Synthesis and Structure-Activity Relationship Studies of Hydrazide-Hydrazones as Inhibitors of Laccase from Trametes versicolor
Abstract
1. Introduction
2. Results and Discussion
2.1. Syntheses and Characterizations
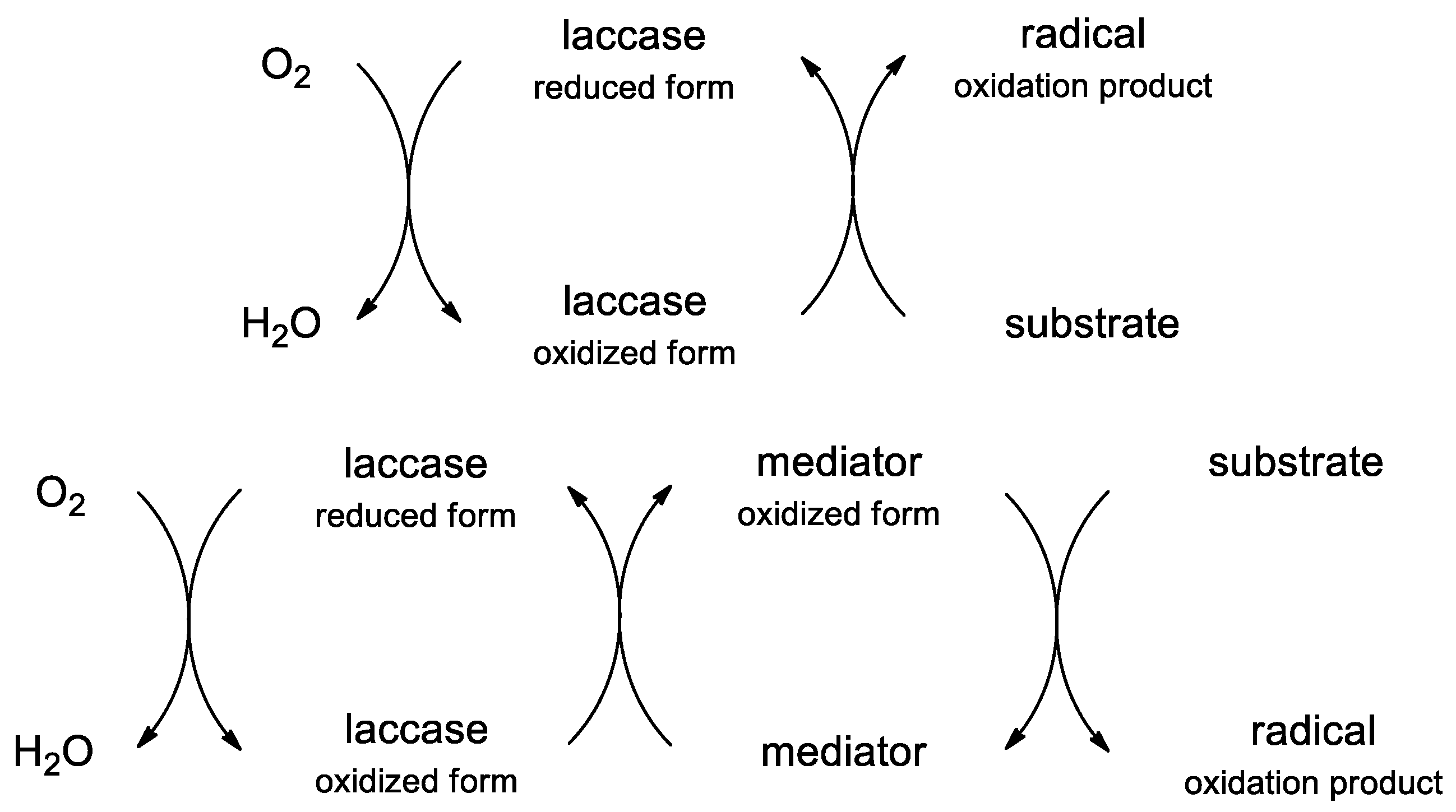
2.2. Kinetic Studies
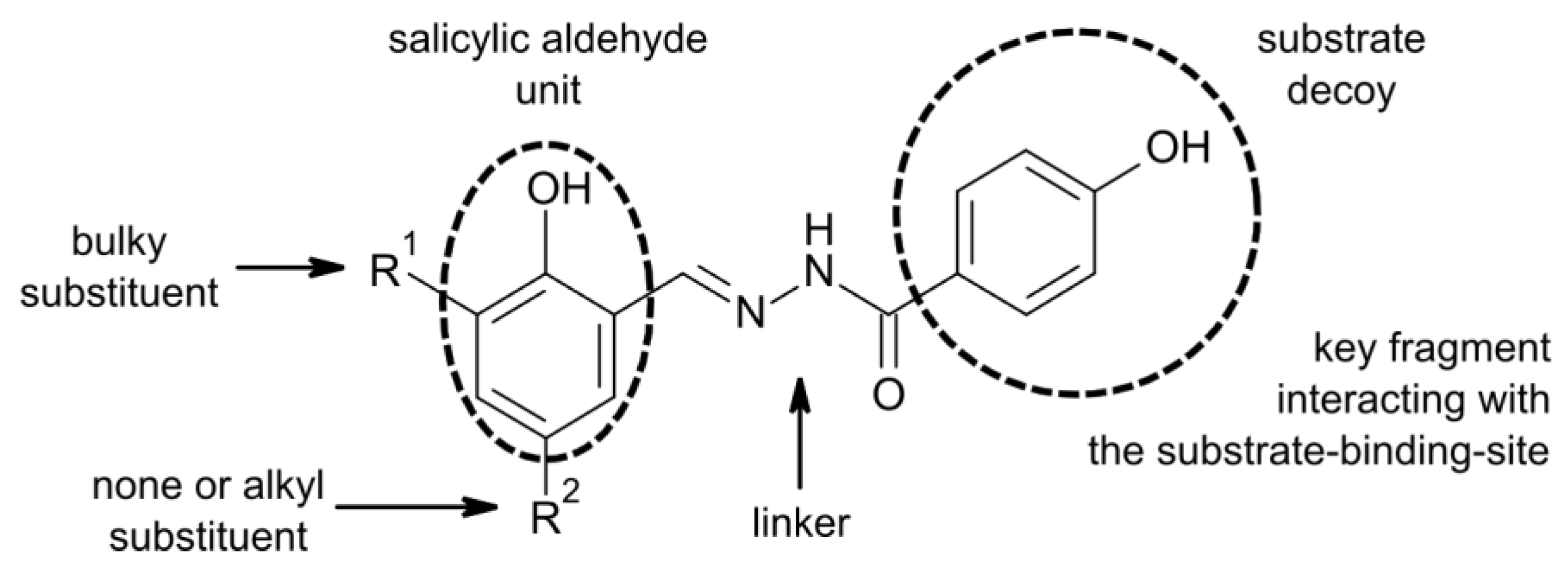
2.3. Structure-Activity Relationship (SAR)
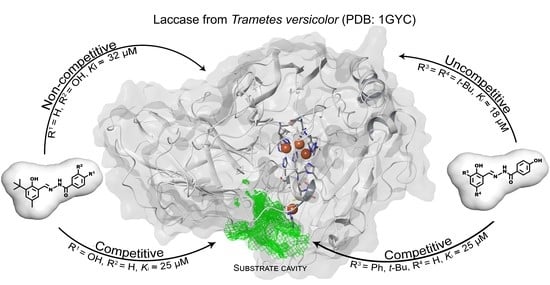
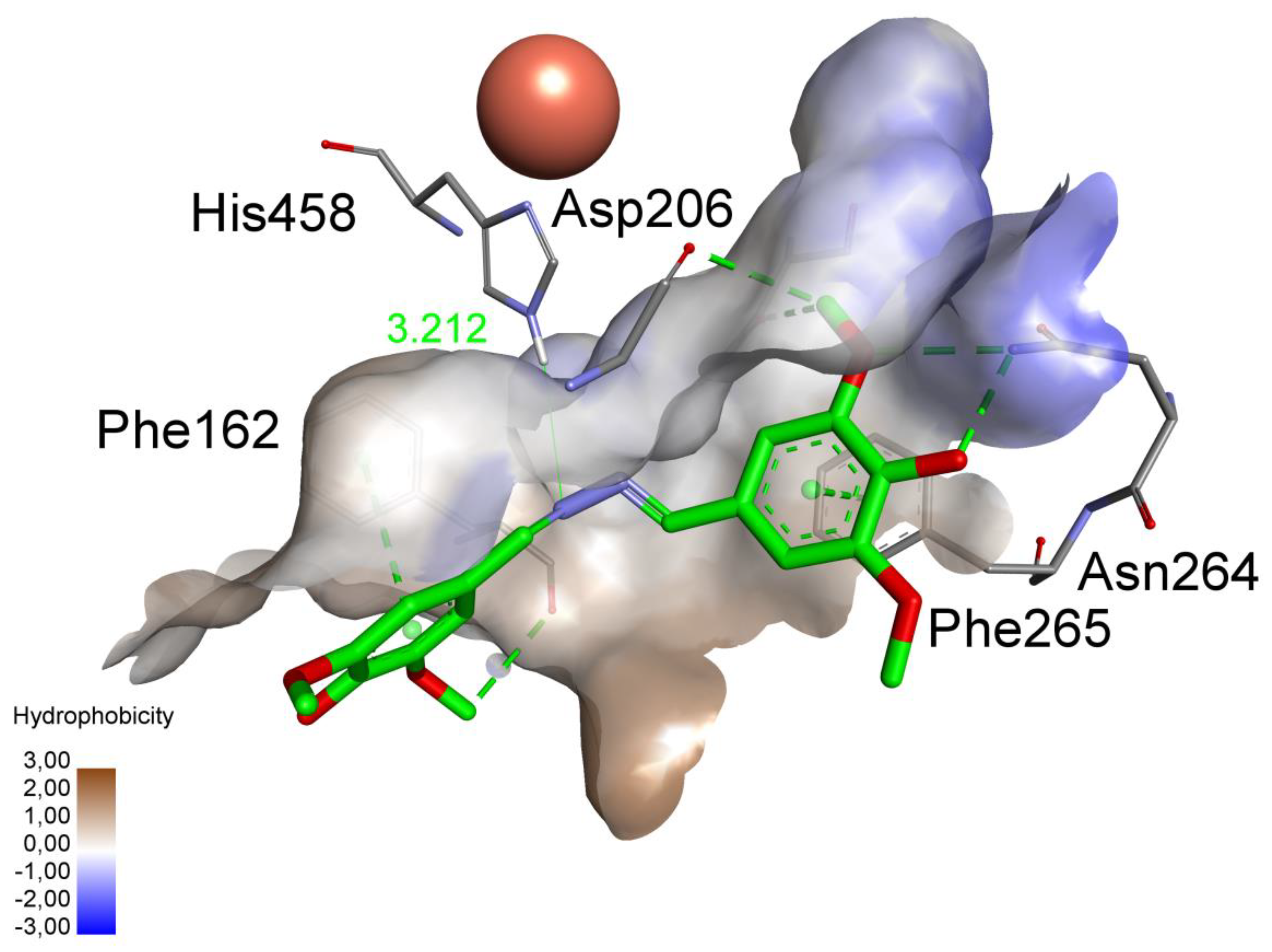
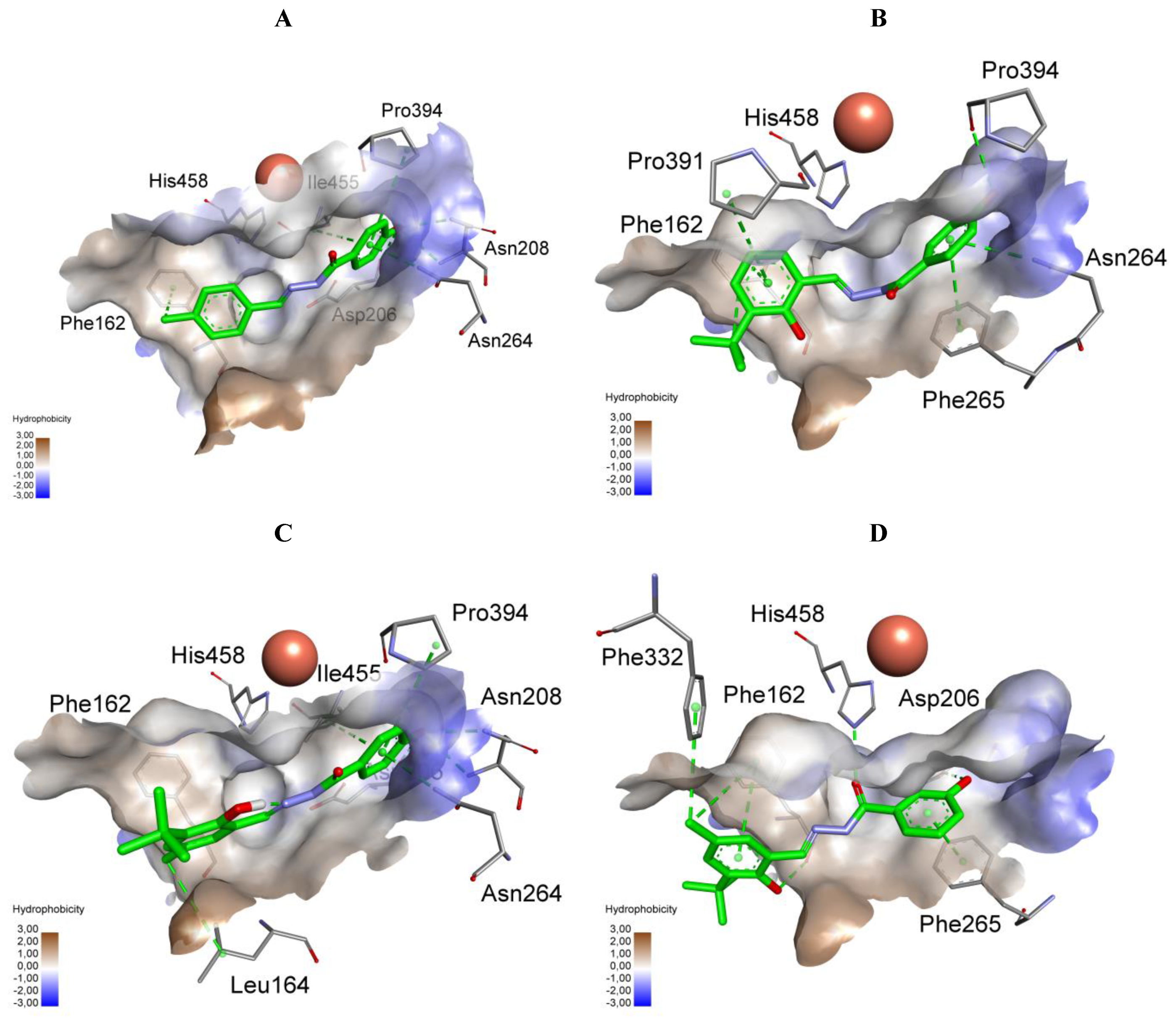
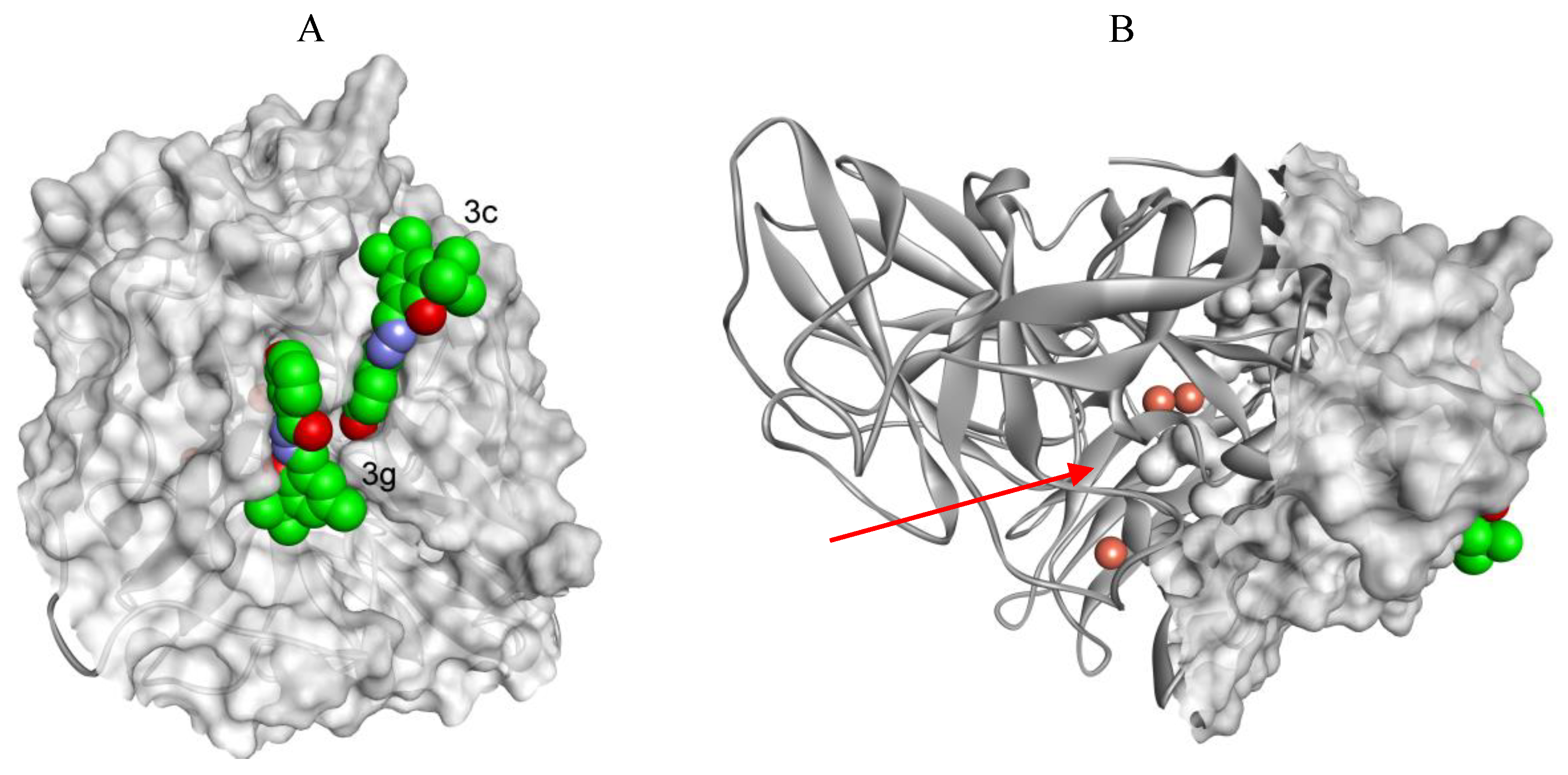
2.4. The Docking Studies
3. Materials and Methods
3.1. Reagents and Materials
3.2. Syntheses
General procedure for the Synthesis of Hydrazide-Hydrazones 1a–j, 2a–h, 3a–g
3.3. Enzyme Inhibition Assay
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dayan, F.E.; Duke, S.O. Natural compounds as next-generation herbicides. Plant Physiol. 2014, 166, 1090–1105. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D. Pesticides and Pest Control. In Integrated Pest Management: Innovation-Development Process; Peshin, R., Dhawan, A.K., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 83–87. ISBN 9781402089923. [Google Scholar] [CrossRef]
- Carvalho, F.P. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Fillinger, S.; Elad, Y. Plant Hosts of Botrytis spp.; Springer: Cham, Switzerland, 2015; ISBN 9783319233710. [Google Scholar] [CrossRef]
- Quijada-Morin, N.; Garcia, F.; Lambert, K.; Walker, A.S.; Tiers, L.; Viaud, M.; Sauvage, F.X.; Hirtz, C.; Saucier, C. Strain effect on extracellular laccase activities from Botrytis cinerea. Aust. J. Grape Wine Res. 2018, 24, 241–251. [Google Scholar] [CrossRef]
- Schwarze, F.W.M.R.; Engels, J.; Mattheck, C. Fungal Strategies of Wood Dacay in Trees; Springer: Berlin/Heidelberg, Germany, 2000; ISBN 978-3-642-57302-6. [Google Scholar] [CrossRef]
- Alford, D.V. Pests of Ornamental Trees, Shrubs and Flowers; Elsevier: London, UK, 2012; ISBN 978-0-12-398515-6. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Madhavi, V.; Lele, S.S. Laccase: Properties and applications. BioResources 2009, 4, 1694–1717. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Mayer, A.M.; Staples, R.C. Laccase: New functions for an old enzyme. Phytochemistry 2002, 60, 551–565. [Google Scholar] [CrossRef]
- Wong, A.-L.; Willetts, H. Polyacrylamide-gel electrophoresis of enzymes during morphogenesis of sclerotia of Sclerotinia sclerotiorum. J. Gen. Microbiol. 1974, 81, 101–109. [Google Scholar] [CrossRef][Green Version]
- Coman, C.; Moţ, A.C.; Gal, E.; Parvu, M.; Silaghi-Dumitrescu, R. Laccase is upregulated via stress pathways in the phytopathogenic fungus Sclerotinia sclerotiorum. Fungal Biol. 2013, 117, 528–539. [Google Scholar] [CrossRef]
- Dittmer, N.T.; Kanost, M.R. Insect multicopper oxidases: Diversity, properties, and physiological roles. Insect Biochem. Mol. Biol. 2010, 40, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef] [PubMed]
- Reinhammar, B.R.M. Oxidation-reduction potentials of the electron acceptors in laccases and stellacyanin. Biochim. Biophisica Acta 1972, 275, 245–259. [Google Scholar] [CrossRef]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef] [PubMed]
- Mot, A.C.; Silaghi-Dumitrescu, R. Laccases: Complex architectures for one-electron oxidations. Biochemistry 2012, 77, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Morozova, O.V.; Shumakovich, G.P.; Shleev, S.V.; Yaropolov, Y.I. Laccase-mediator systems and their applications: A review. Appl. Biochem. Microbiol. 2007, 43, 523–535. [Google Scholar] [CrossRef]
- Cañas, A.I.; Camarero, S. Laccases and their natural mediators: Biotechnological tools for sustainable eco-friendly processes. Biotechnol. Adv. 2010, 28, 694–705. [Google Scholar] [CrossRef]
