Synthesis and Bioactivity Assessment of Novel Spiro Pyrazole-Oxindole Congeners Exhibiting Potent and Selective in vitro Anticancer Effects
Abstract
1. Introduction
2. Results and Discussion
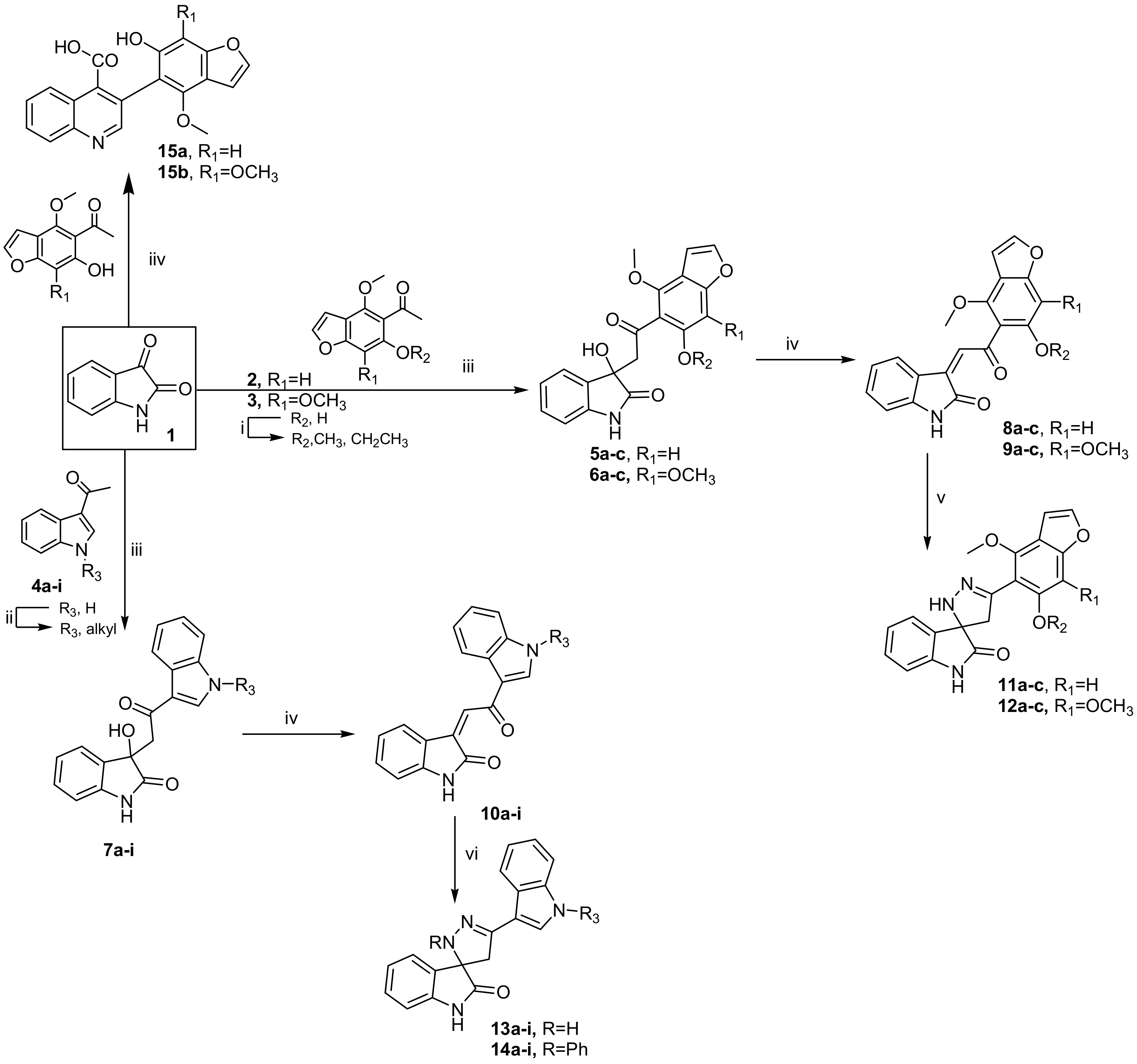
2.1. Chemistry
2.2. Biological Activity
2.2.1. Antimicrobial Activity
2.2.2. Antioxidant Activity
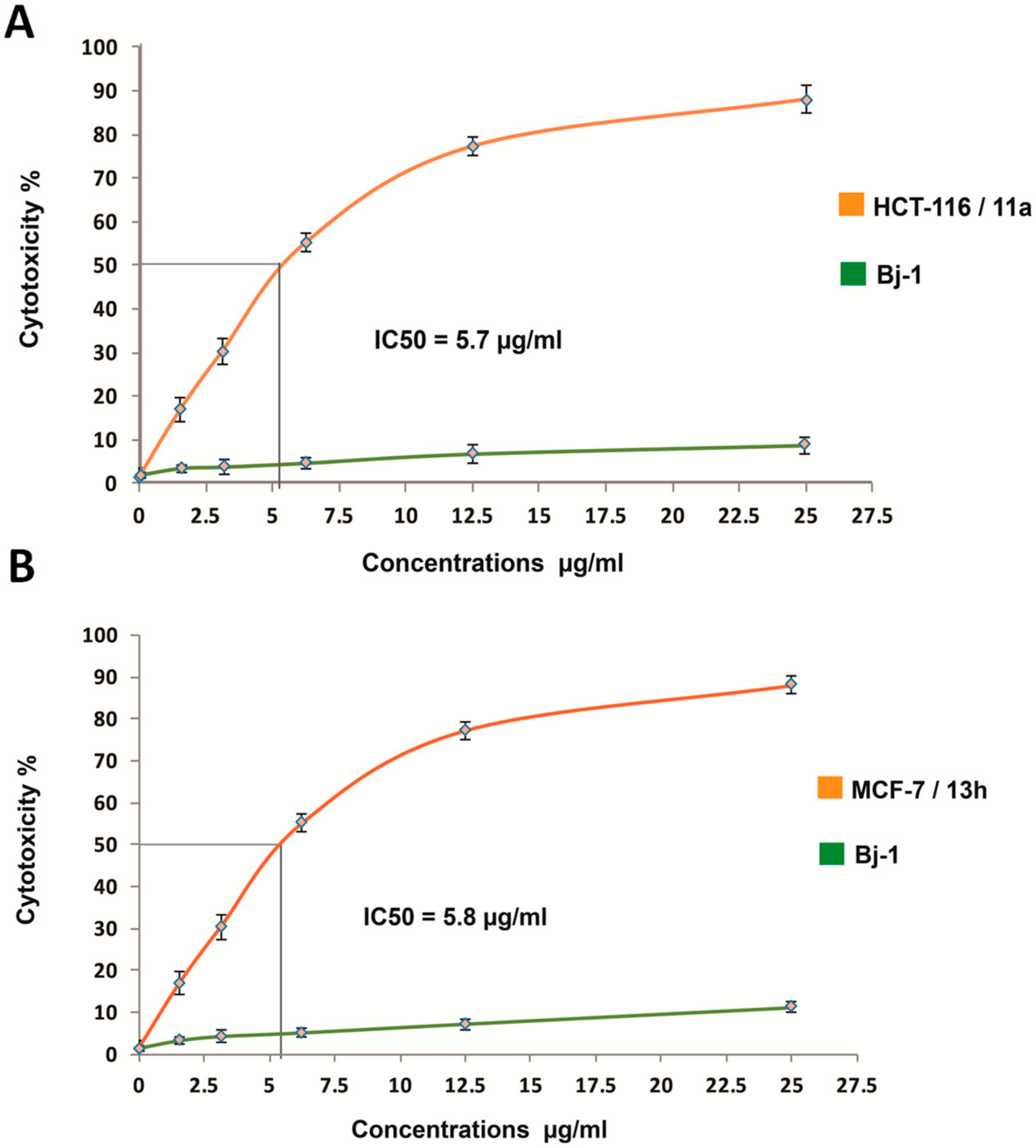
2.2.3. Evaluation of In Vitro Anti-Proliferative Activity
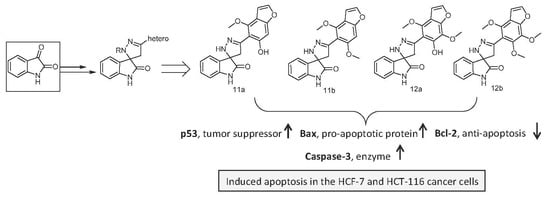
2.2.4. Apoptosis Induction in MCF-7and HCT-116 Cancer Cells
2.2.5. Effect of the Newly Synthesized Compounds on Key Pro- and Antiapoptotic Markers
3. Materials and Methods
3.1. Chemicals and Supplies
3.2. Chemical and Physical Characterization of Synthesized Spiro Pyrazole-Oxindole Congeners
3.3. Synthesis
3.3.1. General Procedure for the Preparation of 3-hydroxy-3-(2-(aryl)-2-oxoethyl)indolin-2-ones 5a–c, 6a–c and 7a–i
3.3.2. General Procedure for the Preparation of 3-(2-(aryl)-2-oxo-ethylidene)indolin-2-ones 8a–c, 9a–c and 10a–i
3.3.3. General Procedure for the Synthesis of 2’,4’-dihydrospiro(indoline-3,3’-pyrazol)-2-one Derivatives 11a–c, 12a–c and 13a–i
3.3.4. General Procedure for the Synthesis of 2’-phenyl-2’,4’-dihydrospiro(indoline-3,3’-pyrazol)-2-ones 14a–i
3.3.5. General Procedure for Synthesis of Quinoline-4-carboxylic acids 15a, b
3.4. Biological Assays
3.4.1. Antimicrobial Evaluation
3.4.2. Antioxidant Evaluation
3.4.3. Anticancer Evaluation
Cell Culture
MTT Cytotoxicity Assay
3.5. Apoptosis Assay
Author Contributions
Funding
Conflicts of Interest
References
- Sagar, S.; Esau, L.; Moosa, B.; Khashab, N.M.; Bajic, V.B.; Kaur, M. Cytotoxicity and apoptosis induced by a plumbagin derivative in estrogen positive MCF-7 breast cancer cells. Anti-Cancer Agents Med. Chem. 2014, 14, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Plackal, B.; George, A.; Abrahamse, H. A Review on Novel Breast Cancer Therapies: Photodynamic Therapy and Plant Derived Agent Induced Cell Death Mechanisms. Anti-Cancer Agents Med. Chem. 2016, 16, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Padma, V.V. An overview of targeted cancer therapy. BioMedicine 2015, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and molecular targeting therapy in cancer. BioMed. Res. Int. 2014, 2014, 150845. [Google Scholar] [CrossRef]
