Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS
Abstract
1. Introduction
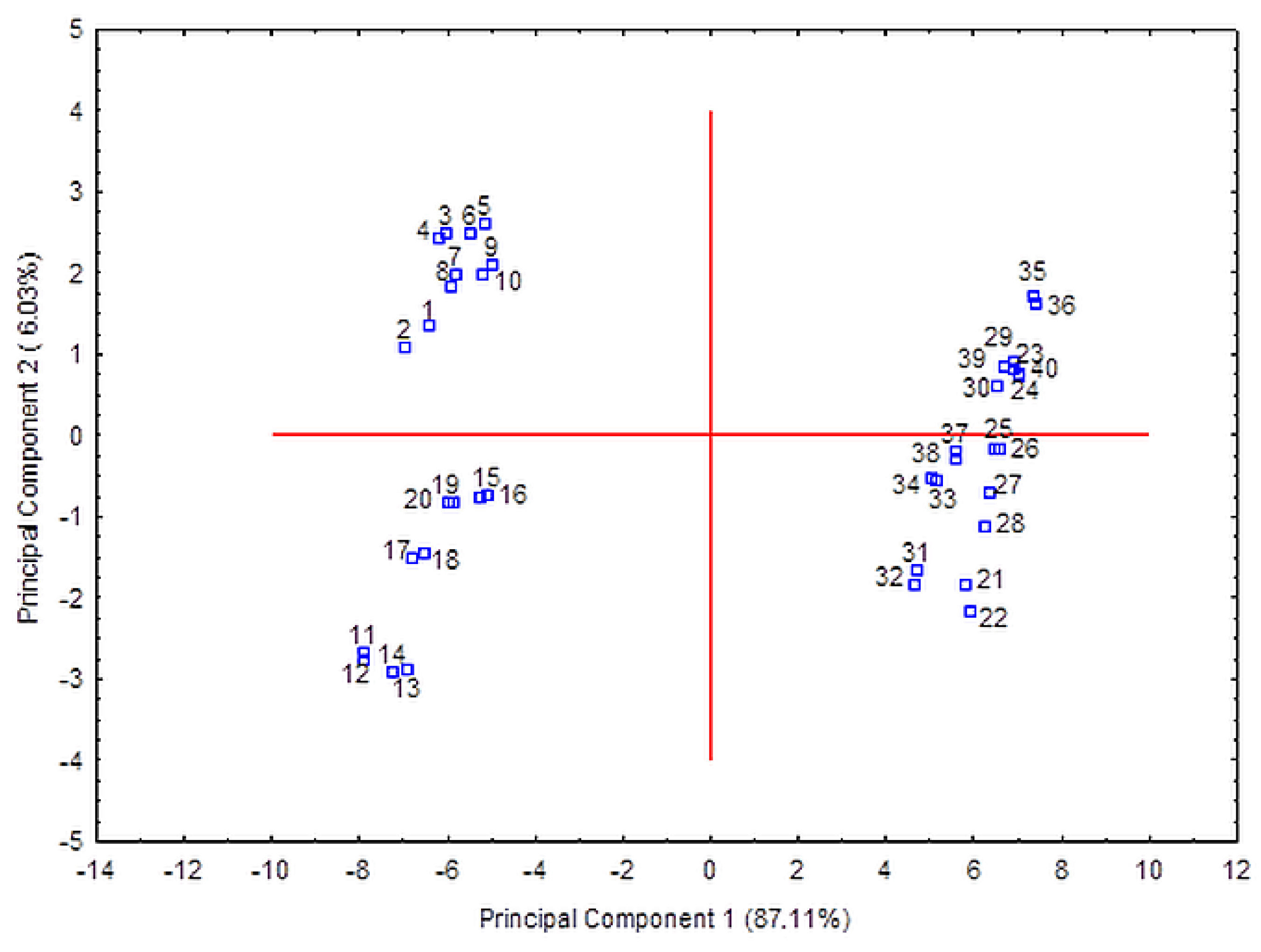
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Winemaking and Wine Samples
3.3. Determination of Oenological Parameters
3.4. Determination of Polyphenolic Compounds
3.5. Chemometric Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uttl, L.; Hurkova, K.; Kocourek, V.; Pulkrabova, J.; Tomaniova, M.; Hajslova, J. Metabolomics-based authentication of wines according to grape variety. Czech. J. Food Sci. 2019, 37, 239–245. [Google Scholar] [CrossRef]
- Geană, E.I.; Ciucure, C.T.; Apetrei, C.; Artem, V. Application of Spectroscopic UV-Vis and FT-IR Screening Techniques Coupled with Multivariate Statistical Analysis for Red Wine Authentication: Varietal and Vintage Year Discrimination. Molecules 2019, 24, 4166. [Google Scholar] [CrossRef] [PubMed]
- Stój, A. Metody wykrywania zafałszowań win. Żywność. Nauka. Technologia. Jakość. 2011, 75, 17–26. [Google Scholar]
- Sun, X.; Li, L.; Ma, T.; Liu, X.; Huang, W.; Zhan, J. Profiles of phenolic acids and flavan-3-ols for select chinese red wines: A comparison and differentiation according to geographic origin and grape variety. J. Food Sci. 2015, 80, C2170–C2179. [Google Scholar] [CrossRef] [PubMed]
- Palade, L.M.; Popa, M.E. Polyphenol fingerprinting approaches in wine traceability and authenticity: Assessment and implications of red wines. Beverages 2018, 4, 75. [Google Scholar] [CrossRef]
- Pereira, L.; Gomes, S.; Barrias, S.; Gomes, E.P.; Baleiras-Couto, M.; Fernandes, J.R.; Martins-Lopes, P. From the Field to the Bottle—An Integrated Strategy for Wine Authenticity. Beverages 2018, 4, 71. [Google Scholar] [CrossRef]
- Alañón, M.E.; Pérez-Coello, M.S.; Marina, M.L. Wine science in the metabolomics era. Trends Anal. Chem. 2015, 74, 1–20. [Google Scholar] [CrossRef]
- Sen, I.; Tokatli, F. Authenticity of wines made with economically important grape varieties grown in Anatolia by their phenolic profiles. Food Control. 2014, 46, 446–454. [Google Scholar] [CrossRef]
- Lorrain, B.; Ky, I.; Pechamat, L.; Teissedre, P.L. Evolution of analysis of polyhenols from grapes, wines, and extracts. Molecules 2013, 18, 1076–1100. [Google Scholar] [CrossRef]
- Silva, C.L.; Pereira, J.; Wouter, V.G.; Giró, C.; Câmara, J.S. A fast method using a new hydrophilic–lipophilic balanced sorbent in combination with ultra-high performance liquid chromatography for quantification of significant bioactive metabolites in wines. Talanta 2011, 86, 82–90. [Google Scholar] [CrossRef]
- Blancquaert, E.H.; Oberholster, A.; Ricardo-da-Silva, J.M.; Deloire, A.J. Effects of abiotic factors on phenolic compounds in the Grape Nerry-a review. S. Afr. J. Enol. Vitic. 2019, 40, 1–14. [Google Scholar]
- Ribereau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology Volume 2 The Chemistry of Wine Stabilization and Treatments, 2nd ed.; Wiley: Chichester, UK, 2006; pp. 141–203. [Google Scholar]
