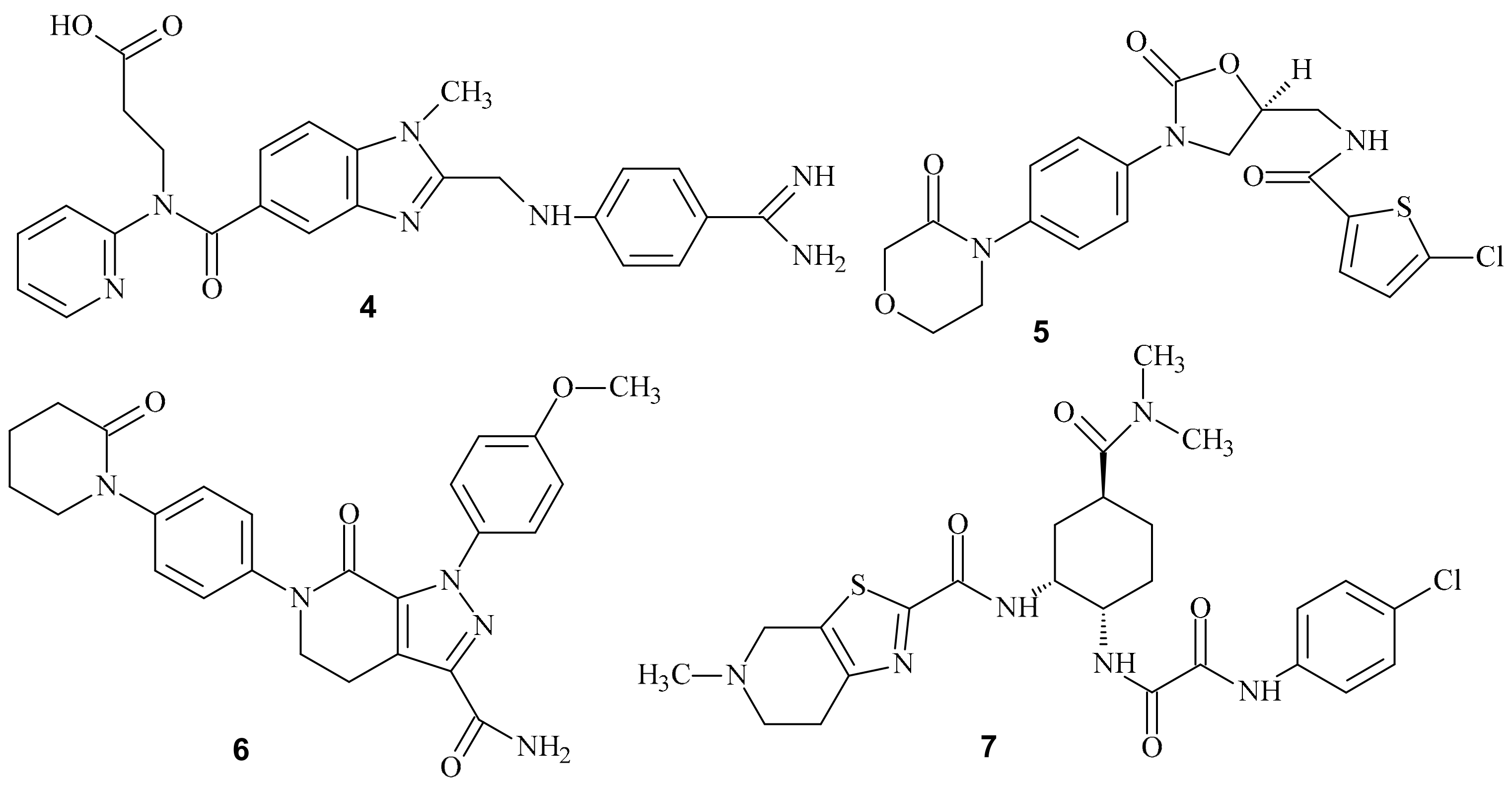
5.2. Analogs of Warfarin
6. Summary
Funding
Conflicts of Interest
References
- Andersson, T.; Magnuson, A.; Bryngelsson, I.L.; Frøbert, O.; Henriksson, K.M.; Edvardsson, N.; Poçi, D. All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995–2008: A Swedish nationwide long-term case - control study. Eur. Heart J. 2013, 34, 1061–1067. [Google Scholar] [CrossRef]
- Crowther, M.A.; Warkentin, T.E. Bleeding risk and the management of bleeding complications in patients undergoing anticoagulant therapy: Focus on new anticoagulant agents. Blood 2008, 111, 4871–4879. [Google Scholar] [CrossRef]
- Ask the expert: Common Questions about Alcohol and Blood Thinners. Available online: https://www.healthline.com/health/high-cholesterol/alcohol-blood-thinners-ate (accessed on 16 December 2019).
- Cropp, J.S.; Bussey, H.I. A review of enzyme induction of warfarin metabolism with recommendations for patient management. Pharmacotherapy 1997, 17, 917–928. [Google Scholar]
- Heit, J.A. The epidemiology of venous thromboembolism in the community. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 370–372. [Google Scholar] [CrossRef]
- Klok, F.A.; van Kralingen, K.W.; van Dijk, A.P.; Heyning, F.H.; Vliegen, H.W.; Kaptein, A.A.; Huisman, M.V. Quality of life in long-term survivors of acute pulmonary embolism. Chest 2010, 138, 1432–1440. [Google Scholar] [CrossRef]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galiè, N.; Gibbs, J.S.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. ESC guidelines for the diagnosis and management of acute pulmonary embolism in 2014. Working Group of the European Society of Cardiology (ESC) for the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3069. [Google Scholar]
- Agnelli, G.; Prandoni, P.; Becattini, C.; Silingardi, M.; Taliani, M.R.; Miccio, M.; Imberti, D.; Poggio, R.; Ageno, W.; Pogliani, E.; et al. Extended oral anticoagulant therapy after a first episode of pulmonary embolism. Ann. Intern. Med. 2003, 139, 19–25. [Google Scholar] [CrossRef]
- Campbel, I.A.; Bentley, D.P.; Prescott, R.J.; Routledge, P.A.; Shetty, H.G.; Williamson, I.J. Anticoagulation for three versus six months in patients with deep vein thrombosis or pulmonary embolism, or both: Randomised trial. BMJ 2007, 334, 674. [Google Scholar] [CrossRef]
- Schulman, S.; Kakkar, A.K.; Goldhaber, S.Z.; Schellong, S.; Eriksson, H.; Mismetti, P.; Christiansen, A.V.; Friedman, J.; Le Maulf, F.; Peter, N.; et al. Treatment of acute venous thromboembolism with dabigatran or warfarin and pooled analysis. Circulation 2014, 129, 764–772. [Google Scholar] [CrossRef]
- Buller, H.R.; Prins, M.H.; Lensin, A.W.; Prins, M.H.; Lensin, A.W.; Decousus, H.; Jacobson, B.F.; Minar, E.; Chlumsky, J.; Verhamme, P.; et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N. Engl. J. Med. 2012, 366, 1287–1297. [Google Scholar]
- Agnelli, G.; Buller, H.R.; Cohen, A.; Curto, M.; Gallus, A.S.; Johnson, M.; Masiukiewicz, U.; Pak, R.; Thompson, J.; Raskob, G.E.; et al. Oral apixaban for the treatment of acute venous thromboembolism. N. Engl. J. Med. 2013, 369, 799–808. [Google Scholar] [CrossRef]
- Buller, H.R.; Decousus, H.; Grosso, M.A.; Mercuri, M.; Middeldorp, S.; Prins, M.H.; Raskob, G.E.; Schellong, S.M.; Schwocho, L.; Segers, A.; et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N. Engl. J. Med. 2013, 369, 1406–1415. [Google Scholar]
- Brandjes, D.P.; Heijboer, H.; Buller, H.R.; de Rijk, M.; Jagt, H.; ten Cate, J.W. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N. Engl. J. Med. 1992, 327, 1485–1489. [Google Scholar] [CrossRef]
- Chugh, S.S.; Havmoeller, R.; Narayanan, K.; Singh, D.; Rienstra, M.; Benjamin, E.J.; Gillum, R.F.; Kim, Y.H.; McAnulty, J.H., Jr.; Zheng, Z.J.; et al. Worldwide epidemiology of atrial fibrillation: A Global Burden of Disease 2010 Study. Circulation 2014, 129, 837–847. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodrigez Muñoz, D.; et al. ESC/EACTS Guidelines for themanagement of valvular heart disease. The Task Force for the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart. J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef]
- Cannegieter, S.C.; Rosendaal, F.R.; Briët, E. Thromboembolic and bleeding complications in patients with mechanical heart valve prostheses. Circulation 1994, 89, 635–641. [Google Scholar] [CrossRef]
- Mok, C.K.; Boey, J.; Wang, R.; Chan, T.K.; Cheung, K.L.; Lee, P.K.; Chow, J.; Ng, R.P.; Tse, T.F. Warfarin versus dipyridamole-aspirin and pentoxifylline-aspirin for the prevention of prosthetic heart valve thromboembolism: A prospective randomized clinical trial. Circulation 1985, 72, 1059–1063. [Google Scholar] [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; Ferreira, R.; Foidart, J.M.; Gibbs, J.S.; Gohlke-Baerwolf, C.; Gorenek, B.; et al. 2018 ESC Guidelines for the management of cardiovascular diseases during pregnancy. Eur. Heart J. 2018, 39, 3165–3241. [Google Scholar] [CrossRef]
- Piccini, J.; Garg, J.; Patel, M.; Lokhnygina, Y.; Goodman, S.; Becker, R.; Berkowitz, S.D.; Breithardt, G.; Hacke, W.; Halperin, J.L.; et al. Management of major bleeding events in patients treated with rivaroxaban versus warfarin: Results from the ROCKET-AF trial. Eur. Heart J. 2014, 35, 1873–1880. [Google Scholar] [CrossRef]
- Connolly, S.J.; Ezekowitz, M.D.; Yusuf, S.; Eikelboom, J.; Oldgren, J.; Parekh, A.; Pogue, J.; Reilly, P.A.; Themeles, E.; Varrone, J.; et al. Dabigatran versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2009, 361, 1139–1151. [Google Scholar] [CrossRef]
- Granger, C.B.; Alexander, J.H.; McMurray, J.J.; Lopes, R.D.; Hylek, E.M.; Hanna, M.; Al-Khalidi, H.R.; Ansell, J.; Atar, D.; Avezum, A.; et al. Apixaban versus Warfarin in Patients with Atrial Fibrillation. N. Engl. J. Med. 2011, 365, 981–992. [Google Scholar] [CrossRef]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. ESC guidelines for the treatment of atrial fibrillation in 2016, developed in collaboration with EACTS. Working Group of the European Society of Cardiology (ESC) for treatment of atrial fibrillation. Eur. Heart. J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin K. In Present Knowledge in Nutrition, 10th ed.; Erdman, J.W., Jr., Macdonald, I.A., Zeisel, S.H., Eds.; Wiley-Blackwell: Ames, IA, USA, 2012; pp. 230–247. [Google Scholar]
- Stepien, E.; Wypasek, E.; Branicka, A.; Undas, A. Optimalisation of treatment with vitamin K antagonists-the role of gene polymorphisms. Cardiol. Pol. 2010, 68, S428–S435. [Google Scholar]
- Natarajan, S.; Ponde, C.K.; Rajani, R.M.; Jijina, F.; Gursahani, R.; Dhairyawan, P.P.; Ashavaid, T.F. Effect of CYP2C9 and VKORC1 genetic variations on warfarin dose requirements in Indian patients. Pharmacol. Rep. 2013, 65, 1375–1382. [Google Scholar] [CrossRef]
- Rishavy, M.A.; Hallgren, K.W.; Wilson, L.A.; Usubalieva, A.; Runge, K.W.; Berkner, K.L. The vitamin K oxidoreductase is a multimer that efficiently reduces vitamin K epoxide to hydroquinone to allow vitamin K-dependent protein carboxylation. J. Biol. Chem. 2013, 288, 31556–31566. [Google Scholar] [CrossRef]
- Tie, J.K.; Jin, D.Y.; Straight, D.L.; Stafford, D.W. Functional study of the vitamin K cycle in mammalian cells. Blood 2011, 117, 2967–2974. [Google Scholar] [CrossRef]
- Rishavy, M.A.; Usubalieva, A.; Hallgren, K.W.; Berkner, K.L. Novel Insight into the Mechanism of the Vitamin K Oxidoreductase (VKOR). J. Biol. Chem. 2011, 286, 7267–7278. [Google Scholar] [CrossRef]
- Sttaford, D.W. The vitamin K cycle. J. Thromb. Haemost. 2005, 3, 1873–1878. [Google Scholar] [CrossRef]
- Watzka, M.; Geisen, C.; Bevans, C.G.; Sittinger, K.; Spohn, G.; Rost, S.; Seifried, E.; Muller, C.R.; Oldenburg, J. Thirteen novel VKORC1 mutations associated with oral anticoagulant resistance: Insights into improved patient diagnosis and treatment. J. Thromb. Haemost. 2011, 9, 109–118. [Google Scholar] [CrossRef]
- Rost, S.; Fregin, A.; Hunerberg, M.; Bevans, C.G.; Muller, C.R.; Oldenburg, J. Site-directed mutagenesis of coumarin-type anticoagulant-sensitive VKORC1: Evidence that highly conserved amino acids define structural requirements for enzymatic activity and inhibition by warfarin. Thromb. Haemost. 2005, 94, 780–786. [Google Scholar]
- Pelz, H.J.; Rost, S.; Hunerberg, M.; Fregin, A.; Heiberg, A.C.; Baert, K.; MacNicoll, A.D.; Prescott, C.V.; Walker, A.S.; Oldenburg, J.; et al. The genetic basis of resistance to anticoagulants in rodents. Genetics 2005, 170, 1839–1847. [Google Scholar] [CrossRef]
- Rost, S.; Fregin, A.; Ivaskevicius, V.; Conzelmann, E.; Hörtnagel, K.; Pelz, H.J.; Lappegard, K.; Seifried, E.; Scharrer, I.; Tuddenham, E.G.; et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature 2004, 427, 537–541. [Google Scholar] [CrossRef]
- The personalized medicine report. Available online: http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/pmc_personalized_medicine_tests.pdf (accessed on 16 August 2016).
