Flexible and Reusable Ag Coated TiO2 Nanotube Arrays for Highly Sensitive SERS Detection of Formaldehyde
Abstract
1. Introduction
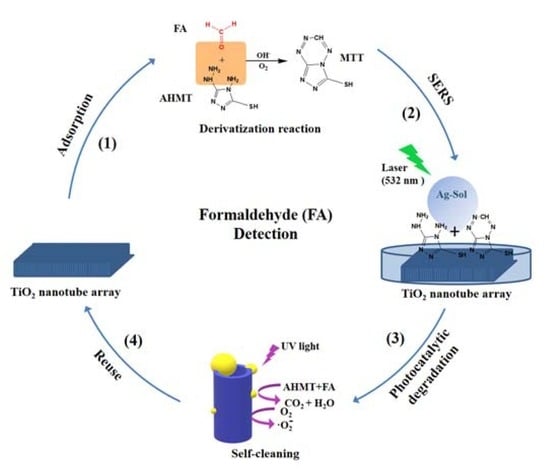
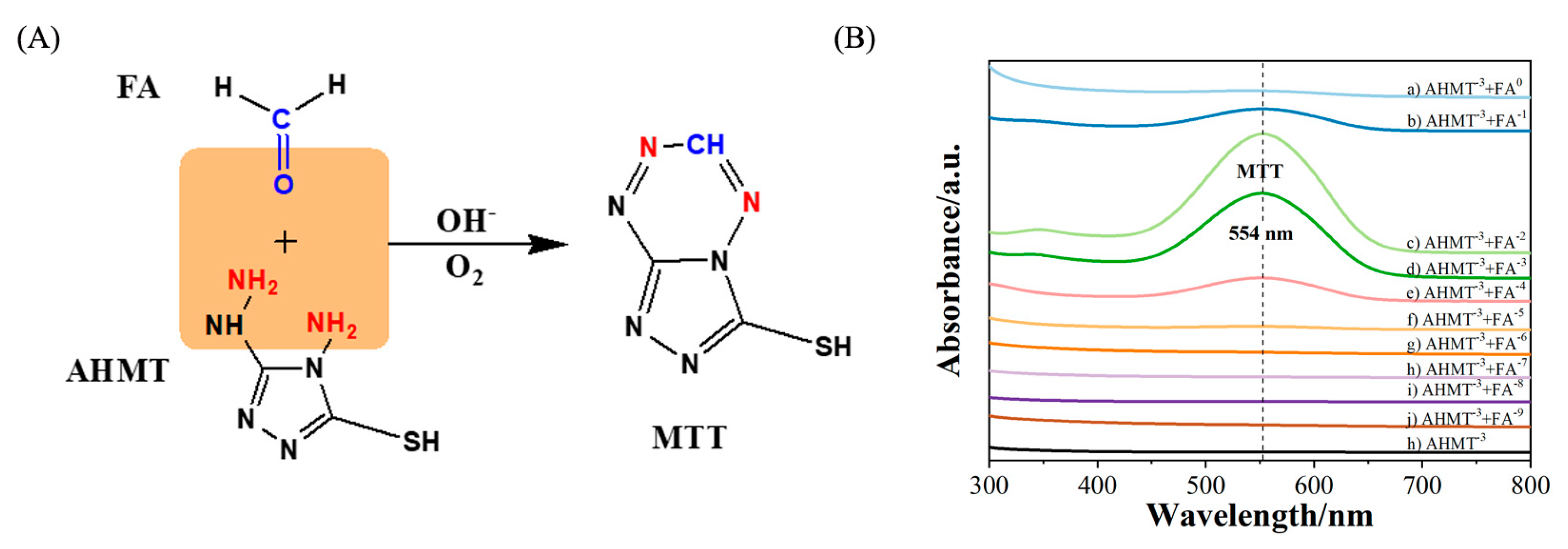
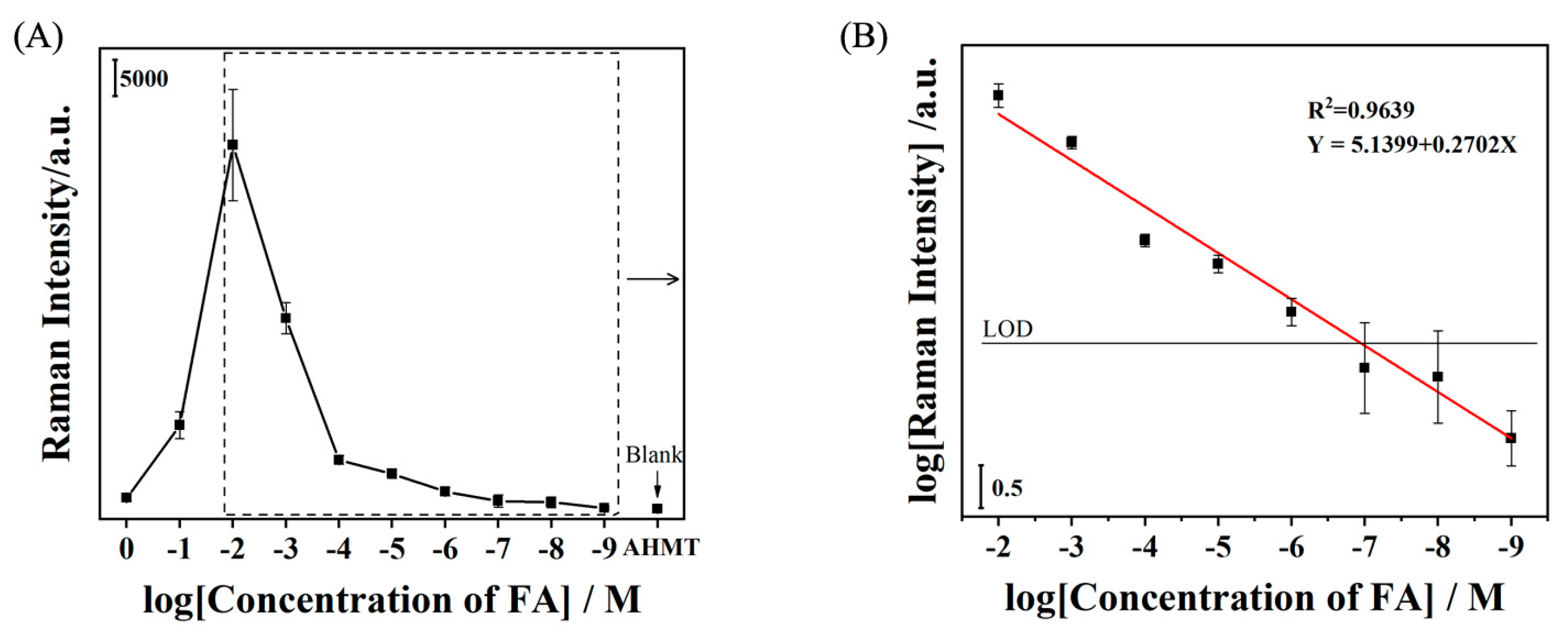
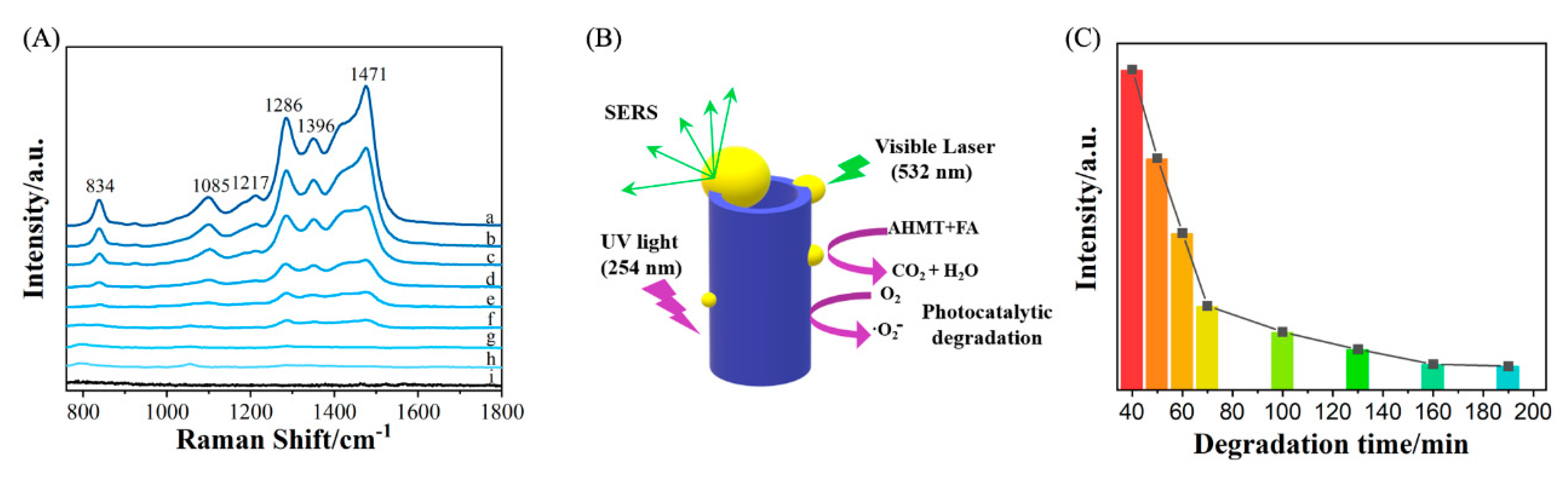
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
3.3. Preparation of Silver Sol
3.4. Preparation of the TNA
3.5. Preparation of the Test Solution
3.6. SERS Detection of FA
3.7. UV Degradability of AHMT-FA with the Ag-TNA
3.8. Recyclable SERS Substrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, H.; Sun, L.; Li, G.; Zou, X. Well-Tuned Surface Oxygen Chemistry of Cation Off-Stoichiometric Spinel Oxides for Highly Selective and Sensitive Formaldehyde Detection. Chem. Mater. 2018, 30, 2018–2027. [Google Scholar] [CrossRef]
- Costa, S.; Costa, C.; Madureira, J.; Valdiglesias, V.; Teixeira-Gomes, A.; Guedes de Pinho, P.; Laffon, B.; Teixeira, J.P. Occupational exposure to formaldehyde and early biomarkers of cancer risk, immunotoxicity and susceptibility. Environ. Res. 2019. [CrossRef]
- Salthammer, T.; Mentese, S.; Marutzky, R. Formaldehyde in the Indoor Environment. Chem. Rev. 2010, 110, 2536–2572. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.; Jiang, H.; Qiu, X.; Yu, D. Smartphone-Based Microfluidic Colorimetric Sensor for Gaseous Formaldehyde Determination with High Sensitivity and Selectivity. Sensors 2018, 18, 3141. [Google Scholar] [CrossRef]
- Wu, K.; Kong, X.; Xiao, K.; Wei, Y.; Zhu, C.; Zhou, R.; Si, M.; Wang, J.; Zhang, Y.; Wen, L. Engineered Smart Gating Nanochannels for High Performance in Formaldehyde Detection and Removal. Adv. Funct. Mater. 2019. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, C.; Li, S.; Fan, J. A hydrophobic deep eutectic solvent based vortex-assisted liquid-liquid microextraction for the determination of formaldehyde from biological and indoor air samples by high performance liquid chromatography. J. Chromatogr. A 2019, 1589, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.; Lin, T.; Chen, C.; Wen, H. Analysis of free and bound formaldehyde in squid and squid products by gas chromatography–mass spectrometry. J. Food Drug Anal. 2013, 21, 190–197. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, J.; Hu, L.; Tan, Y.; Liu, S.; Yin, J. Recent advances in formaldehyde-responsive fluorescent probes. Chin. Chem. Lett. 2017, 28, 1935–1942. [Google Scholar] [CrossRef]
- Ji, W.; Li, L.; Song, W.; Wang, X.; Zhao, B.; Ozaki, Y. Enhanced Raman Scattering by ZnO Superstructures: Synergistic Effect of Charge Transfer and Mie Resonances. Angew. Chem. Int. Ed. Engl. 2019, 58, 14452–14456. [Google Scholar] [CrossRef]
- Lombardi, J.R. Enhanced by organic surfaces. Nat. Mater. 2017, 16, 878–880. [Google Scholar] [CrossRef]
- Gao, Y.; Yin, P. Effect of Bond Dispersion on Raman Spectra Shift in II–VI Semiconductor Nanocrystals. Inorg. Chem. 2019, 58, 4859–4868. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Liu, S.; Zheng, N.; Liu, Y.; Han, X.; He, C.; Lu, H.; Zhao, B. Frequency Shifts in Surface-Enhanced Raman Spectroscopy-Based Immunoassays: Mechanistic Insights and Application in Protein Carbonylation Detection. Anal. Chem. 2019, 91, 9376–9381. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhao, B.; Wang, C.; Ozaki, Y.; Lu, X. Functional nanomaterials with unique enzyme-like characteristics for sensing applications. J. Mater. Chem. B 2019, 7, 850–875. [Google Scholar] [CrossRef]
- Su, H.; Feng, H.; Zhao, Q.; Zhang, X.; Sun, J.; He, Y.; Huang, S.; Huang, T.; Zhong, J.; Wu, D.; et al. Probing the Local Generation and Diffusion of Active Oxygen Species on a Pd/Au Bimetallic Surface by Tip-Enhanced Raman Spectroscopy. J. Am. Chem. Soc. 2020, 142, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shi, W.; Wang, S.; Zhao, H.; Lin, J.; Yang, Z.; Chen, M.; Guo, L. Two-Dimensional Amorphous TiO2 Nanosheets Enabling High-Efficiency Photoinduced Charge Transfer for Excellent SERS Activity. J. Am. Chem. Soc. 2019, 141, 5856–5862. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Ozaki, Y.; Xu, Z.; Zhao, B. Effect of TiO2 on Altering Direction of Interfacial Charge Transfer in a TiO2-Ag-MPY-FePc System by SERS. Angew. Chem. Int. Ed. Engl. 2019, 58, 8172–8176. [Google Scholar] [CrossRef]
- Wang, Y.; Le, J.; Li, W.; Wei, J.; Radjenovic, P.M.; Zhang, H.; Zhou, X.; Cheng, J.; Tian, Z.; Li, J.-F. In situ Spectroscopic Insight into the Origin of the Enhanced Performance of Bimetallic Nanocatalysts towards the Oxygen Reduction Reaction (ORR). Angew. Chem. Int. Ed. Engl. 2019, 58, 16062–16066. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, M.; Ma, H.; Zhang, H.; Cheng, W.; Li, J.; Cai, L.; Han, X.; Zhao, B. Redox-State-Mediated Regulation of Cytochrome c Release in Apoptosis Revealed by Surface-Enhanced Raman Scattering on Nickel Substrates. Angew. Chem. Int. Ed. 2019, 58, 16499–16503. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Han, X.; Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor-enhanced Raman scattering: Active nanomaterials and applications. Nanoscale 2017, 9, 4847–4861. [Google Scholar] [CrossRef]
- Ji, W.; Zhao, B.; Ozaki, Y. Semiconductor materials in analytical applications of surface-enhanced Raman scattering. J. Raman Spectrosc. 2016, 47, 51–58. [Google Scholar] [CrossRef]
- Yang, B.; Jin, S.; Guo, S.; Park, Y.; Chen, L.; Zhao, B.; Jung, Y.M. Recent Development of SERS Technology: Semiconductor-Based Study. Acs Omega 2019, 4, 20101–20108. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, G.; Yang, L.; Jin, Z.; Liu, J. Multifunctional Au-Coated TiO2 Nanotube Arrays as Recyclable SERS Substrates for Multifold Organic Pollutants Detection. Adv. Funct. Mater. 2010, 20, 2815–2824. [Google Scholar] [CrossRef]
