Investigation of a Medical Plant for Hepatic Diseases with Secoiridoids Using HPLC and FT-IR Spectroscopy for a Case of Gentiana rigescens
Abstract
1. Introduction
2. Materials and Methods
2.1. Meterial and Reagents
2.2. HPLC Analysis
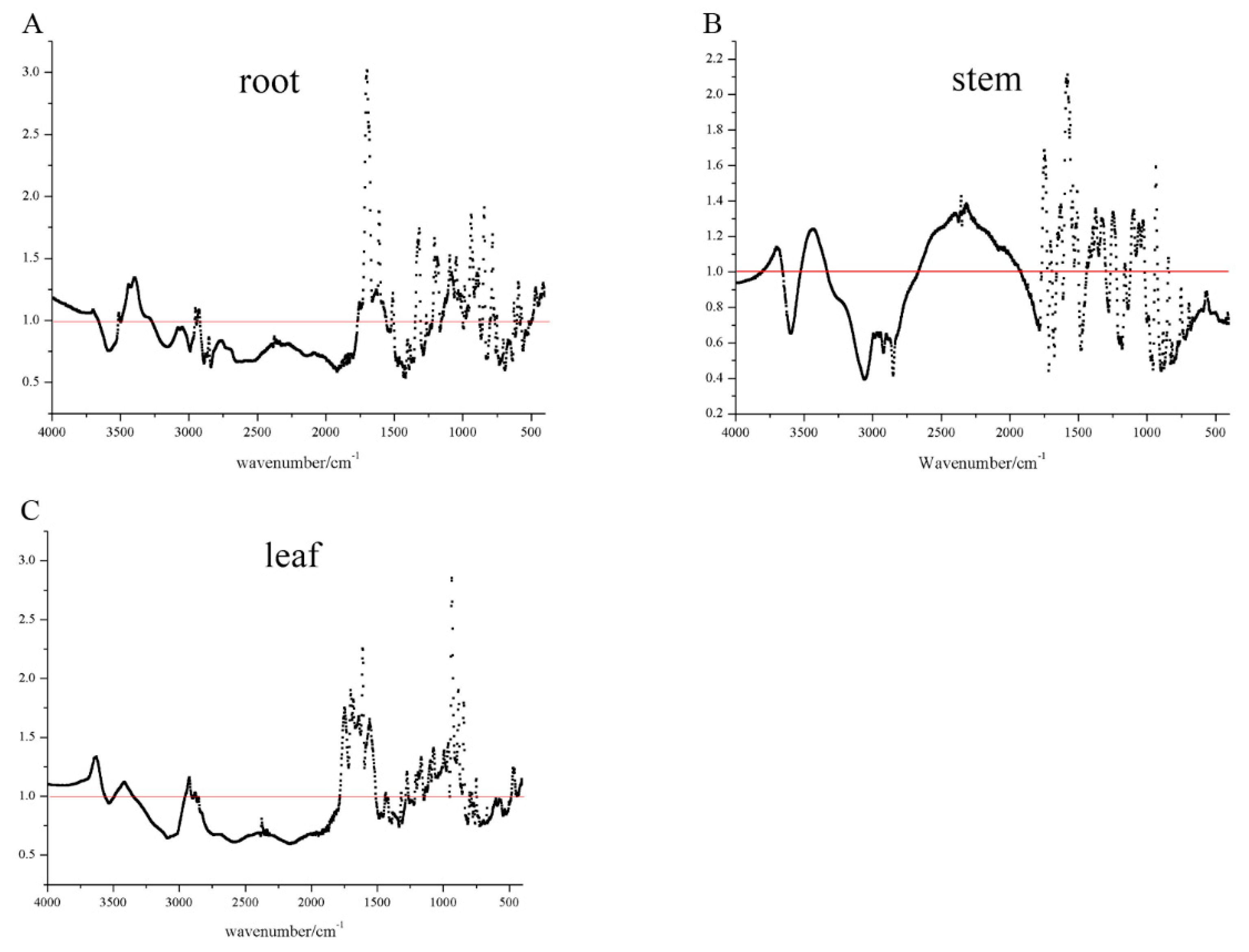
2.3. FT-IR Spectroscopy
2.4. Method Validation
2.5. PLSR
2.5.1. Data Partition of Calibration and Validation Set
2.5.2. Variable Selected
2.5.3. Model Evaluation
2.6. Data Analysis
3. Results and Discussion
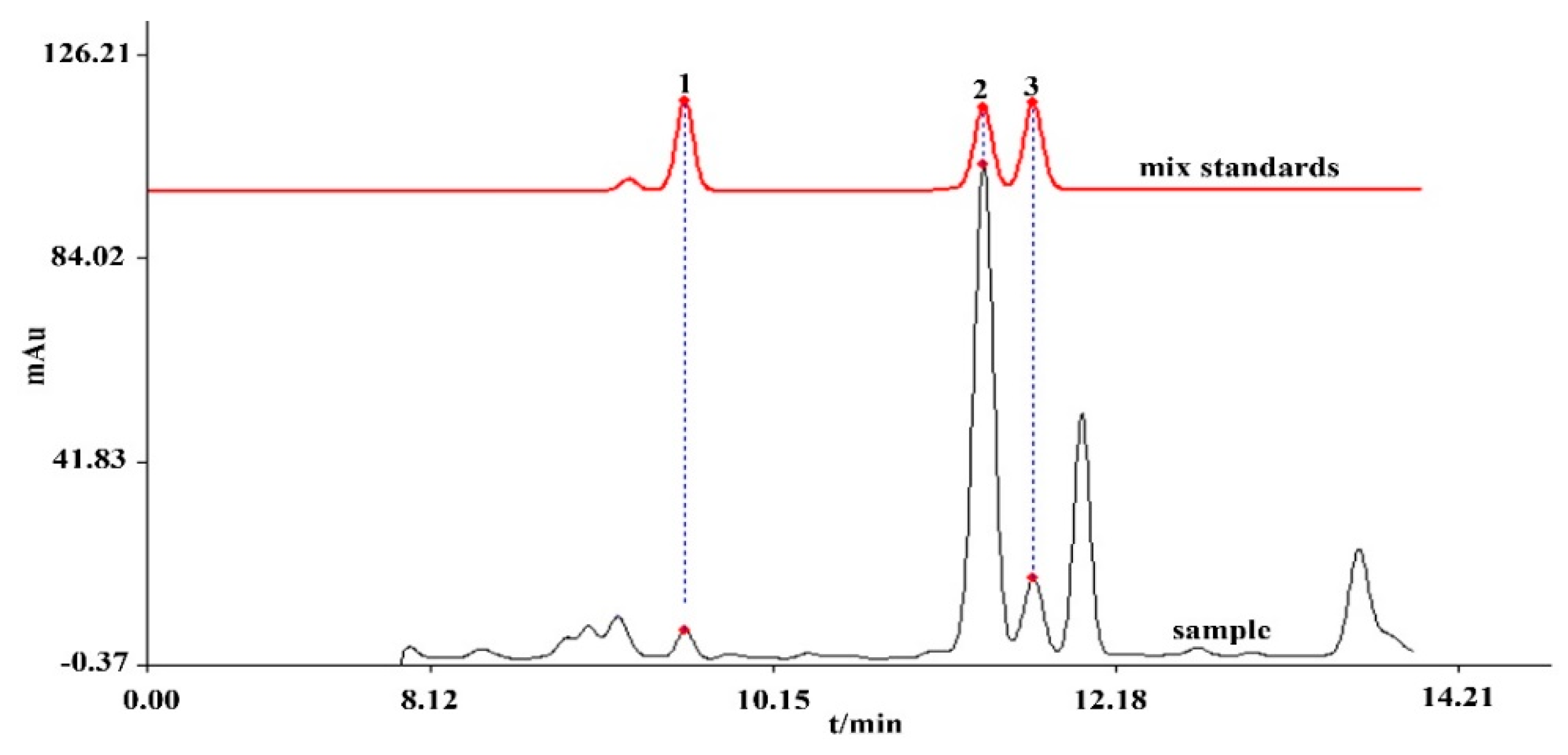
3.1. Optimization of HPLC Conditions
3.2. Method Validation
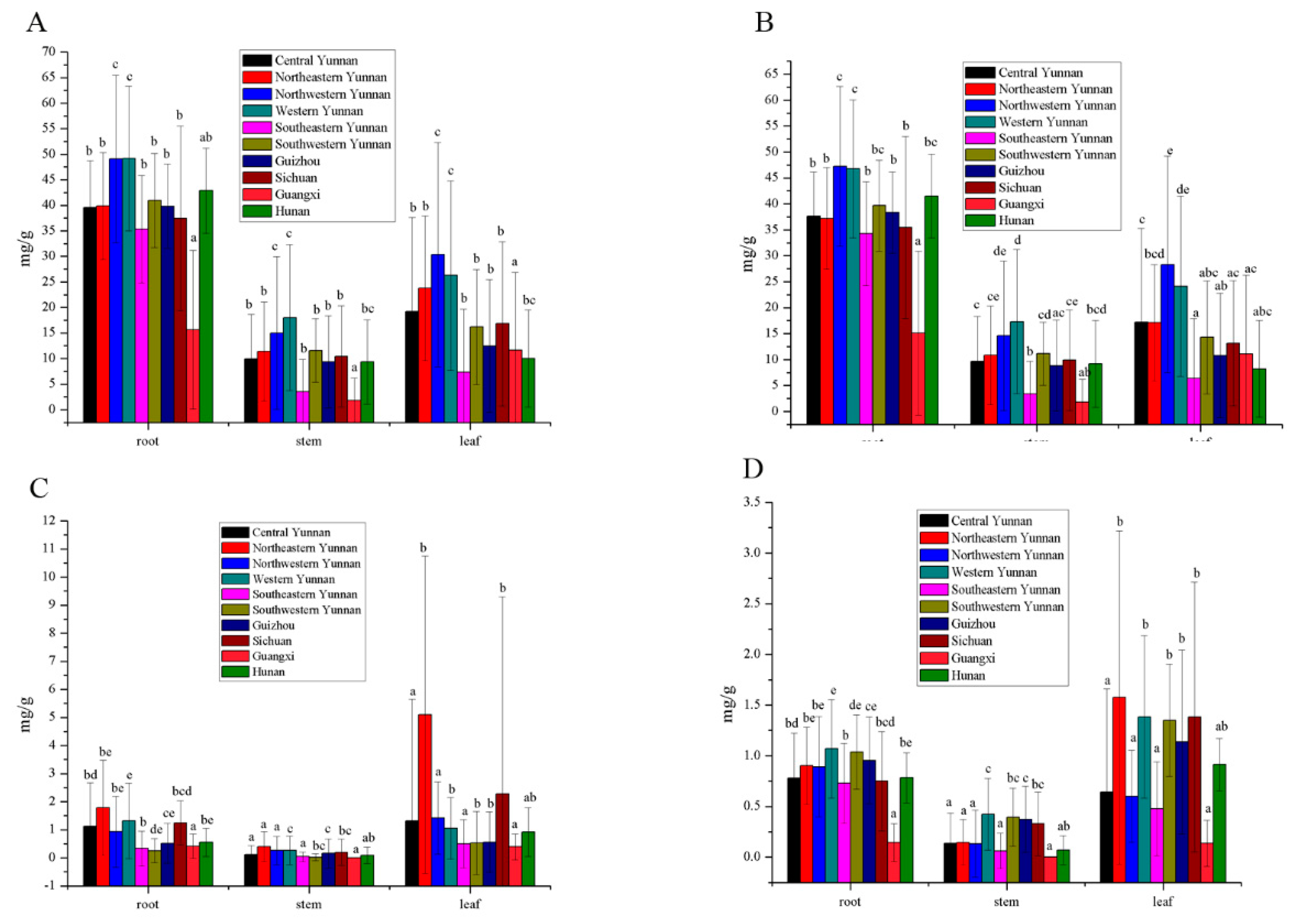
3.3. Secoiridoids Origin from Southwestern China
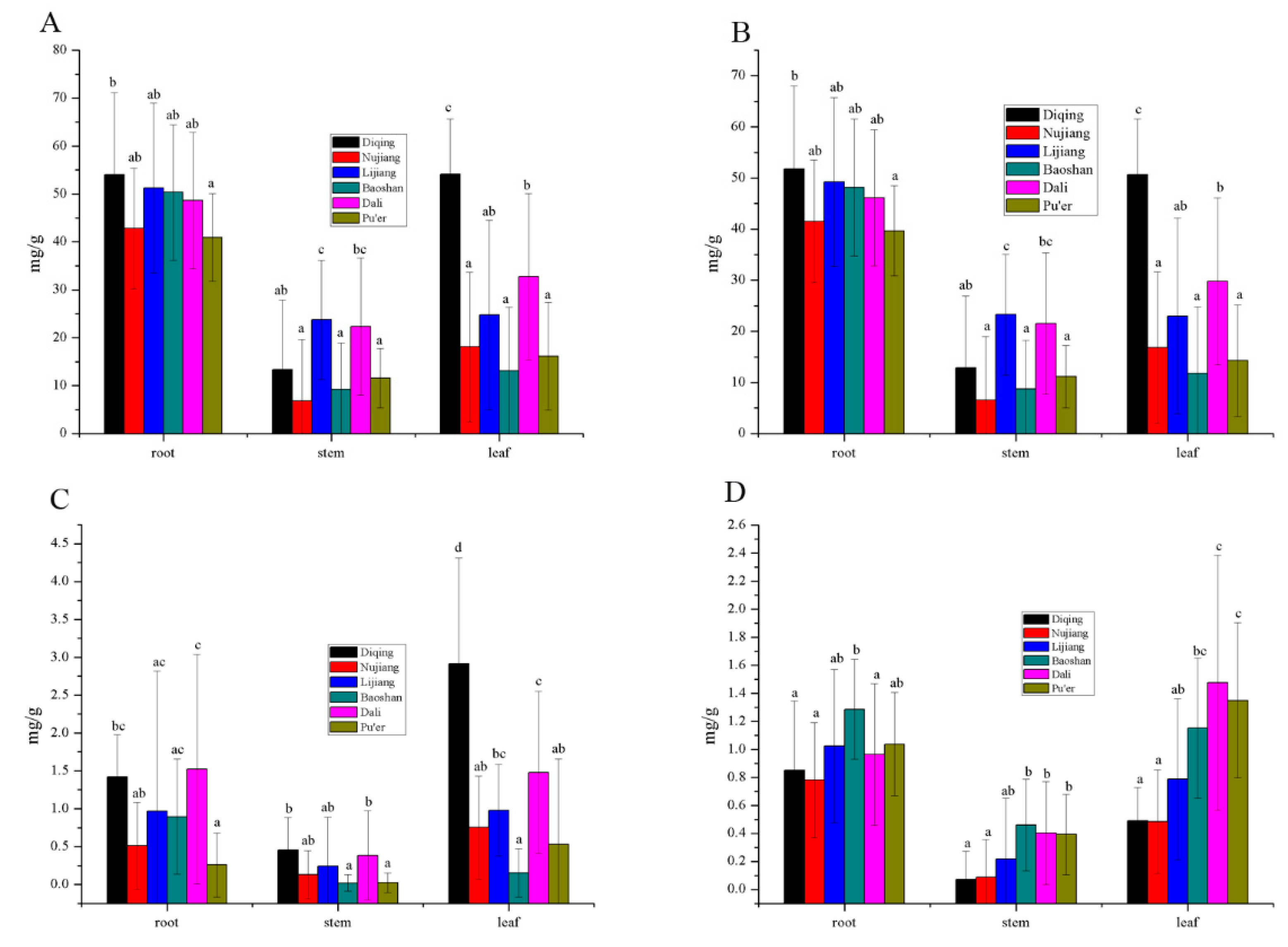
3.4. Secoiridoids Origin from Northwestern and Western Yunnan Province
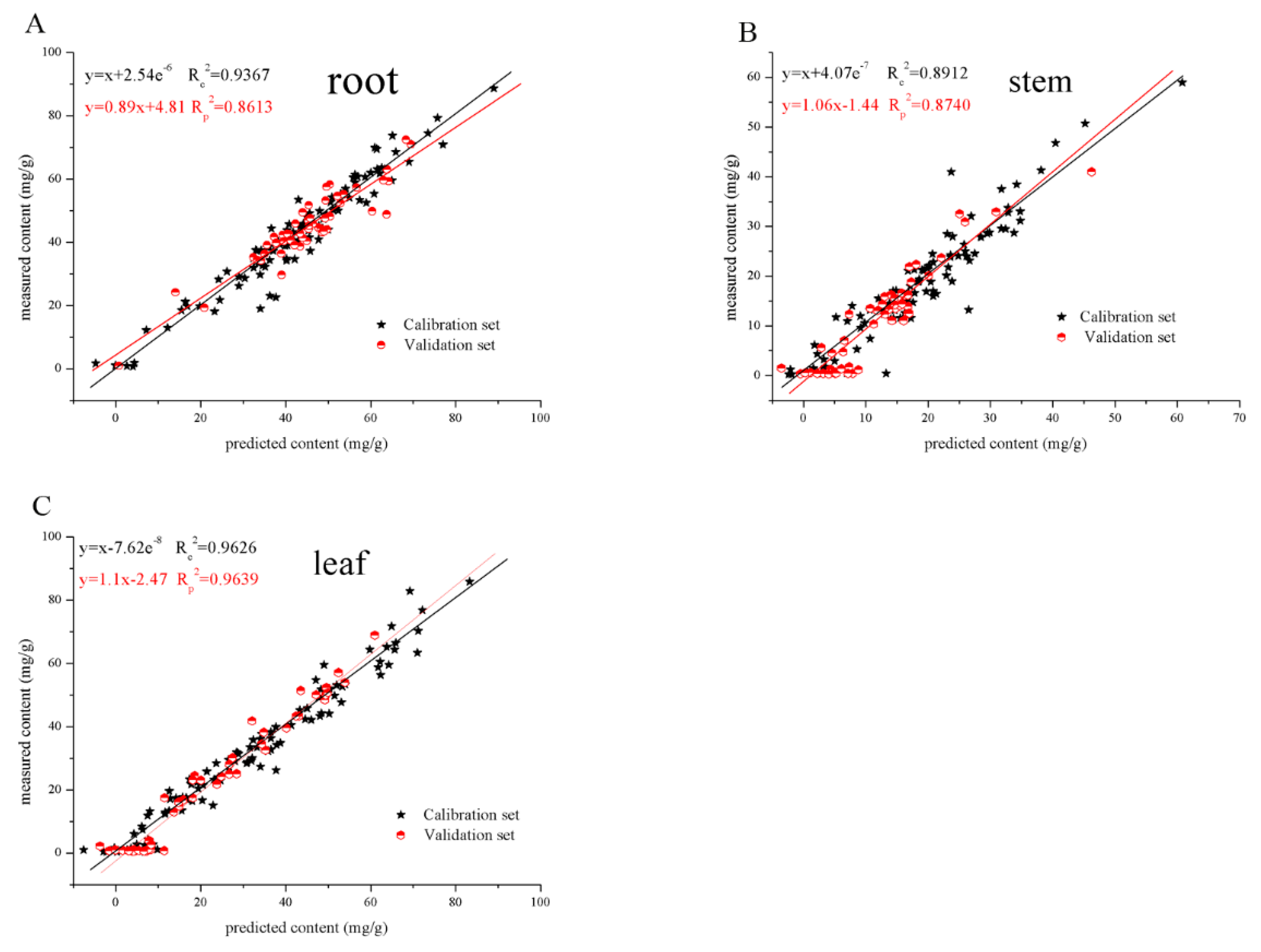
3.5. PLSR for Total Secoiridoids
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Pan, Y.; Zhao, Y.; Zhang, J.; Li, W.; Wang, Y. Phytochemistry and pharmacological activities of the Genus Gentiana (Gentianaceae). Chem. Biodivers. 2016, 13, 107. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Ye, J.; Wu, G.T.; Peng, X.J.; Xia, P.F.; Ren, Y. Gentiopicroside prevents interleukin-1 beta induced inflammation response in rat articular chondrocyte. J. Ethnopharmacol. 2015, 172, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Gu, Y.; Li, L. The anti-hyperplasia, anti-oxidative and anti-inflammatory properties of Qing Ye Dan and swertiamarin in testosterone-induced benign prostatic hyperplasia in rats. Toxicol. Lett. 2017, 265, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.H.; Wu, Y.L.; Wan, Y.; Li, X.; Xie, W.X.; Nan, J.X. Anti-apoptotic activity of gentiopicroside in d-galactosamine/lipopolysaccharide-induced murine fulminant hepatic failure. Chem-Biol. Interact. 2010, 188, 127–133. [Google Scholar] [CrossRef]
- Jaishree, V.; Badami, S. Antioxidant and hepatoprotective effect of swertiamarin from Enicostemma axillare against D-galactosamine induced acute liver damage in rats. J. Ethnopharmacol. 2010, 130, 103–106. [Google Scholar] [CrossRef]