- Johannes, C.; Majcherczyk, A. Natural mediators in the oxidation of polycyclic aromatic hydrocarbons by laccase mediator systems. Appl. Environ. Microbiol. 2000, 66, 524–528. [Google Scholar] [CrossRef]
- Morozova, O.V.; Shumakovich, G.P.; Gorbacheva, M.A.; Shleev, S.V.; Yaropolov, A.I. “Blue” laccases. Biochemistry 2007, 72, 1136–1150. [Google Scholar] [CrossRef]
- Christopher, L.P.; Yao, B.; Ji, Y. Lignin biodegradation with laccase-mediator systems. Front. Energy Res. 2014, 2, 12. [Google Scholar] [CrossRef]
- Węglarz-Tomczak, E.; Burda-Grabowska, M.; Giurg, M.; Mucha, A. Identification of methionine aminopeptidase 2 as a molecular target of the organoselenium drug ebselen and its derivatives/analogues: Synthesis, inhibitory activity and molecular modeling study. Bioorg. Med. Chem. Lett. 2016, 26, 5254–5259. [Google Scholar] [CrossRef]
- Węglarz-Tomczak, E.; Talma, M.; Giurg, M.; Westerhoff, H.V.; Janowski, R.; Mucha, A. Neutral metalloaminopeptidases APN and MetAP2 as newly discovered anticancer molecular targets of actinomycin D and its simple analogs. Oncotarget 2018, 9, 29365–29378. [Google Scholar] [CrossRef]
- Talma, M. Phosphinic dehydrodipeptides: Diversification of the P1′ residue with the Morita–Baylis–Hillman acetates and inhibition of alanyl aminopeptidases. Int. J. Pept. Res. Ther. 2020, in press. [Google Scholar] [CrossRef]
- Helios, K.; Maniak, H.; Sowa, M.; Zierkiewicz, W.; Wąsińska-Kałwa, M.; Giurg, M.; Drożdżewski, P.; Trusek-Hołownia, A.; Malik, M.; Krauze, K. Silver(I) complex with 2-amino-4,4α-dihydro-4α,7-dimethyl-3H-phenoxazin-3-one (Phx-1) ligand: Crystal structure, vibrational spectra and biological studies. J. Coord. Chem. 2017, 70, 3471–3487. [Google Scholar] [CrossRef]
- Giurg, M.; Gołąb, A.; Suchodolski, J.; Kaleta, R.; Krasowska, A.; Piasecki, E.; Piętka-Ottlik, M. Reaction of bis[(2-chlorocarbonyl)phenyl] diselenide with phenols, aminophenols, and other amines towards diphenyl diselenides with antimicrobial and antiviral properties. Molecules 2017, 22, 974. [Google Scholar] [CrossRef] [PubMed]
- Nagender, P.; Kumar, R.N.; Reddy, G.M.; Swaroop, D.K.; Poornachandra, Y.; Kumar, C.G.; Narsaiah, B. Synthesis of novel hydrazone and azole functionalized pyrazolo[3,4-b]pyridine derivatives as promising anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4427–4432. [Google Scholar] [CrossRef]
- Ahmad, M.F.; Alam, I.; Huff, S.E.; Pink, J.; Flanagan, S.A.; Shewach, D.; Misko, T.A.; Oleinick, N.L.; Harte, W.E.; Viswanathan, R. Potent competitive inhibition of human ribonucleotide reductase by a nonnucleoside small molecule. Proc. Natl. Acad. Sci. USA 2017, 114, 8241–8246. [Google Scholar] [CrossRef]
- Reis, J.S.; Corrêa, M.A.; Chung, M.C.; Dos Santos, J.L. Synthesis, antioxidant and photoprotection activities of hybrid derivatives useful to prevent skin cancer. Bioorg. Med. Chem. 2014, 22, 2733–2738. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, M.B.; Dhumal, S.T.; Deshmukh, A.R.; Nawale, L.U.; Khedkar, V.; Sarkar, D.; Mane, R.A. New bithiazolyl hydrazones: Novel synthesis, characterization and antitubercular evaluation. Bioorg. Med. Chem. Lett. 2017, 27, 288–294. [Google Scholar] [CrossRef]
- Zha, G.F.; Leng, J.; Darshini, N.; Shubhavathi, T.; Vivek, H.K.; Asiri, A.M.; Marwani, H.M.; Rakesh, K.P.; Mallesha, N.; Qin, H.L. Synthesis, SAR and molecular docking studies of benzo[d]thiazole-hydrazones as potential antibacterial and antifungal agents. Bioorg. Med. Chem. Lett. 2017, 27, 3148–3155. [Google Scholar] [CrossRef]
- Popiołek, Ł. Hydrazide–hydrazones as potential antimicrobial agents: Overview of the literature since 2010. Med. Chem. Res. 2017, 26, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Chen, M.; Wang, J.; Peng, Y.; Li, L.; Xie, Z.Z.; Deng, B.; Chen, S.; Li, W. Synthesis, biological evaluation and molecular docking studies of chromone hydrazone derivatives as α-glucosidase inhibitors. Bioorg. Med. Chem. Lett. 2017, 27, 2957–2961. [Google Scholar] [CrossRef]
