Natural Mordenite from Spain as Pozzolana
Abstract
1. Introduction
2. Results
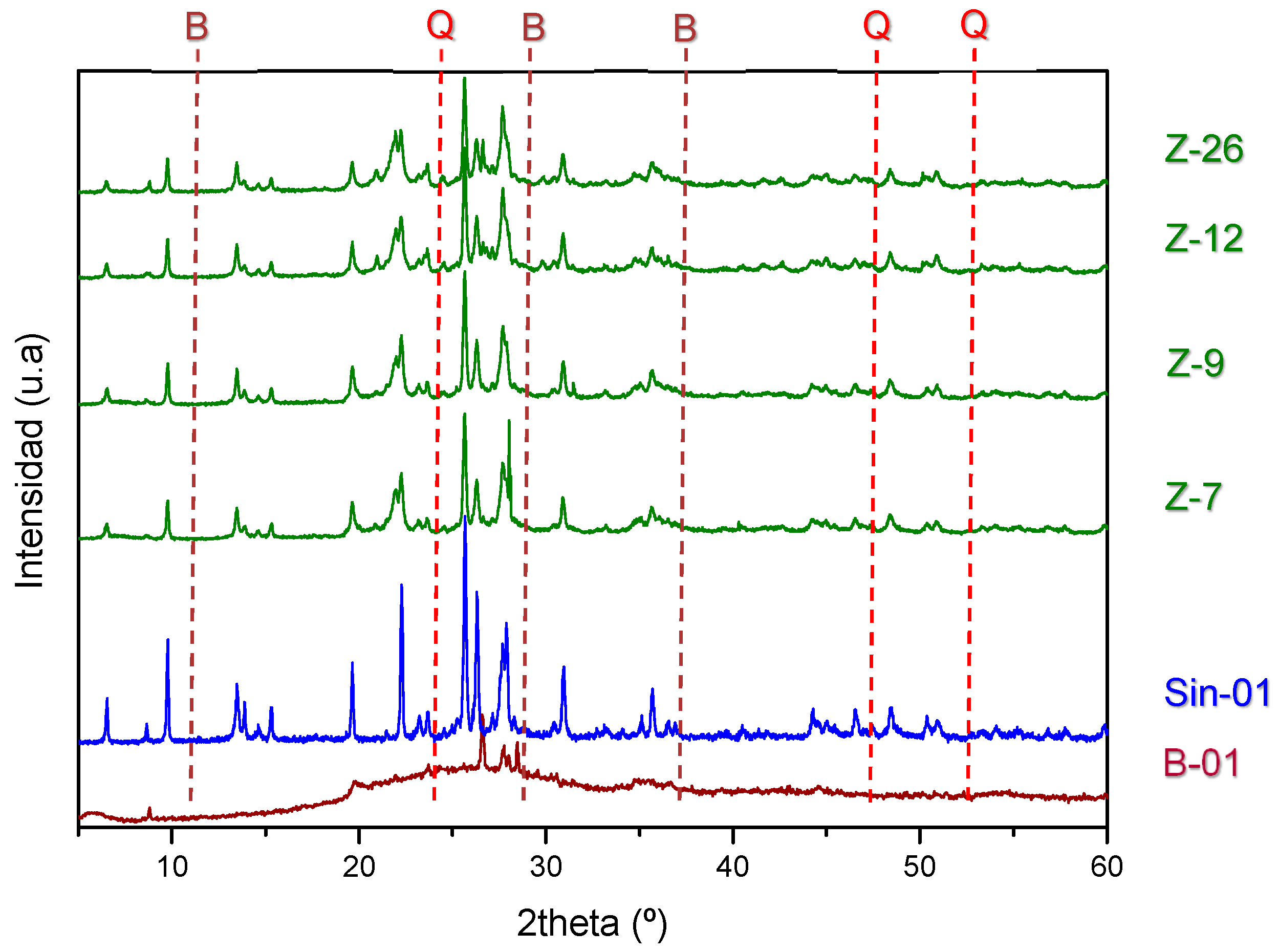
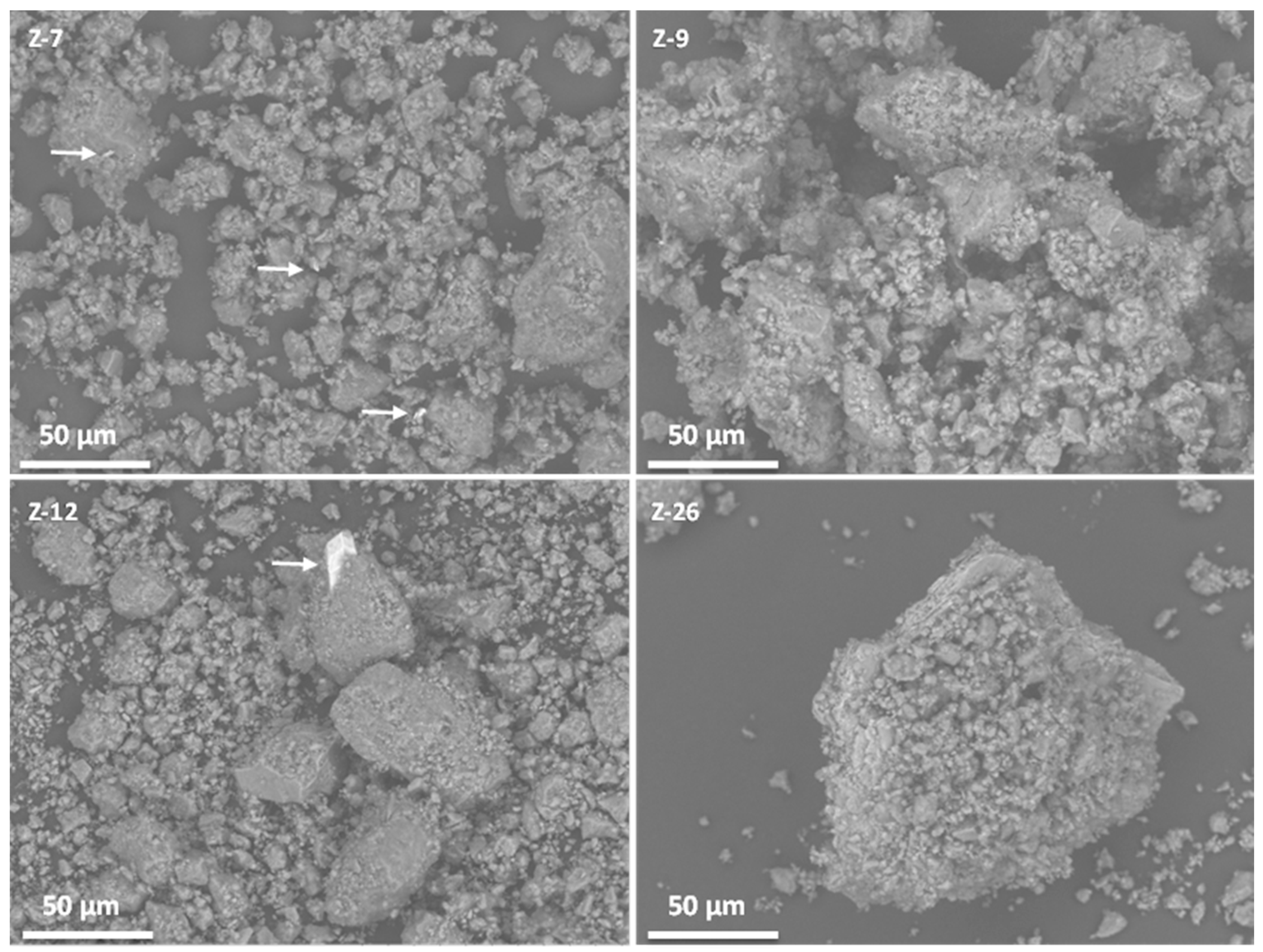
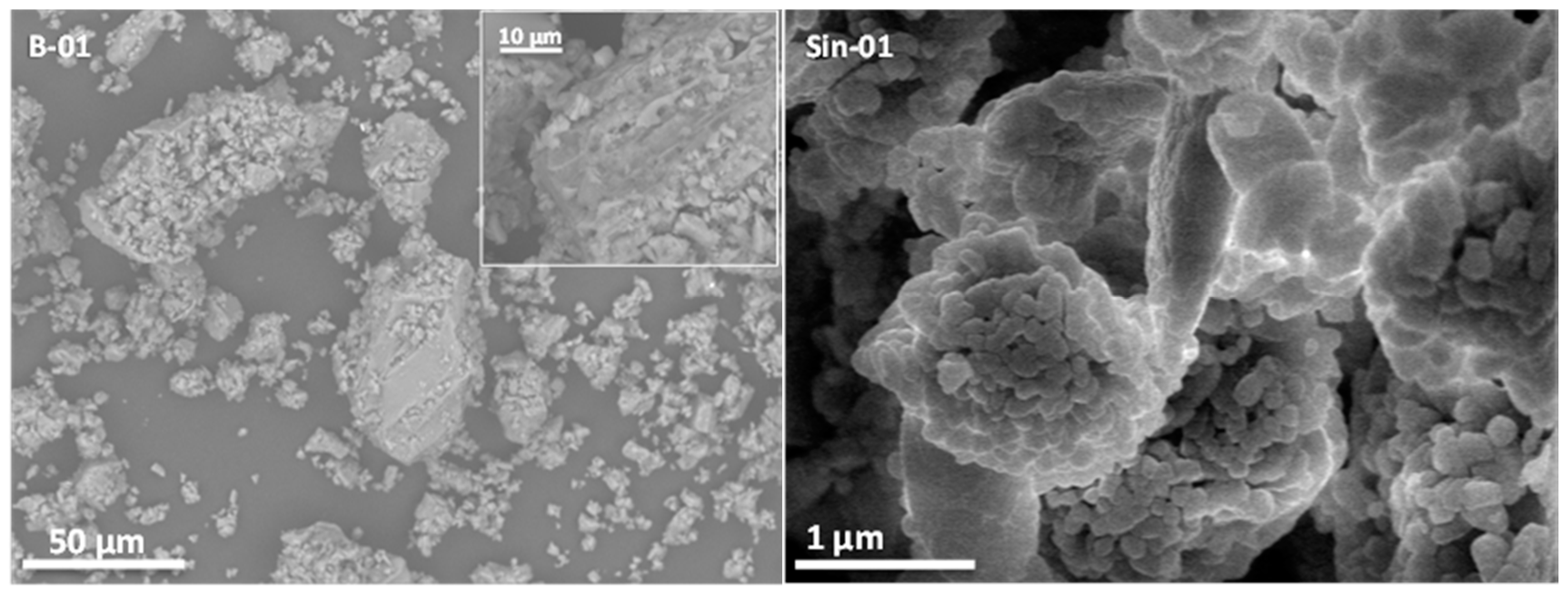
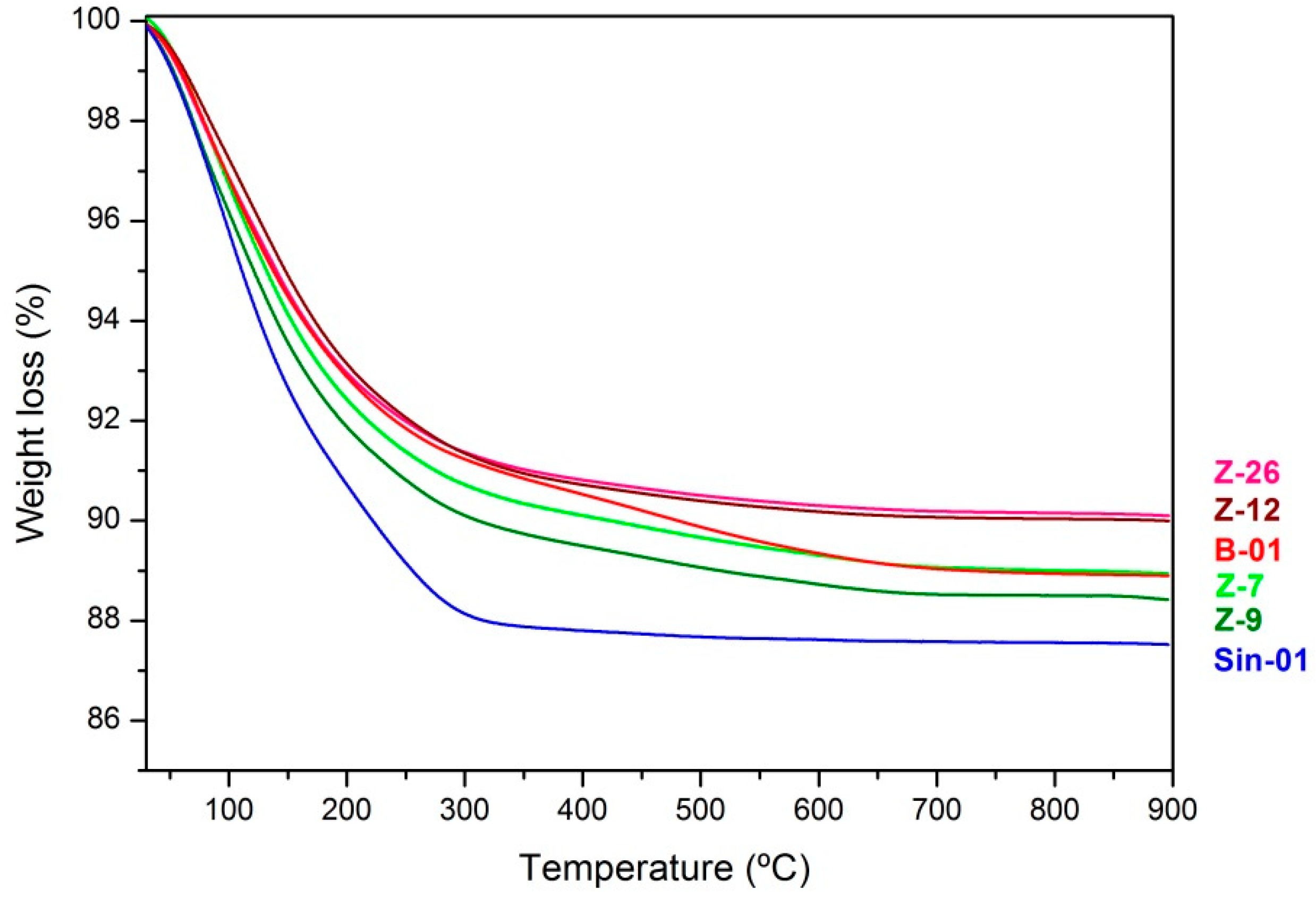
2.1. Characterization of Natural and Synthetic Mordenites
2.2. Characterization of the Pozzolanic Cement
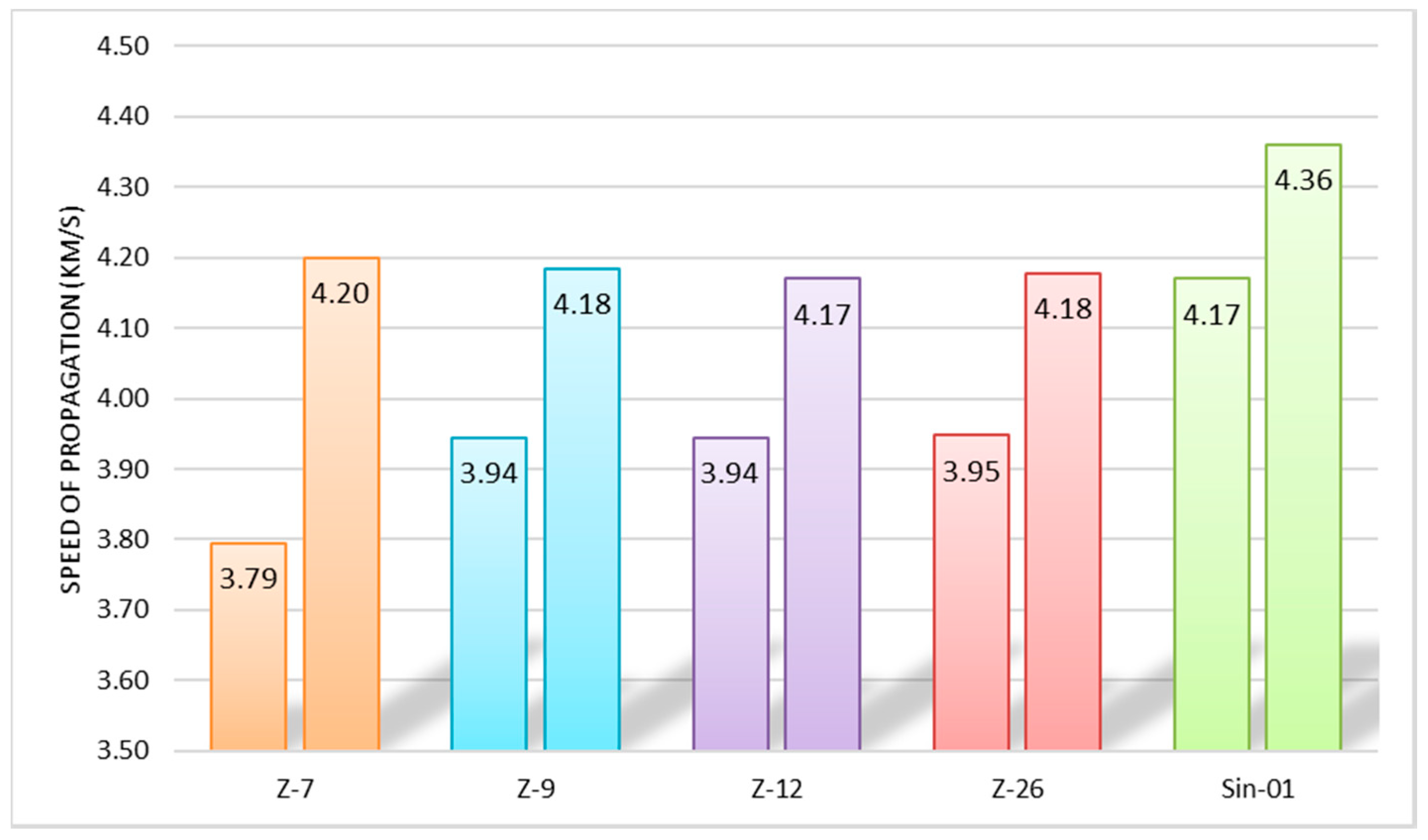
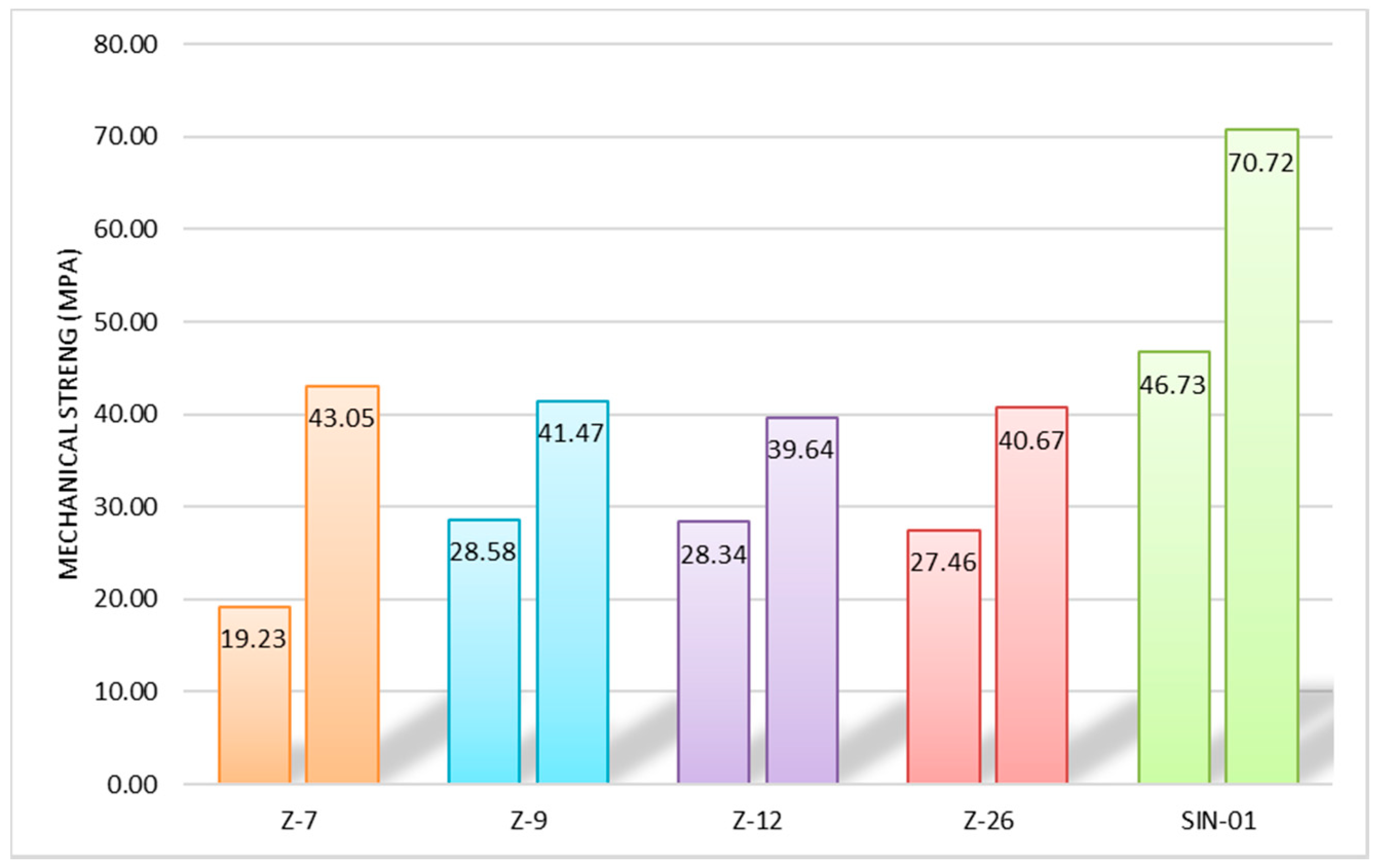
2.3. Appplication as Concrete
3. Discussion
4. Materials and Methods
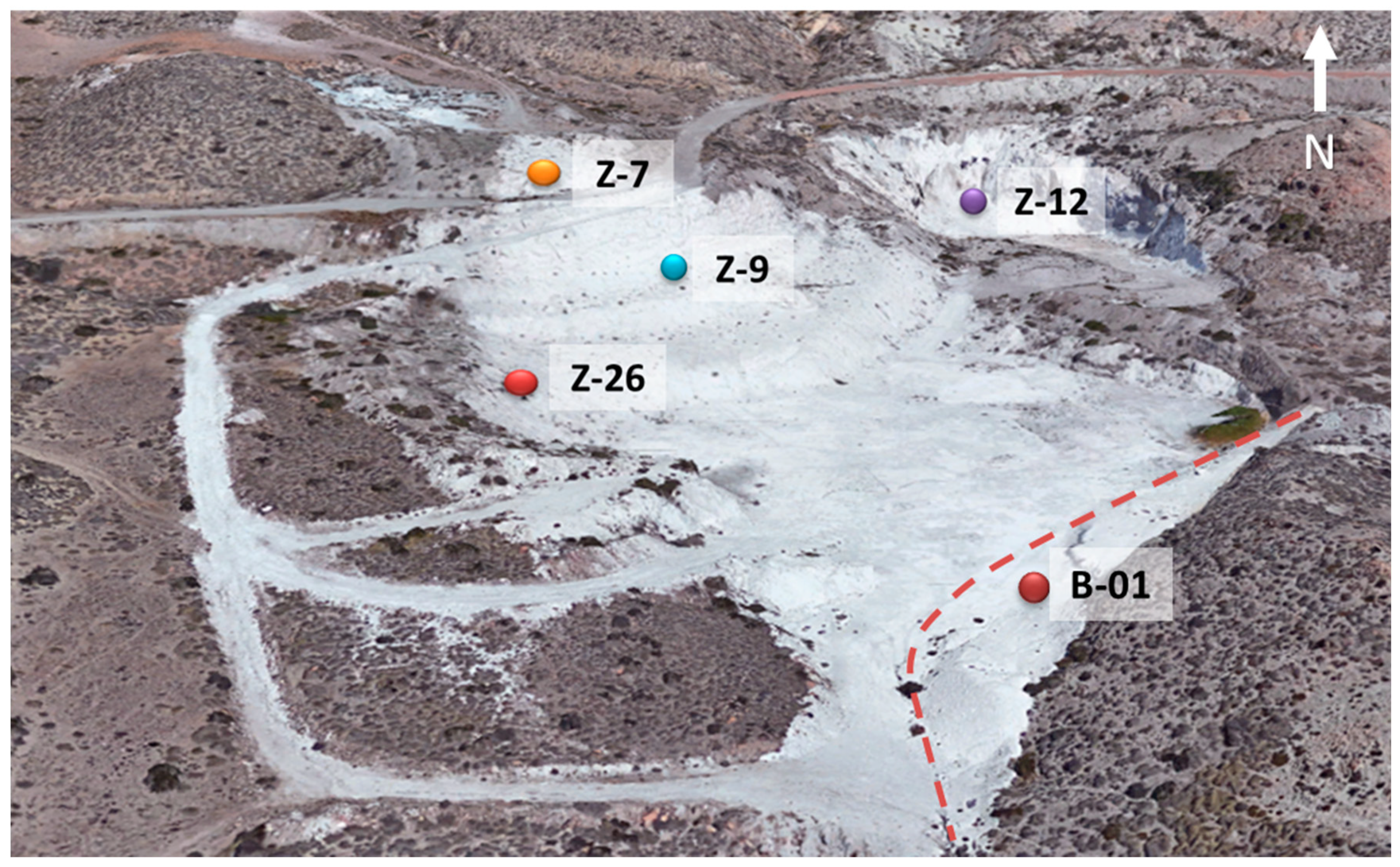
4.1. Description and Characterization of Mordenite Samples
4.2. Characterization Techniques
4.3. Characterization of the Pozzolanic Cements
4.4. Appplication as Concrete
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nagrockiene, D.; Girskas, G. Research into properties of concrete modified with natural zeolite addition. Constr. Build. Mater. 2016, 113, 964–969. [Google Scholar] [CrossRef]
- Ulloa, N.A.; Baykara, H.; Cornejo, M.H.; Rigail, A.; Paredes, C.; Villalba, J.L. Application-oriented mix design optimization and characterization of zeolite-based geopolymer mortars. Constr. Build. Mater. 2018, 174, 138–149. [Google Scholar] [CrossRef]
