Performance Recovery after Contamination with Nitrogen Dioxide in a PEM Fuel Cell
Abstract
:1. Introduction
2. Results and Discussion
2.1. Performance of the Contaminated MEA
2.2. Recovery at Different Air Flow Rates
2.3. Recovery at Different Current Densities
2.4. Other Types of Recovery
2.5. Comparison of Two Operation Techniques
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Das, H.S.; Tan, C.W.; Yatim, A.H.M. Fuel Cell Hybrid Electric Vehicles: A Review on Power Conditioning Units and Topologies. Renew. Sustain. Energy Rev. 2017, 76, 268–291. [Google Scholar] [CrossRef]
- Das, V.; Padmanaban, S.; Venkitusamy, K.; Selvamuthukumaran, R.; Blaabjerg, F.; Siano, P. Recent Advances and Challenges of Fuel Cell Based Power System Architectures and Control – A Review. Renew. Sustain. Energy Rev. 2017, 73, 10–18. [Google Scholar] [CrossRef]
- Daud, W.R.W.; Rosli, R.E.; Majlan, E.H.; Hamid, S.A.A.; Mohamed, R.; Husaini, T. PEM Fuel Cell System Control: A review. Renew. Energy 2017, 113, 620–638. [Google Scholar] [CrossRef]
