New Symmetrical U- and Wavy-Shaped Supramolecular H-Bonded Systems; Geometrical and Mesomorphic Approaches
Abstract
1. Introduction
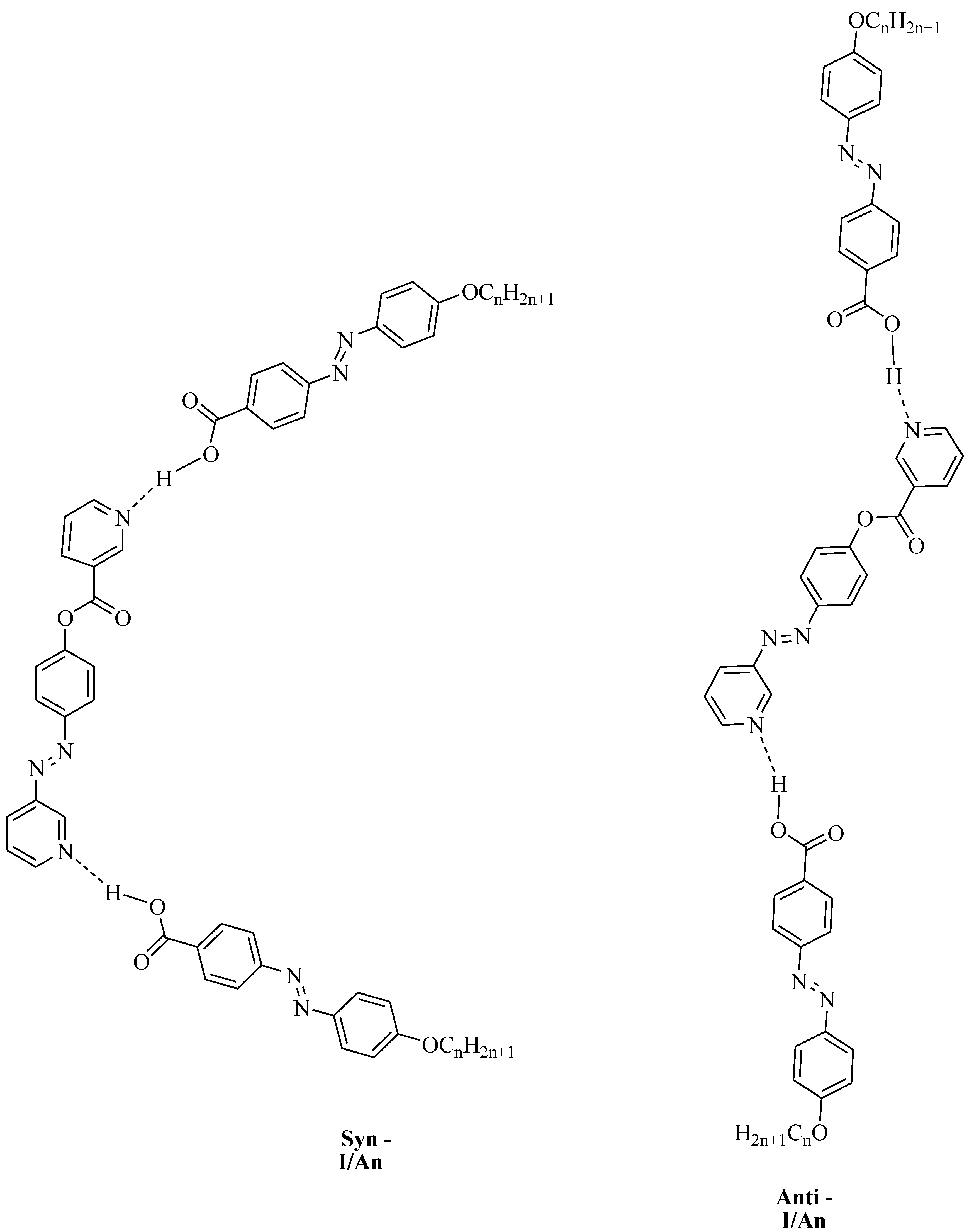
2. Experimental Section
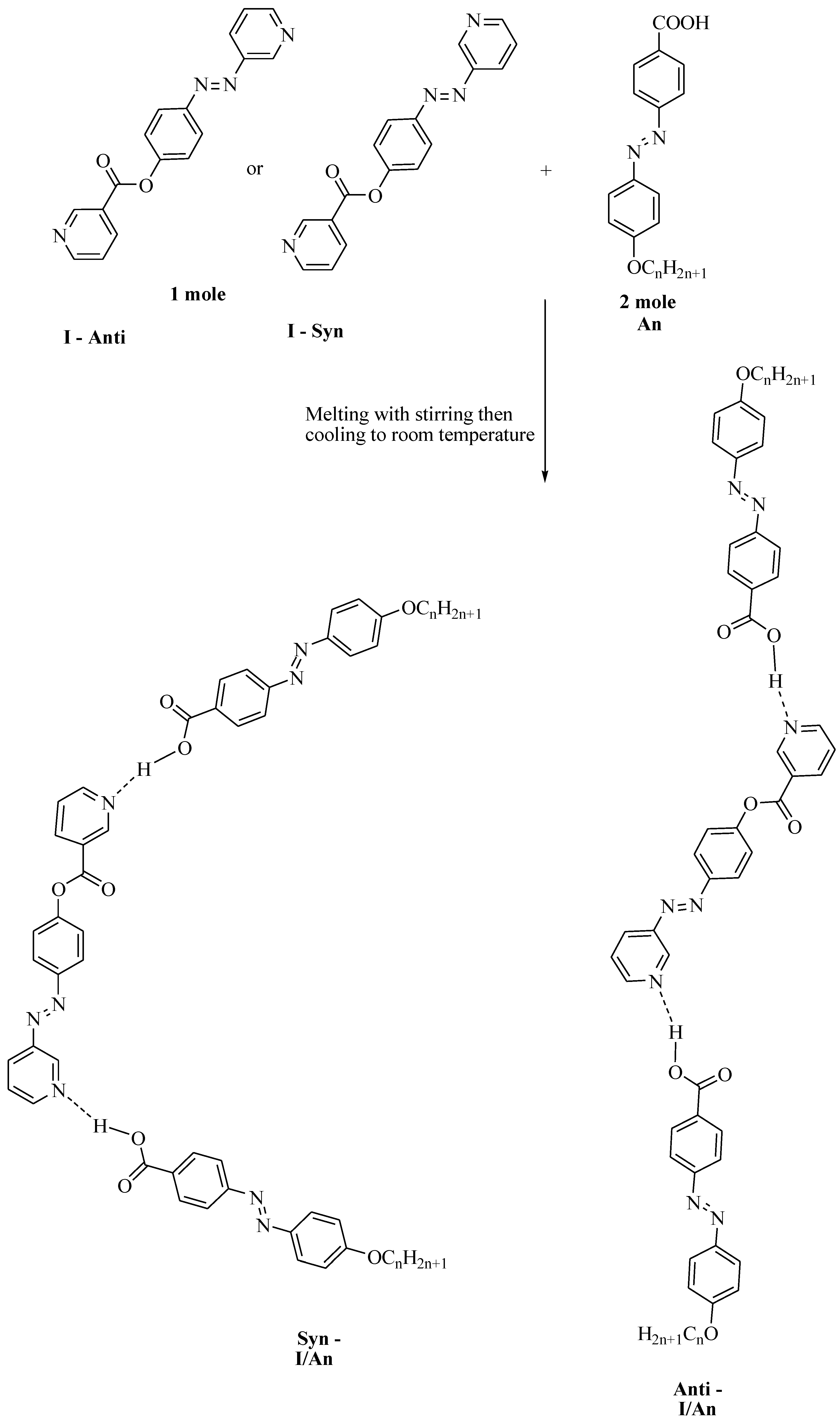
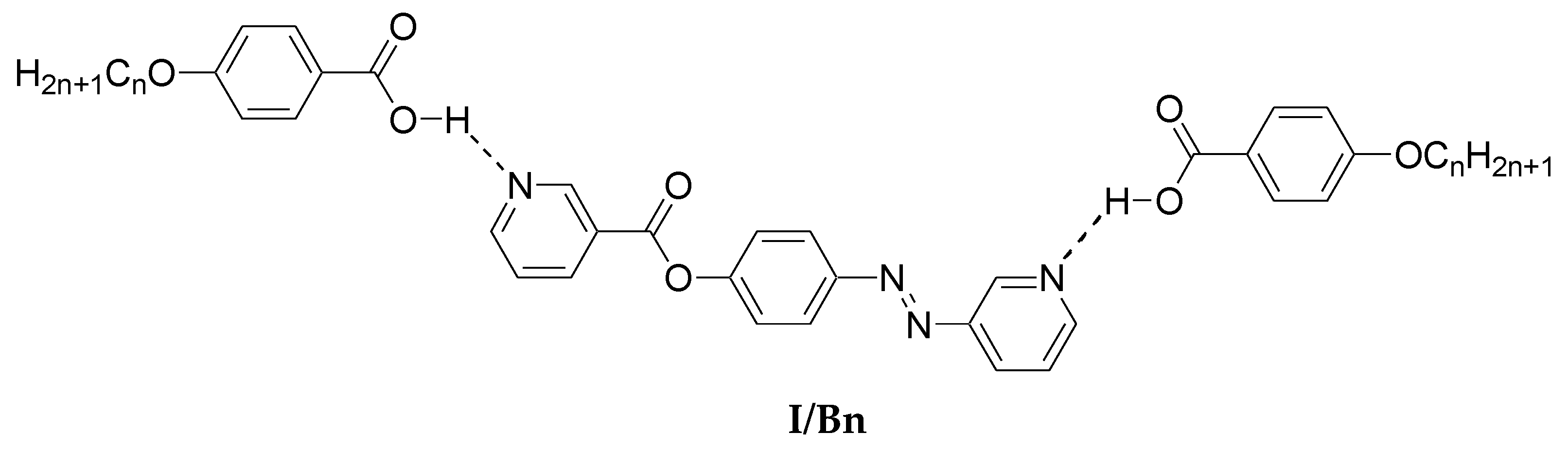
Preparation of 2:1 Symmetrical Supramolecular Complexes
3. Results and Discussion
3.1. Theoretical DFT Calculations
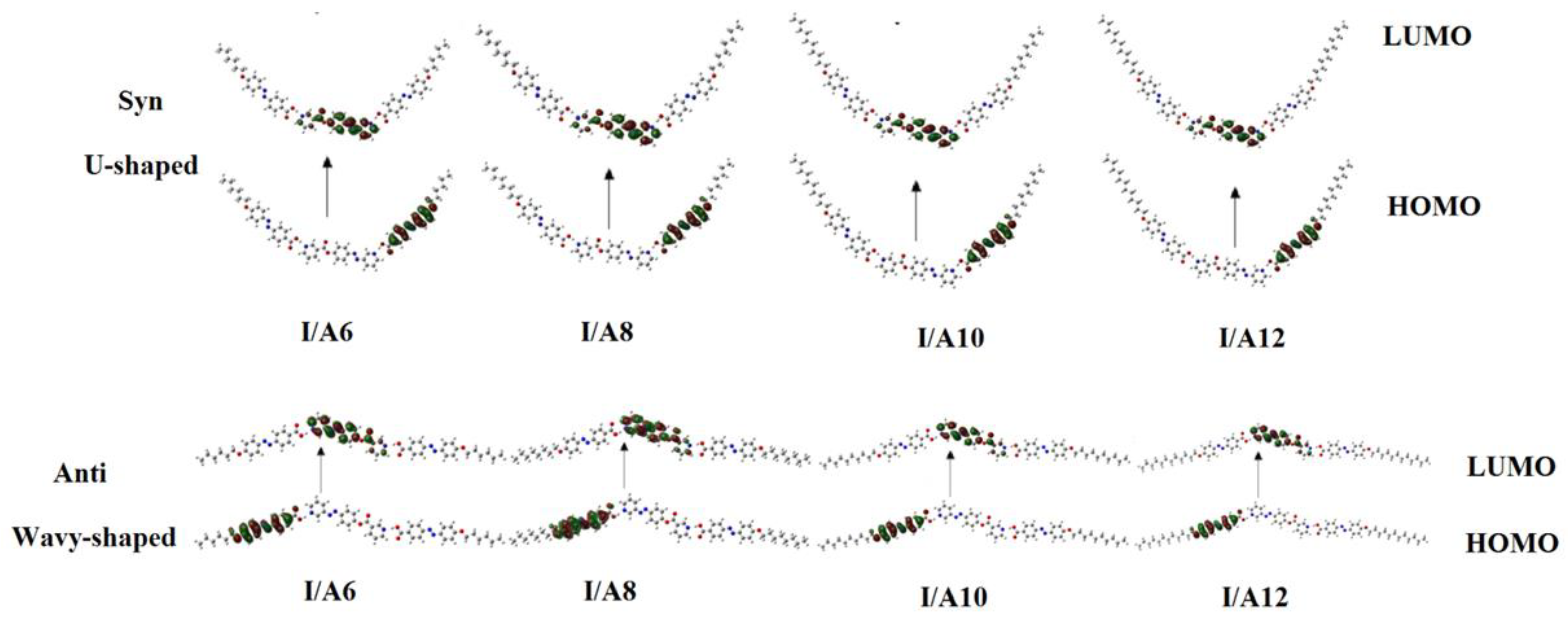
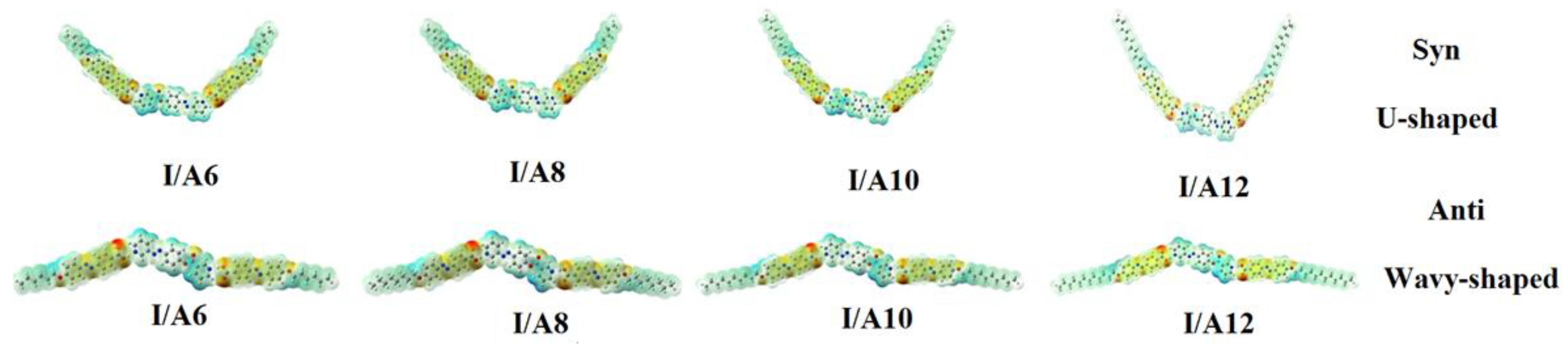
3.1.1. Molecular Geometry
3.1.2. Thermal Parameters
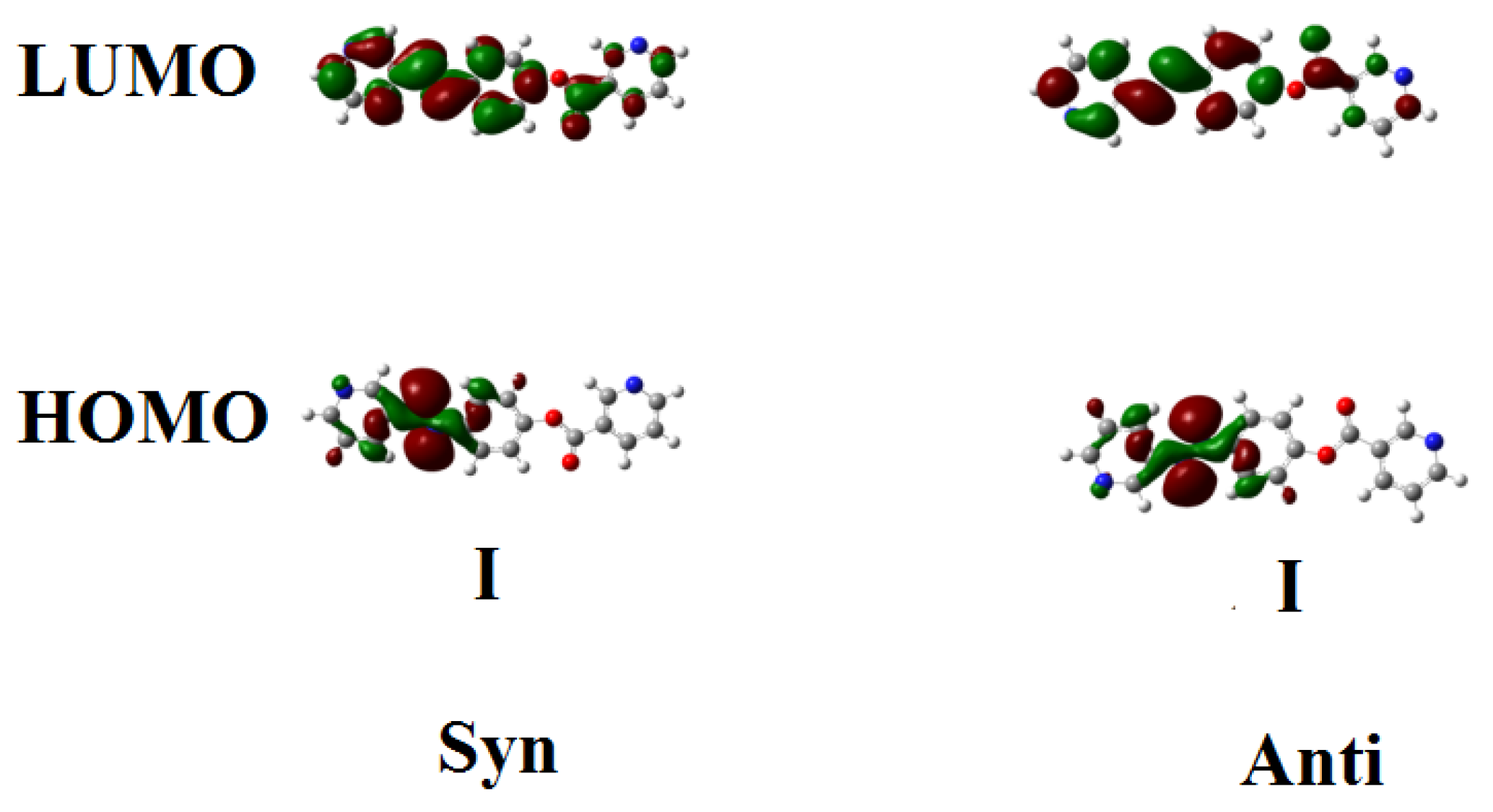
3.1.3. Frontier Molecular Orbitals Dipole Moment and Polarizability
3.1.4. Molecular Electrostatic Potential
3.2. Experimental Results
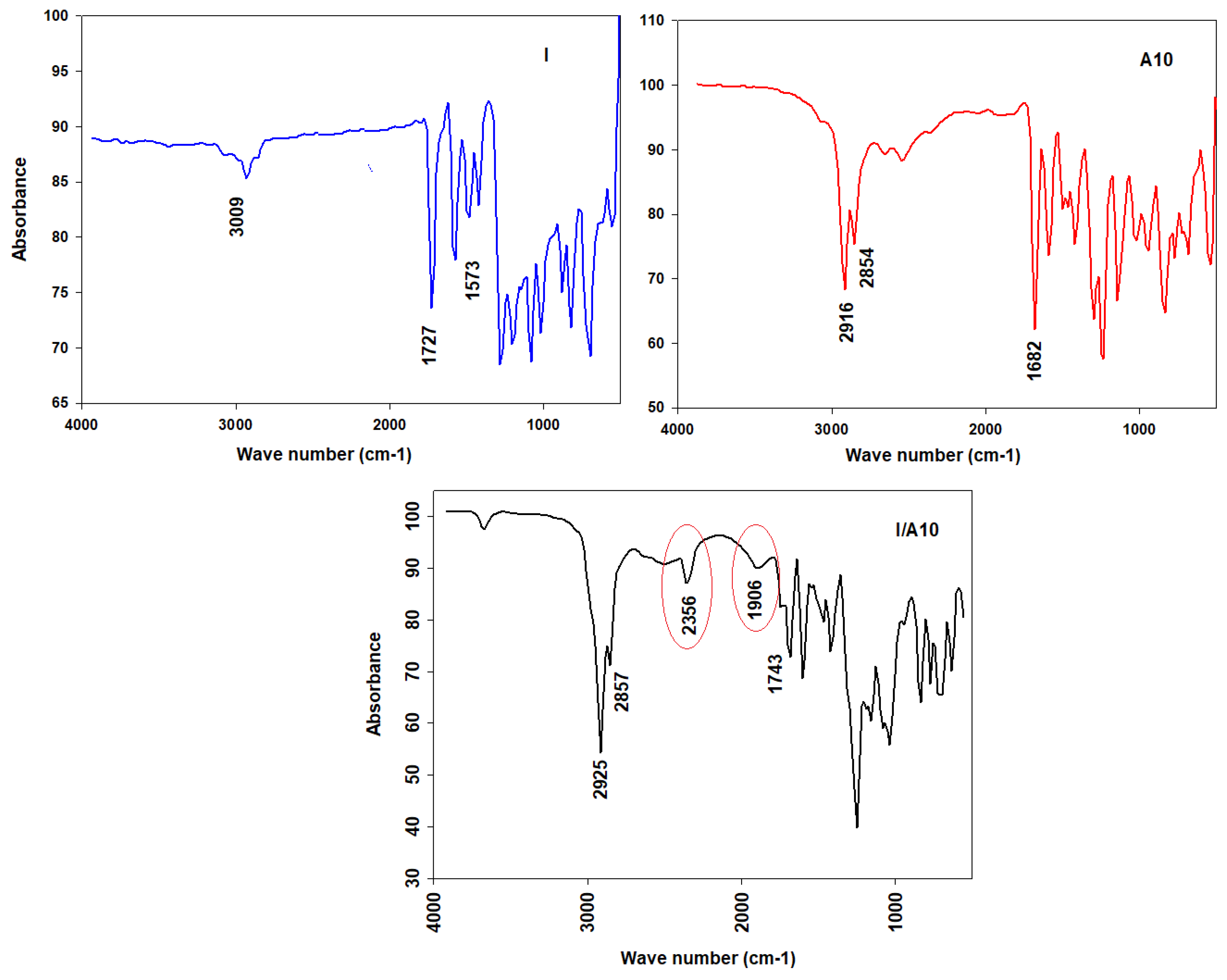
3.2.1. FT-IR Conformation of Prepared SMHBLCs
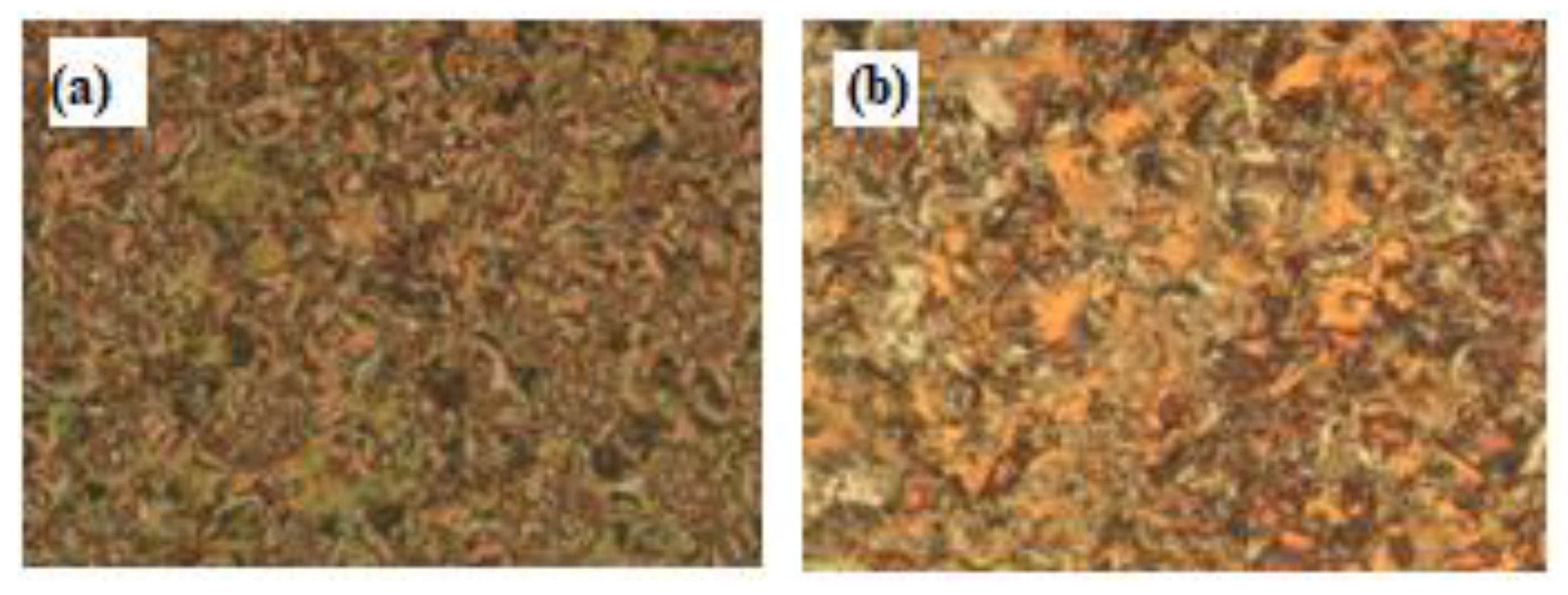
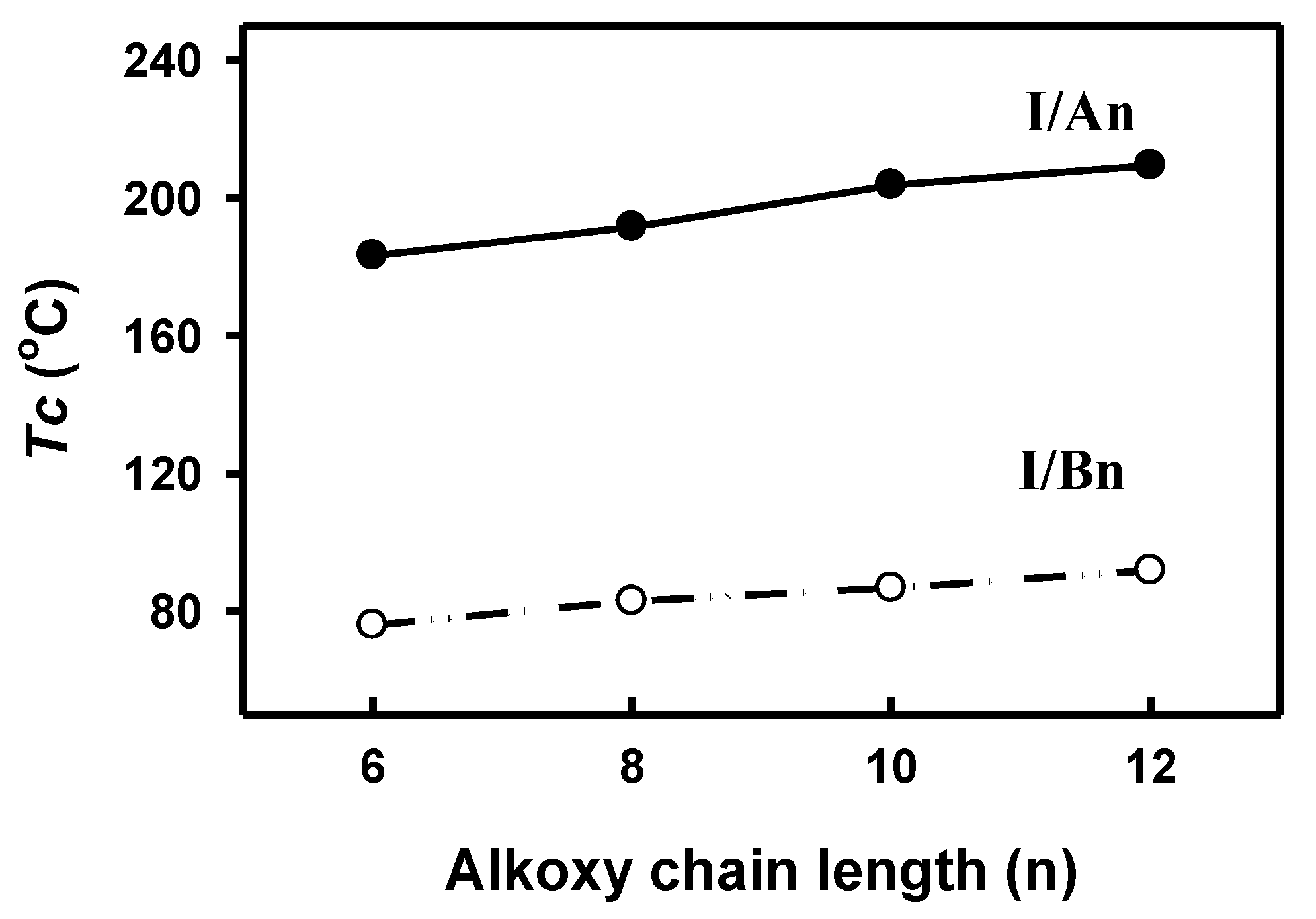
3.2.2. Mesomorphic and Optical Investigations
3.2.3. Entropy Changes
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hagar, M.; Ahmed, H.; Saad, G. New calamitic thermotropic liquid crystals of 2-hydroxypyridine ester mesogenic core: Mesophase behaviour and DFT calculations. Liq. Cryst. 2019, 47, 114–124. [Google Scholar] [CrossRef]
- Chen, K.-Y. Crystal Structure, Hydrogen-Bonding Properties, and DFT Studies of 2-((2-(2-Hydroxyphenyl)benzo[ d ]thiazol-6-yl)methylene)malononitrile. Mol. Cryst. Liq. Cryst. 2015, 623, 285–296. [Google Scholar] [CrossRef]
- Shoji, M.; Tanaka, F. Theoretical Study of Hydrogen-Bonded Supramolecular Liquid Crystals. Macromolecules 2002, 35, 7460–7472. [Google Scholar] [CrossRef]
- Sundaram, S.; Jayaprakasam, R.; Dhandapani, M.; Senthil, T.; Vijayakumar, V. Theoretical (DFT) and experimental studies on multiple hydrogen bonded liquid crystals comprising between aliphatic and aromatic acids. J. Mol. Liq. 2017, 243, 14–21. [Google Scholar] [CrossRef]
- Alhaddad, O.; Ahmed, H.A.; Hagar, M. Experimental and Theoretical Approaches of New Nematogenic Chair Architectures of Supramolecular H-Bonded Liquid Crystals. Molecules 2020, 25, 365. [Google Scholar] [CrossRef]
- Bradfield, A.; Jones, B. Two apparent cases of liquid crystal formation. J. Chem. Soc 1929, 2660–2661. [Google Scholar] [CrossRef]