- Perraki, T.; Kontori, E.; Tsivilis, S.; Kakali, G. The effect of zeolite on the properties and hydration of blended cements. Cement Concrete Comp. 2010, 32, 128–133. [Google Scholar] [CrossRef]
- Ahmadi, B.; Shekarchi, M. Use of natural zeolite as a suplementary cementitious material. Cement Concrete Comp. 2010, 32, 134–141. [Google Scholar] [CrossRef]
- Lilkov, V.; Petrov, O.; Tzvetanova, Y. Rheological porosimetric and SEM studies of cements with additions of natural zeolites. Clay Miner. 2011, 46, 225–232. [Google Scholar] [CrossRef]
- Karakurt, C.; Topçu, I.B. Effect of blended cements produced with natural zeolite and industrial by-products on alkali-silica reaction and sulfate resistance of concrete. Constr. Build. Mater. 2011, 25, 1789–1795. [Google Scholar] [CrossRef]
- Valipour, M.; Pargar, F.; Shekarchi, M.; Khani, S. Comparing a natural pozzolan, zeolite, to matakaolin and silica fume in terms of their effect on the durability characteristics of concrete: A laboratory study. Constr. Build. Mater. 2013, 41, 879–888. [Google Scholar] [CrossRef]
- Colella, C. Chapter 27—Natural zeolites and environment. In Introduction to Zeolite Science and Practice, 3rd ed.; Cejka, J., van Bekkum, H., Corma, A., Schüth, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 999–1035. [Google Scholar]
- Uzal, B.; Turanli, L.; Yucel, H.; Goncuoglu, M.C.; Culfaz, A. Pozzolanic activity of clinoptilolite: A comparative study with silica fume, fly ash and a non-zeolitic natural pozzolan. Cement Concr. Res. 2010, 40, 398–404. [Google Scholar] [CrossRef]
- Najimi, M.; Sobhani, J.; Ahmadi, B.; Shekarchi, M. An experimental study on durability properties of concrete containing zeolite as a highly reactive natural pozzolan. Constr. Build. Mater. 2012, 35, 1023–1033. [Google Scholar] [CrossRef]
- Uzal, B.; Turanli, L. Blended cements containing high volume of natural zeolites: Properties, hydration and paste microstructure. Constr. Build. Mater. 2012, 34, 101–109. [Google Scholar] [CrossRef]
- Özen, S.; Göncüoğlu, M.C.; Liguori, B.; de Gennaro, B.; Cappelletti, P.; Gatta, G.D.; Iucolano, F.; Colella, C. A comprehensive evaluation of sedimentary zeolites from Turkey as pozzolanic addition of cement- and lime-based binders. Constr. Build. Mater. 2016, 105, 46–61. [Google Scholar] [CrossRef]
