Abstract
The ω-hydroxyl-panaxytriol (1) and ω-hydroxyl-dihydropanaxytriol (2)—are rare examples of polyacetylene metabolism by microbial transformation, and these new metabolites (1, 2) from fermented red ginseng (FRG) by solid co-culture induction of two Chaetomium globosum should be the intermediates of biotransformation of panaxylactone (metabolite A). The metabolic pathway of panaxylactone was also exhibited. The ingredients of red ginseng (RG) also induced the production of rare 6/5/5 tricyclic ring spiro-γ-lactone skeleton (3). The ω-hydroxylation of new intermediates (1, 2) decreases cytotoxicity and antifungal activity against C. globosum compared with that of its bioprecursor panaxytriol. Additionally, compounds 1 and 2 indicated obvious inhibition against nitric oxide (NO) production, with ratios of 44.80 ± 1.37 and 23.10 ± 1.00% at 50 μM. 1 has an equivalent inhibition of NO production compared with the positive drug. So, the microbial biotransformation that occurred in FRG fermented by gut C. globosum can change the original bioactivity of polyacetylene, which gave a basis about the metabolic modification of red ginseng by intestinal fungus fermentation.
1. Introduction
As one of the most popular herbal medicines, red ginseng (RG) has been studied for its pharmacological activities, and it is also a widely used functional food [1]. The gut microbiota has an application for medical treatment and drug discovery over distinct pathema indications [2]. It has been reported that ginseng was processed by fungal and bacterial fermentation, which can cause an increase in the bioactivity and biotransform ginsenosides into aglycone forms [3,4]. The fermentation process had the potential for biotransformation of active metabolites into a high-value product, and it can also contribute to the improvement of biological activities of the medicinal plant [5]. Co-culture technology has appeared as a new approach to activating the biosynthesis of natural products [6]. The natural world was the co-culture system, not single cultures, so the co-culture system can imitate the interaction between different strains. Chaetomium globosum, belonging to the Chaetomiaceae family, is commonly found in terrestrial and marine habitats, and it was reported to cause infections in humans [7]. C. globosum was also found residing in Epinephelus drummondhayi guts [8]. On the positive side, the Chaetomium genus afforded a rich source of unique bioactive metabolites [9]. The emergence of antibiotic-resistant pathogenic microorganisms poses a threat to human health [10]. Most of the drug resistance was about the chemicals; the natural medicine was not studied in-depth study. In this work, a pathogen resistance against natural medicine was found by chance. Our previous work led to a rare example of polyacetylene biotransformation and a new polyacetylene, panaxylactone (metabolite A) transformed from panaxytriol in fermented red ginseng (FRG) by process of C. globosum [11]. The biotransformation process was not clear, so the reinvestigation of the polyacetylenes from FRG by solid co-culture of two C. globosum from different sources was carried out. Two new intermediates (1, 2) relevant to the biotransformation of panaxylactone were isolated, along with a microbial metabolite having a novel spiro-γ-lactone skeleton (chaetoginsin, 3) (Figure 1). The metabolic modification of active polyacetylenes from RG was investigated by these compounds from FRG by pathogen C. globosum.
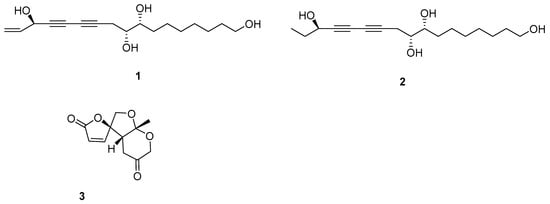
Figure 1.
The structures of isolated compounds.
2. Results and Discussion
Compound 1 was gained as an oil and the formula was identified to be C17H26O4 by its Distortionless Enhancement by Polarization Transfer (DEPT) spectrum and HR-ESI-MS (m/z 317.1735 [M + Na]+). Analysis of its 13C-NMR data exhibited the presence of the panaxytriol skeleton. The analysis of correlation spectroscopy (COSY) spectroscopic data revealed the C8-C9-C10-C11-C12-C13-C14-C15-C16-C17 structural connection. The heteronuclear multiple bond correlation (HMBC) correlations from H-3 to C-1, C-2, and C-4; H-8 to C-6, C-7, C-9 and C-10; H-17 to C-15 and C-16 elucidate the structure of compound 1 (Figure 2). There is a high similarity between the chemical skeletons of compound 1 and known compound panaxytriol (PXT) [12,13]; therefore, a polyacetylene with cytotoxicity previously isolated in ginseng, the configuration of compound 1 at C-3 was identified by comparing the NMR data and the optical rotation (OR) values with those of panaxytriol and metabolite A found from FRG in our previous work [11]. The absolute configuration of 1 was confirmed by comparing OR value at −24.0 and circular dichroism (CD) spectrum with those of known panaxytriol [11,12]. The structure of compound 2 was determined as the dihydrogen at C-1, C-2 derived from 1 by DEPT spectrum, HRESI-MS, 2D-NMR, optical rotation value, and CD spectrum.