- Bennett, G.M.; Jones, B. 94. Mesomorphism and polymorphism of some p-alkoxybenzoic and p-alkoxycinnamic acids. J. Chem. Soc. 1939, 420–425. [Google Scholar] [CrossRef]
- Gray, G.W.; Jones, B. The Mesomorphic Transition Points of the Para-normal-alkoxybenzoic Acids-a Correction; Royal Soc Chemistry Thomas Graham House: Cambridge, UK, 1953; pp. 4179–4180. [Google Scholar]
- Dong, R.; Zhou, Y.; Huang, X.; Zhu, X.; Lu, Y.; Shen, J. Functional Supramolecular Polymers for Biomedical Applications. Adv. Mater. 2014, 27, 498–526. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446. [Google Scholar] [CrossRef]
- Liu, K.; Kang, Y.; Wang, Z.; Zhang, X. 25th Anniversary Article: Reversible and Adaptive Functional Supramolecular Materials: “Noncovalent Interaction” Matters. Adv. Mater. 2013, 25, 5530–5548. [Google Scholar] [CrossRef]
- Yan, X.; Wang, F.; Zheng, B.; Huang, F. Stimuli-responsive supramolecular polymeric materials. Chem. Soc. Rev. 2012, 41, 6042. [Google Scholar] [CrossRef] [PubMed]
- González-Rodríguez, D.; Schenning, A. Hydrogen-bonded Supramolecular π-Functional Materials†. Chem. Mater. 2011, 23, 310–325. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Kumar, U.; Kato, T.; Frechet, J. Use of intermolecular hydrogen bonding for the induction of liquid crystallinity in the side chain of polysiloxanes. J. Am. Chem. Soc. 1992, 114, 6630–6639. [Google Scholar] [CrossRef]
- Kato, T.; Uryu, T.; Kaneuchi, F.; Jin, C.; Frechet, J. Hydrogen-bonded liquid crystals built from hydrogen-bonding donors and acceptors. Infrared study on the stability of the hydrogen bond between carboxylic acid and pyridyl moieties. Liq. Cryst. 1993, 14, 1311–1317. [Google Scholar] [CrossRef]
- Kato, T.; Kihara, H.; Kumar, U.; Uryu, T.; Frechet, J. A Liquid-Crystalline Polymer Network Built by Molecular Self-Assembly through Intermolecular Hydrogen Bonding. Angew. Chem. Int. Ed. 1994, 33, 1644–1645. [Google Scholar] [CrossRef]
- Alaasar, M.; Tschierske, C.; Prehm, M. Hydrogen-bonded supramolecular complexes formed between isophthalic acid and pyridine-based derivatives. Liq. Cryst. 2011, 38, 925–934. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M. Mesophase behaviour of azobenzene-based angular supramolecular hydrogen-bonded liquid crystals. Liq. Cryst. 2015, 43, 1–14. [Google Scholar] [CrossRef]