- Xu, W.; Chen, J.J.; Wei, J.; Zhang, B.; Yuan, X.; Xu, P.; Yu, Q.; Ren, J. Evaluation of inherent factors on flowability, cohesiveness and strength of cementitious mortar in presence of zeolite powder. Constr. Build. Mater. 2019, 214, 61–73. [Google Scholar] [CrossRef]
- Wise, W.S.; Colella, C. Handbook of Natural Zeolites; De Frede Editore: Napoli, Italy, 2013. [Google Scholar]
- Kitsopoulos, K.P.; Dunham, A.C. Heulandite and mordenite-rich tuffs from Greece: a potential source for pozzolanic materials. Mineral. Deposita 1996, 31, 576–583. [Google Scholar] [CrossRef]
- Baykara, H.; Cornejo, M.H.; Murillo, R.; Gavilanes, A.; Paredes, C.; Elsen, J. Preparation, characterization and reaction kinetics of green cement: Ecuadorian natural mordenite-based geopolymers. Mater. Struct. 2017, 50, 188. [Google Scholar] [CrossRef]
- Azad, A.; Saeedian, A.; Mousavi, S.F.; Karami, H.; Farzin, S.; Singh, V.P. Effect of zeolite and pumice powders on the environmental and physical characteristics of green concrete filters. Constr. Build. Mater. 2020, 240, 117931. (in press). [CrossRef]
- UNE-EN 196-1:2018. Methods of Testing Cements—Part 1: Determination of Mechanical Resistance; The European Committee for Standardization: Brussels, Belgium, 2018. [Google Scholar]