- Wojdyło, A.; Samoticha, J.; Nowicka, P.; Chmielewska, J. Characterisation of (poly)phenolic constituents of two interspecific red hybrids of Rondo and Regent (Vitis vinifera) by LC-PDA-ESI-MS QTof. Food Chem. 2018, 239, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, B.F.; Bunea, C.I.; Călugăr, A.; Donici, A. Phenolic, anthocyanin composition and color measurement at red wines from Dealu Bujorului vineyard. Agricultura 2019, 109–110, 14–28. [Google Scholar]
- Li, X.X.; He, F.; Wang, J.; Li, Z.; Pan, Q.H. Simple rain-shelter cultivation prolongs accumulation period of anthocyanins in wine grape berries. Molecules 2014, 19, 14843–14861. [Google Scholar] [CrossRef]
- Urcan, D.E.; Lung, M.L.; Giacosa, S.; Torchio, F.; Ferrandino, A.; Vincenzi, S.; Río Segade, S.; Pop, N.; Rolle, L. Phenolic substances, flavor compounds, and textural properties of three native Romanian wine grape varieties. Int. J. Food Prop. 2016, 19, 76–98. [Google Scholar] [CrossRef]
- Wu, Y.Y.; Xing, K.; Zhang, X.X.; Wang, H.; Wang, Y.; Wang, F.; Li, J.M. Influence of freeze concentration technique on aromatic and phenolic compounds, color attributes, and sensory properties of Cabernet Sauvignon wine. Molecules 2017, 22, 899. [Google Scholar] [CrossRef]
- Coletta, A.; Trani, A.; Faccia, M.; Punzi, R.; Dipalmo, T.; Crupi, P.; Antonacci, D.; Gambacorta, G. Influence of viticultural practices and winemaking technologies on phenolic composition and sensory characteristics of Negroamaro red wines. Int. J. Food Sci. Tech. 2013, 48, 2215–2227. [Google Scholar] [CrossRef]
- Fanzone, M.; Peña-Neira, A.; Jofré, V.; Assof, M.; Zamora, F. Phenolic characterization of Malbec wines from Mendoza province (Argentina). J. Agric. Food Chem. 2010, 58, 2388–2397. [Google Scholar] [CrossRef]
- Jaitz, L.; Siegl, K.; Eder, R.; Rak, G.; Abranko, L.; Koellensperger, G.; Hann, S. LC–MS/MS analysis of phenols for classification of red wine according to geographic origin, grape variety and vintage. Food Chem. 2010, 122, 366–372. [Google Scholar] [CrossRef]
- Pisano, P.L.; Silva, M.F.; Olivieri, A.C. Anthocyanins as markers for the classification of Argentinean wines according to botanical and geographical origin. Chemometric modeling of liquid chromatography–mass spectrometry data. Food Chem. 2015, 175, 174–180. [Google Scholar] [CrossRef]
- Žurga, P.; Vahčić, N.; Pasković, I.; Banović, M.; Staver, M.M. Croatian wines from native grape varieties have highly distinct phenolic (nutraceutic) profiles than wines from non-native varieties with same geographic origin. Chem. Biodivers. 2019, 16, e1900218. [Google Scholar] [CrossRef] [PubMed]
- Rastija, V.; Srečnik, G.; Medić-Šarić, M. Polyphenolic composition of Croatian wines with different geographical origins. Food Chem. 2009, 115, 54–60. [Google Scholar] [CrossRef]