- Ghobrial, I.M.; Witzig, T.E.; Adjei, A.A. Targeting Apoptosis Pathways in Cancer Therapy. CA Cancer J. Clin. 2005, 55, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ma, S.; Liu, B.; Liu, J.; Zhu, R.; Li, M. Chrysin induces cell apoptosis via activation of the p53/Bcl-2/caspase-9 pathway in hepatocellular carcinoma cells. Exp. Ther. Med. 2016, 12, 469–474. [Google Scholar] [CrossRef]
- He, Z.; Ma, W.-Y.; Hashimoto, T.; Bode, A.M.; Yang, C.S.; Dong, Z. Induction of apoptosis by caffeine is mediated by the p53, Bax, and Caspase 3 pathways. Cancer Res. 2003, 63, 4396–4401. [Google Scholar]
- Medvedev, A.; Buneeva, O.; Gnedenko, O.; Ershov, P.; Ivanov, A. Isatin, an endogenous nonpeptide biofactor: A review of its molecular targets, mechanismsof actions, and their biomedical implications. BioFactors 2018, 44, 95–108. [Google Scholar] [CrossRef]
- Singh, G.S.; Desta, Z.Y. Isatins as privileged molecules in design and synthesis of spiro-fused cyclic frameworks. Chem. Rev. 2012, 112, 6104–6155. [Google Scholar] [CrossRef]
- Ziarani, G.M.; Gholamzadeh, P.; Lashgari, N.; Hajiabbasia, P. Oxindole as starting material in organic synthesis. ARKIVOC 2013, 470–535. [Google Scholar] [CrossRef]
- Kozielewicz, P.; Paradowska, K.; Eric, S.; Wawer, I.; Zloh, M. Insights into mechanism of anticancer activity of pentacyclic oxindole alkaloids of Uncaria tomentosa by means of a computational reverse virtual screening and molecular docking approach. Monatsh. Chem. 2014, 145, 1201–1211. [Google Scholar] [CrossRef]