- Mamo, W.; Awoke, Y.; Chebude, Y.; Díaz, I. Mild modification methods for the generation of mesoporosity in synthetic and natural mordenite. Bull. Chem. Soc. Ethiop. 2015, 29, 95–103. [Google Scholar] [CrossRef]
- Wahono, S.K.; Stalin, J.; Addai-Mensah, J.; Skinner, W.; Vinu, A.; Vasilev, K. Physico-chemical modification of natural mordenite-clinoptilolite zeolites and their enhanced CO2 adsorption capacity. Microporous Mesoporous Mater. 2020, 294, 109871. [Google Scholar] [CrossRef]
- Benito, R.; García-Guinea, J.; Valle Fuente, F.J.; Recio, P. Mineralogy geochemistry and uses of the mordenite-bentonite ash-tuff beds of Los Escullos. Almeria. Spain. J. Geochem. Explor. 1998, 62, 229–240. [Google Scholar] [CrossRef]
- Costafreda Mustelier, J.L. Geología, caracterización y aplicaciones de las rocas zeolíticas del 20complejo volcánico de Cabo de Gata (Almería). Ph.D. Thesis, Escuela Técnica Superior de Ingenieros de Minas y Energía, Universidad Politécnica de Madrid, Madrid, Spain, 2008. [Google Scholar]
- Guesh, K.; López-Muñoz, M.J.; Márquez-Álvarez, C.; Chebude, Y.; Diaz, I. Ethiopian Natural Zeolites for Photocatalysis. Bull. Chem. Soc. Ethiop. 2015, 29, 431–440. [Google Scholar] [CrossRef]