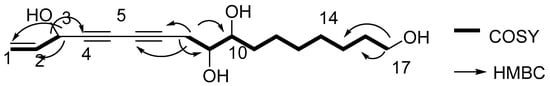
Figure 2.
Correlation spectroscopy (COSY) correlations and heteronuclear multiple bond correlation (HMBC) correlations of new compound 1.
Compound 3 was determined to be C11H12O5 from its NMR spectrum and HR-ESI-MS. Analysis of its DEPT data exhibited one lactone carbonyl (δC 168.7), one ketone (δC 204.9), and two olefinic carbons (δC 124.1 and 152.3), so 3 has a tricyclic ring system. The structure of 3 (Figure 3) was confirmed by HMBC spectrum from H-10 to C-5, C-9; from H-8, H-6,H-11 to C-9; from H-11 to C-4; from H-5, H-6, H-8 to C-7; from H-5, H-11 to C-3; from H-2, H-3 to C-1. The relative configurations of 3 at H-5 and H-9 were determined by the nuclear overhauser enhancement spectroscopy (ROESY) correlations between H-5 and H-10. The absolute configuration of 3 was determined by comparing the circular dichroism spectrum with that of its analog, streptoglyceride A [14].
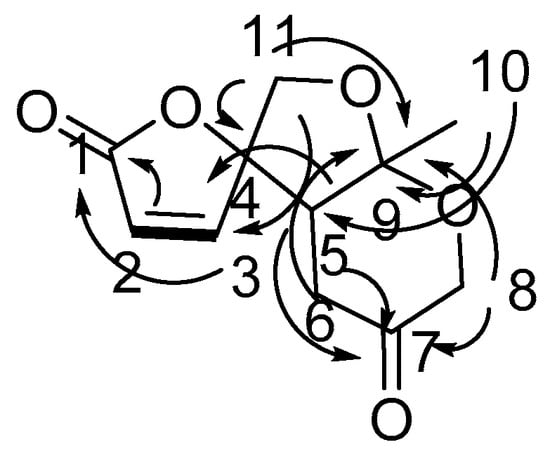
Figure 3.
COSY correlations and HMBC correlations of chaetoginsin (3).
In our previous work, some active compounds such as panaxytriol from RG indicated inhibition against the merisis of C. globosum, and C. globosum may own a detoxication mechanism against the antifungal activity of active compounds [11]. Panaxylactone (metabolite A) biotransformed from panaxytriol exhibited no antifungal activity, suggesting that C. globosum can transform panaxytriol to panaxylactone with less antifungus activity than can panaxytriol for its own merits. The biotransformation of panaxylactone was not clear, so the investigation of the intermediates in the biotransformation of natural polyacetylene by adjustment of fermentation mode was carried out. Two new key intermediates (1, and 2) were isolated, and the metabolism process of panaxylactone was shown in Figure 4. The natural 1-hydroxydihydropanaxacol from ginseng like the structure of 2, except for one more ω-hydroxy at 2, had S configuration at C-3 [15], and 2 had R configuration at C-3, so 2 was mostly transformed from 1. Compound 1, 2, and 3 also exhibited no antifungal activity with MICs > 256 μg/mL against C. globosum, which gave more proof on the detoxication mechanism of C. globosum against the antifungal activity of polyacetylenes from RG. Numerous bioactive polyacetylenes were found in the plant Panax ginseng. Among these compounds, PXT was shown in vitro cytotoxic activity against a series of human tumor cells [13]. Compound 1, 2 indicated no cytotoxicity with a concentration of 40 μM. Based on the LC-MS test (Figure S25), the new compound 2 was exhibited to be non-mark in RG extract, but mark in FRG, so compound 2 was obtained by microbial fermentation. Through these results, it was found that the changes in the chemical structure of PXT can decrease the antifungal activity and cytotoxicity. Compounds 1 and 2 indicated significant inhibition against NO production, with ratios of 44.80 ± 1.37 and 23.10 ± 1.00% at 50 μM. So, pathogen C. globosum might possess the potential of bioactivity modification upon the polyacetylenes from RG. The novel skeleton, like 3, was only found in basidiomycete Lenzites betulinusrare and Streptomyces, but not from plants [14,16]. 3 was positive in FRG by LC-MS, but negative in RG and liquid medium of C. globosum, so 3 should be produced by C. globosum, and this skeleton was first found in the generous Chaetomium metabolites. Thus, the chemical constituents of RG extract can induce the production of rare 6/5/5 tricyclic ring spiro-γ-lactone skeleton (3) from Chaetomium.