- Shu, L.; Wang, Q.; Tao, Y.; Liu, C. Swertiamarin attenuates experimental rat hepatic fibrosis by suppressing angiotensin II-angiotensin type 1 receptor-extracellular signal-regulated kinase signaling. J. Pharmacol. Exp. Th. 2016, 359, 247. [Google Scholar]
- Tang, X.; Yang, Q.; Yang, F.; Gong, J.; Han, H.; Yang, L.; Wang, Z. Target profiling analyses of bile acids in the evaluation of hepatoprotective effect of gentiopicroside on ANIT-induced cholestatic liver injury in mice. J. Ethnopharmacol. 2016, 194, 63–71. [Google Scholar] [CrossRef]
- Yang, Q.; Yang, F.; Gong, J.; Tang, X.; Wang, G.; Wang, Z.; Yang, L. Sweroside ameliorates α-naphthylisothiocyanate-induced cholestatic liver injury in mice by regulating bile acids and suppressing pro-inflammatory responses. Acta Pharmacol. Sin. 2016, 37, 1218–1228. [Google Scholar] [CrossRef]
- Qi, F.; Wang, Z.; Cai, P.; Zhao, L.; Gao, J.; Kokudo, N.; Li, A.; Han, J.; Tang, W. Traditional Chinese medicine and related active compounds: A review of their role on hepatitis B virus infection. Drug Discoveries Ther. 2013, 7, 212–224. [Google Scholar] [CrossRef]
- State Pharmacopoeia Commission. Chinese Pharmacopoeia; Chemistry and Industry Press, 2015; p. 261. [Google Scholar]
- Pan, Y.; Shen, T.; Pan, J.; Xiao, D.; Li, Z.; Li, W.; Wang, Y. Development and validation of a UPLC-MS/MS method for the simultaneous determination and detection of four neuritogenic compounds in different parts of Gentiana rigescens Franch using multiple reaction monitoring and precursor ion scanning. Anal. Methods-UK 2014, 6, 1782–1787. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, H.; Zhang, J.; Zhang, J.; Wang, Y. Quantitative evaluation and discrimination of wild Paris polyphylla var. yunnanensis (Franch.) Hand.-Mazz from three regions of Yunnan Province using UHPLC-UV-MS and UV spectroscopy couple with partial least squares discriminant analysis. J. Nat. Med. 2017, 71, 148–157. [Google Scholar] [CrossRef]