- Naoum, M.M.; Fahmi, A.A.; Refaie, A.A.; Alaasar, M. Novel hydrogen-bonded angular supramolecular liquid crystals. Liq. Cryst. 2012, 39, 47–61. [Google Scholar] [CrossRef]
- Sherif, S.; Nafee, H.A.A. Mohamed Hagar New architectures of supramolecular H-bonded liquid crystal complexes based on dipyridine Derivatives. Liq. Cryst. 2020, 1–12. [Google Scholar]
- Walker, R.; Pociecha, D.; Abberley, J.P.; Martinez-Felipe, A.; Paterson, D.; Forsyth, E.; Lawrence, G.B.; Henderson, P.; Storey, J.M.D.; Gorecka, E.; et al. Spontaneous chirality through mixing achiral components: A twist-bend nematic phase driven by hydrogen-bonding between unlike components. Chem. Commun. 2018, 54, 3383–3386. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef] [PubMed]
- Concellon, A.; Schenning, A.; Romero, P.; Marcos, M.; Serrano, J.L. Size-Selective Adsorption in Nanoporous Polymers from Coumarin Photo-Cross-Linked Columnar Liquid Crystals. Macromolecules 2018, 51, 2349–2358. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alaasar, M.; Naoum, M. Wide nematic phases induced by hydrogen-bonding. Liq. Cryst. 2018, 46, 550–559. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Phase Behavior and DFT Calculations of Laterally Methyl Supramolecular Hydrogen-Bonding Complexes. Crystals 2019, 9, 133. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Aljuhani, A. Mesophase behavior of new linear supramolecular hydrogen-bonding complexes. RSC Adv. 2018, 8, 34937–34946. [Google Scholar] [CrossRef]
- Ahmed, H.; Naoum, M.; Saad, G. Mesophase behaviour of 1:1 mixtures of 4-n-alkoxyphenylazo benzoic acids bearing terminal alkoxy groups of different chain lengths. Liq. Cryst. 2016, 43, 1259–1267. [Google Scholar] [CrossRef]
- Zhang, G.; Ma, J.; Wen, J. Interchain Impacts on Electronic Structures of Heterocyclic Oligomers and Polymers Containing Group 14, 15, and 16 Heteroatoms: Quantum Chemical Calculations in Combination with Molecular Dynamics Simulations. J. Phys. Chem. B 2007, 111, 11670–11679. [Google Scholar] [CrossRef]