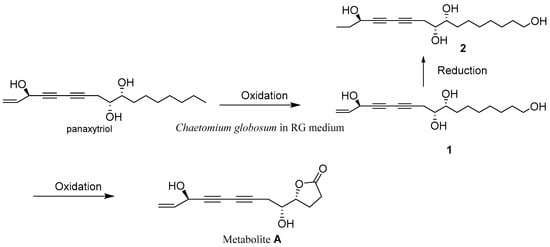
Figure 4.
The plausible biotransformation of panaxylactone (metabolite A).
3. Materials and Methods
3.1. General Experimental Procedure
Silica gel (200–300 mesh), Lichroprep RP-18, and Sephadex LH-20 were applied to column chromatography. The TLC plate was purchased from Qingdao Marine Chemical Group Co. and used for chromatography analysis. One-dimensional and two-dimensional NMR data were acquired on a Bruker AVANCE-600 MHz NMR instrument (Bruker, Karlsruhe, Germany). Mass spectra were measured by spectrometers of Agilent G3250AA (Agilent, Santa Clara, CA, USA) and AutoSpec Premier P776 (Waters, Milford, MA, USA). Optical rotations and circular dichroism spectra were measured on a polarimeter (Jasco P-1020) and an Applied Photophysics Chirascan spectrometer (Applied Photophysics Ltd., Surrey, British). LC-MS analyses were performed on an instrument (LTQ ORBITRAP XL).
3.2. Fungus Material and Cultivation Condition
Red ginseng was purchased from the Juhuacuen medicinal market of Kunming in Yunnan Province in China. The fungi were obtained from Dendrobium officinale gathered in Honghe of Yunnan Province and Hydrocharis dubia collected at Lijiang in Yunnan Province, China. The species were determined as C. globosum (JC-H8, YNH-16) on the analysis of morphological and genetic (ITS) properties. The voucher specimens were conserved in the analytical chemistry key laboratory of universities in Yunnan Province. The fermentation of two C. globosum were put into effect in 500 mL Erlenmeyer flasks consisting of 0.12 L of potato dextrose broth (PDB) with potato infusion of 0.024 kg of fresh potato, 1.8 g of dextrose, 120 mL of distilled H2O, pH 7.0, at 150 rpm and 28 °C for three days for a seed medium. Each 20–25 mL of seed medium was put into a 0.35 L microbiological culture vessel containing 0.12 kg of cooked RG. A total of 7 kg RG was fermented by C. globosum at 28 °C for thirty days.
3.3. Extraction and Separation Methods
All 7000 g of the solid medium was extracted with methanol for three times, the methanol extract was partitioned with ethyl acetate to give an EtOAc fraction and then dried under vacuum to obtain a dark extract (52.3 g). The extract was applied to column chromatography on silica gel (7 cm × 20 cm, 200 to 300 mesh, 400 g) and was eluted with a solvent system of chloroform-methanol from 1:0 to 10:1 (v/v) to give four fractions (A–D). Fraction A (13.3 g) was purified by using Lichroprep RP-18 (3 cm × 10 cm, CH3OH-H2O at 20% to 80%), Sephadex LH-20 (2 cm × 100 cm, chloroform-methanol at 1:1) to get 2 (3 mg). Fraction B (13.2 g) was separated by Sephadex LH-20 (2 cm × 150 cm, chloroform-methanol at 1:1), Lichroprep RP-18column chromatography (3 cm × 12 cm, CH3OH-H2O at 10% to 80%) to get 1 (4 mg), and chaetoginsin (3, 6 mg).