- UNE-EN 196-2:2014. Methods of Testing Cements—Part 2: Chemical Analysis of Cement; The European Committee for Standardization: Brussels, Belgium, 2014. [Google Scholar]
- UNE-EN 197-1:2011. Methods of Testing Cements—Part 1: Composition, Specifications and Conformity Criteria of Common Cements; The European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- UNE-EN 196-5:2011. Methods of Testing Cements—Part 5: Pozzolanicity Test for Pozzolanic Cements; The European Committee for Standardization: Brussels, Belgium, 2011. [Google Scholar]
- UNE-EN ISO 16810:2014. Non-Destructive Testing—Ultrasonic Testing—General Principles; The European Committee for Standardization: Brussels, Belgium, 2014. [Google Scholar]
- UNE-EN 196-3:2017. Methods of Testing Cement—Part 3: Determination of Setting Times and Soundness; The European Committee for Standardization: Brussels, Belgium, 2017. [Google Scholar]
- Stamatakis, M.G.; Regueiro, M.; Calvo, J.P.; Fragoulis, D.; Stamatakis, G. A study of zeolitic tuffs associated with bentonite deposits from Almeria, Spain and Kimolos Island, Greece and their industrial potential as pozzolanas in the cement industry. Hell J. Geosci. 2010, 45, 283–292. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