- Zajac, M.; Hrobarik, P.; Magdolen, P.; Foltínová, P.; Zahradnik, P. Donor–π-acceptor benzothiazole-derived dyes with an extended heteroaryl-containing conjugated system: Synthesis, DFT study and antimicrobial activity. Tetrahedron 2008, 64, 10605–10618. [Google Scholar] [CrossRef]
- Acharya, R.; Cekli, S.; Zeman, C.J.; Altamimi, R.M.; Schanze, K.S.; Acharya, U.R. Effect of Selenium Substitution on Intersystem Crossing in π-Conjugated Donor–Acceptor–Donor Chromophores: The LUMO Matters the Most. J. Phys. Chem. Lett. 2016, 7, 693–697. [Google Scholar] [CrossRef]
- Raychev, D.; Guskova, O.; Seifert, G.; Sommer, J.-U. Conformational and electronic properties of small benzothiadiazole-cored oligomers with aryl flanking units: Thiophene versus Furan. Comput. Mater. Sci. 2017, 126, 287–298. [Google Scholar] [CrossRef]
- Kobilka, B.M.; Hale, B.; Ewan, M.D.; Dubrovskiy, A.V.; Nelson, T.L.; Duzhko, V.; Jeffries-El, M. Influence of heteroatoms on photovoltaic performance of donor–acceptor copolymers based on 2,6-di(thiophen-2-yl)benzo[1,2-b:4,5-b′]difurans and diketopyrrolopyrrole. Polym. Chem. 2013, 4, 5329. [Google Scholar] [CrossRef]
- Kato, T.; Wilson, P.G.; Fujishima, A.; Fréchet, J.M. Hydrogen-bonded liquid crystals. A novel mesogen incorporating nonmesogenic 4, 4′-bipyridine through selective recognition between hydrogen bonding donor and acceptor. Chem. Lett. 1990, 19, 2003–2006. [Google Scholar] [CrossRef]
- Kato, T.; Fréchet, J.M.J.; Wilson, P.G.; Saito, T.; Uryu, T.; Fujishima, A.; Jin, C.; Kaneuchi, F. Hydrogen-bonded liquid crystals. Novel mesogens incorporating nonmesogenic bipyridyl compounds through complexation between hydrogen-bond donor and acceptor moieties. Chem. Mater. 1993, 5, 1094–1100. [Google Scholar] [CrossRef]
- Kato, T.; Fréchet, J.M. In Hydrogen Bonding and the Self-assembly of Supramolecular Liquid-crystalline Materials, Macromolecular Symposia; Wiley Online Library: Hoboken, NJ, USA, 1995; pp. 311–326. [Google Scholar]
- Kang, Y.-S.; Kim, H.; Zin, W.-C. Phase behaviour of hydrogen-bonded liquid crystalline complexes of alkoxycinnamic acids with 4,4’-bipyridine. Liq. Cryst. 2001, 28, 709–715. [Google Scholar] [CrossRef]