- Lacy, A.; O’ Kennedy, R. Studies on Coumarins and Coumarin-Related Compounds to Determine their Therapeutic Role in the Treatment of Cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef]
- Pechmann, V.H.; Duisberg, C. Neue Bildungsweise der Cumarine. Synthese des Daphnetins. Chem. Ber. 1884, 17, 929–979. [Google Scholar] [CrossRef]
- Murray, R.D.H. Naturally occurring plant coumarins. Prog. Chem. Org. Nat. Prod. 2002, 83, 1–119. [Google Scholar]
- Haskins, F.A.; Gorz, H.J. Glucosides of Coumarinic and o-Coumarinic Acids in the Tonka Bean. Science 1963, 139, 496–497. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef]
- Skoczyńska, A.; Pastuszko, A.; Budzisz, E. Coumarins – properties and application in cosmetology and medicine. Pol. J. Cosmetol. 2014, 17, 2–13. [Google Scholar]
- Bourgaud, F.; Hehn, A.; Larbat, R.; Doerper, S.; Gontier, E.; Kellner, S.; Matern, U. Biosynthesis of coumarins in plants: A major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem. Rev. 2006, 5, 293–308. [Google Scholar] [CrossRef]
- Hariprassad, V.; Talele, T.T.; Kulkarni, V.M. Design and synthesis of a novel series of nonpeptidic HIV-1 protease inhibitors. Pharm. Pharmacol. Commun. 1998, 4, 365–372. [Google Scholar]
- Kaye, P.T.; Musa, M.A.; Nichinda, A.T.; Nocanda, X.W. Novel Heterocyclic Analogues of the HIV-1 Protease Inhibitor, Ritonavir. Synth. Commun. 2004, 34, 2575–2589. [Google Scholar] [CrossRef]
- Tang, J.; Jones, S.A.; Jeffrey, J.L.; Miranda, S.R.; Galardi, C.M.; Irlbeck, D.M.; Brown, K.W.; McDanal, C.B.; Johns, B.A. Discovery of a novel and potent class of anti-HIV-1 maturation inhibitors with improved virology profile against gag polymorphisms. Bioorg. Med. Chem. Lett. 2017, 27, 2689–2694. [Google Scholar] [CrossRef]
- Xi, G.L.; Liu, Z.Q. Coumarin-Fused Coumarin: Antioxidant Story from N,N-Dimethylamino and Hydroxyl Groups. J. Agric. Food Chemistry. 2015, 63, 3516–3523. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Chatterjee, M.; Rao, K.B.; Singh, S.; Verma, A.K.; Palit, G. Discovery and synthesis of novel 3-phenylcoumarin derivatives as antidepressant agents. Bioorg. Med. Chem. Lett. 2011, 21, 1937–1941. [Google Scholar] [CrossRef]
- Rollinger, J.M.; Hornick, A.; Langer, T.; Stuppner, H.; Prast, H. Acetylcholinesterase inhibitory activity of scopolin and scopoletin discovered by virtual screening of natural products. J. Med. Chem. 2004, 47, 6248–6254. [Google Scholar] [CrossRef]
- Chen, S.; Cho, M.; Karlsberg, K.; Zhou, D.; Yuan, Y.C. Biochemical and biological characterization of a novel anti-aromatase coumarin derivative. J. Biol. Chem. 2004, 279, 48071–48078. [Google Scholar] [CrossRef]
- Cravotto, G.; Balliano, G.; Robaldo, B.; Oliaro-Bosso, S.; Chimichi, S.; Boccalini, M. Farnesyloxycoumarins, a new class of squalene-hopene cyclase inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 1931–1934. [Google Scholar] [CrossRef]
- Sardari, S.; Nishibe, S.; Daneshtalab, M. Coumarins, the bioactive structures with antifungal property. Stud. Nat. Prod. Chem. 2000, 23, 335–393. [Google Scholar]
- Klenkar, J.; Molnar, M. Natural and synthetic coumarins as potential anticancer agents. J. Chem. Pharm. Res. 2015, 7, 1223–1238. [Google Scholar]
- Refouvelet, B.; Guyon, C.; Jacquot, Y.; Girard, C.; Fein, H.; Bevalot, F.; Robert, J.-F.; Heyd, B.; Mantion, G.; Richert, L.; et al. Synthesis of 4-hydroxycoumarin and 2,4-quinolinediol derivatives and evaluation of their effects on the viability of HepG2 cells and human hepatocytes culture. Eur. J. Med. Chem. 2004, 39, 931–937. [Google Scholar] [CrossRef]
- Clark, G.S. Coumarin. An aroma chemical profile. Perfum. Flavor. 1995, 20, 23–34. [Google Scholar]
- Sekar, N. Coumarin dyes in laser technology. Colourage 2003, 50, 55–56. [Google Scholar]
- Brun, M.-P.; Bischoff, L.; Garbay, C. A very short route to enantiomerically pure coumarin-bearing fluorescent amino acids. Angew. Chem. Int. Ed. 2004, 43, 3432–3436. [Google Scholar] [CrossRef]
- Nian, L.; Zhang, W.; Zhu, N.; Liu, L.; Xie, Z.; Wu, H.; Wurthner, F.; Ma, Y. Photoconductive Cathode Interlayer for Highly Efficient Inverted Polymer Solar. Cells 2015, 137, 6995–6998. [Google Scholar] [CrossRef]
- Panetta, A.; Rapoport, H. New syntheses of coumarins. J. Org. Chem. 1982, 47, 946–950. [Google Scholar] [CrossRef]
- Jones, F.; Piermatti, O.; Pizzo, F. Simple and Efficient One-Pot Preparation of 3-Substituted Coumarins in Water. Heterocycles 1996, 43, 1257–1266. [Google Scholar]
- Maercker, A. The Wittig Reaction. Org. React. 1965, 14, 270–291. [Google Scholar]
- Mali, R.S.; Tilve, S.G. Useful Synthesis of Coumestans. Synth. Commun. 1990, 20, 1781–1791. [Google Scholar] [CrossRef]
- Upadhyay, P.K.; Kumar, P. A novel synthesis of coumarins employing triphenyl(α-carboxymethylene)- phosphorane imidazolide as a C-2 synthon. Tetrahedron Lett. 2009, 50, 236–238. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Adamopoulos, S.G.; Lefkaditis, D.A.; Litinas, K.E. Reactions of ortho-Quinones with Ethoxycarbonylmethylene(triphenyl)-phosphorane. Trapping of the ortho- Quinone Methanide Intermediates. J. Chem. Soc. Perkin Trans. 1990, 1, 2127–2132. [Google Scholar] [CrossRef]
- Nicolaides, D.N.; Gautam, D.R.; Litinas, K.E.; Papamehael, T. Synthesis of some 3,4-dihydro-2H-benzo[f]pyrano[2,3-h]chromen-6-one derivatives. J. Chem. Soc. Perkin Trans. 2002, 1, 1455–1460. [Google Scholar] [CrossRef]
- Perkin, W.H. On propionic coumarin and some of its derivatives. J. Chem. Soc. 1875, 28, 10–11. [Google Scholar] [CrossRef]
- Shriner, R.L. The Reformatsky Reaction. Org. React. 1942, 1, 15–46. [Google Scholar]
- Kostanecki, S.; Różycki, A. A formation of chromone derivatives. Ber. Dtsch. Chem. Ges. 1901, 34, 102–112. [Google Scholar]
- Heravi, M.M.; Khaghaninejad, S.; Mostofi, M. Pechmann reaction in the synthesis of coumarin derivatives. J. Adv. Heterocycl. Chem. 2014, 112, 1–50. [Google Scholar]
- Tyndall, S.; Fai, W.K.; VanAlstine-Parris, M.A. Insight into the Mechanism of the Pechmann Condensation Reaction Using NMR. J. Org. Chem. 2015, 80, 8951–8953. [Google Scholar] [CrossRef]
- Khalafi-Nezhad, A.; Haghighi, S.M.; Panahi, F. Nano-TiO2 on dodecylsulfated silica: As an efficient heterogeneous Lewis acid-surfactant combined catalyst (HLASC) for reaction in aqueous media. ACS Sustain. Chem. Eng. 2013, 1, 1015–1023. [Google Scholar] [CrossRef]