- Whatmore, J.L.; Swann, E.; Barraja, P.; Newsome, J.J.; Bunderson, M.; Beall, H.D.; Tooke, J.E.; Moody, C.J. Comparative study of isoflavone, quinoxaline and oxindole families of anti-angiogenic agents. Angiogenesis 2002, 5, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Giménez, D.G.; Prado, G.E.; Rodríguez, S.T.; Arche, F.A.; La Puerta, R. Cytotoxic effect of the pentacyclic oxindole alkaloid mitraphylline isolated from Uncaria tomentosa bark on human Ewing’s sarcoma and breast cancer cell lines. Planta Med. 2010, 76, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yu, D.-Q.; Liu, H.-M. Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem. 2015, 97, 673–698. [Google Scholar] [CrossRef]
- Gupta, A.K.; Bharadwaj, M.; Kumar, A.; Mehrotra, R. Spiro-oxindoles as a Promising class of small molecule inhibitors of p53–MDM2 interaction useful in targeted cancer therapy. Top. Curr. Chem. 2017, 375, 3. [Google Scholar] [CrossRef]
- Eldehna, W.M.; Almahli, H.; Al-Ansary, G.H.; Ghabbour, H.A.; Aly, M.H.; Ismael, O.E.; Al-Dhfyan, A.; Abdel-Aziz, H.A. Synthesis and in vitro anti-proliferative activity of some novel isatins conjugated with quinazoline/phthalazine hydrazines against triple-negative breast cancer MDA-MB-231 cells as apoptosis inducing agents. J. Enzyme Inhib. Med. Chem. 2017, 32, 600–613. [Google Scholar] [CrossRef]
- Moghaddam, M.N.; Jalal, R.; Zeraatkar, Z. Synthesis and antiproliferative and apoptosis-inducing activity of novel 3-substituted-3-hydroxy-2-oxindole compounds. In Vitro Cell Dev. Biol. Anim. 2018, 54, 61–70. [Google Scholar] [CrossRef]
- Bacher, N.; Tiefenthaler, M.; Sturm, S.; Stuppner, H.; Ausserlechner, M.J.; Kofler, R.; Konwalinka, G. Oxindole alkaloids from Uncaria tomentosa induce apoptosis in proliferating, G0/G1-arrested and bcl-2-expressing acute lymphoblastic leukaemia cells. Br. J. Haematol. 2006, 132, 615–622. [Google Scholar] [CrossRef]
- Schonberg, A.; Sina, A. Khellin and allied compounds. J. Am. Chem. Soc. 1950, 72, 1611–1615. [Google Scholar] [CrossRef]
- Schonberg, A.; Badran, N.; Starkowsky, N.A. Furo-chromones and –coumarins. VII. Degradation of visnagin, khellin and related substances; experiments withchromic acid and hydrogen peroxide; and a synthesis of Eugenitin. J. Am. Chem. Soc. 1953, 75, 4992–4995. [Google Scholar] [CrossRef]
