Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
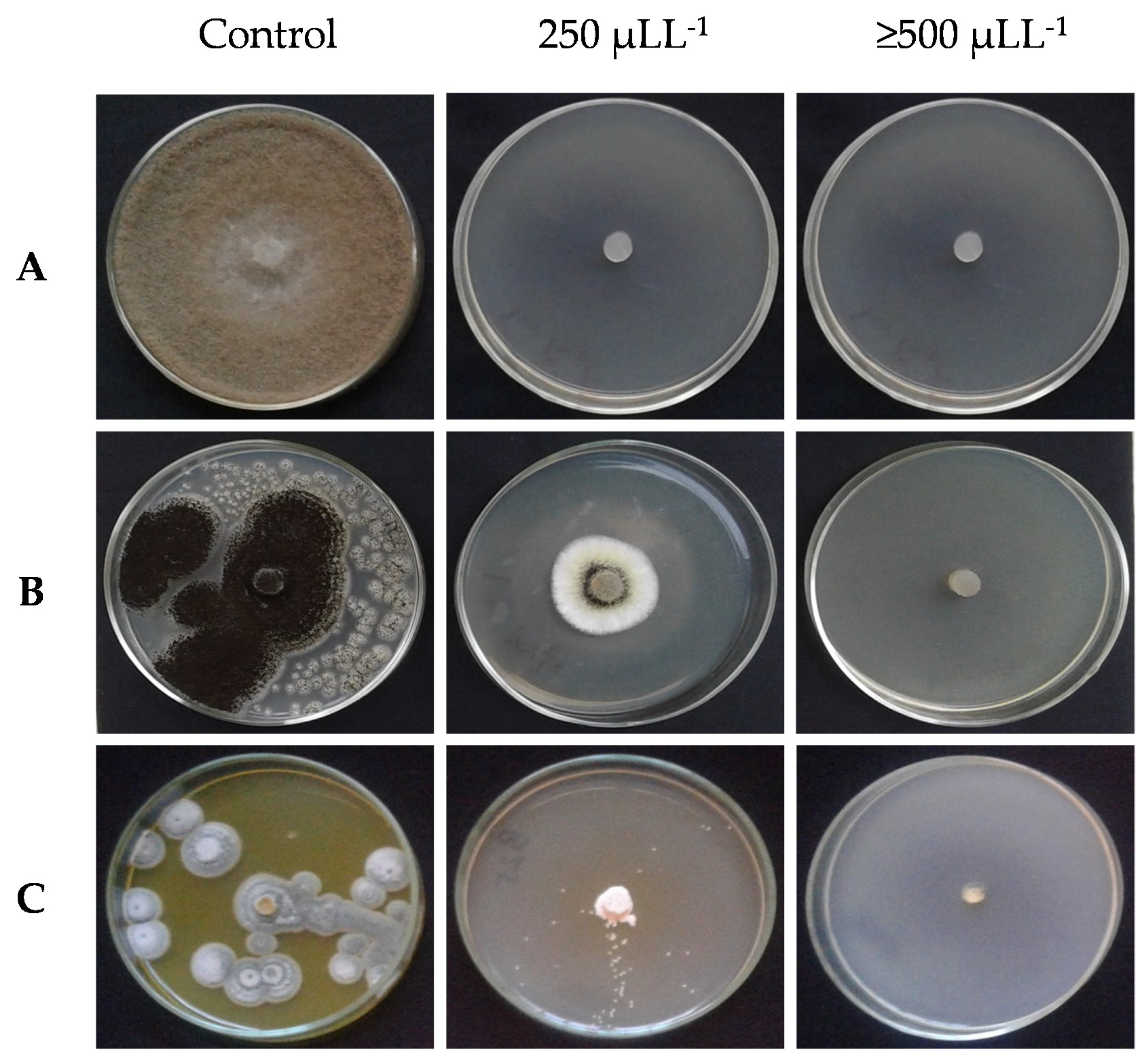
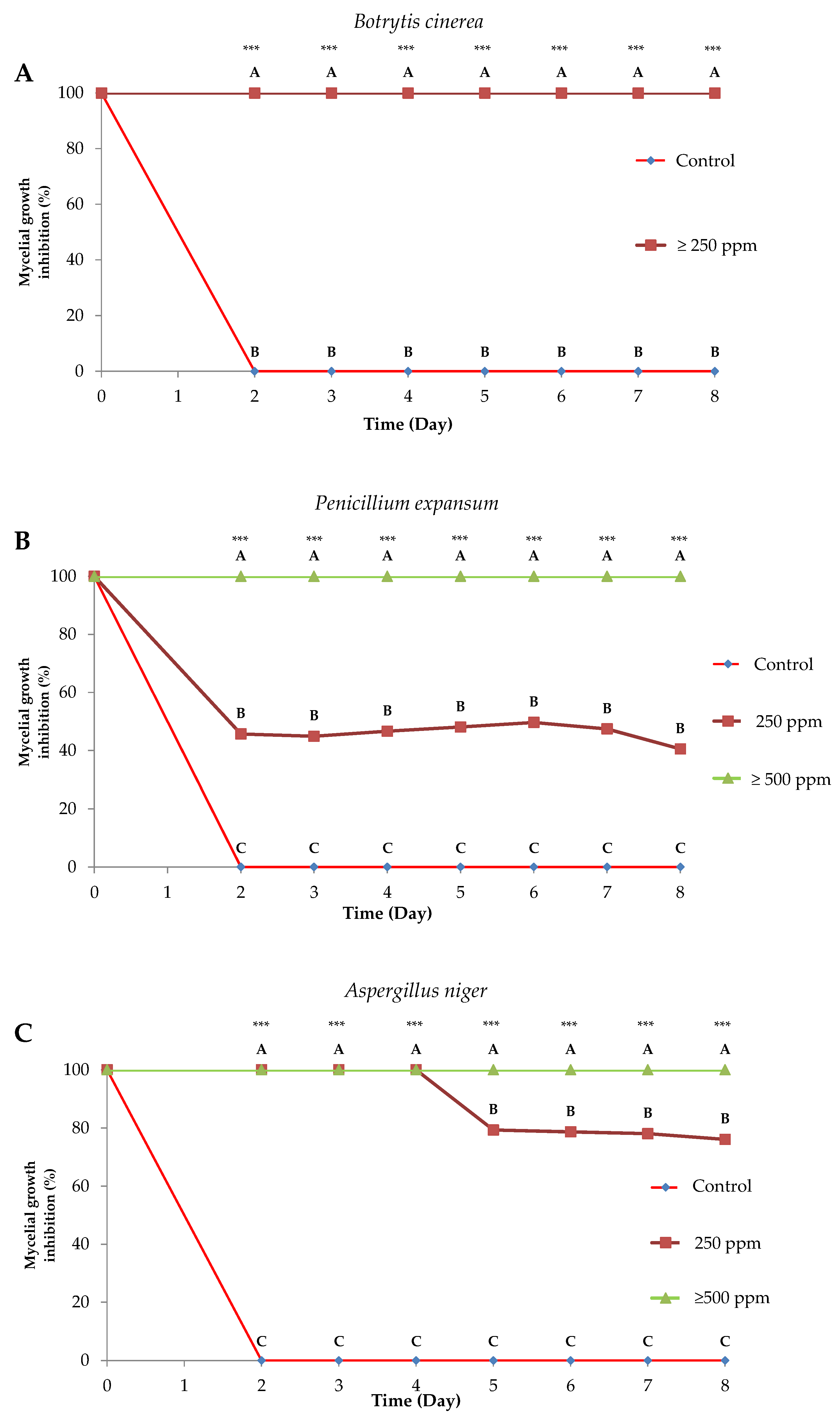
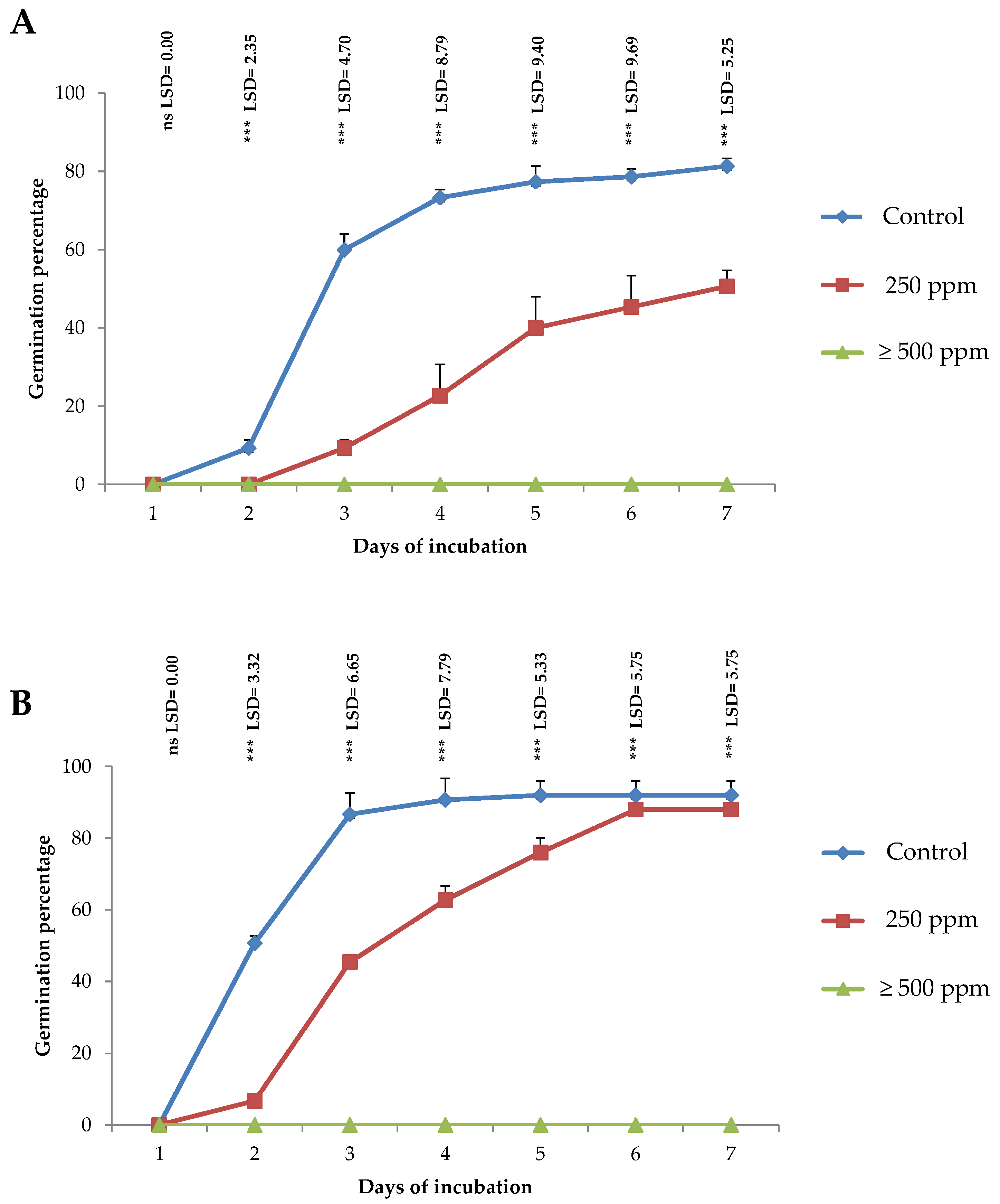
2.2. Antifungal Activity
2.3. Phytotoxic Activity
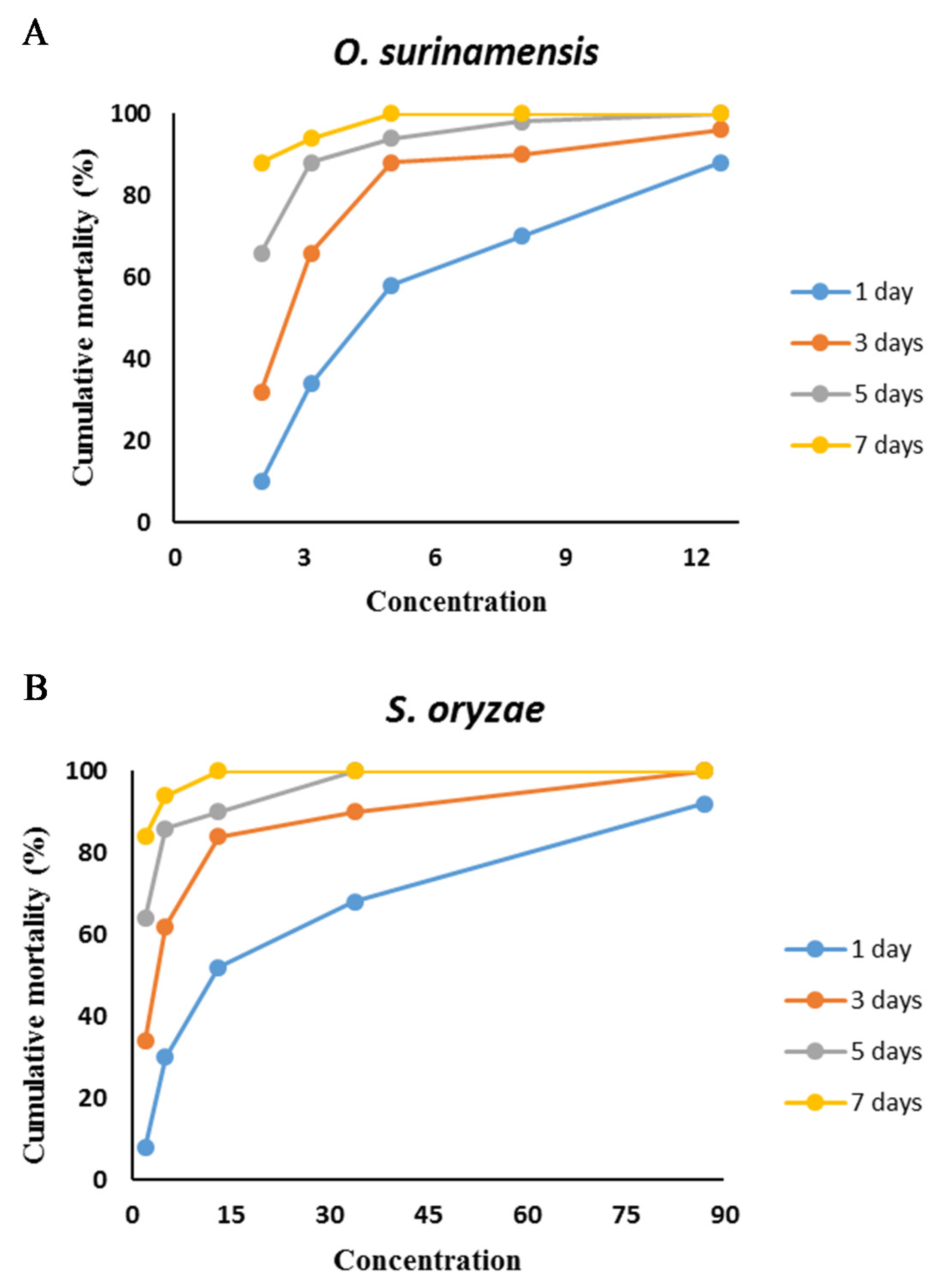
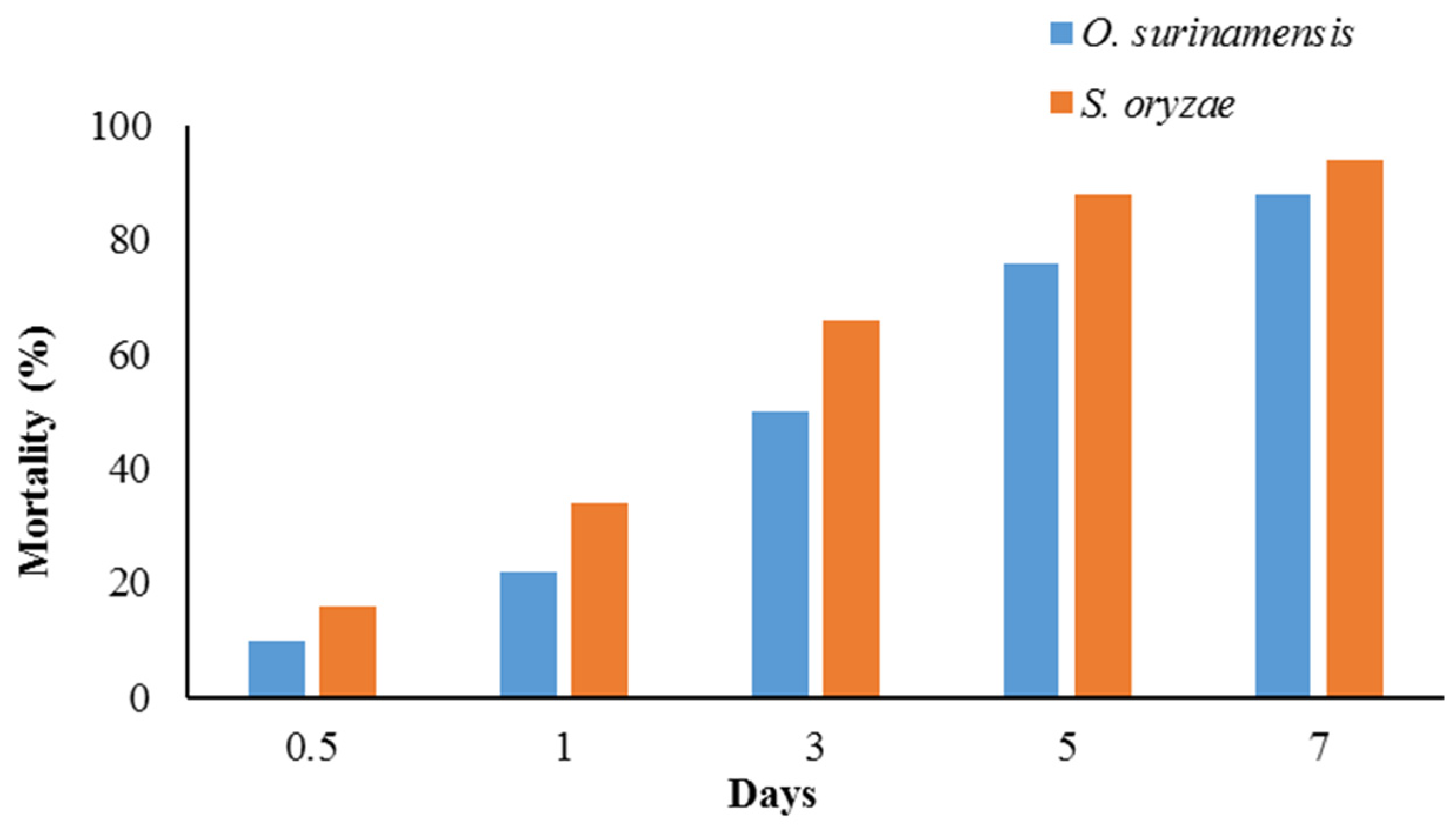
2.4. Insecticidal Activity
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction of Essential Oil
3.3. GC and GC-MS Analysis
3.4. Fungal Isolates
3.5. In vitro Antifungal Assays
3.6. Phytotoxic Activity
3.7. Insecticidal Activity
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenzo, J.M.; Mousavi-Khaneghah, A.; Gavahian, M.; Marszałek, K.; Es, I.; Munekata, P.E.S.; Ferreira, I.C.F.R.; Barba, F.J. Understanding the potential benefits of thyme and its derived products for food industry and consumer health: From extraction of value-added compounds to the evaluation of bioaccessibility, bioavailability, anti-inflammatory, and antimicrobial activities. Crit. Rev. Food Sci. Nutr. 2019, 59, 2879–2895. [Google Scholar] [CrossRef] [PubMed]
- Morales, R. The history, botany and taxonomy of the genus Thymus. In Thyme the Genus Thymus-Medicinal and Aromatic Plants; Stahl-Biskup, E., Saez, F., Eds.; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–43. ISBN 9780203216859. [Google Scholar]
- Moradi, P.; Falsafi, T.; Saffari, N.; Rahimi, E.; Momtaz, H.; Hanedi, B. Chemical composition and antimicrobial effects of Thymus daenensis on Helicobacter pylori. Biosci. Biotechnol. Res. Commun. 2017, 10, 139–144. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils-A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Miguel, M.G. Antioxidant and anti-inflammatory activities of essential oils: A short review. Molecules 2010, 15, 9252–9287. [Google Scholar] [CrossRef]
- Chou, S.T.; Lai, C.C.; Lai, C.P.; Chao, W.W. Chemical composition, antioxidant, anti-melanogenic and anti-inflammatory activities of Glechoma hederacea (Lamiaceae) essential oil. Ind. Crops Prod. 2018, 122, 675–685. [Google Scholar] [CrossRef]
- Zhiani, R.; Dolatabadi, S.; Imani, H.; Moradi, M.; Emrani, S. A Comparison of the chemical composition of flowering shoot Thymus kotschyanus and Thymus vulgaris by using GC-mass and antimicrobial effects of the bacteria Staphylococcus aureus and Pseudomonas aeruginosa. J. Essent. Oil-Bear. Plants 2016, 19, 1639–1647. [Google Scholar] [CrossRef]
- Sefidkon, F.; Jamzad, Z.; Yavari-Behrouz, R.; Nouri Shargh, D. Essential oil composition of Thymus kotschyanus boiss. And hohen from Iran. J. Essent. Oil Res. 1999, 11, 459–460. [Google Scholar] [CrossRef]
- Sefidkon, F.; Dabiri, M.; Mirmostafa, S.A. The essential oil of Thymus persicus (Ronniger ex Rech. f.) Jalas from Iran. J. Essent. Oil Res. 2002, 14, 351–352. [Google Scholar] [CrossRef]