- Fu, L.; Sokiransky, M.M.; Wang, J.; Lai, G.; Yu, A. Development of Ag dendrites-reduced graphene oxide composite catalysts via galvanic replacement reaction. Phys. E: Low-Dimens. Syst. Nanostruct. 2016, 83, 146–150. [Google Scholar] [CrossRef]
- Wang, W.; Li, Z.; Gu, B.; Zhang, Z.; Xu, H. Ag@SiO2 Core−Shell Nanoparticles for Probing Spatial Distribution of Electromagnetic Field Enhancement via Surface-Enhanced Raman Scattering. Acs Nano 2009, 3, 3493–3496. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, C.; Kei, C.; Hsueh, Y.; Cho, W.; Perng, T. Photocatalysis of Ag-Loaded TiO2 Nanotube Arrays Formed by Atomic Layer Deposition. J. Phys. Chem. C 2011, 115, 9498–9502. [Google Scholar] [CrossRef]
- Bao, Z.Y.; Liu, X.; Dai, J.; Wu, Y.; Tsang, Y.H.; Lei, D.Y. In situ SERS monitoring of photocatalytic organic decomposition using recyclable TiO2-coated Ag nanowire arrays. Appl. Surf. Sci. 2014, 301, 351–357. [Google Scholar] [CrossRef]
- Chang, Y.; Wu, S. Bi-functional Al-doped ZnO@SnO2 heteronanowires as efficient substrates for improving photocatalytic and SERS performance. J. Ind. Eng. Chem. 2019, 76, 333–343. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J.; Wei, W.; Wu, Y.; Li, T. Synthesis, characterization and application of TiO2/Ag recyclable SERS substrates. Rsc Adv. 2017, 7, 26704–26709. [Google Scholar] [CrossRef]
- Jiang, X.; Sun, X.; Yin, D.; Li, X.; Yang, M.; Han, X.; Yang, L.; Zhao, B. Recyclable Au-TiO2 nanocomposite SERS-active substrates contributed by synergistic chargetransfer effect. Phys. Chem. Chem. Phys. 2017, 19, 11212–11219. [Google Scholar] [CrossRef]
- Yan, X.; Xu, Y.; Tian, B.; Lei, J.; Zhang, J.; Wang, L. Operando SERS self-monitoring photocatalytic oxidation of aminophenol on TiO2 semiconductor. Appl. Catal. B-Environ. 2018, 224, 305–309. [Google Scholar] [CrossRef]