- Vanhaelen, M.; Vanhaelen-Fastre, R. Quantitative determination of biologically active constituents in medicinal plant crude extracts by thin-layer chromatography-densitometry: I. Aesculus hippocastaneum L., Arctostaphyllos uva-ursi Spreng, Fraxinus excelsior L., Gentiana lutea L., Glycyrrhiza glabra L., Hamamelis virginiana L., Hypericum perforatum L., Olea europea L., Salix alba L. and Silybum marianum Gaertn. J. Chromatogr. A 1983, 281, 263–271. [Google Scholar]
- Chu, B.; Zhang, J.; Li, Z.; Zhao, Y.; Zuo, Z.; Wang, Y.; Li, W. Evaluation and quantitative analysis of different growth periods of herb-arbor intercropping systems using HPLC and UV-vis methods coupled with chemometrics. J. Nat. Med. 2016, 70, 803–810. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, Q.; Chen, X.; Hu, Z. Separation and determination of gentiopicroside and swertiamarin in Tibetan medicines by micellar electrokinetic electrophoresis. Biomed. Chromatogr. 2004, 18, 10–15. [Google Scholar]
- Pan, Y.; Zhang, J.; Zhao, Y.L.; Wang, Y.Z.; Huang, H.Y. Investigation of metabolites accumulation in medical plant Gentiana rigescens during different growing stage using LC-MS/MS and FT-IR. Bot. Stud. 2015, 56, 14. [Google Scholar] [CrossRef]
- Qi, L.M.; Zhang, J.; Zuo, Z.T.; Zhao, Y.L.; Wang, Y.Z.; Jin, H. Determination of iridoids in Gentiana Rigescens by infrared spectroscopy and multivariate analysis. Anal. Lett. 2017, 50, 389–401. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Zhang, J.; Wang, Y. Quality assessment of Gentiana rigescens from different geographical origins using FT-IR spectroscopy combined with HPLC. Molecules 2017, 22, 1238. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Li, T.; Liu, H.; Li, J.; Wang, Y. Geographical traceability of wild Boletus edulis based on data fusion of FT-MIR and ICP-AES coupled with data mining methods (SVM). Spectrochim. Acta Part A 2017, 177, 20–27. [Google Scholar] [CrossRef]