| Sample | MOR (%) | Impurities (%) | a × b × c | Surface Area (m2/g) |
|---|---|---|---|---|
| Z-7 | 74 | 26 | 18.09 × 20.48 × 7.51 | 77 |
| Z-9 | 71 | 29 | 18.10 × 20.47 × 7.51 | |
| Z-12 | 71 | 29 | 18.07 × 20.40 × 7.51 | |
| Z-26 | 67 | 33 | 18.08 × 20.46 × 7.51 | |
| Sin-01 | 100 | 18.02 × 20.40 × 7.51 | 425 |
| Sample | % Oxides Weight | Wt los 1% | Si/Al | Si/ (Al + Fe) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | CaO | Na2O | K2O | MgO | Fe2O3 | TiO2 | ||||
| Z-7 | 68.30 | 11.95 | 1.15 | 2.89 | 1.38 | 1.27 | 1.56 | 0.08 | 11.06 | 5.0 | 4.3 |
| Z-9 | 68.42 | 9.64 | 1.2 | 3.63 | 2.14 | 1.09 | 1.27 | 0.11 | 11.58 | 5.13 | 4.5 |
| Z-12 | 67.04 | 10.09 | 2.3 | 2.05 | 1.98 | 1.56 | 1.56 | 0.11 | 10.01 | 5.3 | 4.3 |
| Z-26 | 63.84 | 13.07 | 1.99 | 2.85 | 2.39 | 1.43 | 1.64 | 0.11 | 9.91 | 5.13 | 4.3 |
| Sin-01 | 12.48 | ||||||||||
| B-01 | 11.11 | ||||||||||
| Sample | Z-7 | Z-9 | Z-12 | Z-26 | Sin-01 |
|---|---|---|---|---|---|
| Total SiO₂ | 71.3 | 72.02 | 72.28 | 71.8 | 74.01 |
| MgO | 0.77 | 0.87 | 0.8 | 0.88 | 0.13 |
| CaO | 0.88 | 1.45 | 1.24 | 1.35 | 0.81 |