- Khaligh, N.G. Ultrasound-assisted one-pot synthesis of substituted coumarins catalyzed by poly(4-vinylpyridinium) hydrogen sulfate as an efficient and reusable solid acid catalyst. Ultrason. Sonochem. 2013, 20, 1062–1068. [Google Scholar] [CrossRef]
- Jadhav, N.H.; Sakate, S.S.; Rasal, N.K.; Shinde, D.R.; Pawar, R.A. Heterogeneously catalyzed Pechmann condensation employing the tailored Zn0.925Ti0.075O NPs: Synthesis of coumarin. ACS Omega 2019, 4, 8522–8527. [Google Scholar] [CrossRef]
- Shirini, F.; Yahyazadeh, A.; Mohammadi, K. A solvent-free synthesis of coumarins using 1,3-disulfonic acid imidazolium hydrogen sulfate as a reusable and effective ionic liquid catalyst. Res. Chem. Intermed. 2015, 41, 6207–6218. [Google Scholar] [CrossRef]
- EL-Dafrawy, S.M.; Hassan, S.M.; Farag, M. Kinetics and mechanism of Pechmann condensation reaction over sulphated zirconia-supported zinc oxide. J. Mater. Res. Technol. 2020, 9, 13–21. [Google Scholar] [CrossRef]
- Singh, V.; Singh, J.; Kaur, P.; Kad, G.L. Acceleration of the Pechmann reaction by microwave irradiation: Application to the preparation of coumarin. J. Chem. Research (S) 1997, 2, 58–59. [Google Scholar] [CrossRef]
- Puri, S.; Kaur, B.; Parmar, A.; Kumar, H. Ultrasound-promoted greener synthesis of 2H-chromen-2-ones catalyzed by copper perchlorate in solventless media. Ultrason. Sonochem. 2009, 16, 705–707. [Google Scholar] [CrossRef]
- Prousis, K.C.; Avlonitis, N.; Heropoulos, G.A.; Calogeropoulou, T. FeCl3-catalysed ultrasonic-assisted, solvent-free synthesis of 4-substituted coumarins. A useful complement to the Pechmann reaction. Ultrason. Sonochem. 2014, 21, 937–942. [Google Scholar] [CrossRef]
- Zambare, A.S.; Khan, F.A.K.; Zambare, S.P.; Shinde, S.D.; Sangshetti, J.N. Recent advances in the synthesis of coumarin derivatives via Pechmann condensation. Curr. Org. Chem. 2016, 20, 798–828. [Google Scholar] [CrossRef]
- Ansary, I.; Taher, A. One-Pot Synthesis of Coumarin Derivatives. In Phytochemicals in Human Health; Rao, V., Mans, D., Rao, L., Eds.; IntechOpen: London, UK, 2019; pp. 1–34. [Google Scholar]
- Sashidhara, K.V.; Kumar, A.; Kumar, M.; Sonkar, R.; Bhatia, G.; Khanna, A.K. Novel coumarin derivatives as potential antidyslipidemic agents. Bioorg. Med. Chem. Lett. 2010, 20, 4248–4251. [Google Scholar] [CrossRef]
- Williams, A.C.; Camp, N. Science of Synthesis: Houben-Weyl Methods of Molecular Transformations; Bellus, D., Ley, S.V., Noyori, R., Regitz, M., Schaumann, E., Shinkai, E., Thomas, E.J., Trost, B.M., Reider, P.J., Thomas, E.J., Eds.; Thieme: Stuttgart, Germany, 2004; pp. 347–438. [Google Scholar]
- Baker, W.; Robinson, R. CCLXVI.-Synthetical Experiments in the isoFlavone Group. Part, I. J. Chem. Soc. 1925, 127, 1981–1986. [Google Scholar] [CrossRef]
- Baker, W.; Eastwood, F.M. Colloidal phenoxides. Part, I. The relation between constitution and colloidal properties in benzo-γ-pyrones. J. Chem. Soc. 1929, 2897–2907. [Google Scholar] [CrossRef]
- Heilbron, I.M.; Hey, D.H.; Lythgoe, B. Studies in the pyrone series. Part III. Influence of the phenyl group in Kostanecki reaction. J. Chem. Soc. 1936, 295–300. [Google Scholar] [CrossRef]
- Neelakantan, S.; Raman, P.V.; Tinabaye, A. A new and convenient synthesis of 4-methyl-3-phenyl-coumarins and 3-phenylcoumarins. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1982, 21, 256–257. [Google Scholar]
- Yamaguchi, S.; Saitoh, M.; Kawase, Y. Some fatty acids having an O-heterocycle in their terminal positions. II. ω-(3-Coumarinyl)alkanoic acids and ω-(2-chromonyl)alkanoic acids. J. Heterocycl. Chem. 1991, 28, 125–127. [Google Scholar] [CrossRef]
- Mentzer, C.; Meunier, P.; Antivitamins, K.I. Hemorrhagic activity and nature of the heterocyclic ring. Bull. Soc. Chim. Fr. 1943, 25, 379–383. [Google Scholar]
- Stahmann, M.A.; Wolff, I.; Link, K.P. Studies on 4-Hydroxycoumarins. I. The Synthesis of 4-Hydroxycoumarins. J. Am. Chem. Soc. 1943, 65, 2285–2287. [Google Scholar] [CrossRef]
- Silverman, R.B. Model studies for a molecular mechanism of action of oral anticoagulants. J. Am. Chem. Soc. 1981, 103, 3910–3915. [Google Scholar] [CrossRef]
- Gandhidasan, R.; Neelakantan, S.; Raman, P.V.; Sripathi, S.K. A new one - step synthesis of 3-aryl-4-hydroxycoumarins. Indian J. Chem. Sect. B Org. Chem. Incl. Med. Chem. 1988, 27, 849. [Google Scholar]
- Lokhande, P.D.; Ghiya, B. Novel method in synthesis of 3-phenyl-4-styryl-hydroxycoumarin and 3-phenyl-4-hydroxycoumarins – formation of 3-phenylacetic acid benzisoxazole from 3-phenyl-4-hydroxycoumarin and NH2OH·HCl. J. Indian Chem. Soc. 1989, 66, 314–315. [Google Scholar] [CrossRef]
- Da Re, P.; Sandri, E. Über eine neue Synthese der 4-Hydroxy-cumarine. Chem. Ber. 1960, 93, 1085–1088. [Google Scholar]
- Chatterjee, A.K.; Toste, F.D.; Goldberg, S.D.; Grubbs, R.H. Synthesis of coumarins by ring-closing metathesis. Pure Appl. Chem. 2003, 75, 421–425. [Google Scholar] [CrossRef][Green Version]
- Nicolaou, K.C.; Pfefferkorn, J.A.; Roecker, A.J.; Cao, G.-Q.; Barluenga, S.; Mitchell, H.J. Natural product-like combinational libraries based on privileged structures 2. Constructions of a 10,000-membered benzopyran library by directed split-and-pool chemistry using NanoKans and optical encoding. J. Am. Chem. Soc. 2000, 122, 9939–9953. [Google Scholar] [CrossRef]
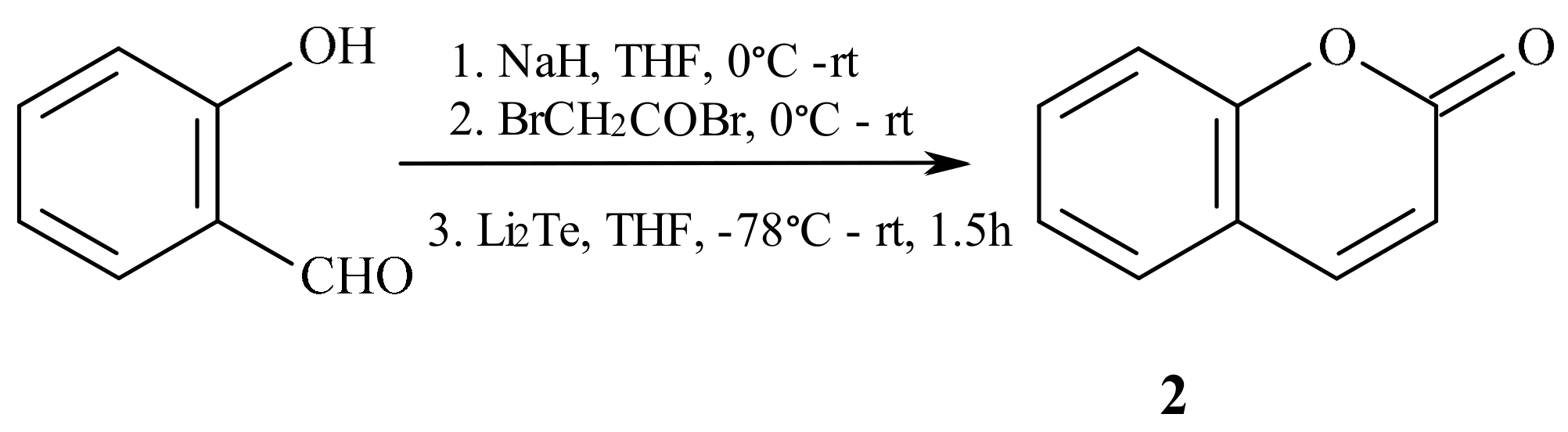
- Ditmer, D.C.; Li, Q.; Avilov, D.V. Synthesis of coumarins, 4-hydroxycoumarins, and 4-hydroxyquinolinones by tellurium-triggered cyclizations. J. Org. Chem. 2005, 70, 4682–4686. [Google Scholar] [CrossRef]
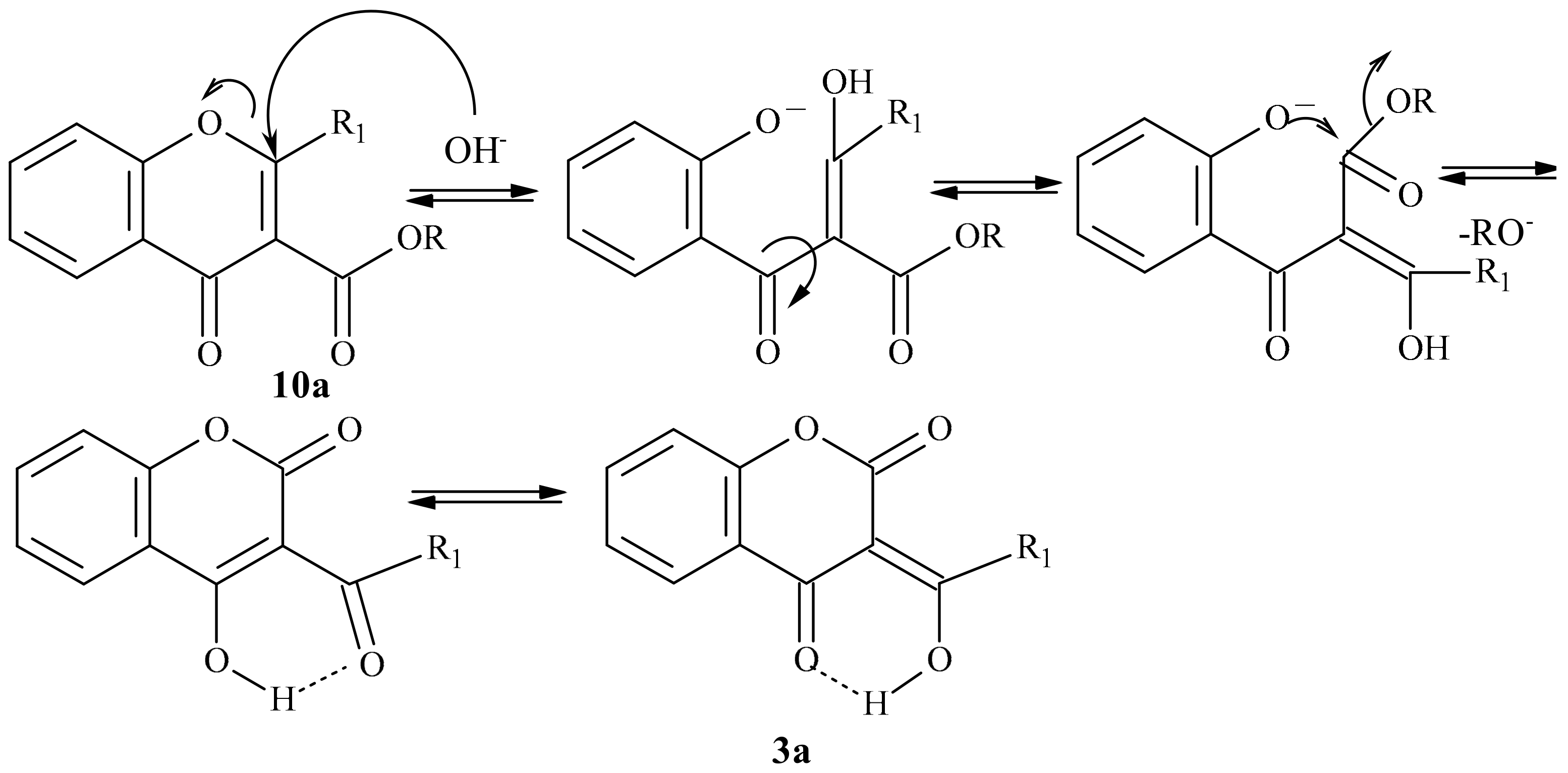
- Klutchko, S.; Shavel, J.; von Strandtmann, M. Base Rearrangement of chromone-3-carboxylic esters to 3-acyl-4-hydroxycoumarins. J. Org. Chem. 1974, 39, 2436–2437. [Google Scholar] [CrossRef]
- Abdou, M.M. 3-Acetyl-4-hydroxycoumarin: Synthesis, reactions and applications. Arabian J. Chem. 2017, 10, 3664–3675. [Google Scholar] [CrossRef]
- Atrial Fibrillation and New Oral Anticoagulant Drugs. Available online: http://www.fda.gov/Drugs/NewsEvents/ucm405148.htm (accessed on 10 March 2016).