ω-Hydroxyl-panaxytriol (1). [α −24.0 (c 0.35, MeOH). HR-ESIMS m/z: 317.1735 [M + Na]+, calcd for C17H26O4Na: 317.1729. 1H-NMR (MeOD, 600 MHz) and 13C-NMR (MeOD, 150 MHz) in Table 1.

Table 1.
13C Nuclear Magnetic Resonance (NMR) and 1H-NMR data of compounds 1–3.
ω-Hydroxyl-dihydropanaxytriol (2). [α −8.6 (c 0.45, MeOH). HR-ESIMS m/z: 319.1879 [M + Na]+, calcd for C17H28O4Na: 319.1885. 1H-NMR (MeOD, 600 MHz) and 13C-NMR (MeOD, 150 MHz) in Table 1.
Chaetoginsin (3). [α −21.6 (c 0.14, MeOH). HR-ESIMS m/z: 247.0578 [M + Na]+, calcd for C11H12O5Na: 247.0582. 1H-NMR (CDCl3, 600 MHz) and 13C-NMR (CDCl3, 150 MHz) in Table 1.
3.4. LC-MS Method
The extracts of RG, and FRG fermentation materials were afforded according to the preparation method exhibited above. Similar sampling volumes of RG, and FRG fermentation materials, and compounds 2, 3 were prepared for application in the LC-MS investigation. A gradient solvent (methanol/0.1% methanoic acid from 40% to 99%) and a velocity of flow at 0.3 mL/min were applied to the investigation. The target ions were extracted by the precise molecular weight of compounds 2 and 3.
3.5. Bioassay Method
The two-fold plate dilution method were applied to the in vitro antifungus experiment [11]. Nystatin as positive drug had a minimum inhibitory concentration (MIC) at 32 μg/mL. The method of 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTT) assay was applied to the investigation of cytotoxicity of compounds 1 and 2 against tumor cell lines [17], A-549, HL-60, MCF-7, SW480, and SMMC-7721. Taxol was the positive drug with the IC50 < 0.008 μM. The NO inhibitory activities of compounds 1,2 were determined using the Griess reagent assay for NO production [11]. The positive control, L-NMMA has inhibitory ratio of 53.52 ± 0.53 % at concentration of 50 μM.
Supplementary Materials
1H and 13C-NMR, HSQC, HMBC, 1H-1H COSY, ROESY, and HR-ESIMS spectra of compounds 1–3. CD spectra of 1, 2, and 3. LC-HRMS finger-prints by ion extraction of co-culture, single strain, the liquid medium of C. globosum fermentation products, red ginseng, and compounds (2, 3). Cytotoxicity of compounds 1, 2 by the 3-(4,5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfopheny)-2H-tetrazolium (MTS) method.
Author Contributions
B.-Y.W. and C.-H.Z. performed the experiments and analyzed the data; X.-Q.Y., M.H., T.-T.X. contributed materials and bioactivity; X.-Y.W., S.Y. tested the NMR experiments; Z.-T.D. designed the experiments; Y.-B.Y. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the National Natural Science Foundation of China (81660582, 81960640, 81560571), a project of the Yunling Scholars of Yunnan Province, and the Program for Changjiang Scholars and Innovative Research Team in University (IRT-17R94).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jin, S.; Jeon, J.H.; Lee, S.; Kang, W.Y.; Seong, S.J.; Yoon, Y.R.; Choi, M.K.; Song, I.S. Detection of 13 ginsenosides (Rb1, Rb2, Rc, Rd, Re, Rf, Rg1, Rg3, Rh2, F1, compound K, 20(S)-protopanaxadiol, and 20(S)-protopanaxatriol) in human plasma and application of the analytical method to human pharma cokinetic studies following two week-repeated administration of red ginseng extract. Molecules 2019, 24, 2618. [Google Scholar]