- Oussama, A.; Elabadi, F.; Platikanov, S.; Kzaiber, F.; Tauler, R. Detection of olive oil adulteration using FT-IR spectroscopy and PLS with variable importance of projection (VIP) scores. J. Am. Oil Chem. Soc. 2012, 89, 1807–1812. [Google Scholar] [CrossRef]
- Farrés, M.; Platikanov, S.; Tsakovski, S.; Tauler, R. Comparison of the variable importance in projection (VIP) and of the selectivity ratio (SR) methods for variable selection and interpretation. J. Chemometr. 2015, 29, 528–536. [Google Scholar] [CrossRef]
- Araújo, M.C.U.; Saldanha, T.C.B.; Galvao, R.K.H.; Yoneyama, T.; Chame, H.C.; Visani, V. The successive projections algorithm for variable selection in spectroscopic multicomponent analysis. Chemometr. Intell. Lab. 2001, 57, 65–73. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, H.; Zhang, J.; Zhang, J.; Wang, Y. Determination of total steroid saponins in different species of Paris using FTIR combined with chemometrics. J. AOAC Int. 2017, 101, 732–738. [Google Scholar] [CrossRef]
- Dhanoa, M.S.; Barnes, R.J.; Lister, S.J. Standard normal variate transformation and de-trending of near-infrared diffuse reflectance spectra. Appl. Spectrosc. 2016, 43, 772–777. [Google Scholar]
- Dhanoa, M.S.; Lister, S.J.; Sanderson, R.; Barnes, R.J. The link between multiplicative scatter correction (MSC) and standard normal variate (SNV) transformations of NIR spectra. J. Near Infrared Spec. 2017, 2, 43–47. [Google Scholar] [CrossRef]
- Zhan, H.; Fang, J.; Tang, L.; Yang, H.; Li, H.; Wang, Z.; Yang, B.; Wu, H.; Fu, M. Application of near-infrared spectroscopy for the rapid quality assessment of Radix Paeoniae Rubra. Spectrochim. Acta A 2017, 183, 75–83. [Google Scholar] [CrossRef]
- Wold, S.; Antti, H.; Lindgren, F.; Ohmanc, J. Orthogonal signal correction of near-infrared spectra. Chemometr. Intell. Lab. 1998, 44, 175–185. [Google Scholar] [CrossRef]
- Xu, M.; Wang, D.; Zhang, Y.J.; Yang, C.R. A new secoiridoidal glucoside from Gentiana rigescens (Gentianaceae). Acta Bot. Yunnanica 2006, 28, 669–672. [Google Scholar]