- Sefidkon, F.; Askari, F.; Ghorbanli, M. Essential oil composition of Thymus pubescens Boiss. et Kotschy ex Celak from Iran. J. Essent. Oil Res. 2002, 14, 116–117. [Google Scholar] [CrossRef]
- Sefidkon, F.; Askari, F.; Mirmostafa, S.A. The essential oil of Thymus carnosus Boiss. From Iran. J. Essent. Oil Res. 2001, 13, 192–193. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; Munekata, P.E.S.; de Melo, M.P. Effects of oregano extract on oxidative, microbiological and sensory stability of sheep burgers packed in modified atmosphere. Food Control. 2016, 63, 65–67. [Google Scholar] [CrossRef]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as potential source of natural additives for meat industry. A Review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Domínguez, R.; Franco, D.; Bermúdez, R.; Trindade, M.A.A.; Lorenzo, J.M. Effect of natural antioxidants in Spanish salchichón elaborated with encapsulated n-3 long chain fatty acids in konjac glucomannan matrix. Meat Sci. 2017, 124, 54–60. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; González-Rodríguez, R.M.; Sánchez, M.; Amado, I.R.; Franco, D. Effects of natural (grape seed and chestnut extract) and synthetic antioxidants (buthylatedhydroxytoluene, BHT) on the physical, chemical, microbiological and sensory characteristics of dry cured sausage “chorizo”. Food Res. Int. 2013, 54, 611–620. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Pugine, S.M.P.; Lima, C.G.; Lorenzo, J.M.; de Melo, M.P. Evaluation of oxidative stability of lamb burger with Origanum vulgare extract. Food Chem. 2017, 233, 101–109. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Lorenzo, J.M.; de Melo, M.P. Assessment of the stability of sheep sausages with the addition of different concentrations of Origanum vulgare extract during storage. Meat Sci. 2018, 137, 244–257. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active packaging films with natural antioxidants to be used in meat industry: A review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Pateiro, M.; Domínguez, R.; Bermúdez, R.; Munekata, P.E.S.; Zhang, W.; Gagaoua, M.; Lorenzo, J.M. Antioxidant active packaging systems to extend the shelf life of sliced cooked ham. Curr. Res. Food Sci. 2019, 1, 24–30. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Batlle, R.; Gómez, M. Extension of the shelf-life of foal meat with two antioxidant active packaging systems. LWT-Food Sci. Technol. 2014, 59, 181–188. [Google Scholar] [CrossRef]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Cunha, L.C.M.; Monteiro, M.L.G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; de Carvalho, F.A.L.; Conte-Junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Pateiro, M.; Gagaoua, M.; Barba, F.J.; Zhang, W.; Lorenzo, J.M. A comprehensive review on lipid oxidation in meat and meat products. Antioxidants 2019, 8, 429. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Giordani, R.; Hadef, Y.; Kaloustian, J. Compositions and antifungal activities of essential oils of some Algerian aromatic plants. Fitoterapia 2008, 79, 199–203. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Veríssimo, A.R.; Faleiro, M.L.; Miguel, M.G. Chemical composition and biological activities of Algerian Thymus oils. Food Chem. 2009, 116, 714–721. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Morteza-Semnani, K.; Mojra, E. Anti-inflammatory and antinociceptive activity of Thymus pubescens extract. Fitoterapia 2008, 79, 361–365. [Google Scholar] [CrossRef]
- Xiao, C.L.; Rogers, J.D. A Postharvest fruit rot in d’Anjou pears caused by Sphaeropsis pyriputrescens sp. nov. Plant. Dis. 2004, 88, 114–118. [Google Scholar] [CrossRef][Green Version]
- Fenoll, J.; Sabater, P.; Navarro, G.; Vela, N.; Pérez-Lucas, G.; Navarro, S. Abatement kinetics of 30 Sulfonylurea herbicide residues in water by photocatalytic treatment with semiconductor materials. J. Environ. Manage. 2013, 130, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, A.O.; Igbinosa, E.O. Chlorophenols and other related derivatives of environmental concern: Properties, distribution and microbial degradation processes. Chemosphere 2011, 83, 1297–1306. [Google Scholar] [CrossRef]
- Janisiewicz, W.J.; Korsten, L. Biological control of postharvest diseases of fruits. Annu. Rev. Phytopathol. 2002, 40, 411–441. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zeng, L.; Sheng, K.; Chen, F.; Zhou, T.; Zheng, X.; Yu, T. γ-Aminobutyric acid induces resistance against Penicillium expansum by priming of defence responses in pear fruit. Food Chem. 2014, 159, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Marín, S.; Sanchis, V.; Ramos, A.J. Plant products in the control of mycotoxins and mycotoxigenic fungi on food commodities. In Natural Products in Plant Pest Management; Dubey, N.K., Ed.; CABI: Oxfordshire, UK, 2010; pp. 21–41. ISBN 9781845936716. [Google Scholar]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phyther. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A.; Koubaa, M.; Barba, F.J.; Abedi, E.; Niakousari, M.; Tavakoli, J. Extraction of essential oil from Aloysia citriodora Palau leaves using continuous and pulsed ultrasound: Kinetics, antioxidant activity and antimicrobial properties. Process. Biochem. 2018, 65, 197–204. [Google Scholar] [CrossRef]
- Giacometti, J.; Bursać Kovaćević, D.; Putnik, P.; Gabrić, D.; Bilušić, T.; Krešić, G.; Stulić, V.; Barba, F.J.; Chemat, F.; Barbosa-Cánovas, G.; et al. Extraction of bioactive compounds and essential oils from mediterranean herbs by conventional and green innovative techniques: A review. Food Res. Int. 2018, 113, 245–262. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathic interactions and allelochemicals: New possibilities for sustainable weed management. CRC. Crit. Rev. Plant. Sci. 2003, 22, 239–311. [Google Scholar] [CrossRef]