- Ivanova-Petropulos, V.; Ricci, A.; Nedelkovski, D.; Dimovska, V.; Parpinello, G.P.; Versari, A. Targeted analysis of bioactive phenolic compounds and antioxidant activity of Macedonian red wines. Food Chem. 2015, 171, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.-T.; Sun, X.-Y.; Gao, G.-T.; Wang, X.-Y.; Liu, X.-Y.; Du, G.-R.; Zhan, J.-C. Phenolic characterisation and antioxidant capacity of young wines made from different grape varieties grown in Helanshan Donglu wine zone (China). S. Afr. J. Enol. Vitic. 2014, 35, 321–331. [Google Scholar] [CrossRef]
- Pereira, V.; Câmara, J.S.; Cacho, J.; Marques, J.C. HPLC-DAD methodology for the quantification of organic acids, furans and polyphenols by direct injection of wine samples. J. Sep. Sci. 2010, 33, 1204–1215. [Google Scholar] [CrossRef]
- Raczkowska, J.; Mielcarz, G.; Howard, A.; Raczkowski, M. UPLC and spectrophotometric analysis of polyphenols in wines available in the Polish market. Int. J. Food Prop. 2011, 14, 514–522. [Google Scholar] [CrossRef]
- Van Leeuw, R.; Kevers, C.; Pincemail, J.; Defraigne, J.O.; Dommes, J. Antioxidant capacity and phenolic composition of red wines from various grape varieties: Specificity of Pinot noir. J. Food Compos. Anal. 2014, 36, 40–50. [Google Scholar] [CrossRef]
- Ragusa, A.; Centonze, C.; Grasso, M.E.; Latronico, M.F.; Mastrangelo, P.F.; Sparascio, F.; Maffia, M. HPLC analysis of phenols in Negroamaro and Primitivo red wines from Salento. Foods 2019, 8, 45. [Google Scholar] [CrossRef]
- Gonçalves, J.; Silva, C.L.; Castilho, P.C.; Câmara, J.S. An attractive, sensitive and high-throughput strategy based on microextraction by packed sorbent followed by UHPLC-PDA analysis for quantification of hydroxybenzoic and hydroxycinnamic acids in wines. Microchem. J. 2013, 106, 129–138. [Google Scholar] [CrossRef]
- Stój, A.; Szwajgier, D.; Baranowska-Wójcik, E.; Domagala, D. Gentisic acid, salicylic acid, total phenolic content and cholinesterase inhibitory activities of red wines made from various grape varieties. S. Afr. J. Enol. Vitic. 2019, 40, 1–12. [Google Scholar] [CrossRef]
- Tomaz, I.; Maslov, L. Simultaneous determination of phenolic compounds in different matrices using phenyl-hexyl stationary phase. Food Anal. Method. 2016, 9, 401–410. [Google Scholar] [CrossRef]
- Valentin, L.; Barroso, L.P.; Barbosa, R.M.; de Paulo, G.A.; Castro, I.A. Chemical typicality of South American red wines classified according to their volatile and phenolic compounds using multivariate analysis. Food Chem. 2020, 302, 125340. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, S.; Perenzoni, D.; Angeli, A.; Stefanini, M.; Rühl, E.; Patz, C.D.; Mattivi, F.; Rauhut, D.; Vrhovsek, U. Metabolite profiling of wines made from disease-tolerant varieties. Eur. Food Res. Technol. 2019, 245, 2039–2052. [Google Scholar] [CrossRef]
- Mayr, C.M.; De Rosso, M.; Dalla Vedova, A.; Flamini, R. High-Resolution mass spectrometry identification of secondary metabolites in four red grape varieties potentially useful as traceability markers of wines. Beverages 2018, 4, 74. [Google Scholar] [CrossRef]