- Chong, I.G.; Jun, C.H. Performance of some variable selection methods when multicollinearity is present. Chemometr. Intell. Lab. 2005, 78, 103–112. [Google Scholar] [CrossRef]
- Mentaschi, L.; Besio, G.; Cassola, F.; Mazzino, A. Problems in RMSE-based wave model validations. Ocean Model. 2013, 72, 53–58. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Elmasry, G.; Sun, D.W.; Allen, P. Non-destructive prediction and visualization of chemical composition in lamb meat using NIR hyperspectral imaging and multivariate regression. Innov. Food Sci. Emerg. 2012, 16, 218–226. [Google Scholar] [CrossRef]
- Fragoso, S.; Aceña, L.; Guasch, J.; Busto, O.; Mestres, M. Application of FT-MIR spectroscopy for fast control of red grape phenolic ripening. J. Agr. Food Chem. 2011, 59, 2175–2183. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y. Characterization of Paris polyphylla var. yunnanensis by infrared and ultraviolet spectroscopies with chemometric data fusion. Analy. Lett. 2018, 51, 1730–1742. [Google Scholar] [CrossRef]
Sample Availability: not available. |


| Min (mg/g) | Max (mg/g) | Mean (mg/g) | SD | ||
|---|---|---|---|---|---|
| root-origin | Calibration set | 0.72 | 88.68 | 43.35 | 18.02 |
| Validation set | 0.97 | 73.68 | 43.76 | 11.55 | |
| root-selected | Calibration set | 0.72 | 88.68 | 42.84 | 18.00 |
| Validation set | 0.97 | 72.43 | 44.73 | 11.82 | |
| root-VIP | Calibration set | 0.72 | 88.68 | 43.36 | 17.98 |
| Validation set | 0.97 | 73.68 | 43.72 | 11.84 | |
| stem-origin | Calibration set | 0.29 | 58.96 | 18.04 | 11.82 |
| Validation set | 0.29 | 33.78 | 11.25 | 9.61 | |
| stem-selected | Calibration set | 0.29 | 58.96 | 17.96 | 11.77 |
| Validation set | 0.30 | 41.01 | 11.41 | 9.84 | |
| stem-VIP | Calibration set | 0.29 | 58.96 | 17.21 | 12.03 |
| Validation set | 0.29 | 41.01 | 12.92 | 10.05 | |
| leaf-origin | Calibration set | 0.34 | 85.88 | 30.95 | 21.63 |