- Arakawa, Y.; Sasaki, Y.; Tsuji, H. Supramolecular hydrogen-bonded liquid crystals based on 4-n-alkylthiobenzoic acids and 4,4′-bipyridine: Their mesomorphic behavior with comparative study including alkyl and alkoxy counterparts. J. Mol. Liq. 2019, 280, 153–159. [Google Scholar] [CrossRef]
- Ebenezer, S.; Muthiah, P.T. Supramolecular architectures in the co-crystals involving carboxylic acids and 1,2-bis(4-pyridyl)ethane, an extended bipyridyl type ligand. J. Mol. Struct. 2011, 990, 281–289. [Google Scholar] [CrossRef]
- Mallia, V.A.; George, M.; Das, S. Photochemical Phase Transition in Hydrogen-Bonded Liquid Crystals. Chem. Mater. 1999, 11, 207–208. [Google Scholar] [CrossRef]
- Balaban, A.T.; Oniciu, D.C.; Katritzky, A.R. Aromaticity as a cornerstone of heterocyclic chemistry. Chem. Rev. 2004, 104, 2777–2812. [Google Scholar] [CrossRef]
- Dave, J.S.; Menon, M. Azomesogens with a heterocyclic moiety. Bull. Mater. Sci. 2000, 23, 237–238. [Google Scholar] [CrossRef]
- Abberley, J.P.; Killah, R.; Walker, R.; Storey, J.M.; Imrie, C.T.; Salamończyk, M.; Zhu, C.; Gorecka, E.; Pociecha, D. Heliconical smectic phases formed by achiral molecules. Nat. Commun. 2018, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.; Crawford, C.A.; Pociecha, D.; Walker, R.; Storey, J.M.; Gorecka, E.; Imrie, C. The role of a terminal chain in promoting the twist-bend nematic phase: The synthesis and characterisation of the 1-(4-cyanobiphenyl-4′-yl)-6-(4-alkyloxyanilinebenzylidene-4′-oxy)hexanes. Liq. Cryst. 2018, 45, 2341–2351. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. Mesomorphic and geometrical orientation study of the relative position of fluorine atom in some thermotropic liquid crystal systems. Liq. Cryst. 2019, 1–10. [Google Scholar] [CrossRef]
- Hagar, M.; Chaieb, K.; Parveen, S.; Ahmed, H.A.; Alnoman, R. N-alkyl 2-pyridone versus O-alkyl 2-pyridol: Ultrasonic synthesis, DFT, docking studies and their antimicrobial evaluation. J. Mol. Struct. 2020, 1199, 126926. [Google Scholar] [CrossRef]
- Nafee, S.S.; Ahmed, H.A.; Hagar, M. Theoretical, experimental and optical study of new thiophene-based liquid crystals and their positional isomers. Liq. Cryst. 2020, 1–12. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Alhaddad, O.A. Experimental and theoretical approaches of molecular geometry and mesophase behaviour relationship of laterally substituted azopyridines. Liq. Cryst. 2019, 46, 1440–1451. [Google Scholar] [CrossRef]