- Kapusta, I.; Cebulak, T.; Oszmiański, J. Characterization of Polish wines produced from the interspecific hybrid grapes grown in south-east Poland. Eur. Food Res. Technol. 2018, 244, 441–455. [Google Scholar] [CrossRef]
- The New York Times. Available online: https://www.nytimes.com/2010/02/19/business/global/19wine.html (accessed on 19 December 2019).
- Dobrowolska-Iwanek, J.; Gąstol, M.; Wanat, A.; Krośniak, M.; Jancik, M.; Zagrodzki, P. Wine of cool-climate areas in South Poland. S. Afr. J. Enol. Vitic. 2014, 35, 1–9. [Google Scholar] [CrossRef]
- Samoticha, J.; Wojdyło, A.; Golis, T. Phenolic composition, physicochemical properties and antioxidant activity of interspecific hybrids of grapes growing in Poland. Food Chem. 2017, 215, 263–273. [Google Scholar] [CrossRef]
- Socha, R.; Gałkowska, D.; Robak, J.; Fortuna, T.; Buksa, K. Characterization of Polish wines produced from the multispecies hybrid and Vitis vinifera L. grapes. Int. J. Food Prop. 2015, 18, 699–713. [Google Scholar] [CrossRef]
- Vitis International Variety Catalogue. Available online: www.vivc.de (accessed on 19 December 2019).
- Liu, J.; Arneborg, N.; Toldam-Andersen, T.B.; Petersen, M.A.; Bredie, W.L. Effect of sequential fermentations and grape cultivars on volatile compounds and sensory profiles of Danish wines. J. Sci. Food Agric. 2017, 97, 3594–3602. [Google Scholar] [CrossRef]
- Lasik-Kurdyś, M.; Gumienna, M.; Nowak, J. Influence of malolactic bacteri, a inoculation scenarios on the efficiency of the vinification process and the quality of grape wine from the Central European region. Eur. Food Res. Technol. 2017, 243, 2163–2173. [Google Scholar] [CrossRef]
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of different techniques of malolactic fermentation induction on diacetyl metabolism and biosynthesis of selected aromatic esters in cool-climate grape wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef] [PubMed]
- International Organisation of Vine and Wine. Compedium of international methods of analysis. Maximum acceptable limits of various substances contained in wine. Available online: http://www.oiv.int/js/lib/pdfjs/web/viewer.html?file=/public/medias/2601/oiv-ma-c1-01.pdf (accessed on 8 January 2020).
- Kapusta, I.; Cebulak, T.; Oszmiański, J. The anthocyanins profile of red grape cultivars growing in south-east Poland (Subcarpathia region). J. Food Meas. Charact. 2017, 11, 1863–1873. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compedium of international methods of analysis. Method OIV-MA-AS313-01. Available online: http://www.oiv.int/public/medias/3731/oiv-ma-as313-01.pdf (accessed on 15 March 2019).
- International Organisation of Vine and Wine. Compedium of international methods of analysis. Method OIV-MA-AS313-15. Available online: http://www.oiv.int/public/medias/2514/oiv-ma-as313-15.pdf (accessed on 15 March 2019).