- Spath, E.; Gruber, W. Die constitution des khelline aus Ammi visnaga. I. Metteil uber naturliche chromone. Berichte 1938, 71B, 106. [Google Scholar]
- Mndzhoyan, A.L.; Papayan, G.L.; Zhuruli, L.D.; Karagezyan, G.; Galstyan, L.S.; Sarafyan, V.G. Synthesis and biological study of hydrazinohydrazones of indole aldehydes and ketons series. Arm. Khim. Zh. 1970, 72, 11189f. [Google Scholar]
- Ottoni, O.; Cruz, R.; Alves, R. Efficient and simple methods for the introduction of the sulfonyl, acyl and alkyl protecting groups on the nitrogen of indole and its derivatives. Tetrahedron 1998, 54, 13915–13928. [Google Scholar] [CrossRef]
- Sangshetti, J.N.; Zambare, A.S.; Gonjari, I.; Shinde, D.B. Pfitzinger reaction in the synthesis of bioactive compounds—A review. Mini-Revi. Org. Chem. 2014, 11, 225–250. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Alenzi, F.Q.; Alenazi, B.Q.; Al-Anazy, F.H.; Mubaraki, A.M.; Salem, M.L.; Al-Jabri, A.A.; Lotfy, M.; Bamaga, M.S.; Alrabia, M.W.; Wyse, R.K. The role of caspase activation and mitochondrial depolarisation in cultured human apoptotic eosinophils. Saudi. J. Biol. Sci. 2010, 17, 29–36. [Google Scholar] [CrossRef][Green Version]
- Lakhani, S.A.; Masud, A.; Kuida, K.; Porter, G.A.; Booth, C.J.; Mehal, W.Z.; Inayat, I.; Flavell, R.A. Caspases 3 and 7: Key mediators of mitochondrial events of apoptosis. Science 2006, 311, 847–851. [Google Scholar] [CrossRef]
- Wolf, B.B.; Schuler, M.; Echeverri, F.; Green, D.R. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 1999, 274, 30651–30656. [Google Scholar] [CrossRef]
- Oren, M. Regulation of the p53 tumor suppressor protein. J. Biol. Chem. 1999, 274, 36031–36034. [Google Scholar] [CrossRef]