- Alnoman, R.B.; Parveen, S.; Hagar, M.; Ahmed, H.A.; Knight, J.G. A new chiral boron-dipyrromethene (BODIPY)-based fluorescent probe: Molecular docking, DFT, antibacterial and antioxidant approaches. J. Biomol. Struct. Dyn. 2019, 1–14. [Google Scholar] [CrossRef]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; El-Shishtawy, R.M.; Raffah, B.M. The Synthesis of New Thermal Stable Schiff Base/Ester Liquid Crystals: A Computational, Mesomorphic, and Optical Study. Molecules 2019, 24, 3032. [Google Scholar] [CrossRef] [PubMed]
- Nafee, S.S.; Hagar, M.; Ahmed, H.A.; Alhaddad, O.; El-Shishtawy, R.M.; Raffah, B.M. New two rings Schiff base liquid crystals; ball mill synthesis, mesomorphic, Hammett and DFT studies. J. Mol. Liq. 2020, 299, 112161. [Google Scholar] [CrossRef]
- Alnoman, R.; Al-Nazawi, F.K.; Ahmed, H.A.; Hagar, M. Synthesis, Optical, and Geometrical Approaches of New Natural Fatty Acids’ Esters/Schiff Base Liquid Crystals. Molecules 2019, 24, 4293. [Google Scholar] [CrossRef]
- Hagar, M.; Ahmed, H.A.; Alhaddad, O.A. New azobenzene-based natural fatty acid liquid crystals with low melting point: Synthesis, DFT calculations and binary mixtures. Liq. Cryst. 2019, 46, 2223–2234. [Google Scholar] [CrossRef]
- Ahmed, N.H.S.; Sadd, G.S.; Ahmed, H.; Hagar, M. New widely stable four ring azo/ester/Schiff base liquid crystals: Synthesis, mesomorphic, photophysical and DFT approaches. RSC Adv. 2020, 10, 9643–9656. [Google Scholar] [CrossRef]
- Meredith, G.R.; VanDusen, J.; Williams, D.J. Optical and nonlinear optical characterization of molecularly doped thermotropic liquid crystalline polymers. Macromolecules 1982, 15, 1385–1389. [Google Scholar] [CrossRef]
- Khoo, I.-C.; Wu, S.-T. Optics and Nonlinear Optics of Liquid Crystals; World Scientific: Shanghai, China, 1993. [Google Scholar]
- Chemla, D.S. Nonlinear Optical Properties of Organic Molecules and Crystals; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Cleland, W.; Kreevoy, M. Low-barrier hydrogen bonds and enzymic catalysis. Science 1994, 264, 1887–1890. [Google Scholar] [CrossRef]