Sample Availability: Not available. |

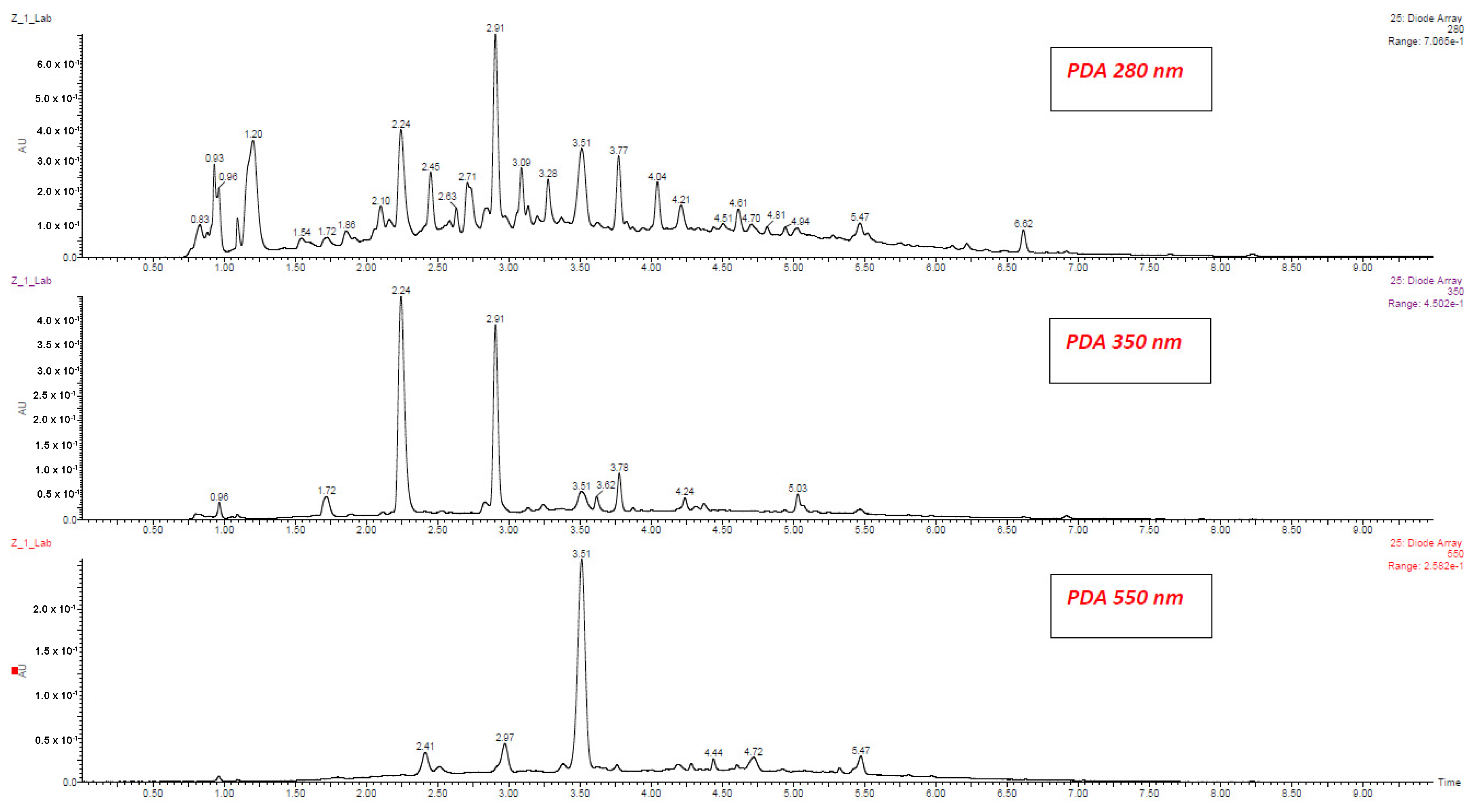
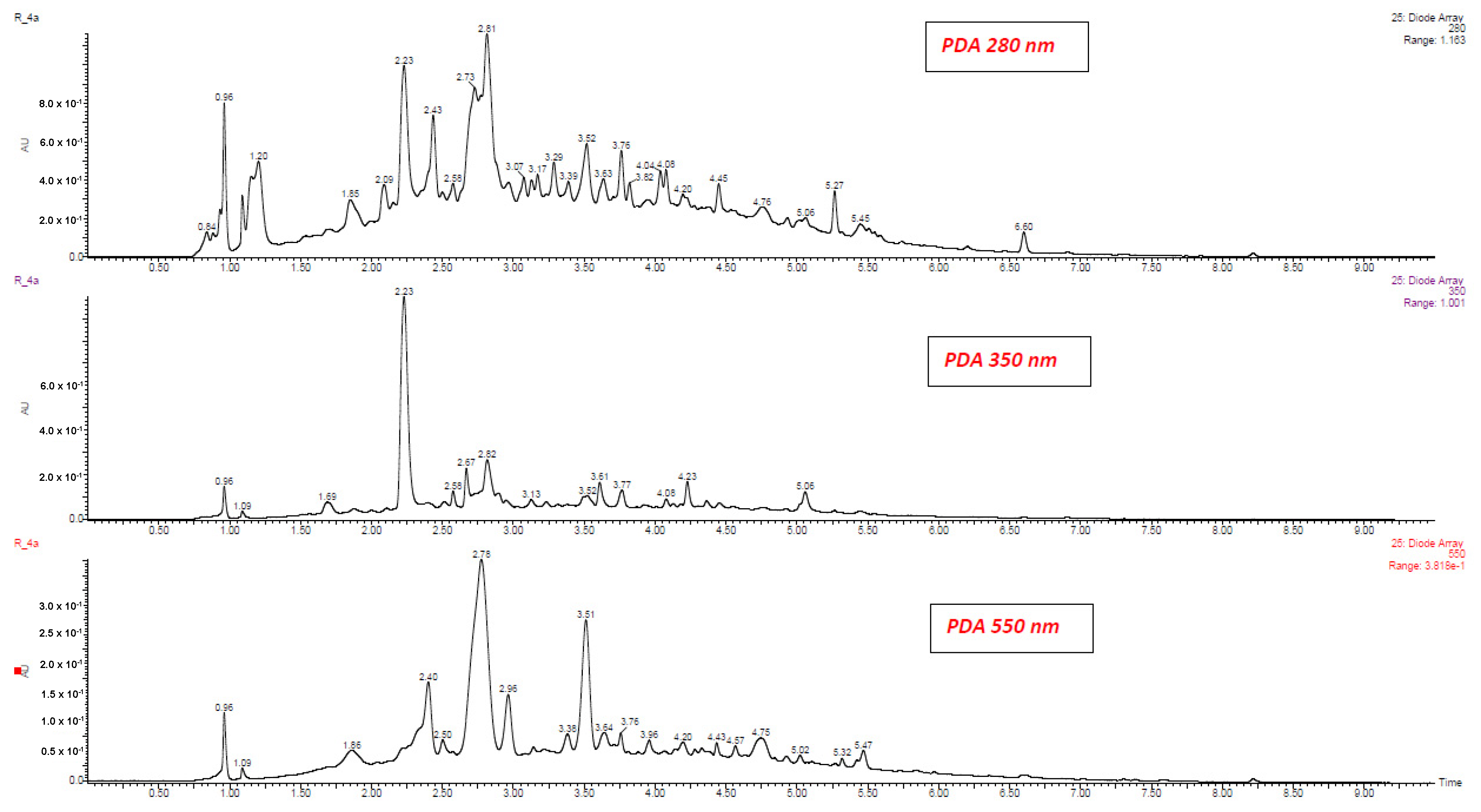


| No | Compound | Abbreviation | RT (min.) | [M − H] (m/z) | Fragment ions (m/z) | λmax (nm) |
|---|---|---|---|---|---|---|
| Anthocyanins | ||||||
| 1 | Delphinidin 3-O-glucoside-5-O-glucoside | 35dGD | 2.04 | 627 | 465, 303 | 277, 525 |
| 2 | Cyanidin 3-O-glucoside-5-O-glucoside | 35dGC | 2.19 | 611 | 449, 287 | 280, 516 |
| 3 | Delphinidin 3-O-glucoside | 3gD | 2.38 | 465 | 303 | 280, 523 |
| 4 | Petunidin 3-O-glucoside-5-O-glucoside | 35dGPet | 2.53 | 641 | 479, 317 | 277, 531 |
| 5 | Peonidin 3-O-glucoside-5-O-glucoside | 35dGPeo | 2.67 | 625 | 463, 301 | 278, 513 |
| 6 | Malvidin 3-O-glucoside-5-O-glucoside | 35dGM | 2.72 | 655 | 493, 331 | 275, 524 |
| 7 | Cyanidin 3-O-glucoside | 3gC | 2.74 | 449 | 287 | 279, 515 |
| 8 | Petunidin 3-O-glucoside | 3gPet | 2.92 | 479 | 317 | 277, 526 |
| 9 | Peonidin 3-O-glucoside | 3gPeo | 3.31 | 463 | 301 | 279, 515 |
| 10 | Malvidin 3-O-glucoside | 3gM | 3.43 | 493 | 331 | 278, 530 |
| 11 | Delphinidin 3-O-(6′′-O-acetyl)-glucoside | 3agD | 3.53 | 507 | 465, 303 | 280, 528 |
| 12 | Cyanidin 3-O-(6′′-O-acetyl)-glucoside | 3agC | 3.95 | 491 | 449, 287 | 283, 522 |
| 13 | Petunidin 3-O-(6′′-O-acetyl)-glucoside | 3agPet | 4.10 | 521 | 317 | 280, 530 |
| 14 | Petunidin 3-O-(6′′-O-acetyl)-glucoside-5-O-glucoside | 3ag5gPet | 4.28 | 787 | 625, 479, 317 | 280, 530 |
| 15 | Delphinidin 3-O-(6′′-O-coumaryl)-glucoside | 3kgD | 4.47 | 611 | 303 | 279, 530 |
| 16 | Malvidin 3-O-(6′′-O-acetyl)-glucoside | 3agM | 4.62 | 535 | 331 | 280, 521 |