- Acevedo Gomez, Y.; Lagergren, C.; Lindbergh, G. Effect of Nitrogen Dioxide Impurities on PEM Fuel Cell Performance. Submitt. Int. J. Hydrog. Energy 2019. [Google Scholar]
- Uribe, F.; Smith, W.; Wilson, M.; Valerio, J.; Rockward, T. Electrodes for Polymer Electrolyte Membrane Operation on Hydrogen/Air and Reformate/Air. Available online: https://pdfs.semanticscholar.org/0de2/236e59c04ef161c5928e751893784784be87.pdf (accessed on 18 September 2018).
- Misz, U.; Talke, A.; Heinzel, A.; Konrad, G. Sensitivity Analyses on the Impact of Air Contaminants on Automotive Fuel Cells. Fuel Cells 2016, 16, 444–462. [Google Scholar] [CrossRef]
- Yang, D.; Ma, J.; Xu, L.; Wu, M.; Wang, H. The Effect of Nitrogen Oxides in Air on the Performance of Proton Exchange Membrane Fuel Cell. Electrochim. Acta 2006, 51, 4039–4044. [Google Scholar] [CrossRef]
- Jing, F.; Hou, M.; Shi, W.; Fu, J.; Yu, H.; Ming, P.; Yi, B. The Effect of Ambient Contamination on PEMFC Performance. J. Power Sources 2007, 166, 172–176. [Google Scholar] [CrossRef]