- Lizu, M.; Lutfor, M.R.; Surugau, N.L.; How, S.E.; Arshad, S.E. Synthesis and Characterization of Ethyl Cellulose–Based Liquid Crystals Containing Azobenzene Chromophores. Mol. Cryst. Liq. Cryst. 2010, 528, 64–73. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Cook, A.G.; Abberley, J.P.; Walker, R.; Storey, J.M.D.; Imrie, C. An FT-IR spectroscopic study of the role of hydrogen bonding in the formation of liquid crystallinity for mixtures containing bipyridines and 4-pentoxybenzoic acid. RSC Adv. 2016, 6, 108164–108179. [Google Scholar] [CrossRef]
- Martinez-Felipe, A.; Imrie, C.T. The role of hydrogen bonding in the phase behaviour of supramolecular liquid crystal dimers. J. Mol. Struct. 2015, 1100, 429–437. [Google Scholar] [CrossRef]
- Ghanem, A.; Noel, C. FTIR Investigation of Two Alkyl-p-Terphenyl-4,4″-Dicarboxylates in Their Crystalline, Smectic and Isotropic Phases. Mol. Cryst. Liq. Cryst. Inc. Nonlinear Opt. 1987, 150, 447–472. [Google Scholar] [CrossRef]
- Paterson, D.; Martinez-Felipe, A.; Jansze, S.; Marcelis, A.T.; Storey, J.M.; Imrie, C. New insights into the liquid crystal behaviour of hydrogen-bonded mixtures provided by temperature-dependent FTIR spectroscopy. Liq. Cryst. 2015, 42, 1–12. [Google Scholar] [CrossRef]
- Ahmed, H.A.; Hagar, M.; Alhaddad, O.A. New chair shaped supramolecular complexes-based aryl nicotinate derivative; mesomorphic properties and DFT molecular geometry. RSC Adv. 2019, 9, 16366–16374. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds IAn are available from the authors. |


| Conformer | Syn | Anti | Syn | Anti | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | I | I | I/A6 | I/A8 | I/A10 | I/A12 | I/A6 | I/A8 | I/A10 | I/A12 |
| Ecorr | 0.262 | 0.262 | 1.0 | 1.1 | 1.3 | 1.4 | 1.0 | 1.1 | 1.3 | 1.4 |
| ZPVE | −1024.112 | −1024.112 | −3168.2 | −3325.3 | −3482.4 | −3639.6 | 3168.2 | −3325.3 | −3482.4 | −3639.6 |
| Etot | −1024.094 | −1024.093 | −3168.1 | −3325.2 | −3482.4 | −3639.5 | −3168.1 | −3325.2 | −3482.4 | −3639.5 |
| H | −1024.093 | −1024.092 | −3168.1 | −3325.2 | −3482.4 | −3639.5 | −3168.1 | −3325.2 | −3482.4 | −3639.5 |