- Mayer, N.; Schweiger, M.; Melcher, M.C.; Fledelius, C.; Zechner, R.; Zimmermann, R.; Breinbauer, R. Structure-activity studies in the development of a hydrazone based inhibitor of adipose-triglyceride lipase (ATGL). Bioorg. Med. Chem. 2015, 23, 2904–2916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ravish, I.; Raghav, N. Synthesis, pharmacological evaluation and molecular docking of some pyrimidinyl hydrazones. Biochem. Anal. Biochem. 2016, s3, 1–6. [Google Scholar] [CrossRef]
- Can, N.Ö; Osmaniye, D.; Levent, S.; Sa Saǧlik, B.N.; Inci, B.; Ilgin, S.; Özkay, Y.; Kaplancikli, Z.A. Synthesis of new hydrazone derivatives for MAO enzymes inhibitory activity. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Hanna, M.L.; Tarasow, T.M.; Perkins, J. Mechanistic differences between in vitro assays for hydrazone-based small molecule inhibitors of anthrax lethal factor. Bioorg. Chem. 2007, 35, 50–58. [Google Scholar] [CrossRef]
- Tisma, M.; Molnar, M.; Skarica, M.; Cacić, M.; Zelić, B. Laccase ihibiting activity of some coumarin derivatives. Lett. Org. Chem. 2004, 11, 583–589. [Google Scholar] [CrossRef]
- Green, D.A.; Wong, S.J.; Chitambar, C.R.; Antholine, W.E.; Richardson, D.R. Inhibition of malignant cell growth by 311, a novel iron chelator of the pyridoxal isonicotinoyl hydrazone class: Effect on the R2 subunit of ribonucleotide reductase. Clin. Cancer Res. 2001, 7, 3574–3579. [Google Scholar]
- Siemann, S.; Evanoff, D.P.; Marrone, L.; Clarke, A.J.; Viswanatha, T.; Dmitrienko, G.I. N-arylsulfonyl hydrazones as inhibitors of IMP-1 metallo-β-lactamase. Antimicrob. Agents Chemother. 2002, 46, 2450–2457. [Google Scholar] [CrossRef]
- Said, S.B.; Elagamey, A.A.; Khadr, R.E. Facile oxidative conversion of aroyl hydrazones into 1,3,4-oxadiazoles. Egypt. J. Chem. 2003, 46, 881–888. [Google Scholar]
- Flagstad, T.; Petersen, M.T.; Nielsen, T.E. A four-component reaction for the synthesis of dioxadiazaborocines. Angew. Chemie Int. Ed. 2015, 54, 8395–8397. [Google Scholar] [CrossRef] [PubMed]
- Barry, F.; Donna, Y. Ligand For Estrogen Related Receptors and Methods for Synthesis of Said Ligands. U.S. Patent 7,544,838 B2, 9 June 2009. [Google Scholar]
- Angelova, V.T.; Vassilev, N.G.; Nikolova-Mladenova, B.; Vitas, J.; Malbaša, R.; Momekov, G.; Djukic, M.; Saso, L. Antiproliferative and antioxidative effects of novel hydrazone derivatives bearing coumarin and chromene moiety. Med. Chem. Res. 2016, 25, 2082–2092. [Google Scholar] [CrossRef]
- Leigh, M.; Raines, D.J.; Castillo, C.E.; Duhme-Klair, A.K. Inhibition of xanthine oxidase by thiosemicarbazones, hydrazones and dithiocarbazates derived from hydroxy-substituted benzaldehydes. ChemMedChem 2011, 6, 1107–1118. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.K.; Bhamana, R.P.; Patel, M.R.; Bellare, R.A.; Deliwala, C.V. Chemotherapy of fungus infections. III. Alkyl or aryl thiosemicarbazones, acid hydrazones, and styryl aryl ketones of 5-bromo- and 5-nitrosalicylaldehydes. Indian J. Chem. 1972, 10, 694–698. [Google Scholar]
- Prachumrat, R.; Kobkeatthawin, T.; Ruanwas, P.; Boonnak, N.; Laphookhieo, S.; Kassim, M.B.; Chantrapromma, S. Synthesis, crystal structure, antioxidant, and α-glucosidase inhibitory activities of methoxy-substituted benzohydrazide derivatives. Crystallogr. Rep. 2018, 63, 405–411. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, Y.; Wang, J.; Peng, Q.; Luo, H.; Zheng, J. Synthesis and biological activities of substituted N’-benzoylhydrazone derivatives. Afr. J. Biotechnol. 2011, 10, 18013–18021. [Google Scholar] [CrossRef]