- Kong, C.H.; Xu, X.H.; Zhang, M.; Zhang, S.Z. Allelochemical tricin in rice hull and its aurone isomer against rice seedling rot disease. Pest. Manag. Sci. 2010, 66, 1018–1024. [Google Scholar] [CrossRef]
- Ben El Hadj Ali, I.; Bahri, R.; Chaouachi, M.; Boussaïd, M.; Harzallah-Skhiri, F. Phenolic content, antioxidant and allelopathic activities of various extracts of Thymus numidicus Poir. organs. Ind. Crops Prod. 2014, 62, 188–195. [Google Scholar] [CrossRef]
- Ben El Hadj Ali, I.; Chaouachi, M.; Bahri, R.; Chaieb, I.; Boussaïd, M.; Harzallah-Skhiri, F. Chemical composition and antioxidant, antibacterial, allelopathic and insecticidal activities of essential oil of Thymus algeriensis Boiss. et Reut. Ind. Crops Prod. 2015, 77, 631–639. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Benabdelkader, T.; Chelghoum, C. Studies on the essential oil composition and antimicrobial activity of Thymus algeriensis Boiss. et Reut. Int. J. Aromather. 2006, 16, 95–100. [Google Scholar] [CrossRef]
- Sefidkon, F.; Dabiri, M.; Rahimi-Bidgoly, A. The effect of distillation methods and stage of plant growth on the essential oil content and composition of Thymus kotschyanus Boiss. and Hohen. Flavour Fragr. J. 1999, 14, 405–408. [Google Scholar] [CrossRef]
- Rasooli, I.; Mirmostafa, S.A. Bacterial susceptibility to and chemical composition of essential oils from Thymus kotschyanus and Thymus persicus. J. Agric. Food Chem. 2003, 51, 2200–2205. [Google Scholar] [CrossRef] [PubMed]
- Nickavar, B.; Mojab, F.; Dolat-Abadi, R. Analysis of the essential oils of two Thymus species from Iran. Food Chem. 2005, 90, 609–611. [Google Scholar] [CrossRef]
- Toroglu, S. In vitro antimicrobial activity and antagonistic effect of essential oils from plant species. J. Environ. Biol. 2007, 28, 551–559. [Google Scholar]
- Amiri, H. Essential oils composition and antioxidant properties of three Thymus species. Evidence-Based Complement. Altern. Med. 2012, 2012, 1–8. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Marchese, A.; Izadi, M.; Curti, V.; Daglia, M.; Nabavi, S.F. Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem. 2015, 173, 339–347. [Google Scholar] [CrossRef]
- Tohidi, B.; Rahimmalek, M.; Arzani, A. Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem. 2017, 220, 153–161. [Google Scholar] [CrossRef]