- Hirsh, J.; Dalen, J.; Anderson, D.R.; Poller, L.; Bussey, H.; Ansell, J.; Deykin, D. Oral anticoagulants: Mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest 2001, 119, 8S–21S. [Google Scholar] [CrossRef]
- Inoue, T.; Nitta, K.; Sugihara, K.; Horie, T.; Kitamura, S.; Ohta, S. CYP2C9-Catalyzed Metabolism of S-Warfarin to 7-Hydroxywarfarin in Vivo and in Vitro in Chimeric Mice with Humanized Liver. Drug. Metab. Dispos. 2008, 36, 2429–2433. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, S.; Chan, E. Effect of Coenzyme Q10 on Warfarin Hydroxylation in Rat and Human Liver Microsomes. Curr. Drug Metab. 2005, 6, 67–81. [Google Scholar] [CrossRef]
- Guasch, L.; Peach, M.L.; Nicklaus, M.C. Tautomerism of Warfarin: Combined Chemoinformatics, Quantum Chemical, and NMR Investigation. J. Org. Chem. 2015, 80, 9900–9909. [Google Scholar] [CrossRef]
- Borges, F.; Roleira, F.; Milhazes, N.; Santana, L.; Uriarte, E. Simple Coumarins and Analogues in Medicinal Chemistry: Occurrence, Synthesis and Biological Activity. Curr. Med. Chem. 2005, 12, 887–916. [Google Scholar] [CrossRef]
- Mayer, G.A.; Conell, W.F. The Anticoagulant Effect of a New Coumarin Derivative—Sintrom (Geigy) and Its Control by Standardized Clotting Time. Can, Med. Assoc. J. 1957, 76, 272–278. [Google Scholar]
- Prior, I.A.M. Anticoagulant Studies with “Marcoumar”, A New Coumarin Derivative. Br. Med. J. 1955, 2, 944–946. [Google Scholar] [CrossRef][Green Version]