| G | −1024.163 | −1024.163 | −3168.3 | −3325.4 | −3482.6 | −3639.7 | −3168.3 | −3325.4 | −3482.6 | −3639.7 |
| Parameter | Syn | Anti | ||||||
|---|---|---|---|---|---|---|---|---|
| I/A6 | I/A8 | I/A10 | I/A12 | I/A6 | I/A8 | I/A10 | I/A12 | |
| ELUMO | −0.12030 | −0.12035 | −0.12055 | −0.12056 | −0.12081 | −0.12080 | −0.12080 | −0.12078 |
| EHOMO | −0.22075 | −0.22073 | −0.22068 | −0.22066 | −0.21410 | −0.22135 | −0.22131 | −0.22126 |
| ΔEHOMO-LUMO | 0.10045 | 0.10038 | 0.10013 | 0.1001 | 0.09329 | 0.10055 | 0.10051 | 0.10048 |
| Total µ | 2.6897 | 2.7634 | 2.7941 | 2.8109 | 1.9935 | 1.4576 | 2.0176 | 2.0260 |
| Polarizability α | 893.01 | 941.85 | 988.48 | 1036.07 | 902.07 | 951.13 | 999.38 | 1047.24 |
| Compound | TCr1-Cr2 | TCr2-SmC | ∆HCr2-SmC | TSmC-I | ∆HSmC-I | ∆S/R |
|---|---|---|---|---|---|---|
| I/A6 | 130.4 | 139.7 | 38.67 | 183.2 | 3.91 | 1.03 |
| I/A8 | 124.0 | 147.0 | 27.21 | 191.5 | 2.98 | 0.77 |
| I/A10 | 150.2 | 184.2 | 21.99 | 203.7 | 2.70 | 0.68 |
| I/A12 | 144.7 | 169.2 | 28.24 | 209.4 | 3.68 | 0.92 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Mutabagani, L.A.; Alshabanah, L.A.; Ahmed, H.A.; Hagar, M.; Al-Ola, K.A.A. New Symmetrical U- and Wavy-Shaped Supramolecular H-Bonded Systems; Geometrical and Mesomorphic Approaches. Molecules 2020, 25, 1420. https://doi.org/10.3390/molecules25061420
Al-Mutabagani LA, Alshabanah LA, Ahmed HA, Hagar M, Al-Ola KAA. New Symmetrical U- and Wavy-Shaped Supramolecular H-Bonded Systems; Geometrical and Mesomorphic Approaches. Molecules. 2020; 25(6):1420. https://doi.org/10.3390/molecules25061420
Chicago/Turabian StyleAl-Mutabagani, Laila A., Latifah Abdullah Alshabanah, Hoda A. Ahmed, Mohamed Hagar, and Khulood A. Abu Al-Ola. 2020. "New Symmetrical U- and Wavy-Shaped Supramolecular H-Bonded Systems; Geometrical and Mesomorphic Approaches" Molecules 25, no. 6: 1420. https://doi.org/10.3390/molecules25061420
APA StyleAl-Mutabagani, L. A., Alshabanah, L. A., Ahmed, H. A., Hagar, M., & Al-Ola, K. A. A. (2020). New Symmetrical U- and Wavy-Shaped Supramolecular H-Bonded Systems; Geometrical and Mesomorphic Approaches. Molecules, 25(6), 1420. https://doi.org/10.3390/molecules25061420