- Khanavi, M.; Hajimehdipoor, H.; Emadi, F.; Khandani, N.K. Essential Oil Compositions of Thymus kotschyanus Boiss. obtained by hydrodistillation and microwave oven distillation. J. Essent. Oil-Bear. Plants 2013, 16, 117–122. [Google Scholar] [CrossRef]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Yoshinari, T.; Rezaee, M.-B.; Jaimand, K.; Nagasawa, H.; Sakuda, S. Inhibitory effects of Satureja hortensis L. essential oil on growth and aflatoxin production by Aspergillus parasiticus. Int. J. Food Microbiol. 2008, 123, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Giweli, A.; Džamić, A.M.; Soković, M.; Ristić, M.S.; Marin, P.D. Antimicrobial and antioxidant activities of essential oils of Satureja thymbra growing wild in Libya. Molecules 2012, 17, 4836–4850. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.; Kunz, B.; Weidenbürner, M. Mycelial deformations of Cladosporium herbarum due to the Application of Eugenol or Carvacrol. J. Essent. Oil Res. 1996, 8, 535–540. [Google Scholar] [CrossRef]
- Adam, K.; Sivropoulou, A.; Kokkini, S.; Lanaras, T.; Arsenakis, M. Antifungal activities of Origanum vulgare subsp. hirtum, Mentha spicata, Lavandula angustifolia, and Salvia fruticosa essential oils against human pathogenic fungi. J. Agric. Food Chem. 1998, 46, 1739–1745. [Google Scholar] [CrossRef]
- Ocak, I.; Çelik, A.; Özel, M.Z.; Korcan, E.; Konuk, M. Antifungal activity and chemical composition of essential oil of Origanum Hypericifolium. Int. J. Food Prop. 2012, 15, 38–48. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Mycol. Med. 2014, 24, e51–e56. [Google Scholar] [CrossRef]
- Šegvić Klarić, M.; Kosalec, I.; Mastelić, J.; Piecková, E.; Pepeljnak, S. Antifungal activity of thyme (Thymus vulgaris L.) essential oil and thymol against moulds from damp dwellings. Lett. Appl. Microbiol. 2006, 44, 36–42. [Google Scholar] [CrossRef]
- Mathur, A.; Singh, R.; Yousuf, S.; Bhardwaj, A.; Satish, K. Antifungal activity of some plant extracts against clinical pathogens. Adv. Appl. Sci. Res. 2011, 2, 260–264. [Google Scholar]
- Campaniello, D.; Corbo, M.R.; Sinigaglia, M. Antifungal activity of eugenol against Penicillium, Aspergillus, and Fusarium species. J. Food Prot. 2010, 73, 1124–1128. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In vitro antifungal activity of terpinen-4-ol, eugenol, carvone, 1,8-cineole (eucalyptol) and thymol against mycotoxigenic plant pathogens. Food Addit. Contam. Part A 2012, 29, 415–422. [Google Scholar]
- Zhang, J.; Ma, S.; Du, S.; Chen, S.; Sun, H. Antifungal activity of thymol and carvacrol against postharvest pathogens Botrytis cinerea. J. Food Sci. Technol. 2019, 56, 2611–2620. [Google Scholar] [CrossRef] [PubMed]
- Chavan, P.S.; Tupe, S.G. Antifungal activity and mechanism of action of carvacrol and thymol against vineyard and wine spoilage yeasts. Food Control. 2014, 46, 115–120. [Google Scholar] [CrossRef]
- Hassan, B.; Soumya, E.; Moulay, S.; Mounyr, B.; Saad, I.K. Antifungal activity and physico-chemical surface properties of the momentaneously exposed Penicillium expansum spores to carvacrol. Res. J. Microbiol. 2016, 11, 178–185. [Google Scholar]