- Abdelhafez, O.M.; Amin, K.M.; Batran, R.Z.; Maher, T.J.; Nada, S.A. Sethumadhavan, S. Synthesis, anticoagulant and PIVKA-II induced by new 4-hydroxycoumarin derivatives. Bioorg. Med. Chem. 2010, 18, 3371–3378. [Google Scholar]
- Garazd, Y.L.; Kornienko, E.M.; Maloshtan, L.N.; Garazd, M.M.; Khilya, V.P. Modified Coumarins. 17. Synthesis and Anticoagulant Activity of 3,4-Cycloannelated Coumarin D-Glycopyranosides. Chem. Nat. Comp. 2005, 41, 508–512. [Google Scholar] [CrossRef]
- Thumber, B.L.; Vasoya, V.G.; Desai, T.R.; Naliapara, Y.T.; Shah, K.V.; Tirgar, P.R. Anticoagulant activity of methylated coumarin derivatives. Pharm. Online 2 2011, 1010–1017. [Google Scholar]
- Kasperkiewicz, K.; Malecka, M.; Ponczek, M.B.; Nowak, P.; Budzisz, E. Design, synthesis, X-ray structures of the new coumarin derivatives and perspectives of binding them to albumin and vitamin K epoxide reductase complex subunit 1. Cryst. Growth Des. 2016, 16, 456–466. [Google Scholar] [CrossRef]
- Lei, L.; Xue, Y.; Liu, Z.; Peng, S.; He, Y.; Zhang, Y.; Fang, R.; Wang, J.P.; Luo, Z.W.; Yao, G.M.; et al. Coumarin derivatives from Ainsliaea fragrans and their anticoagulant activity. Sci. Rep. 2015, 5, 13544. [Google Scholar] [CrossRef]
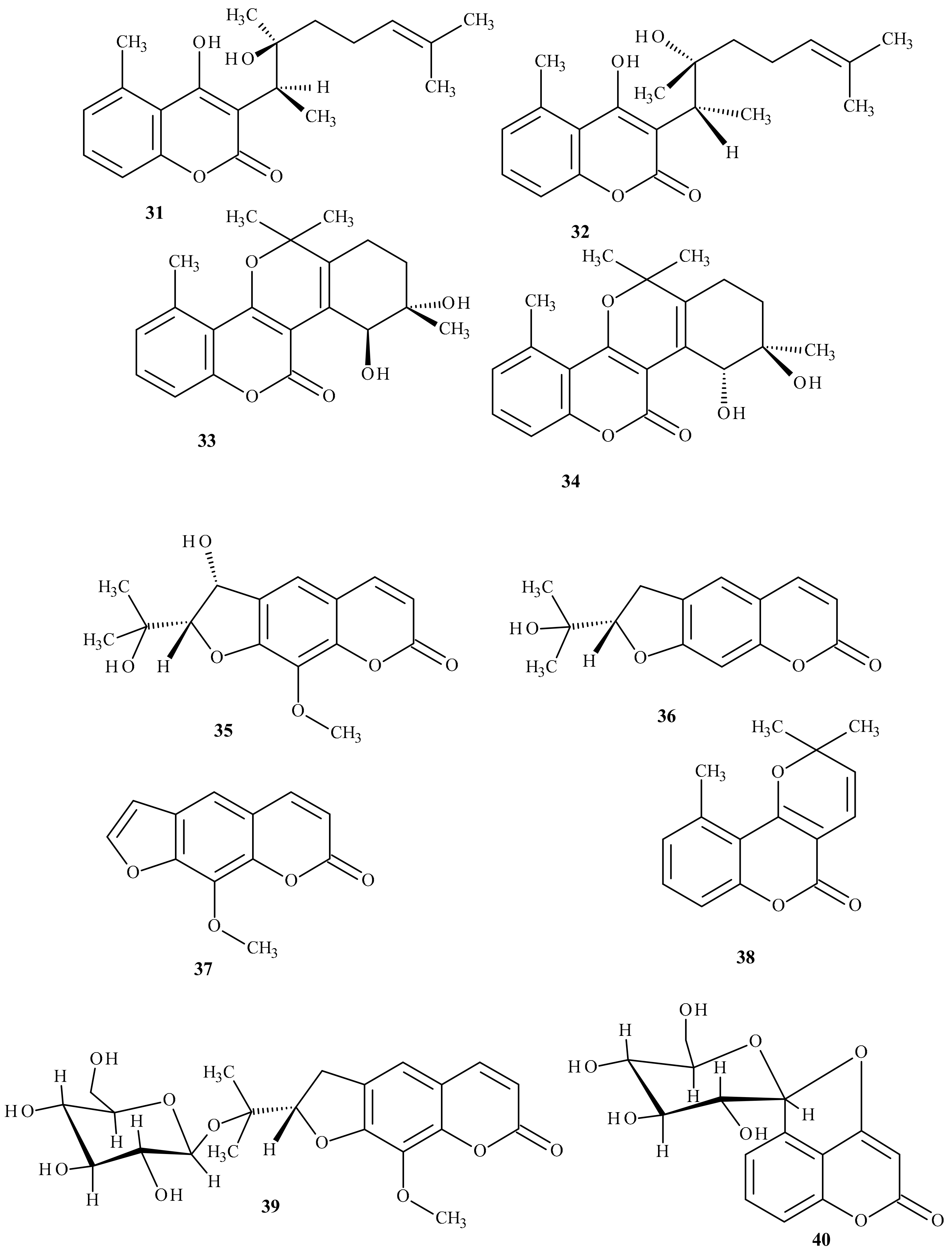
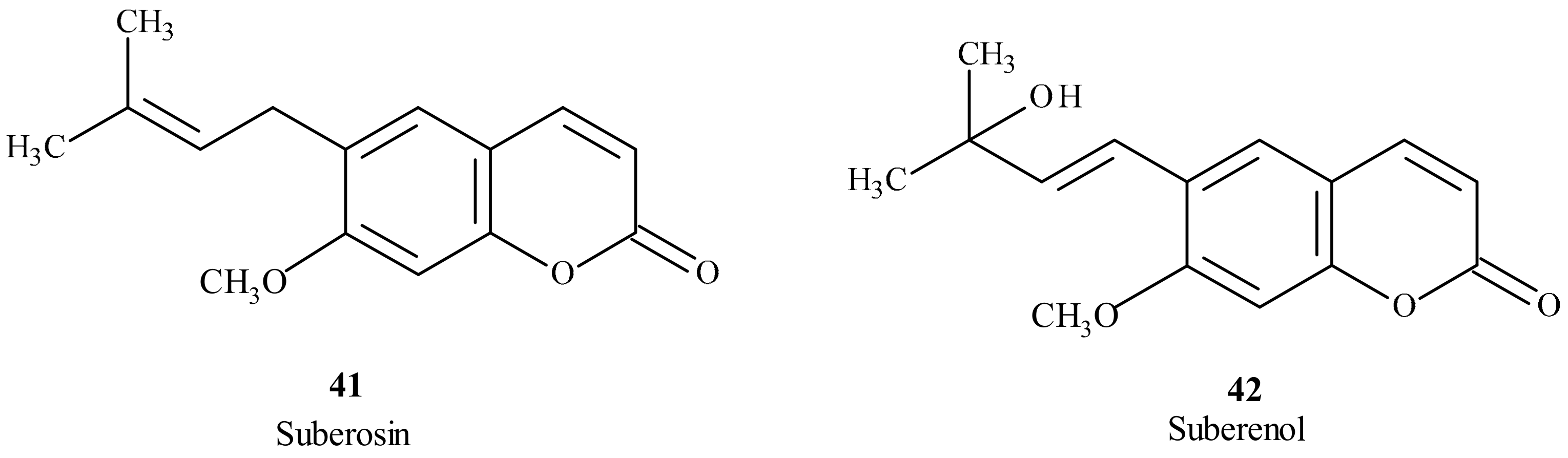
- Golfakhrabadi, F.; Abdollahi, M.; Ardakani, M.R.; Saeidnia, S.; Akbarzadeh, T.; Ahmadabadi, A.N.; Ebrahimi, A.; Yousefbeyk, F.; Hassanzadeh, A.; Khanavi, M. Anticoagulant activity of isolated coumarins (suberosin and suberenol) and toxicity evaluation of Ferulago carduchorum in rats. Pharm. Biol. 2014, 52, 1335–1340. [Google Scholar] [CrossRef]