- Zimmermann, M.; Zimmermann-Kogadeeva, M.; Wegmann, R.; Goodman, A.L. Mapping human microbiome drug metabolism by gut bacteria and their genes. Nature 2019, 570, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Kim, H.B.; Lee, K.H.; Choi, Y.R.; Kim, H.J.; Shin, S.I.; Gyoung, Y.S.; Joo, S.S. Steam-dried ginseng berry fermented with lactobacillus plantarum controls the increase of blood glucose and body weight in type 2 obese diabetic db/db mice. J. Agric. Food Chem. 2012, 60, 5438–5445. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.K.; Paik, H.D. Bioconversion using lactic acid bacteria: Ginsenosides, GABA, and phenolic Compounds. J. Microbiol. Biotechnol. 2017, 27, 869–877. [Google Scholar] [CrossRef] [PubMed]
- Parshikov, I.A.; Woodling, K.A.; Sutherland, J.B. Biotransformations of organic compounds mediated by cultures of Aspergillus niger. Appl. Microbiol. Biot. 2015, 99, 6971–6986. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.F.; Zhao, S.J.; Wang, Z.T.; Koffas, M.A. Recent advances in modular co-culture engineering for synthesis of natural products. Curr. Opin. Biotech. 2020, 62, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.W.; Lombard, L.; Groenewald, J.Z.; Li, J.; Videira, S.I.R.; Samson, R.A.; Liu, X.Z.; Crous, P.W. Phylogenetic reassessment of the Chaetomium globosum species complex. Persoonia 2016, 36, 83–133. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Ge, H.M.; Wang, G.; Jiang, N.; Mei, Y.N.; Jiang, R.; Li, S.J.; Chen, C.J.; Jiao, R.H.; Xu, Q.; et al. Pictet–Spengler reaction-based biosynthetic machinery in fungi. PNAS. 2014, 111, 18138–18143. [Google Scholar] [CrossRef] [PubMed]
- Fatima, N.; Muhammad, S.A.; Khan, I.; Qazi, M.A.; Shahzadi, I.; Mumtaz, A.; Hashmi, M.A.; Khan, A.K.; Ismail, T. Chaetomium endophytes: a repository of pharmacologically active metabolites. Acta Physiol. Plant. 2016, 38, 136. [Google Scholar] [CrossRef]
- Chang, Z.; Yadav, V.; Lee, S.C.; Heitman, J. Epigenetic mechanisms of drug resistance in fungi. Fungal Genet. Biol. 2019, 132, 103253. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.; Yang, X.Q.; Hu, M.; Shi, L.J.; Yin, H.Y.; Wu, Y.M.; Yang, Y.B.; Zhou, H.; Ding, Z.T. Biotransformation of natural polyacetylene in red ginseng by Chaetomium globosum. J. Ginseng Res. 2019. [Google Scholar] [CrossRef]
- Satoh, M.; Ishii, M.; Watanabe, M.; Isobe, K.; Uchiyama, T.; Fujimoto, Y. Absolute structure of panaxytriol. Chem. Pharm. Bull. 2002, 50, 126–128. [Google Scholar] [CrossRef][Green Version]
- Gurjar, M.K.; Kumar, V.S.; Rao, B.V. Synthesis of a new type of antitumour agent panaxytriol: Synthesis of its four diastereomers. Tetrahedron 1999, 55, 12563–12576. [Google Scholar] [CrossRef]
- Choi, B.K.; Park, S.Y.; Choi, D.K.; Shin, B.; Shin, Y.H.; Oh, D.C.; Lee, H.S.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; et al. Streptoglycerides A−D with a rare 6/5/5 tricyclic ring skeleton from a marine actinomycete Streptomyces species. Org. Lett. 2018, 20, 6037–6040. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, Y.; Satoh, M.; Takeuchi, N.; Kirisawa, M. Synthesis and absolute configurations of the cytotoxic polyacetylenes isolated from the callus of Panax ginseng. Chem. Pharm. Bull. 1990, 38, 1447–1450. [Google Scholar] [CrossRef] [PubMed]
- Wen, C.N.; Chen, H.P.; Zhao, Z.Z.; Hu, D.B.; Li, Z.H.; Feng, T.; Liu, J.K. Two new γ-lactones from the cultures of basidiomycete Lenzites betulinus. Phytochemistry Lett. 2017, 20, 9–12. [Google Scholar] [CrossRef]
- Szoka, L.; Isidorov, V.; Nazaruk, J.; Stocki, M.; Siergiejczyk, L. Cytotoxicity of triterpene seco-acids from Betula pubescens buds. Molecules 2019, 24, 4060. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–3 are available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).