- Shalash, M.; Salhin, A.; Adnan, R.; Yeap, C.S.; Fun, H.-K. (E)-4-Hydroxy-N′-(4-hydroxy-3-methoxybenzylidene)benzohydrazide. Acta Crystallogr. Sect. E 2010, E66, o3126–o3127. [Google Scholar] [CrossRef]
- Nurkenov, O.A.; Satpaeva, Z.B.; Schepetkin, I.A.; Khlebnikov, A.I.; Turdybekov, K.M.; Seilkhanov, T.M.; Fazylov, S.D. Synthesis and biological activity of hydrazones of o- and p-hydroxybenzoic acids. Spatial structure of 5-bromo-2-hydroxybenzylidene-4-hydroxybenzohydrazide. Russ. J. Gen. Chem. 2017, 87, 2299–2306. [Google Scholar] [CrossRef]
- Nomura, N.; Ishii, R.; Yamamoto, Y.; Kondo, T. Stereoselective ring-opening polymerization of a racemic lactide by using achiral salen- and homosalen-aluminum complexes. Chem. A Eur. J. 2007, 13, 4433–4451. [Google Scholar] [CrossRef]
- Zhang, S.; Wan, C.; Wang, Q.; Zhang, B.; Gao, L.; Zha, Z.; Wang, Z. Synthesis of chromones through LiOtBu/air-mediated oxidation and regioselective cyclization of o-hydroxyphenyl propargyl carbinols. Eur. J. Org. Chem. 2013, 2013, 2080–2083. [Google Scholar] [CrossRef]
- Luehr, G.; Anik, S.T.; Peng, G.; Dostenko, I.; Phiasivongsa, P.; Romani, D. Pegylated Carfilzomb Compounds. U.S. Patent WO 2017/205392 A1, 30 November 2017. [Google Scholar]
- Haight, A.R.; Bailey, A.E.; Baker, W.S.; Cain, M.H.; Copp, R.R.; Demattei, J.A.; Ford, K.L.; Henry, R.F.; Hsu, M.C.; Keyes, R.F.; et al. A scaleable synthesis of fiduxosin. Org. Process Res. Dev. 2004, 8, 897–902. [Google Scholar] [CrossRef]
- Ogawa, A.; Oohora, K.; Hayashi, T. Synthesis and characterization of meso-substituted cobalt tetradehydrocorrin and evaluation of its electrocatalytic behavior toward CO2 reduction and H2 evolution. Inorg. Chem. 2018, 57, 14644–14652. [Google Scholar] [CrossRef] [PubMed]
- Serra, S.; Alouane, A.; Le Saux, T.; Huvelle, S.; Plasson, R.; Schmidt, F.; Jullien, L.; Labruère, R. A chemically encoded timer for dual molecular delivery at tailored ranges and concentrations. Chem. Commun. 2018, 54, 6396–6399. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, G.; Casnati, G.; Puglia, G.; Sartori, G.; Terenghi, G. Selective reactions between phenols and formaldehyde. A novel route to salicylaldehydes. J. Chem. Soc. Perkin Trans. 1 1980, 1980, 1862–1865. [Google Scholar] [CrossRef]
- Skarżewski, J.; Ostrycharz, E.; Siedlecka, R.; Zielińska-Blajet, M.; Pisarski, B. Substituted N-salicylidene β-aminoalcohols: Preparation and use as chiral ligands in enantioselective sulfoxidation and conjugate addition. J. Chem. Res. Part S 2001, 263–264. [Google Scholar] [CrossRef]
- Kauch, M.; Hoppe, D. Efficient two-step synthesis of salicylaldehydes via directed ortho-lithiation of in situ N-silylated O-aryl N-isopropylcarbamates. Synthesis 2006, 1575–1577. [Google Scholar] [CrossRef]
- Grimster, N.P.; Connelly, S.; Baranczak, A.; Dong, J.; Krasnova, L.B.; Sharpless, K.B.; Powers, E.T.; Wilson, I.A.; Kelly, J.W. Aromatic sulfonyl fluorides covalently kinetically stabilize transthyretin to prevent amyloidogenesis while affording a fluorescent conjugate. J. Am. Chem. Soc. 2013, 135, 5656–5668. [Google Scholar] [CrossRef]
- Rajput, J.D.; Bagul, S.D.; Hosamani, A.A.; Patil, M.M.; Bendre, R.S. Synthesis, characterizations, biological activities and docking studies of novel dihydroxy derivatives of natural phenolic monoterpenoids containing azomethine linkage. Res. Chem. Intermed. 2017, 43, 5377–5393. [Google Scholar] [CrossRef]
- Ndikuryayo, F.; Kang, W.M.; Wu, F.X.; Yang, W.C.; Yang, G.F. Hydrophobicity-oriented drug design (HODD) of new human 4-hydroxyphenylpyruvate dioxygenase inhibitors. Eur. J. Med. Chem. 2019, 166, 22–31. [Google Scholar] [CrossRef]
- Couto, S.R.; Toca, J.L. Inhibitors of laccases: A review. Curr. Enzym. Inhib. 2006, 2, 343–352. [Google Scholar] [CrossRef]