- Kashkooli, A.B.; Saharkhiz, M.J. Essential Oil Compositions and Natural Herbicide Activity of Four Denaei Thyme (Thymus daenensis Celak.) Ecotypes. J. Essent. Oil Bear. Plants 2014, 17, 859–874. [Google Scholar] [CrossRef]
- Synowiec, A.; Kalemba, D.; Drozdek, E.; Bocianowski, J. Phytotoxic potential of essential oils from temperate climate plants against the germination of selected weeds and crops. J. Pest. Sci. 2017, 90, 407–419. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Ozer, H.; Cakmakci, R.; Kesdek, M.; Mete, E. Antifungal, phytotoxic and insecticidal properties of essential oil isolated from Turkish Origanum acutidens and its three components, carvacrol, thymol and p-cymene. Bioresour. Technol. 2008, 99, 8788–8795. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Sutay, S. Inhibitory effects of monoterpenes on seed germination and seedling growth. Z. Naturforsch. C. 2007, 62, 207–214. [Google Scholar] [CrossRef]
- Mutlu, S.; Atici, Ö.; Esim, N.; Mete, E. Essential oils of catmint (Nepeta meyeri Benth.) induce oxidative stress in early seedlings of various weed species. Acta Physiol. Plant. 2011, 33, 943–951. [Google Scholar] [CrossRef]
- Nishida, N.; Tamotsu, S.; Nagata, N.; Saito, C.; Sakai, A. Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 2005, 31, 1187–1203. [Google Scholar] [CrossRef]
- Lee, B.-H.; Choi, W.-S.; Lee, S.-E.; Park, B.-S. Fumigant toxicity of essential oils and their constituent compounds towards the rice weevil, Sitophilus oryzae (L.). Crop. Prot. 2001, 20, 317–320. [Google Scholar] [CrossRef]
- Rattan, R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop. Prot. 2010, 29, 913–920. [Google Scholar] [CrossRef]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and Carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of essential oil components by gas chromatography/quadrupole mass spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 2005, 16, 1902–1903. [Google Scholar] [CrossRef]
- Jiménez, M.; Mateo, J.; Hinojo, M.; Mateo, R. Sugars and amino acids as factors affecting the synthesis of fumonisins in liquid cultures by isolates of the Gibberella fujikuroi complex. Int. J. Food Microbiol. 2003, 89, 185–193. [Google Scholar] [CrossRef]
- Tian, J.; Ban, X.; Zeng, H.; Huang, B.; He, J.; Wang, Y. In vitro and in vivo activity of essential oil from dill (Anethum graveolens L.) against fungal spoilage of cherry tomatoes. Food Control 2011, 22, 1992–1999. [Google Scholar] [CrossRef]
- Ellis, R.H.; Roberts, E.H. The quantification of ageing and survival in orthodox seeds. Seed Sci. Technol. 1981, 9, 373–409. [Google Scholar]
- Kulkarni, M.G.; Street, R.A.; Van Staden, J. Germination and seedling growth requirements for propagation of Dioscorea dregeana (Kunth) Dur. and Schinz—A tuberous medicinal plant. S. Afr. J. Bot. 2007, 73, 131–137. [Google Scholar] [CrossRef]