- Degenhardt, K.; Chen, G.; Lindsten, T.; White, E. BAX and BAK mediate p53 independent suppression of tumorigenesis. Cancer Cell 2002, 2, 193–203. [Google Scholar] [CrossRef]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Truck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Lebensm. Wiss. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assays for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Aboul-Soud, M.A.M.; Al-Amri, M.Z.; Kumar, A.; Al-Sheikh, Y.A.; Ashour, A.E.; El-Kersh, T.A. Specific Cytotoxic Effects of Parasporal Crystal Proteins Isolated from Native Saudi Arabian Bacillus thuringiensis Strains against Cervical Cancer Cells. Molecules 2019, 24, 506. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Hirao, A.; Kong, Y.Y.; Matsuoka, S.; Wakeham, A.; Ruland, J.; Yoshida, H.; Liu, D.; Elledge, S.J.; Mak, T.W. DNA damage-induced activation of p53 by checkpoint kinase Chk2. Science 2000, 287, 1824–1827. [Google Scholar] [CrossRef]
- Chen, C.J.; Makino, S. Murine coronavirus replication induces cell cycle arrest in G0/G1 phase. J. Virol. 2004, 78, 5658–5669. [Google Scholar] [CrossRef]
Sample Availability: Samples of the synthesized spiro pyrazole-oxindole compounds are available from the authors upon request and MTA formulation. |
| Compd. No b | Inhibition Zone (mm) | ||||||
|---|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | Yeast | Fungi | ||||
| S. aureus (ATCC 6538) | B. subtilis (ATCC 6633) | P. aeruginosa (ATCC 27853) | E. coli (DSMZ 1058) | C. albicans (ATCC 10231) | S. cerevisiae (ATCC 9080) | A. niger (NRRL A-326) | |
| 11a | - | 12 | 8 | - | 20 | 16 | - |
| 11b | - | 12 | 12 | - | 18 | 18 | - |
| 11c | - | 10 | 10 | - | 20 | 25 | - |
| 12a | 8 | 16 | 18 | - | 12 | 10 | - |
| 12b | 10 | 18 | 24 | - | 10 | 10 | 8 |
| 12c | 10 | 18 | 20 | - | 10 | 10 | - |
| 13a | - | - | - | - | 18 | 20 | - |
| 15a | 8 | 16 | 24 | - | 12 | - | 10 |
| 15b | 8 | 10 | - | - | 8 | - | - |
| Amoxicillin | 25.6 | 28.4 | - | - | - | - | - |
| Ciprofloxacin | - | - | 30.2 | 25.8 | - | - | - |
| Amphotericin B | - | - | - | - | 24.8 | 23.5 | 26.7 |
| Compd. No | Scavenging Activity (%) a at Different Time (min) | |||
|---|---|---|---|---|
| 15 | 30 | 45 | 60 | |
| 11a | 6.86 ± 1.17 | 10.26 ± 1.37 | 17.73 ± 1.28 | 26.87 ± 1.56 |