| Validation set | 0.57 | 68.89 | 21.56 | 20.28 | |
| leaf-selected | Calibration set | 0.34 | 85.88 | 31.55 | 22.58 |
| Validation set | 0.41 | 53.33 | 20.37 | 17.37 | |
| leaf-VIP | Calibration set | 0.34 | 85.88 | 32.78 | 21.70 |
| Validation set | 0.43 | 53.33 | 17.90 | 17.72 |
| Analyte | Regression Equation | Linearity Range (μg/mL) | r2 | LOD (μg/mL) | LOQ (μg/mL) | Inter-Day | Intra-Day |
|---|---|---|---|---|---|---|---|
| gentiopicroside | Y = 5826.56946x + 130.94542 | 24.42–3600.00 | 0.9998 | 52.58 | 175.25 | 1.54 | 1.89 |
| swertiamarin | Y = 8161.85182x + 11.73637 | 1.79–184.47 | 0.9995 | 1.45 | 4.83 | 2.32 | 1.62 |
| sweroside | Y = 4129.62105x + 2.48267 | 1.75–343.00 | 0.9987 | 3 | 9.98 | 2.92 | 2.74 |
| Original Amount (mg/g) | Added Amount (mg/g) | Measured Amount (mg/g) | Recovery Rate | RSD | |
|---|---|---|---|---|---|
| 0.5 | 1.71 | 96% | 2.33% | ||
| gentiopicroside | 1.23 | 1 | 2.21 | 98% | 2.68% |
| 1.5 | 2.72 | 99.33% | 2.42% | ||
| 24 | 72.78 | 101.08% | 1.90% | ||
| swertiamarin | 48.52 | 48 | 95.76 | 98.42% | 0.85% |
| 60 | 107.74 | 98.70% | 1.68% | ||
| 1 | 2.88 | 99% | 1.19% | ||
| sweroside | 1.89 | 1.5 | 3.34 | 96.67% | 2.88% |
| 3 | 4.83 | 98% | 1.80% |
| LV | RMSEE | RMSEP | Rc2 | Rp2 | RPD | ||
|---|---|---|---|---|---|---|---|
| root-origin | original | 8 | 8.5139 | 6.2829 | 0.7964 | 0.7038 | 1.7020 |
| SNV | 7 | 7.8257 | 5.8600 | 0.8243 | 0.7472 | 1.8820 | |
| MSC | 7 | 7.7996 | 5.7331 | 0.8254 | 0.7544 | 1.9240 | |
| MSC+OSC | 1 | 5.3947 | 4.3041 | 0.9112 | 0.8592 | 2.5492 | |
| MSC+OSC+1st | 2 | 4.3754 | 4.8940 | 0.9422 | 0.8443 | 2.4392 | |
| MSC+OSC+2st | 2 | 4.4507 | 4.7914 | 0.9402 | 0.8546 | 2.4626 | |
| root-selected | original | 6 | 8.5225 | 7.7012 | 0.7893 | 0.6156 | 1.5250 |
| SNV | 5 | 8.2827 | 7.6118 | 0.7968 | 0.6508 | 1.5844 | |
| MSC | 5 | 8.1629 | 7.6997 | 0.8107 | 0.5557 | 1.3806 | |
| MSC+OSC | 2 | 5.2748 | 4.6584 | 0.9159 | 0.8635 | 2.5795 | |
| MSC+OSC+1st | 2 | 4.5626 | 4.6172 | 0.9379 | 0.8497 | 2.5132 | |