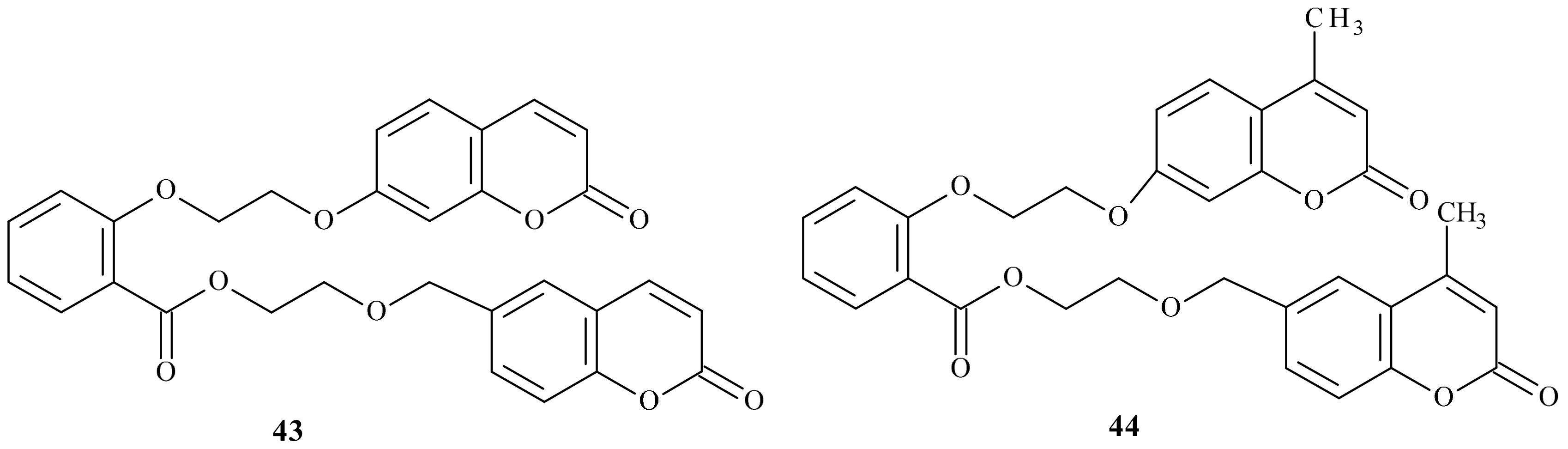
- Bang, N.C.; Abyshev, A.Z.; Ivkin, D.Y. Synthesis and in vivo evaluation of new coumarin conjugates as potential indirect-action anticoagulants. Pharm. Chem. J. 2019, 53, 419–422. [Google Scholar] [CrossRef]
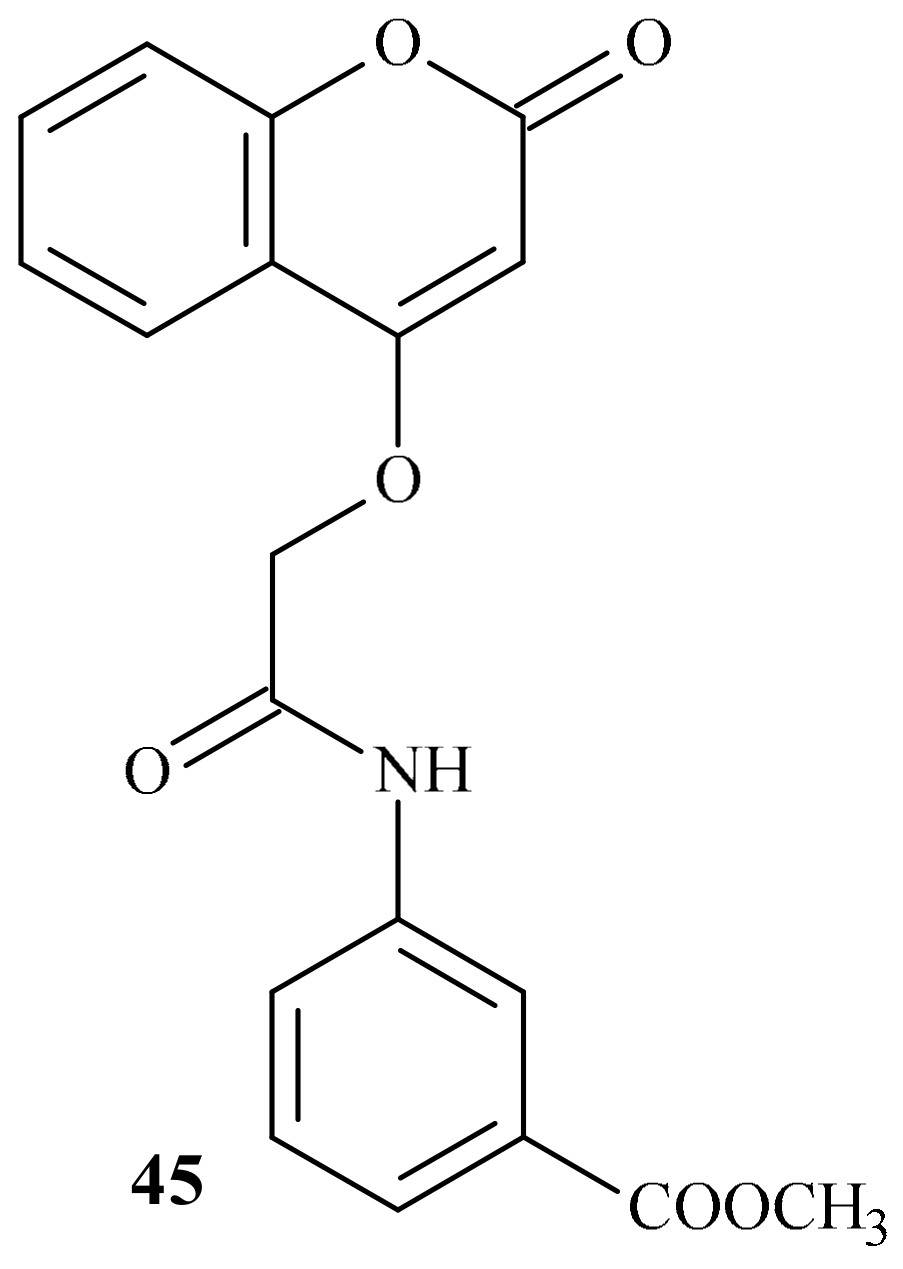
- Venkatramaiah, T.; Prashantha Kumar, B.R. Design, synthesis and anticoagulant activity of some novel coumarins. Asian J. Pharm. Anal. Med. Chem. 2018, 6, 105–122. [Google Scholar]
- Govindappa, M.; Chandrashekhar, N.; Channabasava, B.P. Anticoagulant activity of partially purified coumarin(s) extracts of Sonchus oleraceus. Adv. Med. Plant Res. 2015, 3, 87–91. [Google Scholar]
- Mira, A.; Alkhiary, W.; Shimizu, K. Antiplatelet and Anticoagulant Activities of Angelica shikokiana Extract and Its Isolated Compounds. Clin. Appl. Thromb/Hemost. 2017, 23, 91–99. [Google Scholar] [CrossRef]
- Hashim, F.J.; Hussain, S.M.; Shawkat, M.S. Separation, Characterization and Anticoagulant Activity of Coumarin and its Derivatives Extracted from Melilotus officinalis. Biosci. Biotech. Res. Asia 2017, 14. [Google Scholar] [CrossRef]
- Durić, K.; Kovac Besovic, E.E.; Niksic, H.; Muratovic, S.; Sofic, E. Anticoagulant activity of some Artemisia dracunculus leaf extracts. Bosn. J. Basic Med. Sci. 2015, 15, 9–14. [Google Scholar] [CrossRef]
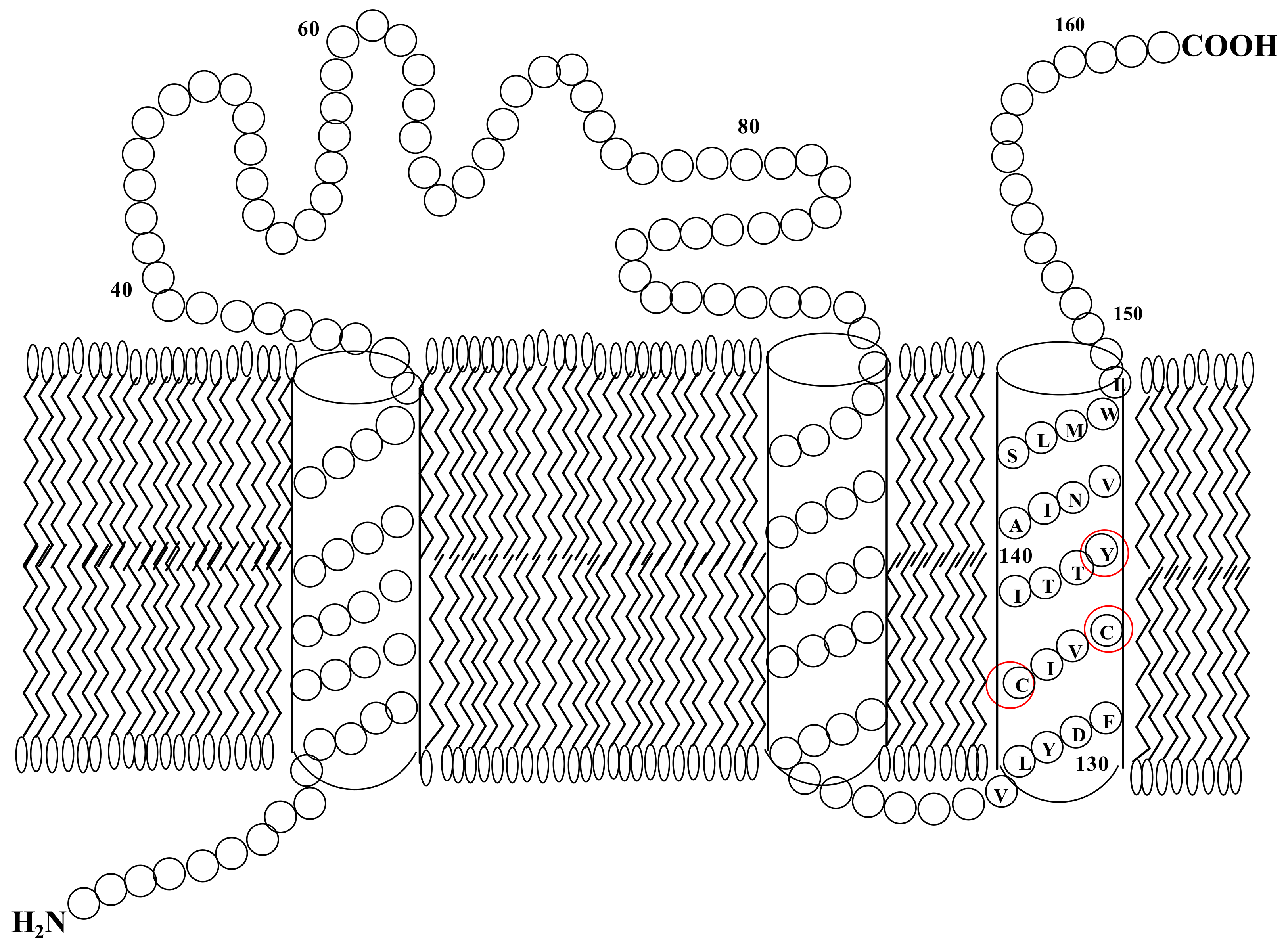