- Mohtadi, R.; Lee, W.K.; Van Zee, J.W. Assessing Durability of Cathodes Exposed to Common Air Impurities. J. Power Sources 2004, 138, 216–225. [Google Scholar] [CrossRef]
- Chen, M.; Du, C.; Zhang, J.; Wang, P.; Zhu, T. Effect, Mechanism and Recovery of Nitrogen Oxides Poisoning on Oxygen Reduction Reaction at Pt/C Catalysts. J. Power Sources 2011, 196, 620–626. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hung, W.-T.; Wu, C.-T.; Ho, K.-C. Electrochemical Reduction of NO2 at a Pt/Membrane Electrode—Application to Amperometric NO2 Sensing. Sens. Actuators B: Chem. 2009, 136, 32–38. [Google Scholar] [CrossRef]
- St-Pierre, J.; Jia, N.; Rahmani, R. PEMFC Contamination Model: Competitive Adsorption Demonstrated with NO2. J. Electrochem. Soc. 2008, 155, B315–B320. [Google Scholar] [CrossRef]
- Villamena, F.A. Chapter 2 - Chemistry of Reactive Species. In Reactive Species Detection in Biology; Villamena, F.A., Ed.; Elsevier: Boston, MA, USA, 2017; pp. 13–64. [Google Scholar]