| MSC+OSC+2st | 2 | 4.5757 | 4.5731 | 0.9367 | 0.8613 | 2.6298 | |
| root-VIP | original | 7 | 8.7822 | 6.5822 | 0.7779 | 0.6893 | 1.6299 |
| SNV | 5 | 8.3730 | 6.0865 | 0.7938 | 0.7406 | 1.8118 | |
| MSC | 5 | 8.2735 | 5.8589 | 0.7967 | 0.7576 | 1.8793 | |
| MSC+OSC | 2 | 5.7787 | 5.4884 | 0.8987 | 0.7898 | 2.0616 | |
| MSC+OSC+1st | 2 | 5.3411 | 5.1036 | 0.9135 | 0.8162 | 2.2285 | |
| MSC+OSC+2st | 2 | 5.4466 | 5.0488 | 0.9100 | 0.8174 | 2.2791 | |
| stem-origin | original | 3 | 10.0467 | 9.7660 | 0.2985 | 0.1919 | 0.8185 |
| SNV | 7 | 7.2516 | 7.8903 | 0.6496 | 0.5301 | 1.1515 | |
| MSC | 7 | 7.2809 | 7.9455 | 0.6466 | 0.5364 | 1.1513 | |
| MSC+OSC | 2 | 5.2292 | 5.3134 | 0.8103 | 0.7316 | 1.7194 | |
| MSC+OSC+1st | 3 | 3.9330 | 3.9500 | 0.8925 | 0.8445 | 2.3729 | |
| MSC+OSC+2st | 2 | 3.0003 | 3.6079 | 0.9368 | 0.8779 | 2.4998 | |
| stem-selected | original | 6 | 7.8168 | 9.4903 | 0.5851 | 0.2504 | 0.9290 |
| SNV | 4 | 7.5040 | 8.2579 | 0.6096 | 0.3795 | 1.0452 | |
| MSC | 4 | 7.4939 | 8.2921 | 0.6149 | 0.3684 | 1.0386 | |
| MSC+OSC | 2 | 5.7072 | 4.6995 | 0.7695 | 0.7855 | 1.9185 | |
| MSC+OSC+1st | 2 | 4.8656 | 4.4198 | 0.8325 | 0.8159 | 2.1008 | |
| MSC+OSC+2st | 2 | 3.9018 | 3.5715 | 0.8912 | 0.8740 | 2.5886 | |
| stem-VIP | original | 5 | 8.7443 | 8.9892 | 0.4925 | 0.3219 | 0.8713 |
| SNV | 2 | 10.0687 | 8.6813 | 0.3130 | 0.3039 | 0.9061 | |
| MSC | 4 | 8.6653 | 8.4545 | 0.5006 | 0.3693 | 1.0021 | |
| MSC+OSC | 2 | 5.4675 | 4.7228 | 0.7974 | 0.7830 | 1.9927 | |
| MSC+OSC+1st | 1 | 5.3223 | 4.3811 | 0.8061 | 0.8187 | 2.1707 | |
| MSC+OSC+2st | 1 | 5.2983 | 4.7727 | 0.8079 | 0.7815 | 1.9946 | |
| leaf-origin | original | 8 | 10.3486 | 9.9320 | 0.7892 | 0.7839 | 1.8854 |
| SNV | 6 | 10.5555 | 8.7191 | 0.7777 | 0.8236 | 2.1817 | |
| MSC | 6 | 10.3409 | 9.0157 | 0.7850 | 0.8221 | 2.1101 | |
| MSC+OSC | 3 | 5.9238 | 6.0009 | 0.9272 | 0.9144 | 3.3800 | |
| MSC+OSC+1st | 3 | 5.3764 | 4.5992 | 0.9400 | 0.9529 | 4.4631 | |