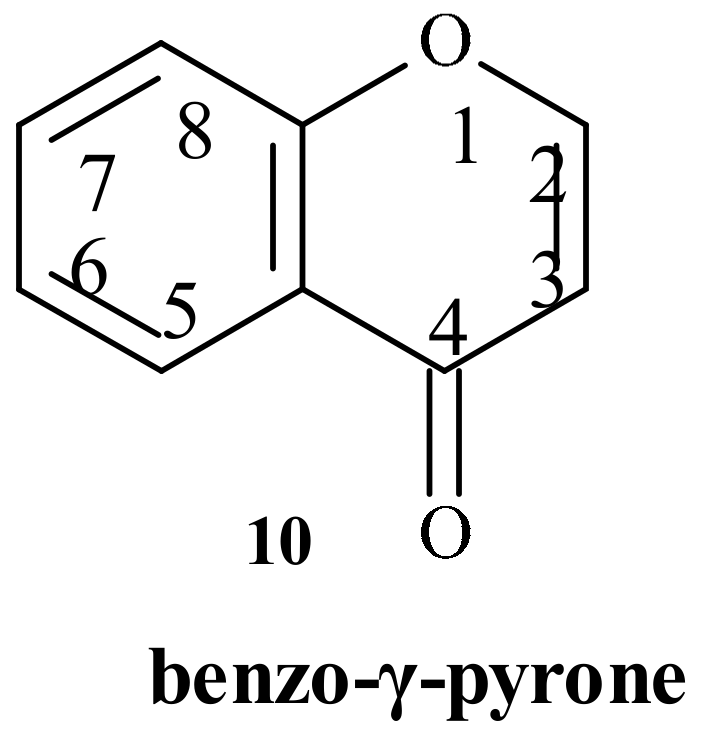
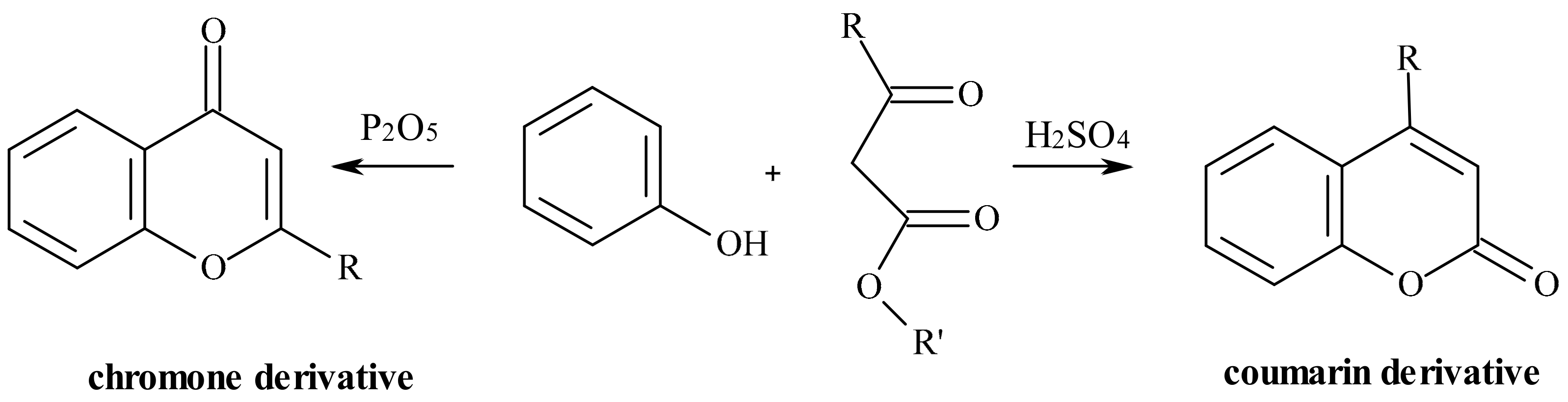

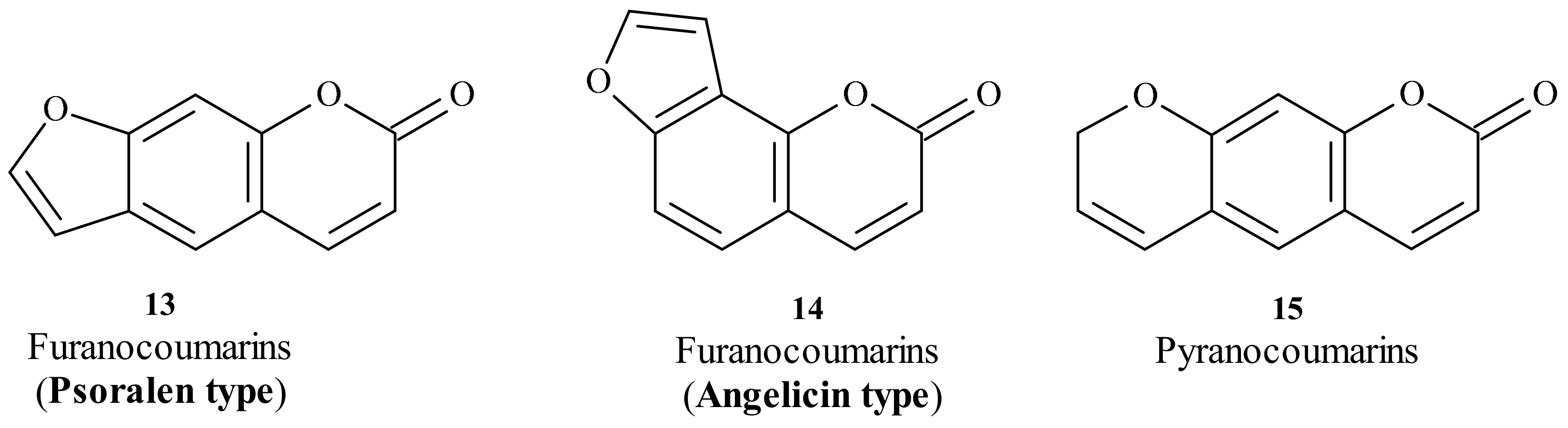
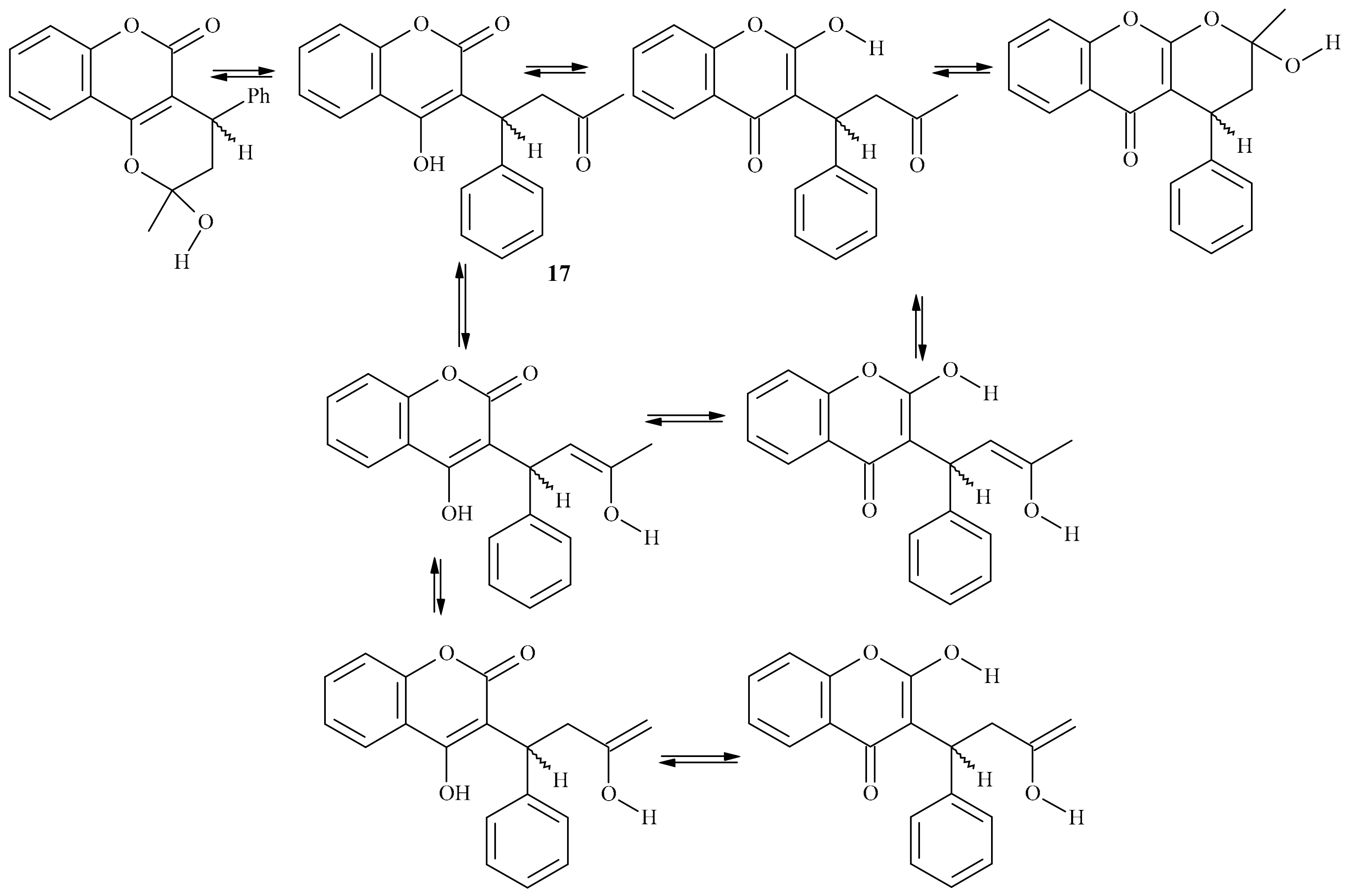
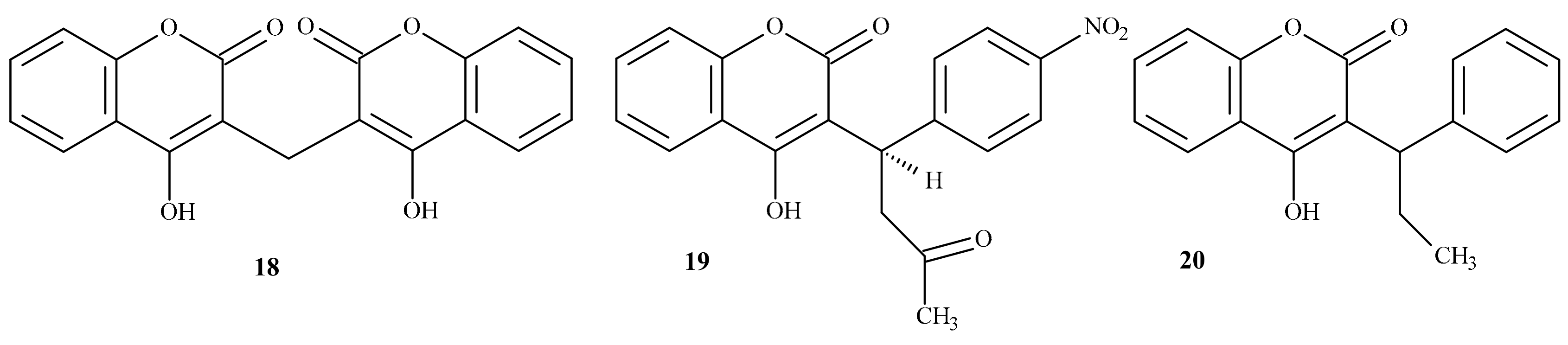
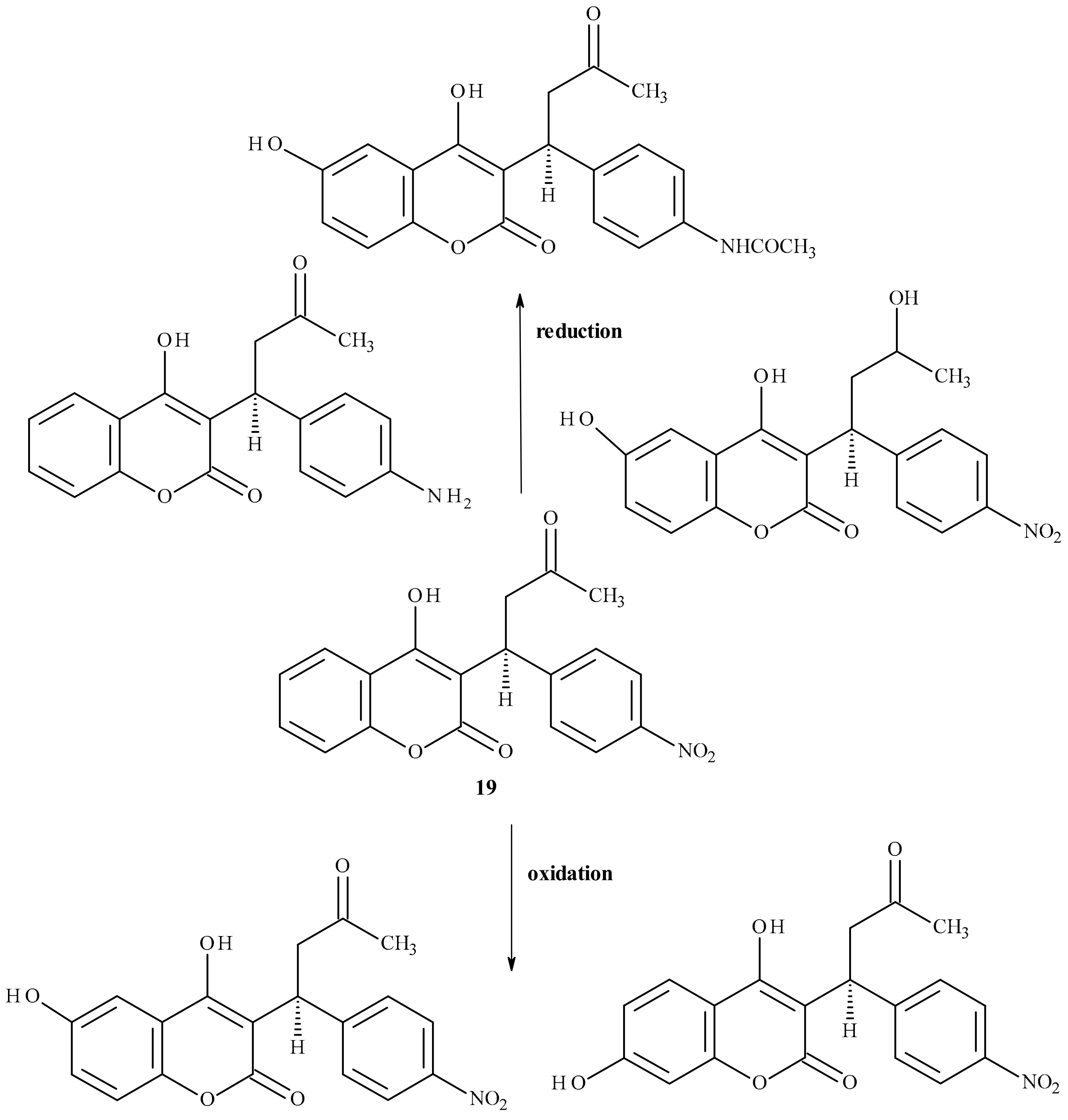
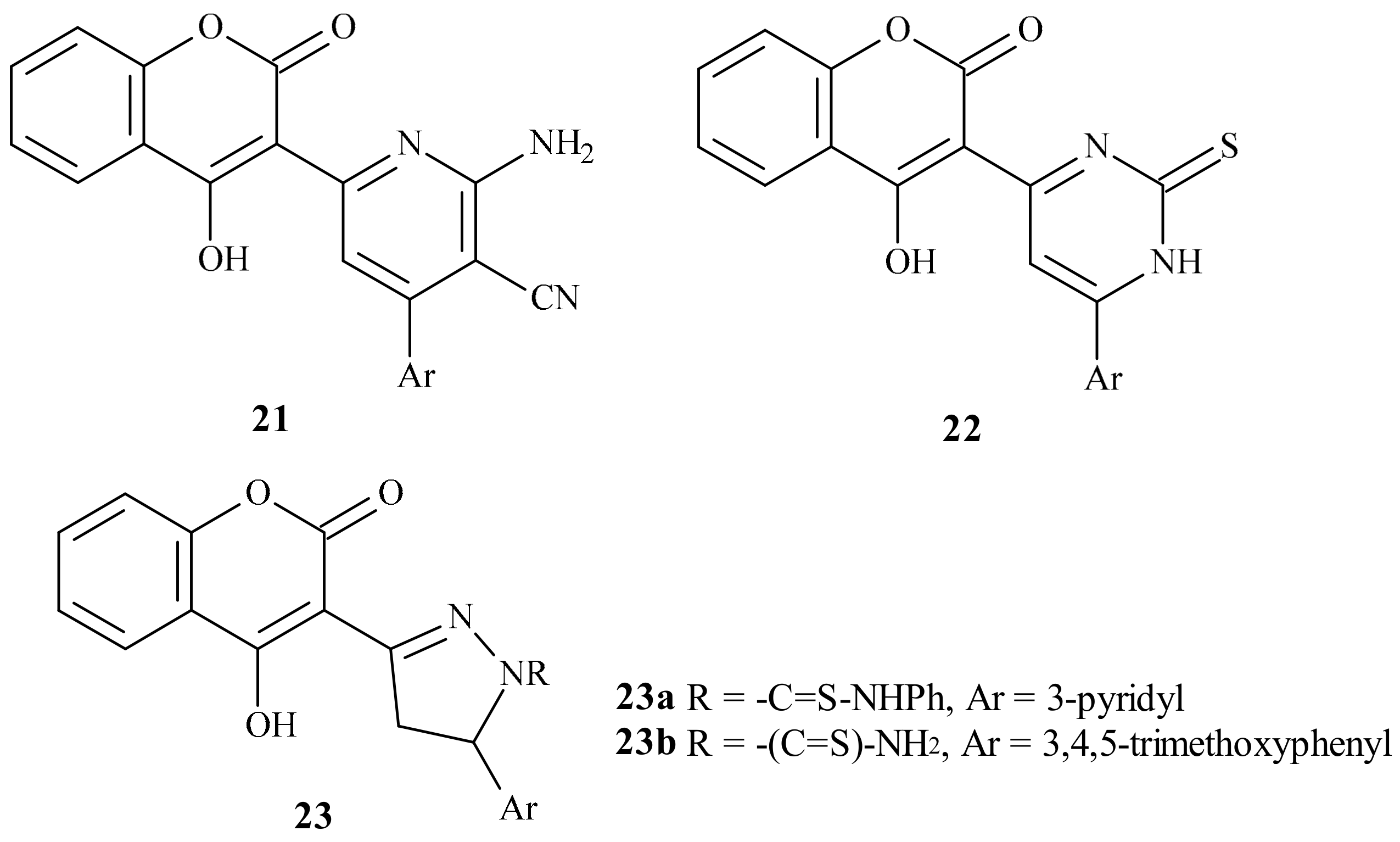
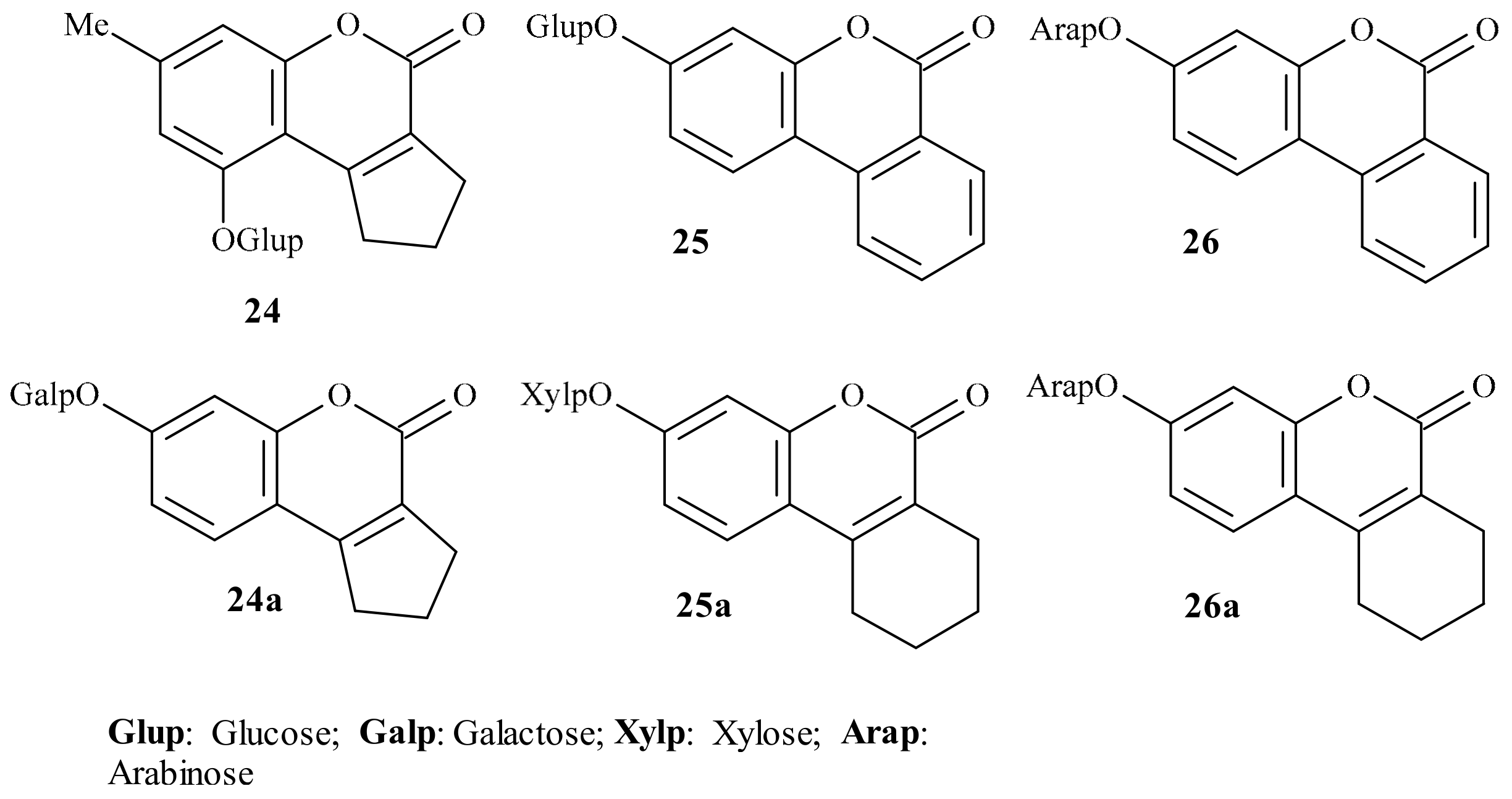
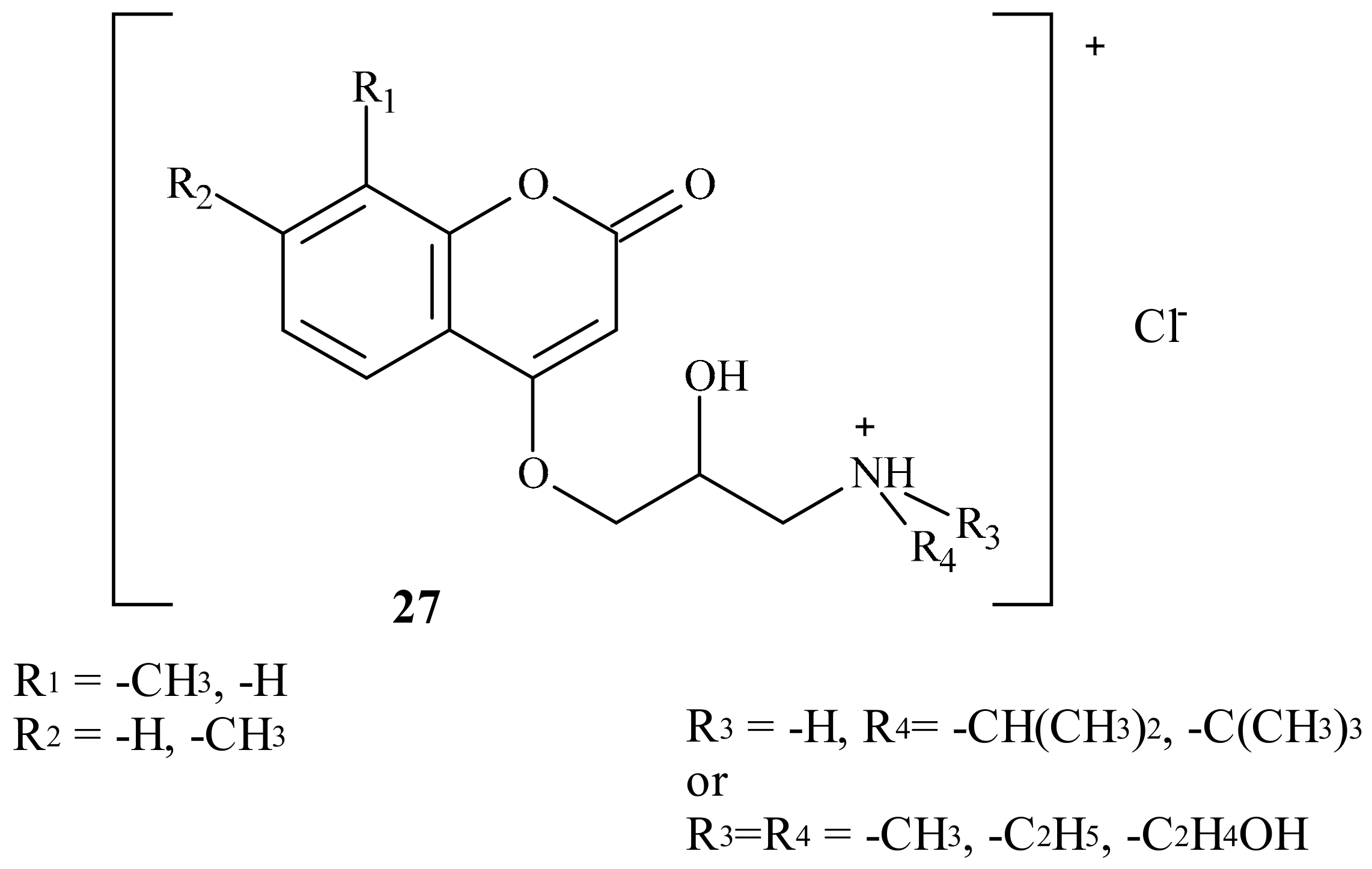
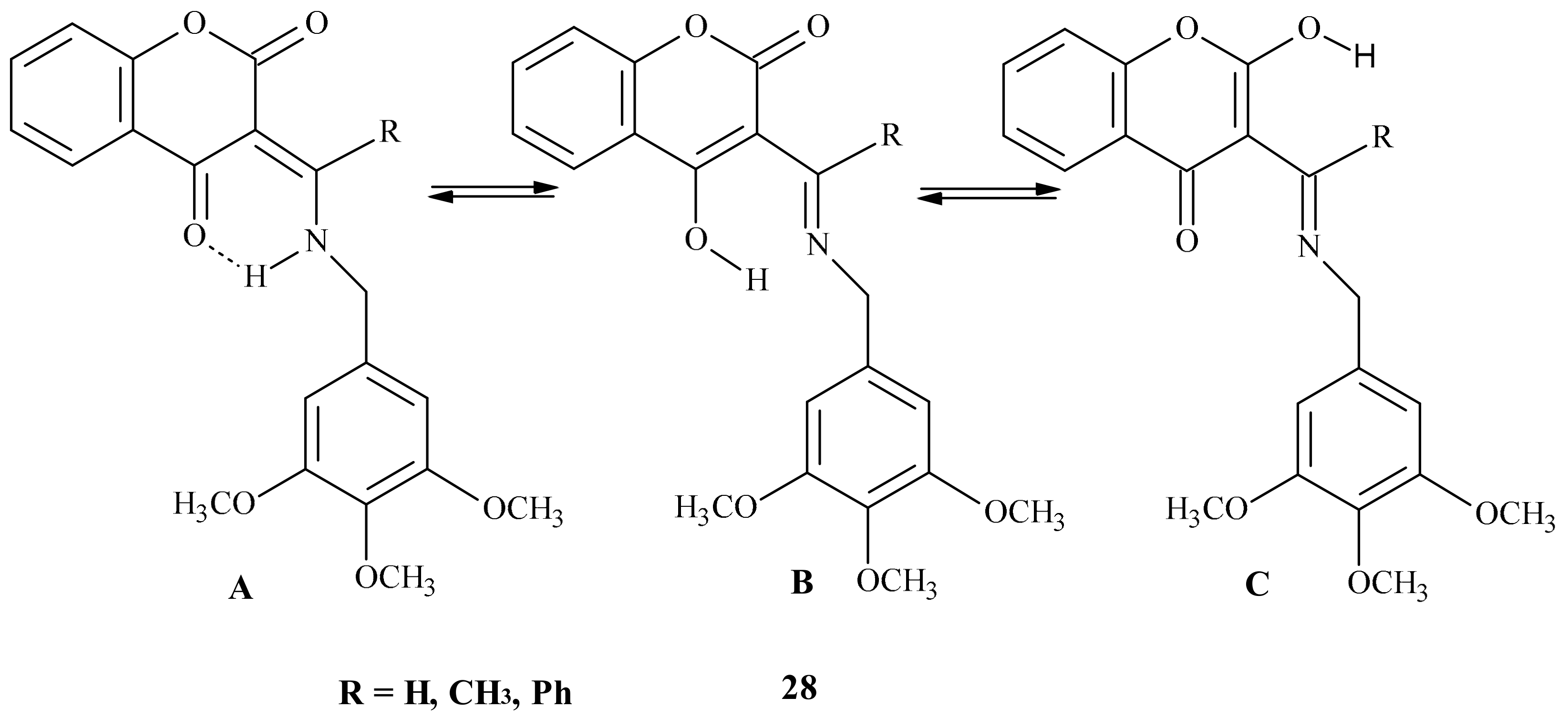
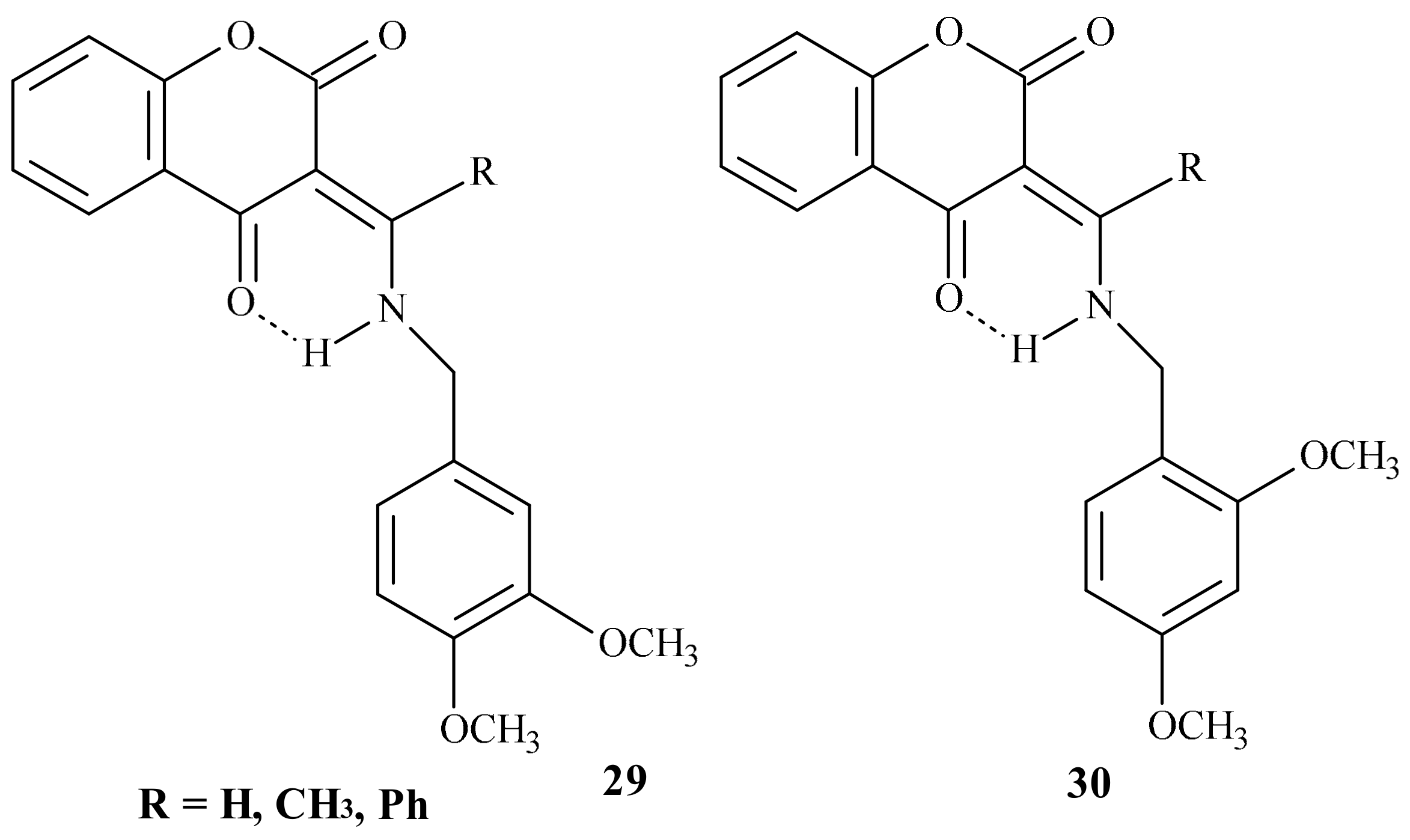
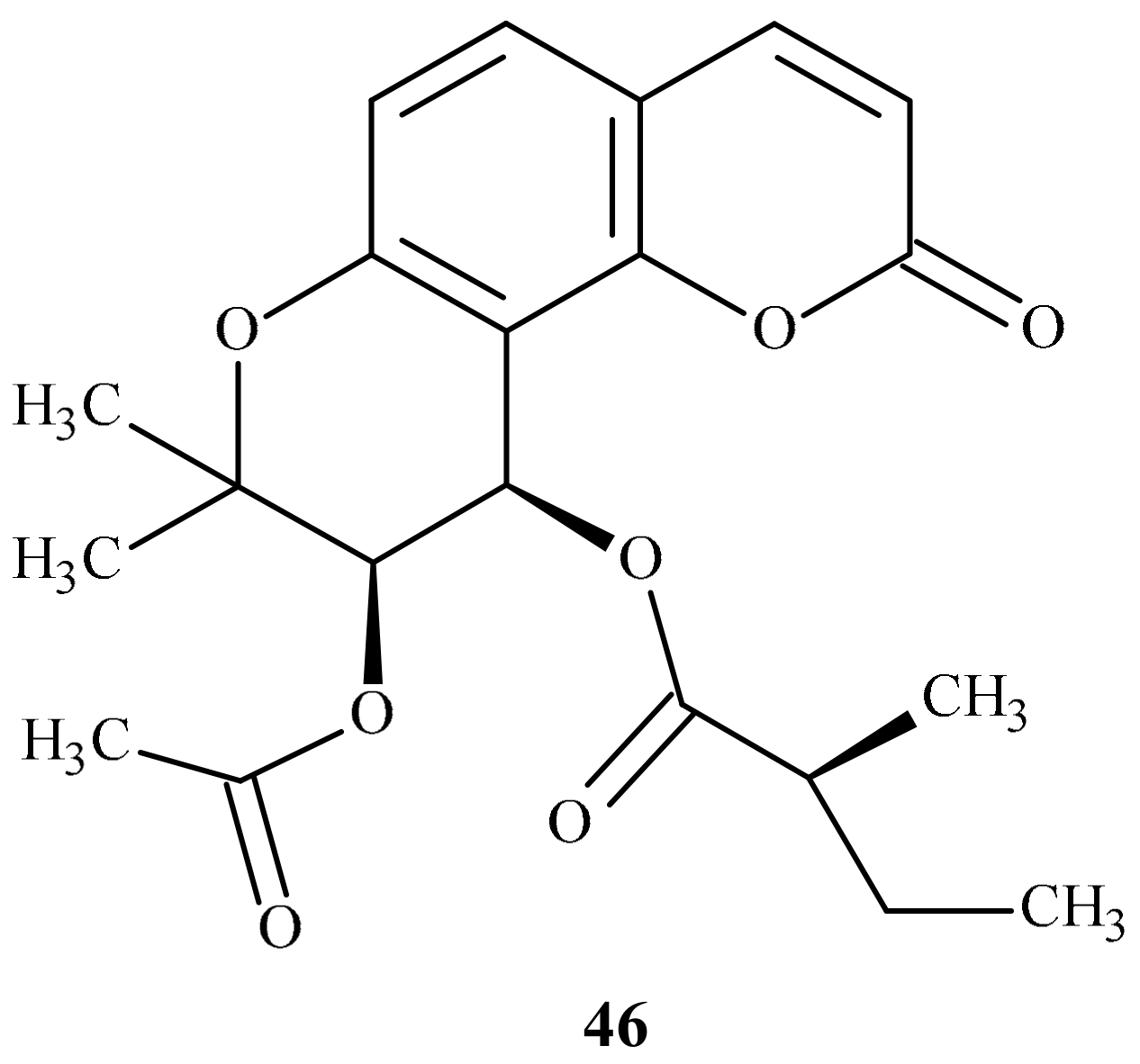
| Position of Amino Acid Residue | Mutation |
|---|---|
| 26 | A → T |
| 29 | V → L |
| 36 | D → G |
| 36 | D → Y |
| 45 | V → A |
| 52 | S → W |
| 56 | S → F |
| 58 | R → G |
| 59 | W → C |
| 59 | W → L |
| 66 | V → G |
| 66 | V → M |
| 71 | G → A |
| 77 | N → S |
| 77 | N → Y |
| 123 | I → N |
| 128 | L → R |
| Position of Amino Acid Residue | Mutation | Enzyme Activity | Coumarin Inhibitory Effect |
|---|---|---|---|
| 16 | C → A or S | Reduced by about 80% | |
| 35 | R → P | Nearly abolished | |
| 43 | C → A or S | Reduced | |
| 51 | C → A or S | Reduced | |
| 56 | S → P | Nearly abolished | |
| 57 | S → A | Nearly abolished | |
| 85 | C → A | Reduced by about 25% | |
| 85 | C → S | Reduced by about 75% | |
| 96 | C → A or S | Reduced by about 70% | |
| 98 | R → D or E | Reduced by about 80%. | Decreased |
| 98 | R → K | No effect | Decreased |
| 120 | L → Q | Decreased moderately. | Decreased |
| 128 | L → Q or S | Decreased by about 80%. | Decreased |