- Milton, R.D.; Minteer, S.D. Investigating the reversible inhibition model of laccase by hydrogen peroxide for bioelectrocatalytic applications. J. Electrochem. Soc. 2014, 161, H3011–H3014. [Google Scholar] [CrossRef][Green Version]
- Xu, F. Effects of redox potential and hydroxide inhibition on the pH activity profile of fungal laccases. J. Biol. Chem. 1997, 272, 924–928. [Google Scholar] [CrossRef] [PubMed]
- Carunchio, F.; Crescenzi, C.; Girelli, A.M.; Messina, A.; Tarola, A.M. Oxidation of ferulic acid by laccase: Identification of the products and inhibitory effects of some dipeptides. Talanta 2001, 55, 189–200. [Google Scholar] [CrossRef]
- Marques De Souza, C.G.; Peralta, R.M. Purification and characterization of the main laccase produced by the white-rot fungus Pleurotus pulmonarius on wheat bran solid state medium. J. Basic Microbiol. 2003, 43, 278–286. [Google Scholar] [CrossRef]
- Zavarzina, A.G.; Leontievsky, A.A.; Golovleva, L.A.; Trofimov, S.Y. Biotransformation of soil humic acids by blue laccase of Panus tigrinus 8/18: An in vitro study. Soil Biol. Biochem. 2004, 36, 359–369. [Google Scholar] [CrossRef]
- Dalisay, D.S.; Saludes, J.P.; Molinski, T.F. Ptilomycalin A inhibits laccase and melanization in Cryptococcus neoformans. Bioorg. Med. Chem. 2011, 19, 6654–6657. [Google Scholar] [CrossRef]
- Martínez-Sotres, C.; López-Albarrán, P.; Cruz-de-León, J.; García-Moreno, T.; Rutiaga-Quiñones, J.G.; Vázquez-Marrufo, G.; Tamariz-Mascarúa, J.; Herrera-Bucio, R. Medicarpin, an antifungal compound identified in hexane extract of Dalbergia congestiflora Pittier heartwood. Int. Biodeterior. Biodegrad. 2012, 69, 38–40. [Google Scholar] [CrossRef]
- Martínez-Sotres, C.; Rutiaga-Quiñones, J.G.; Herrera-Bucio, R.; Gallo, M.; López-Albarrán, P. Molecular docking insights into the inhibition of laccase activity by medicarpin. Wood Sci. Technol. 2015, 49, 857–868. [Google Scholar] [CrossRef]
- Rodakiewicz-Nowak, J.; Kasture, S.M.; Dudek, B.; Haber, J. Effect of various water-miscible solvents on enzymatic activity of fungal laccases. J. Mol. Catal. B Enzym. 2000, 11, 1–11. [Google Scholar] [CrossRef]
- Wilson, J.L. Biochemistry, 3rd ed.; (Stryer, Lubert), Freeman: New York, NY, USA. J. Chem. Educ. 1988, 65, A337. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Lubert, S. Biochemia; Wydawnictwo Naukowe PWN: Warsaw, Poland, 2009; ISBN 978-83-01-15811-8. [Google Scholar]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Alagesan, M.; Bhuvanesh, N.S.P.; Dharmaraj, N. Potentially cytotoxic new copper(II) hydrazone complexes: Synthesis, crystal structure and biological properties. Dalton Trans. 2013, 42, 7210–7223. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zhao, S.S.; Liu, Y.P.; Man, Z.W.; Wang, P.; Wang, L.N.; Xu, Y.; Zhu, H.L. Preparations, characterization, and biological features of mononuclear Cu(II) complexes based on hydrazone ligands. Bioorg. Med. Chem. Lett. 2016, 26, 4925–4929. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.M.; Teng, H.B.; Ke, X.B.; Xu, W.J.; Su, J.T.; Liang, S.C.; Hu, X.M. Copper(II) complexes of salicylaldehyde hydrazones: Synthesis, structure, and DNA interaction. Chem. Biodivers. 2007, 4, 2198–2209. [Google Scholar] [CrossRef]
- Hayes, M.H.B.; Swift, R.S. An appreciation of the contribution of Frank Stevenson to the advancement of studies of soil organic matter and humic substances. J. Soils Sediments 2018, 18, 1212–1231. [Google Scholar] [CrossRef]
- Lisov, A.V.; Trubitsina, L.I.; Lisova, Z.A.; Trubitsin, I.V.; Zavarzina, A.G.; Leontievsky, A.A. Transformation of humic acids by two-domain laccase from Streptomyces anulatus. Process Biochem. 2019, 76, 128–135. [Google Scholar] [CrossRef]
- Johannes, C.; Majcherczyk, A. Laccase activity tests and laccase inhibitors. J. Biotechnol. 2000, 78, 193–199. [Google Scholar] [CrossRef]