- Wang, L.; Li, R.; Liu, J.; Han, J.; Huang, M. Synthesis of Au@CdS core-shell nanoparticles and their photocatalytic capacity researched by SERS. J. Mater. Sci. 2017, 52, 1847–1855. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Liu, R.; Wang, X.; Zhu, Y.; Zhang, J. SERS and the photo-catalytic performance of Ag/TiO2/graphene composites. Opt. Mater. Express 2018, 8, 704–717. [Google Scholar] [CrossRef]
- Sinha, G.; Depero, L.E.; Alessandri, I. Recyclable SERS Substrates Based on Au-Coated ZnO Nanorods. Acs Appl. Mater. Interfaces 2011, 3, 2557–2563. [Google Scholar] [CrossRef]
- Ko, Y.C.; Fang, H.Y.; Chen, D.H. Fabrication of Ag/ZnO/reduced graphene oxide nanocomposite for SERS detection and multiway killing of bacteria. J. Alloys Compd. 2017, 695, 1145–1153. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, F.; Zhao, X.; Wang, B.; Shi, Y.; Zhang, L.; Liu, J. In Situ SERS Detection of Photocatalytic Degradation of Aminothiophenol on Carbon-Nanotubes/CoPt Hollow Nanoparticles Composite. Langmuir 2019, 35, 11629–11634. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, Y.; Nie, W.; Chen, P. One-step synthesis of Ag2S/Ag@MoS2 nanocomposites for SERS and photocatalytic applications. J. Nanopart. Res. 2018, 20, 1–13. [Google Scholar] [CrossRef]
- Cao, Q.; Che, R.; Chen, N. Facile and rapid growth of Ag2S microrod arrays as efficient substrates for both SERS detection and photocatalytic degradation of organic dyes. Chem. Commun. 2014, 50, 4931–4933. [Google Scholar] [CrossRef]
- Huang, Q.; Li, J. Enhanced photocatalytic and SERS properties of ZnO/Ag hierarchical multipods-shaped nanocomposites. Mater. Lett. 2017, 204, 85–88. [Google Scholar] [CrossRef]
- Sheng, P.; Li, W.; Du, P.; Cao, K.; Cai, Q. Multi-functional CuO nanowire/TiO2 nanotube arrays photoelectrode synthesis, characterization, photocatalysis and SERS applications. Talanta 2016, 160, 537–546. [Google Scholar] [CrossRef]