| 132 | C → S | Nearly abolished | |
| 135 | C → S | Nearly abolished | |
| 139 | Y → C, G or S | Decreased moderately | Strongly decreased |
| 139 | Y → F | No effect | Strongly decreased |
| Coumarin Derivatives | Structures |
|---|---|
| 7,8-Dihydroxy-6-methoxy-coumarin | 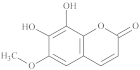 |
| Umbeliferone | 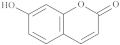 |
| 3-Hydroxycoumarin | 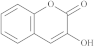 |
| Scopoletin | 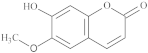 |
| 6,7-Dihydroxycoumarin | 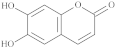 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasperkiewicz, K.; Ponczek, M.B.; Owczarek, J.; Guga, P.; Budzisz, E. Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules 2020, 25, 1465. https://doi.org/10.3390/molecules25061465
Kasperkiewicz K, Ponczek MB, Owczarek J, Guga P, Budzisz E. Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules. 2020; 25(6):1465. https://doi.org/10.3390/molecules25061465
Chicago/Turabian StyleKasperkiewicz, Kinga, Michał B. Ponczek, Jacek Owczarek, Piotr Guga, and Elżbieta Budzisz. 2020. "Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives" Molecules 25, no. 6: 1465. https://doi.org/10.3390/molecules25061465
APA StyleKasperkiewicz, K., Ponczek, M. B., Owczarek, J., Guga, P., & Budzisz, E. (2020). Antagonists of Vitamin K—Popular Coumarin Drugs and New Synthetic and Natural Coumarin Derivatives. Molecules, 25(6), 1465. https://doi.org/10.3390/molecules25061465