| 11b | 9.60 ± 1.77 | 14.28 ± 1.52 | 20.44 ± 1.65 | 30.76 ± 2.04 |
| 11c | 40.65 ± 1.28 | 40.65 ± 1.81 | 40.65 ± 1.67 | 40.65 ± 1.35 |
| 12a | 56.43 ± 1.08 | 67.09 ± 1.45 | 76.92 ± 1.51 | 81.41 ± 1.37 |
| 12b | 17.29 ± 1.53 | 23.46 ± 1.64 | 30.56 ± 1.49 | 40.65 ± 1.46 |
| 12c | 9.76 ± 1.76 | 10.24 ± 1.26 | 11.39 ± 1.53 | 13.97 ± 1.55 |
| 13a | 41.14 ±1.25 | 45.77 ± 1.36 | 56.55 ± 1.99 | 64.08 ± 2.01 |
| 13b | 43.52± 1.98 | 47.05 ± 1.81 | 56.33 ± 1.29 | 65.55 ± 1.55 |
| 13c | 13.97± 1.36 | 20.97 ± 1.26 | 23.96 ± 1.51 | 35.13 ± 1.61 |
| 13d | 19.32± 1.24 | 31.32 ± 1.81 | 25.92 ± 1.24 | 39.70 ± 1.99 |
| 13e | 9.10 ± 1.38 | 26.15 ± 1.26 | 36.70 ± 1.62 | 48.13 ± 1.82 |
| 13f | 17.90 ± 1.62 | 28.28 ± 1.61 | 34.28 ± 1.27 | 40.74 ± 1.54 |
| 13g | 25.95 ± 1.85 | 44.00 ± 1.46 | 49.25 ± 1.77 | 53.67 ± 1.81 |
| 13h | 15.22 ± 1.98 | 16.31 ± 1.96 | 20.58 ± 1.48 | 35.71 ± 1.91 |
| 14i | 45.88 ± 1.05 | 54.32 ± 1.08 | 60.60 ± 1.64 | 69.18 ± 1.65 |
| 14a | 41.09 ± 2.05 | 51.52 ± 1.91 | 53.98 ± 2.06 | 59.22 ± 2.14 |
| 14b | 42.36 ± 1.45 | 58.08 ± 1.28 | 60.27 ± 1.66 | 65.96 ± 1.64 |
| 14c | 14.85 ± 1.36 | 22.56 ± 1.79 | 26.29 ± 1.23 | 39.48 ± 1.61 |
| 14d | 39.91 ± 1.75 | 41.82 ± 1.49 | 44.96 ± 1.37 | 55.44 ± 1.33 |
| 14e | 17.89 ± 1.23 | 28.18 ± 1.27 | 30.87 ± 1.98 | 34.89 ± 1.72 |
| 14f | 9.05 ± 1.63 | 10.15 ± 1.83 | 16.19 ± 1.21 | 22.93 ± 1.62 |
| 14g | 8.39 ± 1.14 | 11.87 ± 1.23 | 14.08 ± 2.01 | 26.55 ± 2.13 |
| 14h | 57.93 ± 1.36 | 67.35 ± 1.35 | 72.00 ± 1.82 | 85.99 ± 2.17 |
| 14i | 39.91 ± 1.87 | 41.82 ± 1.09 | 44.96 ± 1.66 | 55.44 ± 1.55 |
| 15a | 9.36 ± 1.25 | 14.15 ± 1.61 | 20.39 ± 1.92 | 24.77 ± 2.05 |
| 15b | 23.60 ± 1.36 | 32.73 ± 1.13 | 39.23 ± 1.29 | 47.48 |
| Negative control | 0 | 0 | 0 | 0 |
| Ascorbic acid | 94.37 ± 1.74 | 97.45 ± 1.32 | 98.78 ± 0.94 | 99.67 ± 0.28 |
| Compd. No. | Growth Inhibition (%) | Growth Inhibition (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HCT-116 | HepG-2 | MCF-7 | BJ-1 | Compd. No. | HCT-116 | HepG-2 | MCF-7 | BJ-1 | |
| 11a | 96.2 | - | 94.3 | 6.2 | 13g | 92.4 | 81.9 | 91.6 | 13.0 |