Sample Availability: Samples of Thymus kotschyanus essential oil are available from the authors. |
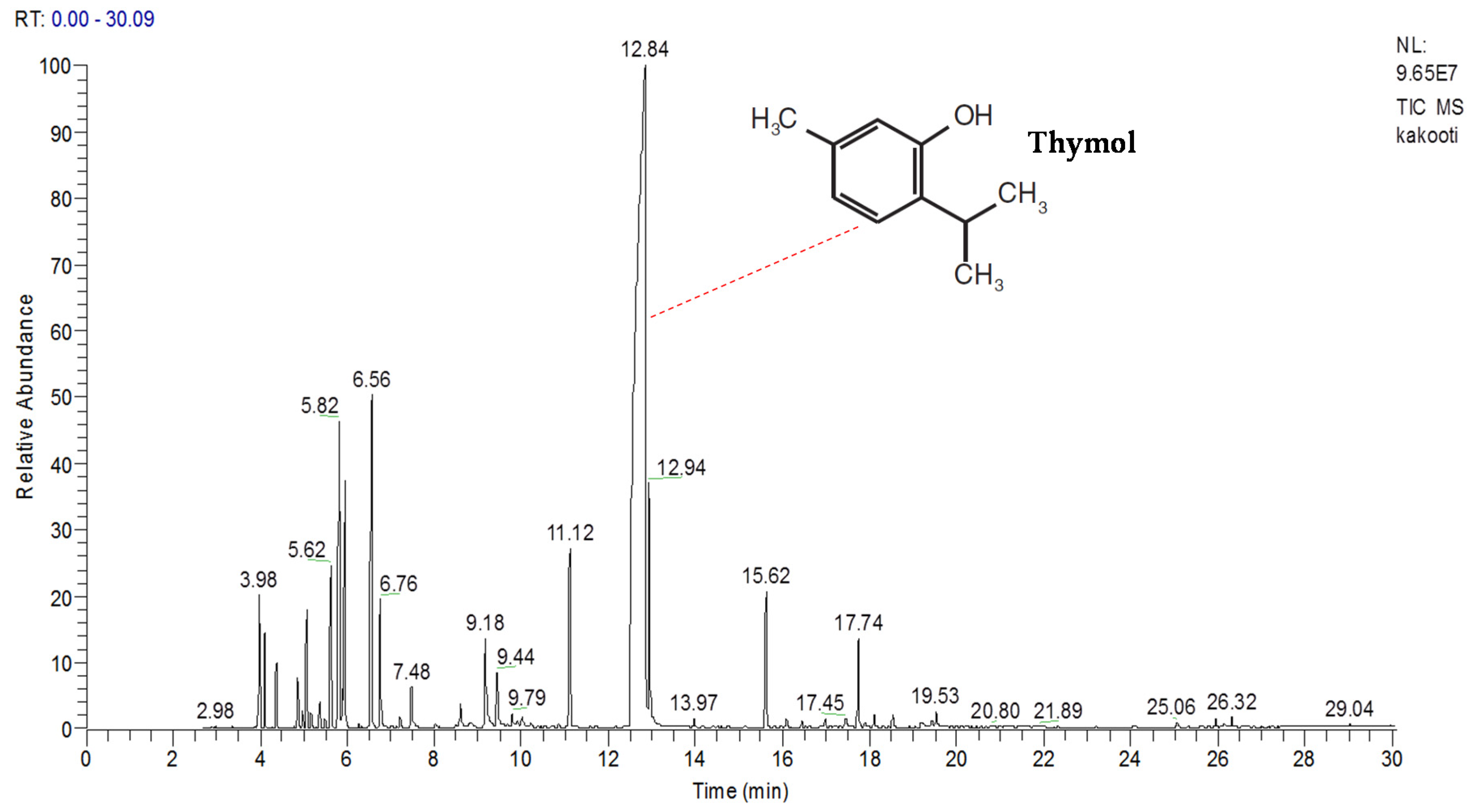
| No | Compounds | Tn | RI | RI-L | Percentage |
|---|---|---|---|---|---|
| 1 | α-Thujene | 3.98 | 926 | 925 | 1.38 ± 0.01 |
| 2 | α-Pinene | 4.11 | 934 | 932 | 0.96 ± 0.00 |
| 3 | Camphene | 4.37 | 949 | 952 | 0.62 ± 0.00 |
| 4 | β-Pinene | 4.86 | 978 | 978 | 0.53 ± 0.00 |
| 5 | 3-Octanone | 4.97 | 984 | 984 | 0.16 ± 0.00 |
| 6 | β-Myrcene | 5.07 | 990 | 991 | 1.36 ± 0.01 |
| 7 | 3-Octanol | 5.16 | 995 | 994 | 0.15 ± 0.00 |
| 8 | α-Phellandrene | 5.37 | 1006 | 1006 | 0.27 ± 0.00 |
| 9 | 3-Carene | 5.49 | 1011 | 1011 | 0.08 ± 0.00 |
| 10 | α-Terpinene | 5.62 | 1017 | 1017 | 2.1 ± 0.01 |
| 11 | p-Cymene | 5.82 | 1026 | 1026 | 5.56 ± 0.02 |
| 12 | Limonene | 5.89 | 1030 | 1030 | 0.52 ± 0.00 |
| 13 | 1,8-Cineol | 5.95 | 1032 | 1033 | 2.82 ± 0.02 |
| 14 | γ-Terpinene | 6.56 | 1060 | 1060 | 6.67 ± 0.03 |
| 15 | cis-Sabinene hydroxide | 6.76 | 1069 | 1070 | 1.66 ± 0.01 |
| 16 | α- Terpinolen | 7.21 | 1090 | 1090 | 0.12 ± 0.00 |
| 17 | trans-Sabinene hydrate | 7.48 | 1102 | 1104 | 0.57 ± 0.00 |
| 18 | Camphor | 8.6 | 1148 | 1148 | 0.36 ± 0.00 |
| 19 | Borneol | 9.18 | 1171 | 1173 | 1.82 ± 0.01 |
| 20 | Terpinen-4-ol | 9.44 | 1182 | 1182 | 0.88 ± 0.00 |
| 21 | α-Terpineol | 9.79 | 1196 | 1195 | 0.17 ± 0.00 |
| 22 | cis-α-terpineol | 10.02 | 1205 | 1209 | 0.13 ± 0.00 |
| 23 | Carvacrol methyl ether | 11.12 | 1247 | 1246 | 2.94 ± 0.01 |
| 24 | Thymol | 12.84 | 1313 | 1311 | 60.48 ± 0.78 |
| 25 | Carvacrol | 12.94 | 1317 | 1316 | 3.02 ± 0.01 |
| 26 | Thymol acetate | 13.97 | 1358 | 1357 | 0.13 ± 0.00 |
| 27 | E-Caryophyllene | 15.62 | 1424 | 1422 | 2.18 ± 0.01 |
| 28 | Aromandendrene | 16.08 | 1443 | 1440 | 0.09 ± 0.00 |
| 29 | α-Humulene | 16.44 | 1458 | 1457 | 0.08 ± 0.00 |
| 30 | γ-Muurolene | 16.99 | 1480 | 1479 | 0.09 ± 0.00 |
| 31 | Virdiflorene | 17.45 | 1499 | 1497 | 0.13 ± 0.00 |
| 32 | β-Bisabolene | 17.74 | 1512 | 1511 | 1.36 ± 0.01 |
| 33 | δ-Cadinene | 18.1 | 1527 | 1526 | 0.16 ± 0.00 |
| 34 | (E)-α-Bisabolene | 18.53 | 1545 | 1545 | 0.17 ± 0.00 |
| 35 | Spathulenol | 19.42 | 1583 | 1582 | 0.08 ± 0.00 |
| 36 | Caryophyllene oxide | 19.53 | 1588 | 1587 | 0.21 ± 0.00 |
| Treatments | GP (%) | MGT (day) | GRI | RL (cm) | ShL (cm) | SLL (cm) | FW (mg) | VI | T50 |
|---|---|---|---|---|---|---|---|---|---|
| Amaranthus retroflexus | |||||||||
| Control | 81.33 ± 2.0 | 3.22 ± 0.31 | 6.50 ± 0.13 | 1.54 ± 0.12 | 1.52 ± 0.00 | 3.05 ± 0.13 | 0.02 ± 0.00 | 248.33 ± 16.81 | 2.57 ± 0.01 |
| 250 ppm | 50.67 ± 4.0 | 4.70 ± 0.28 | 2.86 ± 0.35 | 0.45 ± 0.12 | 0.00 | 0.46 ± 0.13 | 0.01 ± 0.00 | 23.52 ± 8.44 | 4.17 ± 0.29 |
| 500 ppm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 750 ppm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1000 ppm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1500 ppm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LSD | 5.25 | 0.45 | 0.43 | 0.19 | 0.016 | 0.20 | 0.01 | 19.3 | 0.33 |