- Ters, T.; Kuncinger, T.; Srebotnik, E. Carboxylic acids used in common buffer systems inhibit the activity of fungal laccases. J. Mol. Catal. B Enzym. 2009, 61, 261–267. [Google Scholar] [CrossRef]
- Patel, H.; Gupte, S.; Gahlout, M.; Gupte, A. Purification and characterization of an extracellular laccase from solid-state culture of Pleurotus ostreatus HP-1. Biotechnology 2014, 4, 77–84. [Google Scholar] [CrossRef]
- Protein Data Bank: 1GYC. Available online: https://www.rcsb.org/structure/1GYC (accessed on 3 November 2018). [CrossRef]
- Mehra, R.; Muschiol, J.; Meyer, A.S.; Kepp, K.P. A structural-chemical explanation of fungal laccase activity. Sci. Rep. 2018, 8, 17285. [Google Scholar] [CrossRef]
- Pereira, T.M.; Vitório, F.; Amaral, R.C.; Zanoni, K.P.S.; Murakami Iha, N.Y.; Kümmerle, A.E. Microwave-assisted synthesis and photophysical studies of novel fluorescent N-acylhydrazone and semicarbazone-7-OH-coumarin dyes. New J. Chem. 2016, 40, 8846–8854. [Google Scholar] [CrossRef]
- Nisa, M.-u.; Munawar, M.A.; Iqbal, A.; Ahmed, A.; Ashraf, M.; Gardener, Q.t.A.A.; Khan, M.A. Synthesis of novel 5-(aroylhydrazinocarbonyl) escitalopram as cholinesterase inhibitors. Eur. J. Med. Chem. 2017, 138, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.S.; Reddy, B.V.S.; Reddy, P.S.R.; Basak, A.K.; Narsaiah, A.V. Efficient halogenation of aromatic systems using N-halosuccinimides in ionic liquids. Adv. Synth. Catal. 2004, 346, 77–82. [Google Scholar] [CrossRef]
- Bieszczad, B.; Barbasiewicz, M. The key role of the nonchelating conformation of the benzylidene ligand on the formation and initiation of Hoveyda-Grubbs metathesis catalysts. Chem. A Eur. J. 2015, 21, 10322–10325. [Google Scholar] [CrossRef] [PubMed]
- Gisch, N.; Balzarini, J.; Meier, C. Studies on enzyme-cleavable dialkoxymethyl-cycloSaligenyl-2′, 3′-dideoxy-2′,3′-didehydrothymidine monophosphates. J. Med. Chem. 2008, 51, 6752–6760. [Google Scholar] [CrossRef] [PubMed]
- Khusnutdinov, R.I.; Shchadneva, N.A.; Mayakova, Y.Y. Methylation of aliphatic and aromatic carboxylic acids with dimethyl carbonate under the influence of manganese and iron carbonyls. Russ. J. Gen. Chem. 2018, 88, 15–19. [Google Scholar] [CrossRef]
- Dias, L.C.; Polo, E.C. Nhatrangin A: Total syntheses of the proposed structure and six of its diastereoisomers. J. Org. Chem. 2017, 82, 4072–4112. [Google Scholar] [CrossRef]
- Bigi, F.; Conforti, M.L.; Maggi, R.; Sartori, G. Trialkylamine controlled phenol-for maldehyde reaction over clay catalysts: Selective and environmentally benign synthesis of salicylic aldehydes. Tetrahedron 2000, 56, 2709–2712. [Google Scholar] [CrossRef]
- Casiraghi, G.; Casnati, G.; Cornia, M.; Pochini, A.; Sartori, G.; Ungaro, R. Selective reactions using metal phenoxides. Part 2. Reaktions with aromatic alcohols. J. Chem. Soc. Perkin Trans. 1 1978, 1972–1999, 322–325. [Google Scholar] [CrossRef]
- Diciccio, A.M.; Longo, J.M.; Rodríguez-Calero, G.G.; Coates, G.W. Development of highly active and regioselective catalysts for the copolymerization of epoxides with cyclic anhydrides: An unanticipated effect of electronic variation. J. Am. Chem. Soc. 2016, 138, 7107–7113. [Google Scholar] [CrossRef]
- Leonowicz, A.; Grzywnowicz, K. Quantitative estimation of laccase forms in some white-rot fungi using syringaldazine as a substrate. Enzym. Microb. Technol. 1981, 3, 55–58. [Google Scholar] [CrossRef]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aid. Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Sherman, W.; Day, T.; Jacobson, M.P.; Friesner, R.A.; Farid, R. Novel procedure for modeling ligand/receptor induced fit effects. J. Med. Chem. 2006, 49, 534–553. [Google Scholar] [CrossRef] [PubMed]
- Schrödinger Release 2018-4; LigPrep, Schrödinger, LLC: New York, NY, USA, 2018.