| 17 | Malvidin 3-O-(6′′-O-coumaryl)-glucoside-5-O-glucoside | 3kg5gM | 4.67 | 801 | 639, 493, 331 | 280, 530 |
| 18 | Peonidin 3-O-(6′′-O-coumaryl)-glucoside-5-O-glucoside | 3kg5gPeo | 4.68 | 771 | 609, 463, 301 | 279, 523 |
| 19 | Peonidin 3-O-(6′′-O-acetyl)-glucoside | 3agPeo | 4.85 | 505 | 463, 301 | 277, 535 |
| 20 | Cyanidin 3-O-(6′′-O-coumaryl)-glucoside | 3kgC | 4.93 | 595 | 287 | 283, 522 |
| 21 | Petunidin 3-O-(6′′-O-coumaryl)-glucoside | 3kgPet | 4.98 | 625 | 317 | 280, 531 |
| 22 | Delphinidin 3-O-(6′′-caffeoyl)-glucoside | 3cafGD | 5.35 | 627 | 465, 303 | 280, 528 |
| 23 | Peonidin 3-O-(6′′-O-coumaryl)-glucoside | 3kgPeo | 5.39 | 609 | 301 | 279, 523 |
| 24 | Malvidin 3-O-(6′′-O-coumaryl)-glucoside | 3kgM | 5.44 | 639 | 331 | 280, 521 |
| Flavonols | ||||||
| 25 | Myricetin-3-O-rutinoside | 3-RutM | 4.08 | 625 | 479, 317 | 255, 353 |
| 26 | Myricetin-3-O-glucoside | 3-GM | 4.24 | 479 | 317 | 255, 353 |
| 27 | Quercetin 3-O-glucuronide | 3-GluQ | 4.48 | 477 | 301 | 255, 356 |
| 28 | Isorhamnetin 3-O-glucoside | 3-Giso | 4.67 | 447 | 315 | 254, 369 |
| 29 | Quercetin 3-O-glucoside | 3-GQ | 4.82 | 463 | 301 | 253, 365 |
| 30 | Quercetin 3-O-rutinoside | 3-RutQ | 4.99 | 609 | 447, 301 | 255, 355 |
| 31 | Dihydroquercetin 3-O-ramnoside | 3-RhadQ | 5.57 | 449 | 303 | 253, 372 |
| Flavan-3-ols | ||||||
| 32 | Procyanidin B1 | ProcB1 | 2.66 | 577 | 425, 285 | 280 |
| 33 | Procyanidin B-type 1 | ProcB-type1 | 2.81 | 577 | 425, 285 | 276 |
| 34 | Procyanidin C1 | ProcC1 | 2.97 | 865 | 577, 285 | 280 |
| 35 | (+) catechin | Cat | 3.01 | 289 | - | 280 |