| 11b | 94.9 | - | 92.3 | 10.1 | 13h | 91.3 | 79.5 | 93.4 | 14.1 |
| 11c | 30.2 | - | 0 | 45.2 | 13i | 97.7 | 96.8 | 97.8 | 69.4 |
| 12a | 96.7 | - | 93.6 | 9.1 | 14a | 91.2 | - | 90.4 | 14.2 |
| 12b | 95.1 | - | 97.4 | 5.0 | 14b | 84.6 | - | 80.3 | 8.1 |
| 12c | 12.4 | - | 6.3 | 62.3 | 14c | 91.8 | - | 92.3 | 13.6 |
| 13a | 96.9 | 89.3 | 96.4 | 11.6 | 14d | 96.2 | - | 94 | 9.5 |
| 13b | 94.5 | 81.4 | 95.4 | 10.4 | 14e | 35.1 | - | 85.3 | 16.6 |
| 13c | 97.9 | 90.9 | 97.8 | 5.1 | 14f | 91.4 | - | 89.3 | 14.5 |
| 13d | 94.6 | 82.7 | 96.4 | 7.0 | 14g | 92.1 | - | 90.6 | 16.0 |
| 13e | 90.4 | 79.8 | 96.4 | 10.3 | 14h | 96.2 | - | 94 | 86.5 |
| 13f | 86.5 | 72.8 | 79.7 | 16.4 | 14i | 35.1 | - | 52.3 | 78.6 |
| Doxorubicin | 100 | 100 | 96.8 | 0 | 100 | 100 | 96.8 | 0 | |
| Compd. No. | IC50ug/ml | ||
|---|---|---|---|
| HCT-116 | HepG-2 | MCF-7 | |
| 11a | 5.7 | - | 21.1 |
| 11b | 16.4 | - | 19.4 |
| 12a | 5.8 | - | 20.2 |
| 12b | 7.9 | - | 16.7 |
| 13a | 31.3 | 60.6 | 32.4 |
| 13b | 21.3 | 48.0 | 24.0 |
| 13c | 14.7 | 27.0 | 24.1 |
| 13d | 26.9 | 60.9 | 25.4 |
| 13e | 35.4 | 67.1 | 31.9 |
| 13f | 28.4 | 83.6 | 32.1 |
| 13g | 35.3 | 72.2 | 43.0 |
| 13h | 20.5 | 19.2 | 5.8 |
| 14a | 36.2 | - | 32.3 |
| 14b | 40.1 | - | 36.3 |
| 14c | 40.5 | - | 32.5 |
| 14d | 30.7 | - | 31.6 |
| 14e | - | - | 54.0 |
| 14f | 35.8 | - | 31.2 |
| 14g | 38.0 | - | 37.4 |
| Doxorubicin | 26.1 | 21.6 | 37.6 |
| Compounds | Caspase-3 Activity % | Bcl-2 (ng/50 mg Protein) | p53 (Pg/50 mg Protein) | Bax (pg/50 mg Protein) |
|---|---|---|---|---|
| Control (MCF-7) | 10.2 ± 2.84 | 25.13 ± 3.45 | 4.18 ± 0.58 | 39.56 ± 4.56 |
| 11a | 16.67 ± 0.67 | 12.3 ± 2.26 | 14.45 ± 0.82 | 122.34 ± 3.45 |
| 11b | 16.19 ± 0.79 | 16.18 ± 0.78 | 5.85 ± 0.75 | 109.35 ± 4.05 |
| 12a | 14.18 ± 0.74 | 13.09 ± 2.27 | 13.31 ± 1.08 | 113.09 ± 1.98 |
| 12b | 47.25 ± 1.89 | 12.87 ± 1.84 | 29.58 ± 2.53 | 167.07 ± 4.83 |
| 13c | 39.07 ± 4.97 | 44.5 ± 4.56 | 7.34 ± 1.5 | 88.34 ± 3.79 |
| Compounds | Caspase-3 Activity % | Bcl-2 (ng/50 mg Protein) | p53 (pg/50 mg Protein) | Bax (pg/50 mg Protein) |