- Tan, X.; Wang, L.; Cheng, C.; Yan, X.; Shen, B.; Zhang, J. Plasmonic MoO3-x@MoO3 nanosheets for highly sensitive SERS detection through nanoshell-isolated electromagnetic enhancement. Chem. Commun. 2016, 52, 2893–2896. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, N.; Deng, D.; Xing, X.; Xiao, X.; Wang, Y. Formaldehyde detection: SnO2 microspheres for formaldehyde gas sensor with high sensitivity, fast response/recovery and good selectivity. Sens. Actuators B: Chem. 2017, 238, 264–273. [Google Scholar] [CrossRef]


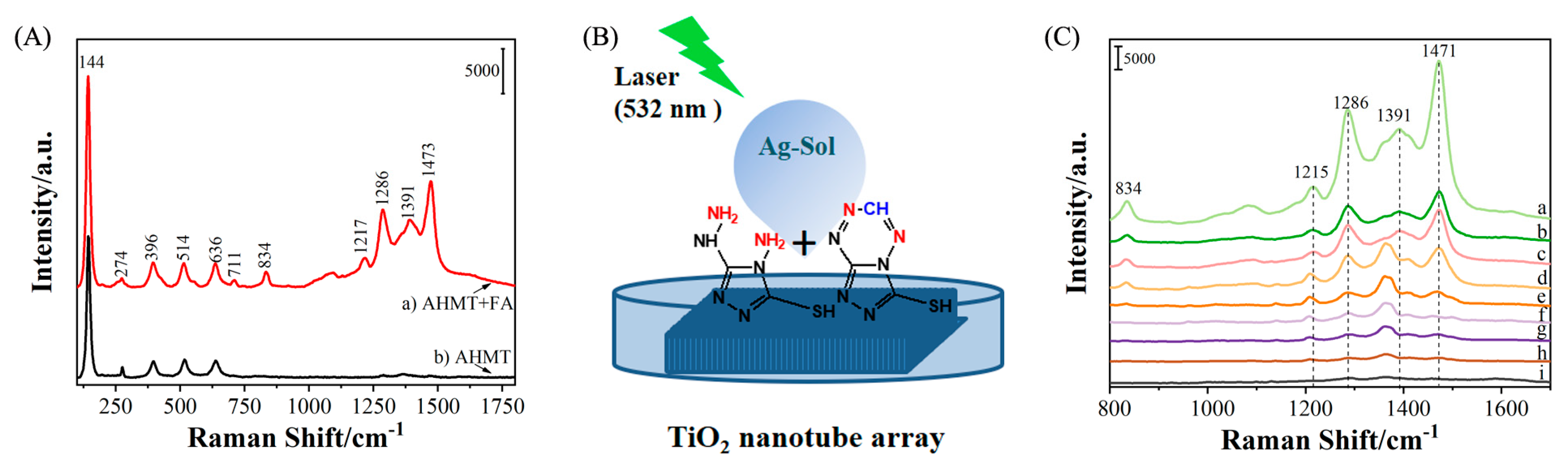

| Functional Group/Vibration | Raman Bands of AHMT-FA |
|---|---|
| Eg | 144, 636 cm−1 |
| B1g | 396 cm−1 |
| A1g/B1g | 514 cm−1 |
| ν(S-C-N) | 710 cm−1 |
| ν(N-C-N) | 832 cm−1 |
| ν(C-C) | 1473 cm−1 |
| Breathing vibration | 1217, 1286, 1391 cm−1 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, T.; Wang, H.; Zang, L.; Jin, S.; Guo, S.; Park, E.; Mao, Z.; Jung, Y.M. Flexible and Reusable Ag Coated TiO2 Nanotube Arrays for Highly Sensitive SERS Detection of Formaldehyde. Molecules 2020, 25, 1199. https://doi.org/10.3390/molecules25051199
Zhu T, Wang H, Zang L, Jin S, Guo S, Park E, Mao Z, Jung YM. Flexible and Reusable Ag Coated TiO2 Nanotube Arrays for Highly Sensitive SERS Detection of Formaldehyde. Molecules. 2020; 25(5):1199. https://doi.org/10.3390/molecules25051199
Chicago/Turabian StyleZhu, Tong, Hang Wang, Libin Zang, Sila Jin, Shuang Guo, Eungyeong Park, Zhu Mao, and Young Mee Jung. 2020. "Flexible and Reusable Ag Coated TiO2 Nanotube Arrays for Highly Sensitive SERS Detection of Formaldehyde" Molecules 25, no. 5: 1199. https://doi.org/10.3390/molecules25051199
APA StyleZhu, T., Wang, H., Zang, L., Jin, S., Guo, S., Park, E., Mao, Z., & Jung, Y. M. (2020). Flexible and Reusable Ag Coated TiO2 Nanotube Arrays for Highly Sensitive SERS Detection of Formaldehyde. Molecules, 25(5), 1199. https://doi.org/10.3390/molecules25051199