| MSC+OSC+2st | 2 | 4.2230 | 4.2420 | 0.9626 | 0.9639 | 4.5041 | |
| leaf-selected | original | 6 | 10.1826 | 9.9332 | 0.8086 | 0.7360 | 1.5651 |
| SNV | 3 | 10.7515 | 10.4739 | 0.7799 | 0.7053 | 1.5485 | |
| MSC | 3 | 10.7445 | 10.5360 | 0.7802 | 0.7058 | 1.5375 | |
| MSC+OSC | 1 | 6.6718 | 6.8725 | 0.9135 | 0.8600 | 2.5923 | |
| MSC+OSC+1st | 2 | 5.0529 | 4.7375 | 0.9509 | 0.9248 | 3.6239 | |
| MSC+OSC+2st | 1 | 5.3409 | 4.9566 | 0.9446 | 0.9294 | 3.5038 | |
| leaf-VIP | original | 9 | 8.9449 | 11.9643 | 0.8453 | 0.6701 | 1.3841 |
| SNV | 6 | 8.7314 | 9.9045 | 0.8478 | 0.7799 | 1.6555 | |
| MSC | 6 | 8.7607 | 9.8223 | 0.8467 | 0.7856 | 1.6692 | |
| MSC+OSC | 2 | 6.4200 | 6.0348 | 0.9142 | 0.9082 | 2.9345 | |
| MSC+OSC+1st | 2 | 5.9801 | 6.4896 | 0.9250 | 0.8860 | 2.7377 | |
| MSC+OSC+2st | 2 | 5.4105 | 6.3621 | 0.9391 | 0.8872 | 2.7910 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhao, Y.; Zuo, Z.; Zhang, J.; Shi, Y.; Wang, Y. Investigation of a Medical Plant for Hepatic Diseases with Secoiridoids Using HPLC and FT-IR Spectroscopy for a Case of Gentiana rigescens. Molecules 2020, 25, 1219. https://doi.org/10.3390/molecules25051219
Yang Y, Zhao Y, Zuo Z, Zhang J, Shi Y, Wang Y. Investigation of a Medical Plant for Hepatic Diseases with Secoiridoids Using HPLC and FT-IR Spectroscopy for a Case of Gentiana rigescens. Molecules. 2020; 25(5):1219. https://doi.org/10.3390/molecules25051219
Chicago/Turabian StyleYang, Yuangui, Yanli Zhao, Zhitian Zuo, Ji Zhang, Yao Shi, and Yuanzhong Wang. 2020. "Investigation of a Medical Plant for Hepatic Diseases with Secoiridoids Using HPLC and FT-IR Spectroscopy for a Case of Gentiana rigescens" Molecules 25, no. 5: 1219. https://doi.org/10.3390/molecules25051219
APA StyleYang, Y., Zhao, Y., Zuo, Z., Zhang, J., Shi, Y., & Wang, Y. (2020). Investigation of a Medical Plant for Hepatic Diseases with Secoiridoids Using HPLC and FT-IR Spectroscopy for a Case of Gentiana rigescens. Molecules, 25(5), 1219. https://doi.org/10.3390/molecules25051219