- Lopes, T.; Chlistunoff, J.; Sansiñena, J.-M.; Garzon, F.H. Oxygen Reduction Reaction on a Pt/Carbon Fuel Cell Catalyst in the Presence of Trace Quantities of Ammonium Ions: An RRDE Study. Int. J. Hydrog. Energy 2012, 37, 5202–5207. [Google Scholar] [CrossRef]
- Ge, J.; St-Pierre, J.; Zhai, Y. PEMFC Cathode Catalyst Contamination Evaluation with a RRDE- Propene and Naphthalene. Electrochim. Acta 2014, 138, 437–446. [Google Scholar] [CrossRef]
- Garsany, Y.; Baturina, O.A.; Swider-Lyons, K.E. Impact of Sulfur Dioxide on the Oxygen Reduction Reaction at Pt/Vulcan Carbon Electrocatalysts. J. Electrochem. Soc. 2007, 154, B670–B675. [Google Scholar] [CrossRef]
Sample Availability: Samples of the tested MEAS are available from the authors. |
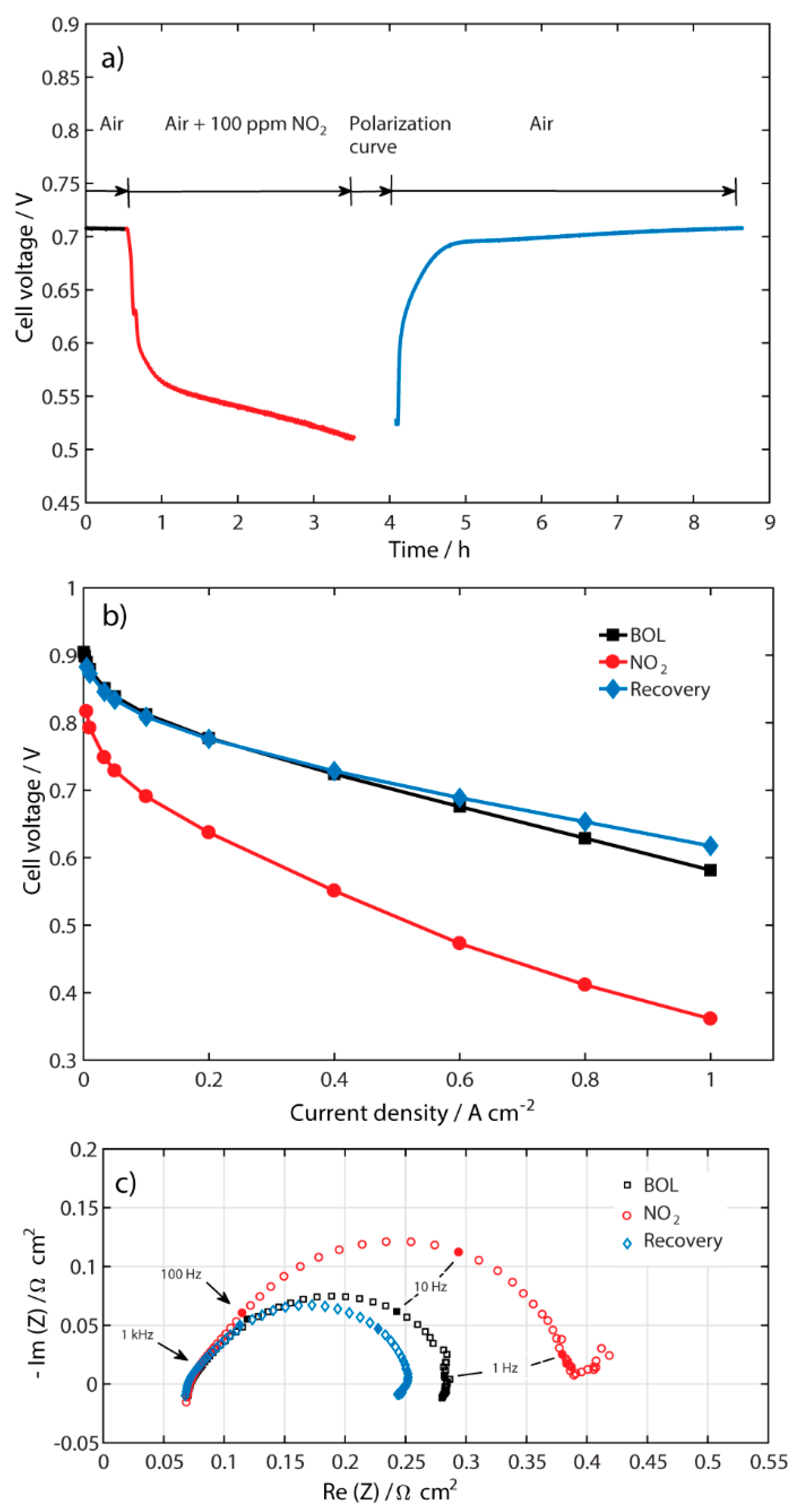
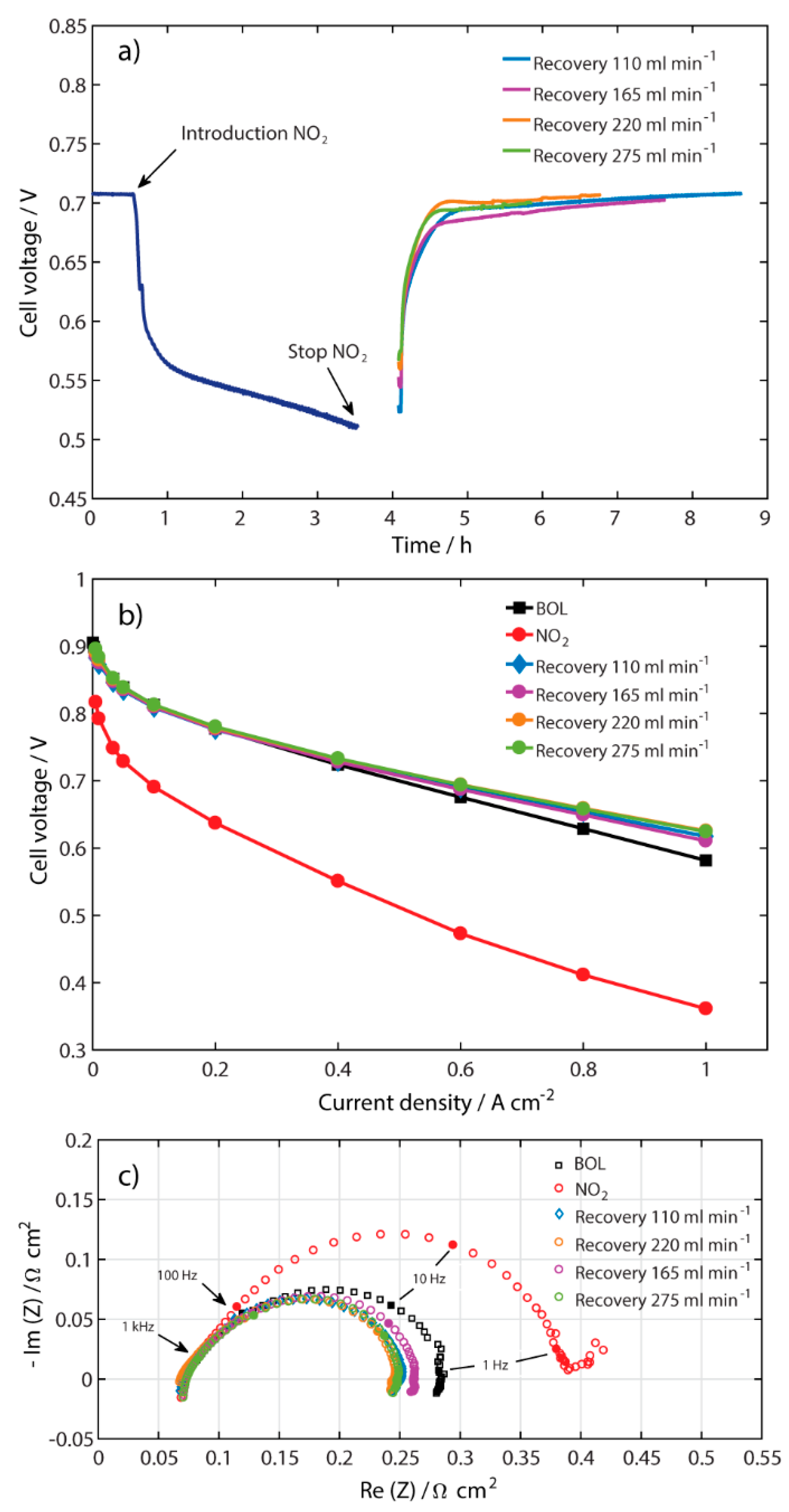

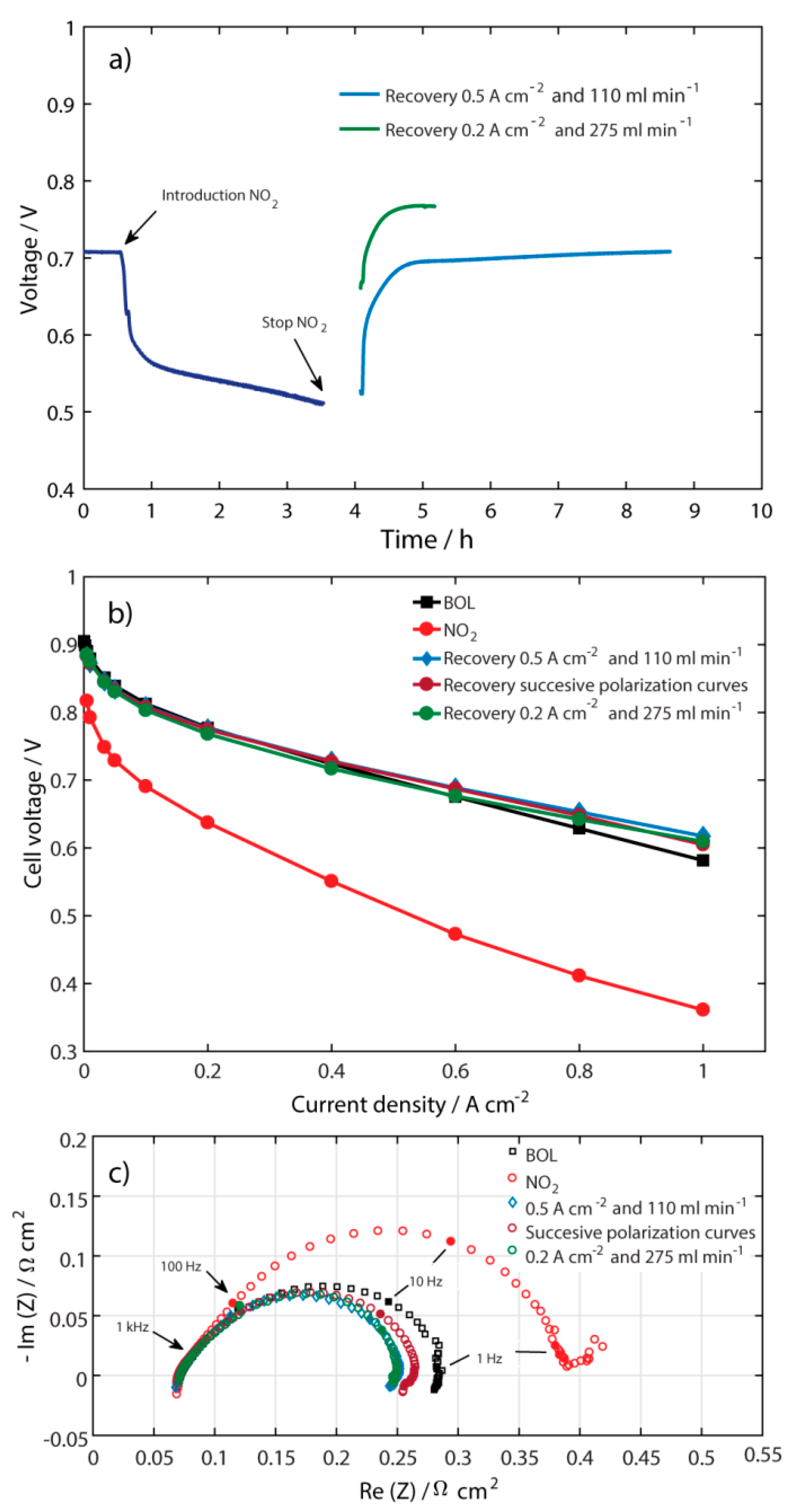
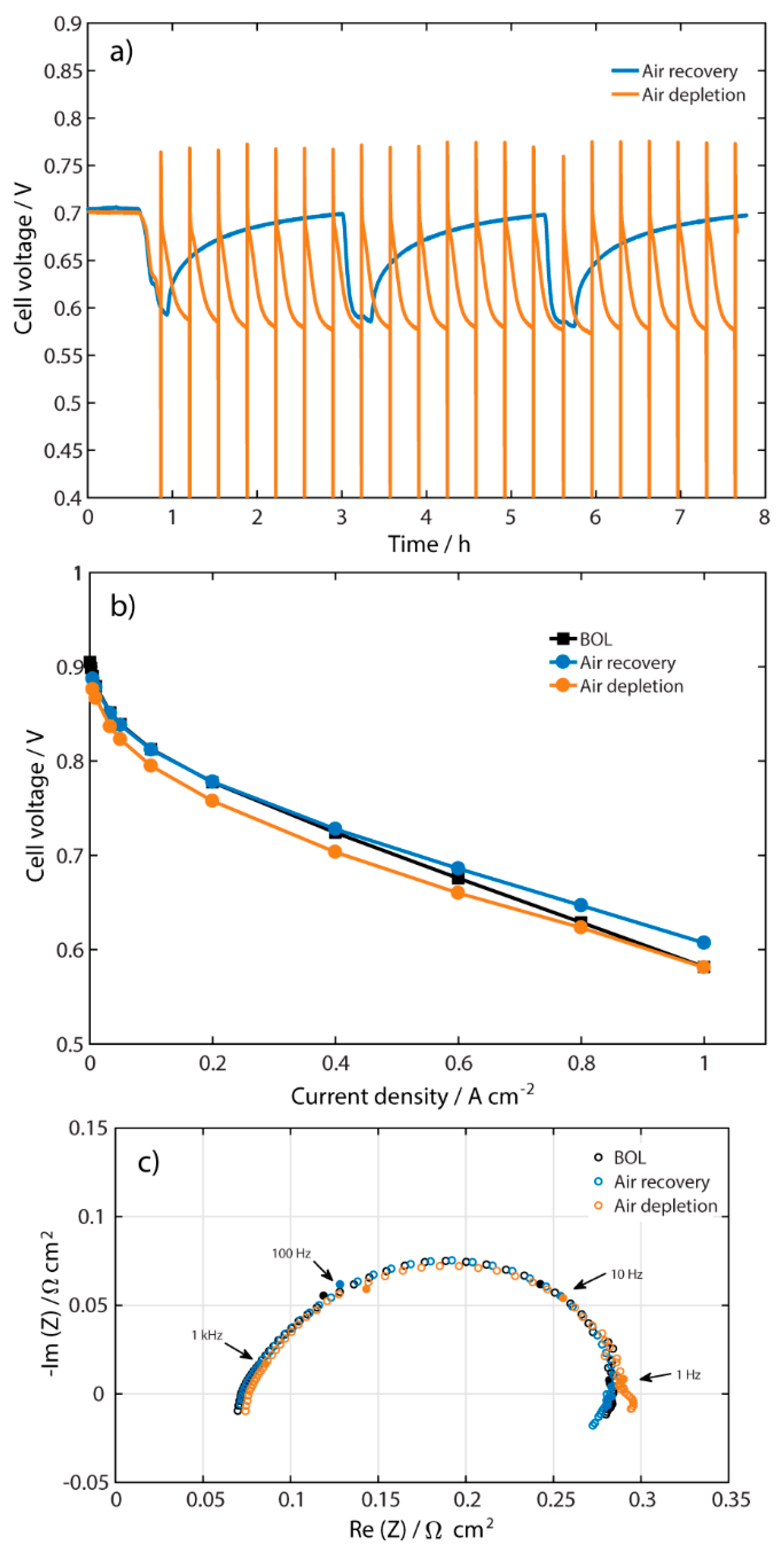
| Recovery at Different Air Flows Rates at 0.5 A cm−2 | Recovery at Different Current Densities at 110 mL min−1 | ||
|---|---|---|---|