| Prob | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Panucum miliaceum | |||||||||
| Control | 92 ± 4.00 | 2.52 ± 0.08 | 9.65 ± 0.28 | 5.54 ± 1.09 | 3.23 ± 0.27 | 8.78 ± 1.36 | 0.12 ± 0.03 | 803.67 ± 91.0 | 1.86 ± 0.03 |
| 250 ppm | 88 ± 4.00 | 3.83 ± 0.15 | 6.30 ± 0.11 | 1.06 ± 0.16 | 0.95 ± 0.10 | 2.01 ± 0.12 | 0.02 ± 0.00 | 176.44 ± 2.1 | 3.06 ± 0.05 |
| 500 ppm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 750 ppm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1000 ppm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1500 ppm | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| LSD | 5.75 | 0.17 | 0.34 | 1.23 | 0.30 | 1.48 | 0.02 | 106.7 | 0.06 |
| Prob | *** | *** | *** | *** | *** | *** | *** | *** | *** |
| Insect | LC50 (µL·L−1) | LC95 (µL·L−1) | Slope ± SE | Intercept ± SE | χ2 (df = 3) | p-Value |
|---|---|---|---|---|---|---|
| O. surinamensis | 4.78 | 17.98 | 2.86 ± 0.34 | −1.94 ± 0.26 | 2.04 | 0.57 |
| (4.12–5.51) | (13.63–27.63) | |||||
| S. oryzae | 13.2 | 150.22 | 1.56 ± 0.18 | −1.75 ± 0.22 | 1.83 | 0.61 |
| (10.08–17.29) | (90.73–320.93) |
| Insect | LT50 (days) | LT95 (days) | Slope ± SE | Intercept ± SE | χ2 (df = 3) | p-Value |
|---|---|---|---|---|---|---|
| O. surinamensis | 1.57 | 9.17 | 2.14 ± 0.23 | −0.42 ± 0.12 | 1.73 | 0.63 |
| (1.26–1.91) | (6.64–14.62) | |||||
| S. oryzae | 2.36 | 14.6 | 2.08 ± 0.23 | −0.78 ± 0.13 | 2.47 | 0.48 |
| (1.93–2.89) | (10.12–25.22) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemi, G.; Alirezalu, A.; Ghosta, Y.; Jarrahi, A.; Safavi, S.A.; Abbas-Mohammadi, M.; Barba, F.J.; Munekata, P.E.S.; Domínguez, R.; Lorenzo, J.M. Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil. Molecules 2020, 25, 1152. https://doi.org/10.3390/molecules25051152
Ghasemi G, Alirezalu A, Ghosta Y, Jarrahi A, Safavi SA, Abbas-Mohammadi M, Barba FJ, Munekata PES, Domínguez R, Lorenzo JM. Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil. Molecules. 2020; 25(5):1152. https://doi.org/10.3390/molecules25051152
Chicago/Turabian StyleGhasemi, Ghader, Abolfazl Alirezalu, Youbert Ghosta, Azadeh Jarrahi, Seyed Ali Safavi, Mahdi Abbas-Mohammadi, Francisco J. Barba, Paulo E. S. Munekata, Rubén Domínguez, and José M. Lorenzo. 2020. "Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil" Molecules 25, no. 5: 1152. https://doi.org/10.3390/molecules25051152
APA StyleGhasemi, G., Alirezalu, A., Ghosta, Y., Jarrahi, A., Safavi, S. A., Abbas-Mohammadi, M., Barba, F. J., Munekata, P. E. S., Domínguez, R., & Lorenzo, J. M. (2020). Composition, Antifungal, Phytotoxic, and Insecticidal Activities of Thymus kotschyanus Essential Oil. Molecules, 25(5), 1152. https://doi.org/10.3390/molecules25051152