|---|---|---|---|---|
| Control (HCT-116) | 13.60 ± 2.45 | 23.56 ± 3.56 | 1.68 ± 0.06 | 118.54 ± 0.83 |
| 11a | 19.12 ± 0.89 | 8.2 ± 1.26 | 16.2 ± 0.56 | 159.26 ± 0.96 |
| 11b | 14.04 ± 0.54 | 7.25 ± 1.45 | 3.25 ± 0.15 | 119.14 ± 1.65 |
| 12a | 17.43 ± 0.58 | 21.13 ± 1.45 | 23.11 ± 1.22 | 124.56 ± 0.95 |
| 12b | 33.12 ± 1.37 | 18.87 ± 1.84 | 48.07 ± 1.94 | 191.07 ± 1.95 |
| 13c | 47.32 ± 6.32 | 57.45 ± 6.45 | 2.34 ± 0.95 | 123.45 ±7.45 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abo-Salem, H.M.; Nassrallah, A.; Soliman, A.A.F.; Ebied, M.S.; Elawady, M.E.; Abdelhamid, S.A.; El-Sawy, E.R.; Al-Sheikh, Y.A.; Aboul-Soud, M.A.M. Synthesis and Bioactivity Assessment of Novel Spiro Pyrazole-Oxindole Congeners Exhibiting Potent and Selective in vitro Anticancer Effects. Molecules 2020, 25, 1124. https://doi.org/10.3390/molecules25051124
Abo-Salem HM, Nassrallah A, Soliman AAF, Ebied MS, Elawady ME, Abdelhamid SA, El-Sawy ER, Al-Sheikh YA, Aboul-Soud MAM. Synthesis and Bioactivity Assessment of Novel Spiro Pyrazole-Oxindole Congeners Exhibiting Potent and Selective in vitro Anticancer Effects. Molecules. 2020; 25(5):1124. https://doi.org/10.3390/molecules25051124
Chicago/Turabian StyleAbo-Salem, Heba M., Amr Nassrallah, Ahmed A.F. Soliman, Manal S. Ebied, Mohamed E. Elawady, Sayeda A. Abdelhamid, Eslam R. El-Sawy, Yazeed A. Al-Sheikh, and Mourad A. M. Aboul-Soud. 2020. "Synthesis and Bioactivity Assessment of Novel Spiro Pyrazole-Oxindole Congeners Exhibiting Potent and Selective in vitro Anticancer Effects" Molecules 25, no. 5: 1124. https://doi.org/10.3390/molecules25051124
APA StyleAbo-Salem, H. M., Nassrallah, A., Soliman, A. A. F., Ebied, M. S., Elawady, M. E., Abdelhamid, S. A., El-Sawy, E. R., Al-Sheikh, Y. A., & Aboul-Soud, M. A. M. (2020). Synthesis and Bioactivity Assessment of Novel Spiro Pyrazole-Oxindole Congeners Exhibiting Potent and Selective in vitro Anticancer Effects. Molecules, 25(5), 1124. https://doi.org/10.3390/molecules25051124