- Schrödinger Release 2018-4: Schrödinger Suite 2018-2 Induced Fit Docking Protocol; Glide, Schrödinger, LLC: New York, NY, USA, 2018.
- Jacobson, M.P.; Pincus, D.L.; Rapp, C.S.; Day, T.J.F.; Honig, B.; Shaw, D.E.; Friesner, R.A. A hierarchical approach to all-atom protein loop prediction. Proteins 2004, 55, 351–367. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |

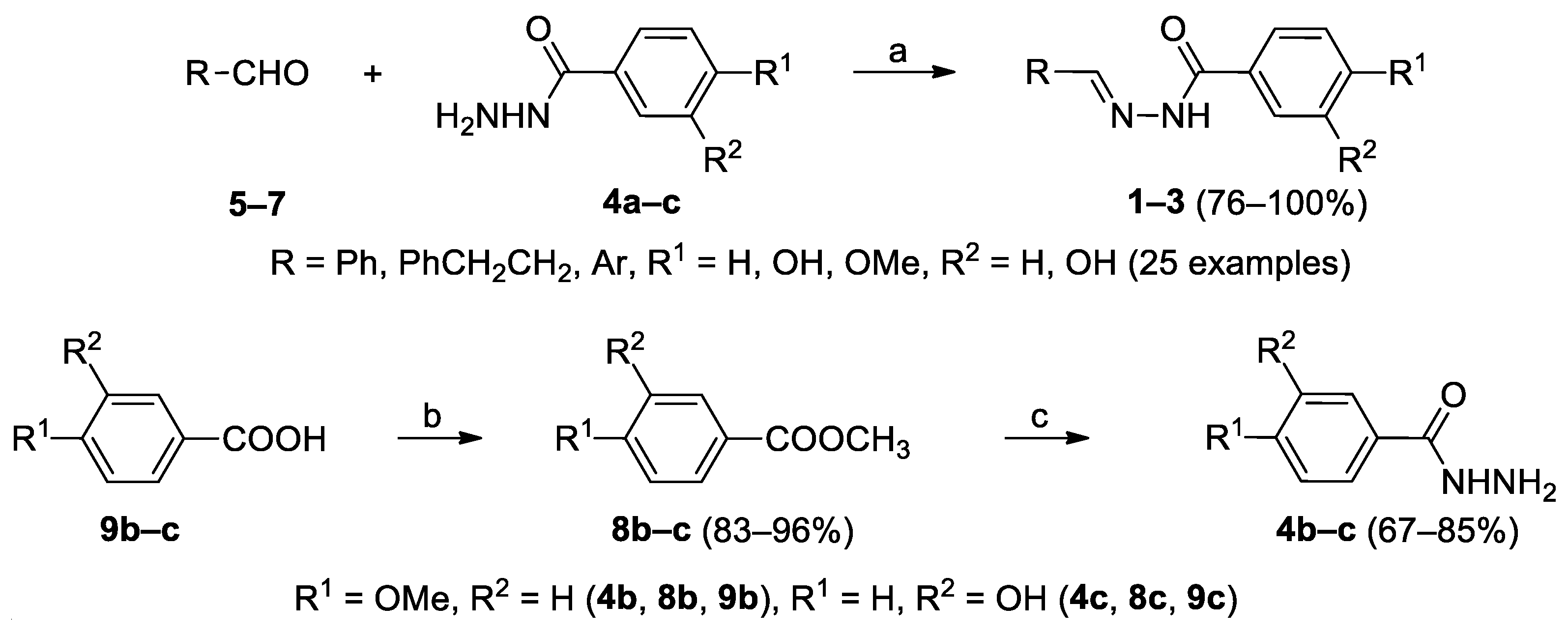
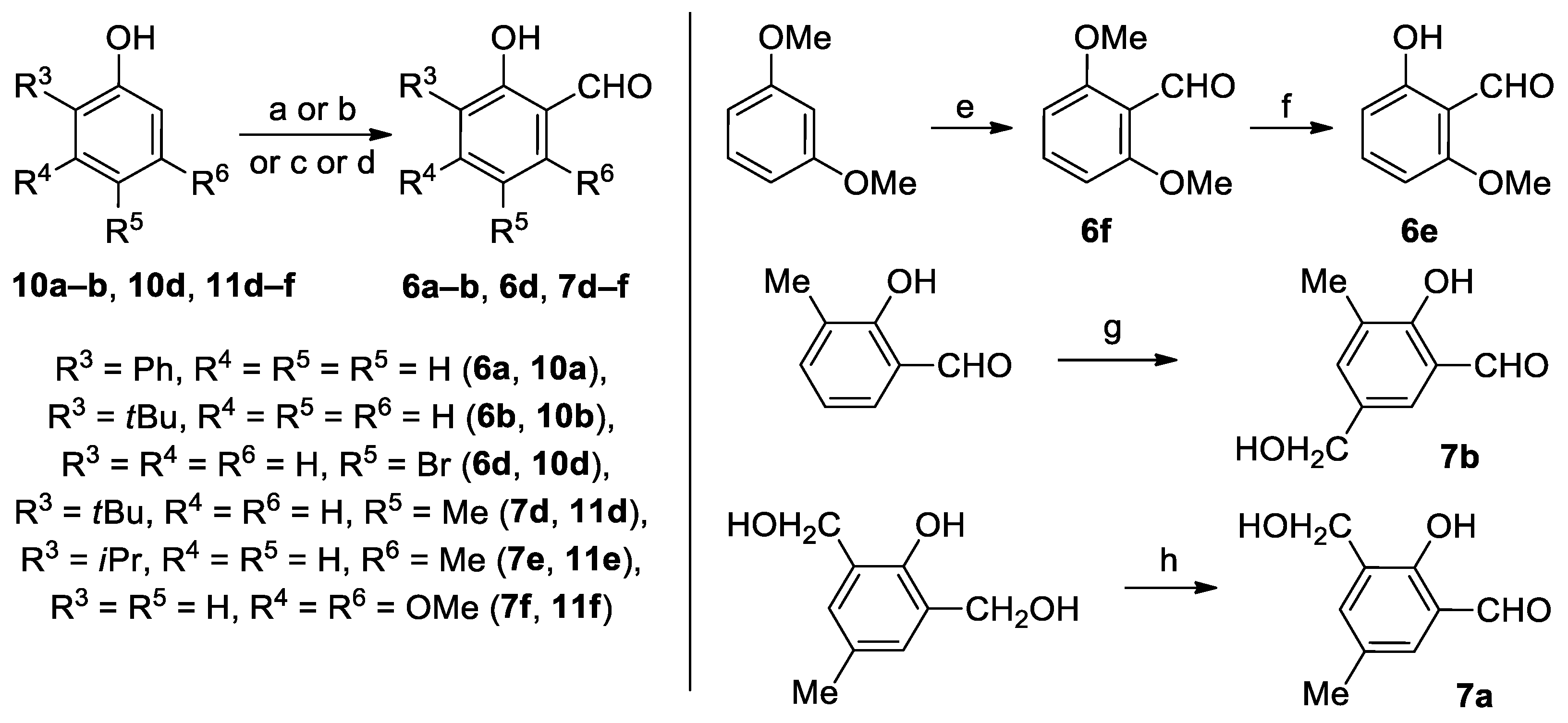

| No. | R | R1 | R2 | Rx time | Yield(%) | Mp (°C) | Mp lit.(°C) | Lit. |
|---|---|---|---|---|---|---|---|---|
| 1a | Ph | OH | H | 2h | 96 | 242.0–244.0 | 240–242 | [44] |
| 1b | C6H5CH2CH2 | OH | H | 6h | 72 | 243.5–244.0 | – | [45] |
| 1c | 4-MeC6H4 | OH | H | 2h | 80 | 246.0–247.0 | 252.6 | [46] |
| 1d | 2-NaSO3C6H4 | OH | H | 2h | 97 | 251 dec. | – | – |
| 1e | 2-HOC6H4 | OH | H | 2h | 86 | 265.0–266.5 | 257–258 | [47] |
| 1f | 3-HOC6H4 | OH | H | 2h | 93 a | 254 dec. | >250 | [48] |
| 1g | 4-HOC6H4 | OH | H | 2h | 96 b | 261 dec. | 265 | [49] |
| 1h | 2-MeOC6H4 | OH | H | 4h | 91 | 236.0–237.0 | 230–231 | [50] |
| 1i | 3-MeOC6H4 | OH | H | 5h | 79 | 210.0–212.0 | 205–206 | [50] |
| 1j | 4-MeOC6H4 | OH | H | 5h | 82 | 223.0–224.0 | 220–221 | [50] |
| 2a | 2-HO-3-PhC6H3 | OH | H | 6h | 85 c | 215.0–218.0 | – | – |
| 2b | 2-HO-3-tBuC6H3 | OH | H | 6h | 93 | 240.0–242.0 | – | – |
| 2c | 2,4-(HO)2C6H3 | OH | H | 2h | 90 | 294 dec. | – | [48] |
| 2d | 2-HO-5-BrC6H3 | OH | H | 3h | 90 | 276.0–278.0 | 279–280 | [49] |
| 2e | 2-HO-6-MeOC6H3 | OH | H | 2h | 71 | 256.5–257.5 | – | – |
| 2f | 2,6-(MeO)2C6H3 | OH | H | 6h | 97 | 214.5–216.0 | – | – |
| 2g | 3-MeO-4-HOC6H3 | OH | H | 3h | 100 | 218.5–220.0 | 226–227 | [51] |
| 2h | 2-HO-3-PhC6H3 | OMe | H | 3h | 84 | 240.0–242.5 | – | – |
| 3a | 2-HO-3-CH2OH-5-MeC6H2 | OH | H | 3h | 77 | 229.0–230.0 | – | – |
| 3b | 2-HO-3-Me-5-CH2OHC6H2 | OH | H | 3h | 86 | 266 dec. | – | – |
| 3c | 2-HO-3,5-(tBu)2C6H2 | OH | H | 6h | 93.5 | 274.5–275.5 | – | – |
| 3d | 2-HO-3-tBu-5-MeC6H2 | OH | H | 2h | 76 | 261.0–262.0 | – | – |
| 3e | 2-HO-3-iPr-6-MeC6H2 | OH | H | 6h | 94.5 | 258.5–260.5 | – | – |
| 3f | 2-HO-4,6-(MeO)2C6H2 | OH | H | 5h | 99 | 231.5–234.5 | – | – |