| mL min−1 | min | A cm−2 | min |
| 110 | 274 | 0.2 | 76 |
| 165 | 213 | 0.5 | 274 |
| 220 | 161 | 0.75 | 217 |
| 275 | 106 | 1 | 181 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo Gomez, Y.; Lindbergh, G.; Lagergren, C. Performance Recovery after Contamination with Nitrogen Dioxide in a PEM Fuel Cell. Molecules 2020, 25, 1115. https://doi.org/10.3390/molecules25051115
Acevedo Gomez Y, Lindbergh G, Lagergren C. Performance Recovery after Contamination with Nitrogen Dioxide in a PEM Fuel Cell. Molecules. 2020; 25(5):1115. https://doi.org/10.3390/molecules25051115
Chicago/Turabian StyleAcevedo Gomez, Yasna, Göran Lindbergh, and Carina Lagergren. 2020. "Performance Recovery after Contamination with Nitrogen Dioxide in a PEM Fuel Cell" Molecules 25, no. 5: 1115. https://doi.org/10.3390/molecules25051115
APA StyleAcevedo Gomez, Y., Lindbergh, G., & Lagergren, C. (2020). Performance Recovery after Contamination with Nitrogen Dioxide in a PEM Fuel Cell. Molecules, 25(5), 1115. https://doi.org/10.3390/molecules25051115