| Fe₂O₃ | 1.15 | 1.21 | 1.24 | 1.39 | 0.48 |
| Al₂O₃ | 11.82 | 11.67 | 11.58 | 11.84 | 9.69 |
| a Reactive SiO₂ | 58.68 | 61.57 | 62.12 | 63.16 | 72.98 |
| b IR | 19.87 | 16.49 | 16.83 | 14.08 | 1.82 |
| c LoI | 5.96 | 5.97 | 6.02 | 6.35 | 7.85 |
| Specimens | Z-7 | Z-9 | Sin-01A | Sin-01B |
|---|---|---|---|---|
| Initial setting | 165 | 195 | 105 | 105 |
| Final setting | 235 | 260 | 180 | 180 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Presa, L.; Costafreda, J.L.; Martín, D.A.; Díaz, I. Natural Mordenite from Spain as Pozzolana. Molecules 2020, 25, 1220. https://doi.org/10.3390/molecules25051220
Presa L, Costafreda JL, Martín DA, Díaz I. Natural Mordenite from Spain as Pozzolana. Molecules. 2020; 25(5):1220. https://doi.org/10.3390/molecules25051220
Chicago/Turabian StylePresa, Leticia, Jorge L. Costafreda, Domingo A. Martín, and Isabel Díaz. 2020. "Natural Mordenite from Spain as Pozzolana" Molecules 25, no. 5: 1220. https://doi.org/10.3390/molecules25051220
APA StylePresa, L., Costafreda, J. L., Martín, D. A., & Díaz, I. (2020). Natural Mordenite from Spain as Pozzolana. Molecules, 25(5), 1220. https://doi.org/10.3390/molecules25051220