| 3g | 2-HO-3-tBu-5-MeC6H2 | H | OH | 2h | 94 | 240.0–242.5 | – | – |
 | |||||
| Nr | R | Ki[µM] | Nr | R | Ki[µM] |
| 1a | Ph | 1468 ± 58 a | 2e | 2-HO-6-MeOC6H3 | ≥1000 c |
| 1b | C6H5CH2CH2 | 1919 ± 163 b | 2f | 2,6-(MeO)2C6H3 | ≥1000 c |
| 1c | 4-MeC6H4 | 251 ± 26 a | 2g | 3-MeO-4-HOC6H3 | 251 ± 38 a |
| 1d | 2-NaSO3C6H4 | ≥2400 c | 2h | – | ≥1000 c |
| 1e | 2-HOC6H4 | 674 ± 10 a | 3a | 2-HO-3-CH2OH-5-MeC6H2 | ≥1000 c |
| 1f | 3-HOC6H4 | 2396 ± 334 a | 3b | 2-HO-3-Me-5-CH2OHC6H2 | ≥1000 c |
| 1g | 4-HOC6H4 | 638 ± 35 b | 3c | 2-HO-3,5-(tBu)2C6H2 | 17.9 ± 1.3 b |
| 1h | 2-MeOC6H4 | 1064 ± 18 a | 3d | 2-HO-3-tBu-5-MeC6H2 | 26.4 ± 3.4 a |
| 1i | 3-MeOC6H4 | 416 ± 1.5 a | 3e | 2-HO-3-iPr-6-MeC6H2 | ≥1000 c |
| 1j | 4-MeOC6H4 | ≥2400 c | 3f | 2-HO-4,6-(MeO)2C6H2 | ≥1000 c |
| 2a | 2-HO-3-PhC6H3 | 25.3 ± 6.9 a | 3g | – | 32.0 ± 8.0 d |
| 2b | 2-HO-3-tBuC6H3 | 24.0 ± 5.9 a | 4b | 4-HBAH | ≥2400 c |
| 2c | 2,4-(HO)2C6H3 | 939 ± 30 b | control | 4-HBA | ≥2400 c |
| 2d | 2-HO-5-BrC6H3 | ≥1000 c | control | NaN3 | 2.72 ± 0.3 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maniak, H.; Talma, M.; Matyja, K.; Trusek, A.; Giurg, M. Synthesis and Structure-Activity Relationship Studies of Hydrazide-Hydrazones as Inhibitors of Laccase from Trametes versicolor. Molecules 2020, 25, 1255. https://doi.org/10.3390/molecules25051255
Maniak H, Talma M, Matyja K, Trusek A, Giurg M. Synthesis and Structure-Activity Relationship Studies of Hydrazide-Hydrazones as Inhibitors of Laccase from Trametes versicolor. Molecules. 2020; 25(5):1255. https://doi.org/10.3390/molecules25051255
Chicago/Turabian StyleManiak, Halina, Michał Talma, Konrad Matyja, Anna Trusek, and Mirosław Giurg. 2020. "Synthesis and Structure-Activity Relationship Studies of Hydrazide-Hydrazones as Inhibitors of Laccase from Trametes versicolor" Molecules 25, no. 5: 1255. https://doi.org/10.3390/molecules25051255
APA StyleManiak, H., Talma, M., Matyja, K., Trusek, A., & Giurg, M. (2020). Synthesis and Structure-Activity Relationship Studies of Hydrazide-Hydrazones as Inhibitors of Laccase from Trametes versicolor. Molecules, 25(5), 1255. https://doi.org/10.3390/molecules25051255