| 36 | Procyanidin C-type 1 | ProcC-type1 | 3.07 | 865 | 577, 285 | 280 |
| 37 | Procyanidin B-type 2 | ProcB-type2 | 3.31 | 577 | 285 | 279 |
| 38 | Procyanidin B2 | ProcB2 | 3.34 | 577 | 285 | 280 |
| 39 | (−) epicatechin | Epicat | 3.68 | 289 | - | 280 |
| 40 | Procyanidin C-type 2 | ProcC-type2 | 3.74 | 865 | 577, 289 | 279 |
| 41 | Procyanidin C-type 3 | ProcC-type3 | 3.84 | 865 | 577, 289 | 280 |
| 42 | Procyanidin B-type 3 | ProcB-type3 | 4.06 | 577 | 289 | 279 |
| 43 | Procyanidin B-type 4 | ProcB-type4 | 4.34 | 577 | 289 | 280 |
| Phenolic acids | ||||||
| 44 | Gallic acid | Gal | 1.47 | 169 | 125 | 272 |
| 45 | Protocatechuic acid | Prot | 2.25 | 153 | 109 | 308 |
| 46 | Caftaric acid | Caft | 2.49 | 311 | 179 | 328, 294 |
| 47 | Coutaric acid | Cout | 3.08 | 295 | 163 | 310 |
| 48 | Caffeic acid | Caff | 3.46 | 153 | 109 | 260, 294 |
| 49 | Ferulic acid | Fer | 4.92 | 193 | 134 | 323, 293 |
| 50 | p-Coumaric acid | p-Coum | 4.39 | 163 | 119 | 308 |
| 51 | Coumaric acid | Coum | 4.82 | 163 | 119 | 310 |
| Stilbenes | ||||||
| 52 | Trans-piceid | Trans-P | 4.75 | 389 | 227 | 327 |
| 53 | Cis-piceid | Cis-P | 5.94 | 389 | 227 | 327 |
| 54 | Trans-resveratrol | Trans-R | 6.24 | 227 | 185 | 327 |
| 55 | Cis-resveratrol | Cis-R | 7.42 | 227 | 143 | 327 |
| Wine Code | Grape Variety | Yeast | Lactic Acid Bacteria |
|---|---|---|---|
| Z1 | Zweigelt | SafŒno ™ SC 22 | - |
| Z1 LAB | Zweigelt | SafŒno ™ SC 22 | Viniflora Oenos |
| Z2 | Zweigelt | SafŒno ™ HD S62 | - |
| Z2 LAB | Zweigelt | SafŒno ™ HD S62 | Viniflora Oenos |
| Z3 | Zweigelt | Essentiale Grand Cru | - |
| Z3 LAB | Zweigelt | Essentiale Grand Cru | Viniflora Oenos |
| Z4 | Zweigelt | Siha Active Yeast 8 | - |
| Z4 LAB | Zweigelt | Siha Active Yeast 8 | Viniflora Oenos |
| Z5 | Zweigelt | Siha Rubino Cru | - |
| Z5 LAB | Zweigelt | Siha Rubino Cru | Viniflora Oenos |
| R1 | Rondo | SafŒno ™ SC 22 | - |
| R1 LAB | Rondo | SafŒno ™ SC 22 | Viniflora Oenos |
| R2 | Rondo | SafŒno ™ HD S62 | - |
| R2 LAB | Rondo | SafŒno ™ HD S62 | Viniflora Oenos |
| R3 | Rondo | Essentiale Grand Cru | - |
| R3 LAB | Rondo | Essentiale Grand Cru | Viniflora Oenos |
| R4 | Rondo | Siha Active Yeast 8 | - |
| R4 LAB | Rondo | Siha Active Yeast 8 | Viniflora Oenos |
| R5 | Rondo | Siha Rubino Cru | - |
| R5 LAB | Rondo | Siha Rubino Cru | Viniflora Oenos |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stój, A.; Kapusta, I.; Domagała, D. Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS. Molecules 2020, 25, 1342. https://doi.org/10.3390/molecules25061342
Stój A, Kapusta I, Domagała D. Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS. Molecules. 2020; 25(6):1342. https://doi.org/10.3390/molecules25061342
Chicago/Turabian StyleStój, Anna, Ireneusz Kapusta, and Dorota Domagała. 2020. "Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS" Molecules 25, no. 6: 1342. https://doi.org/10.3390/molecules25061342
APA StyleStój, A., Kapusta, I., & Domagała, D. (2020). Classification of Red Wines Produced from Zweigelt and Rondo Grape Varieties Based on the Analysis of Phenolic Compounds by UPLC-PDA-MS/MS. Molecules, 25(6), 1342. https://doi.org/10.3390/molecules25061342







