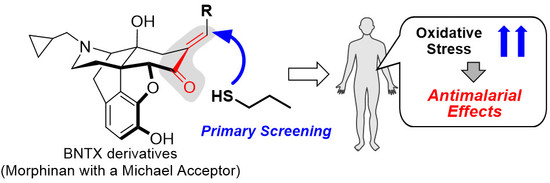
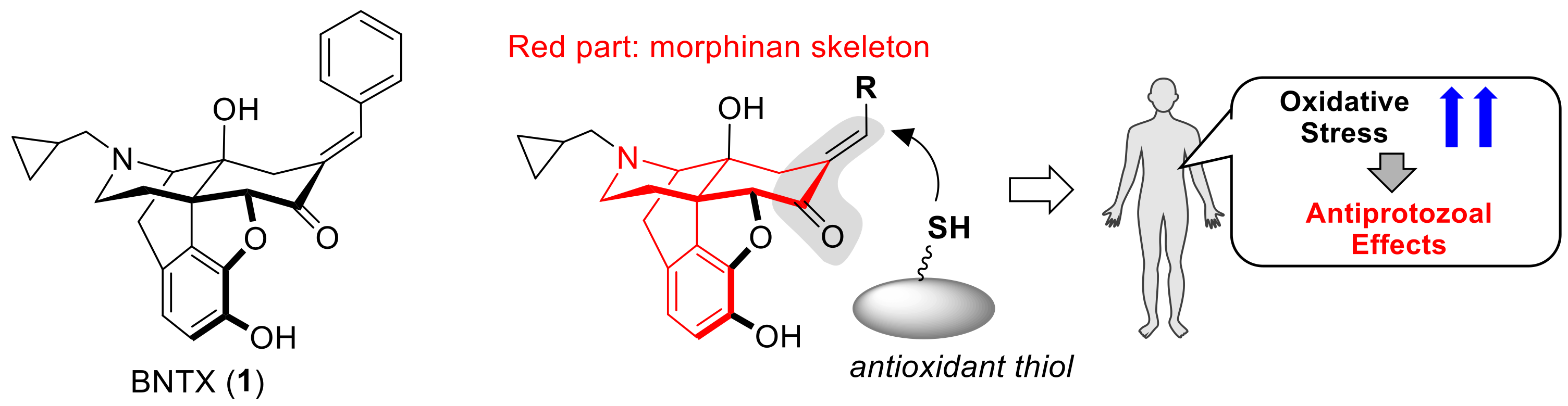
Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities
Abstract
1. Introduction
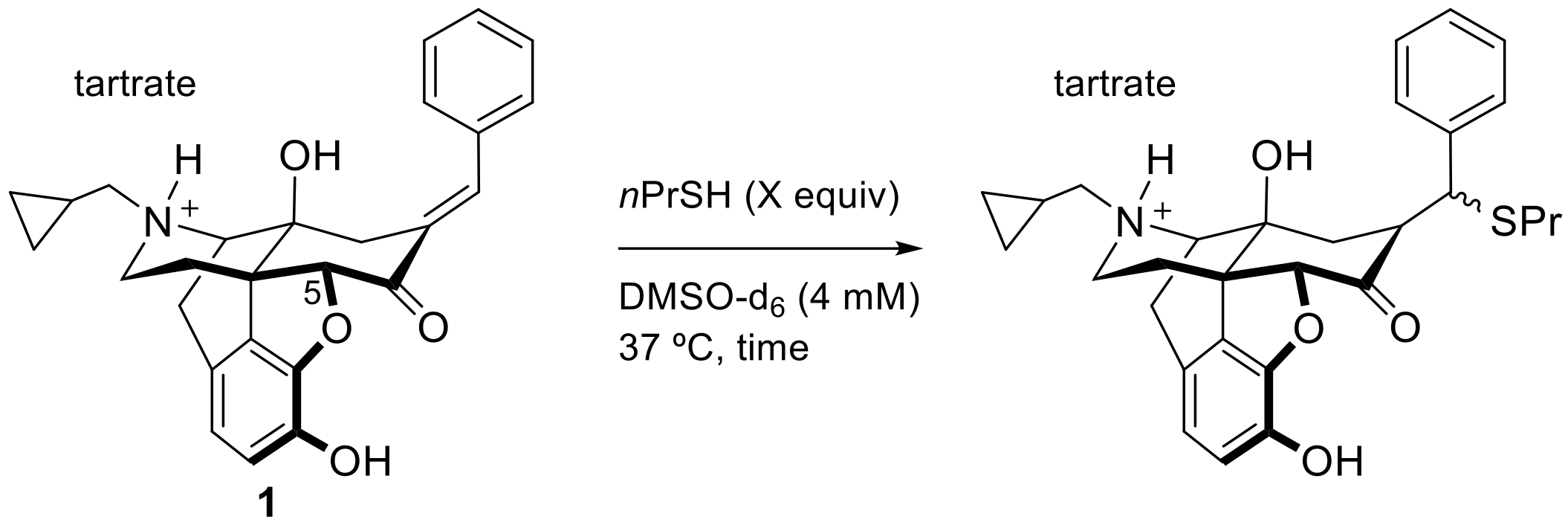
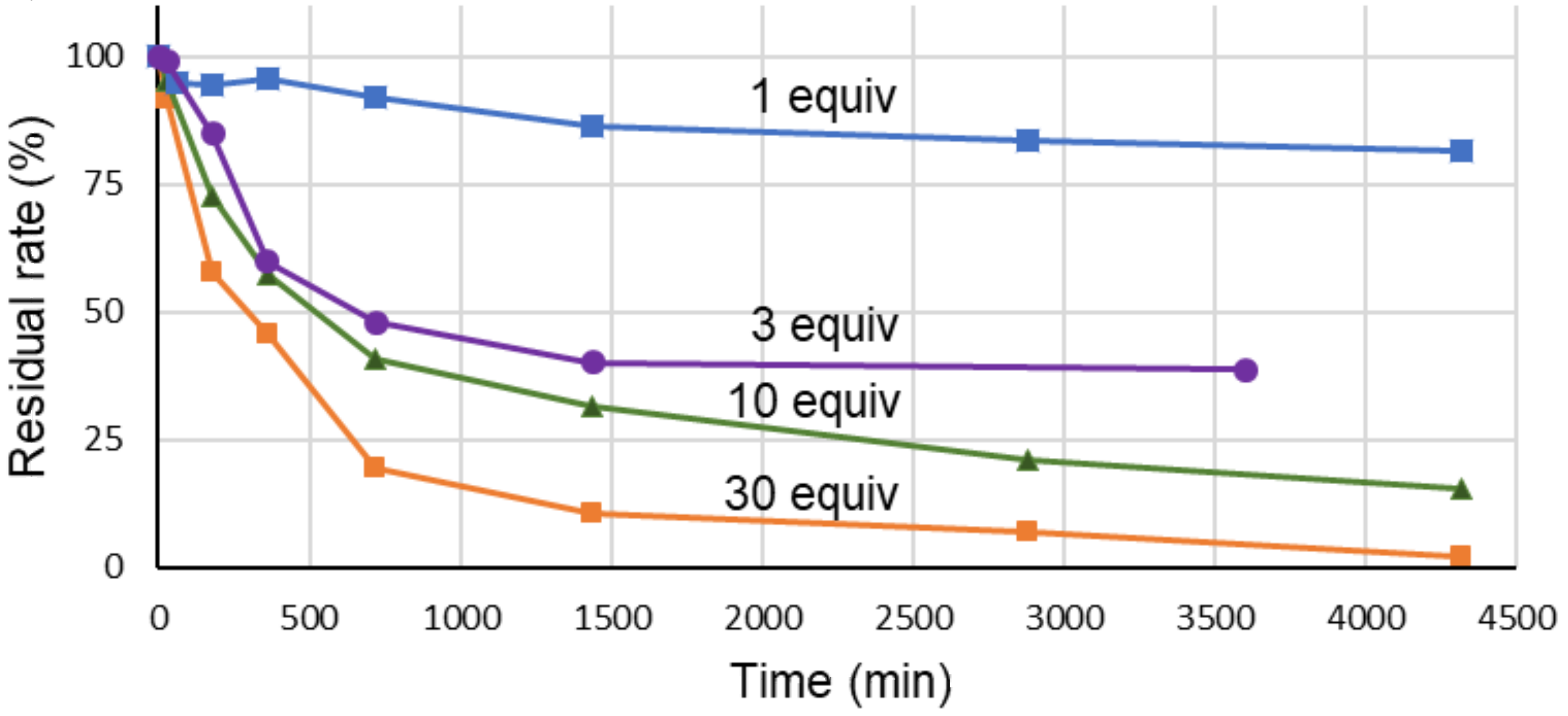
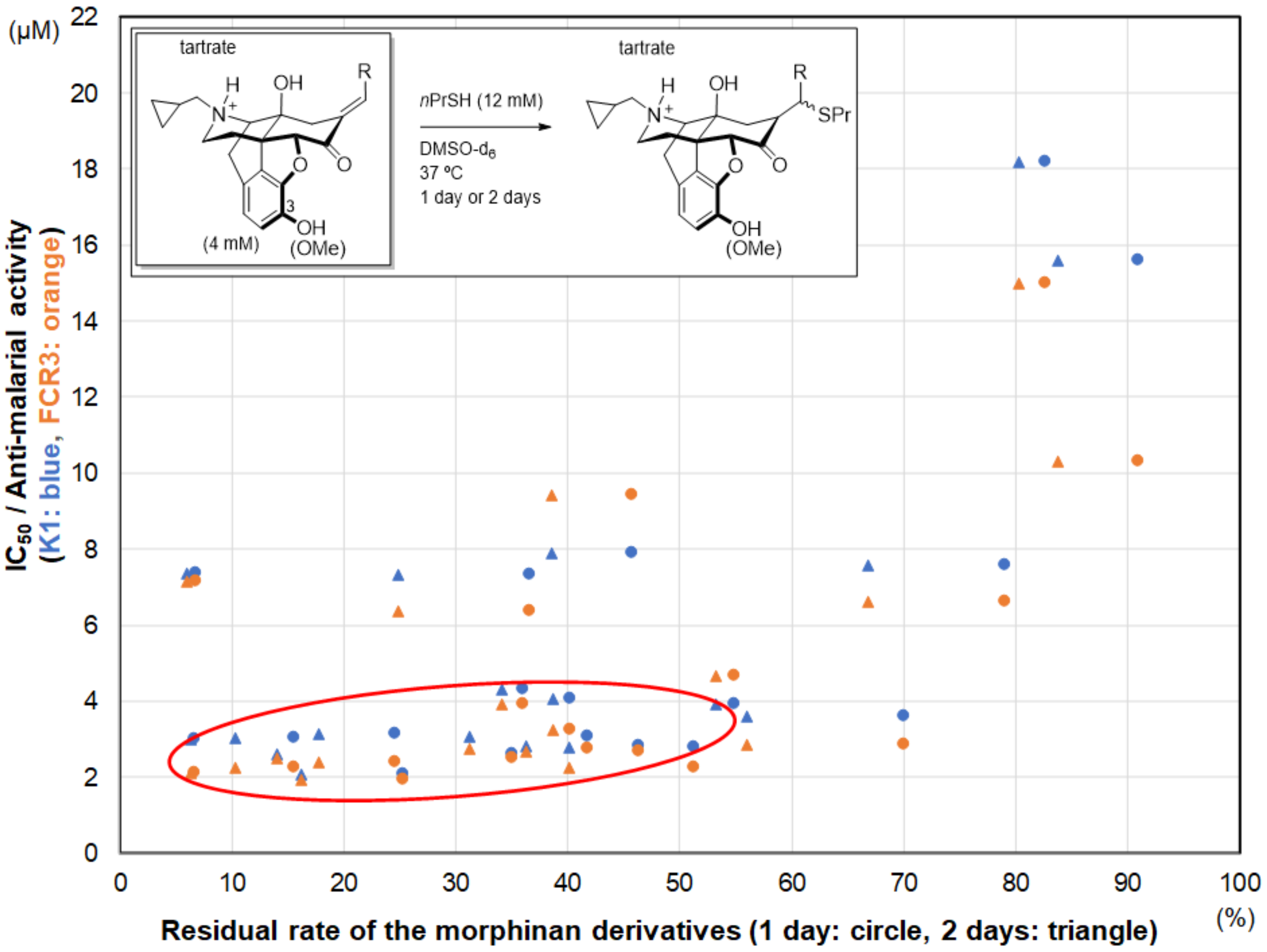
2. Results and Discussion
3. Materials and Methods
3.1. Experimental Data
3.2. Antimalarial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Malaria. Available online: https://www.who.int/news-room/fact-sheets/detail/malaria (accessed on 4 October 2019).
- Beare, N.A.V.; Lewallen, S.; Taylor, T.E.; Molyneux, M.E. Redefining cerebral malaria by including malaria retinopathy. Future Microbiol. 2011, 6, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Bartoloni, A.; Zammarchi, L. Clinical aspects of uncomplicated and severe malaria. Mediterr. J. Hematol. Infect. Dis. 2012, 4, e2012026. [Google Scholar] [CrossRef] [PubMed]
- Taylor, W.R.J.; Hanson, J.; Turner, G.D.H.; White, N.J.; Dondorp, A.M. Respiratory manifestations of malaria. Chest 2012, 142, 492–505. [Google Scholar] [CrossRef] [PubMed]
- Wongsrichanalai, C.; Meshnick, S.R. Declining artesunate-mefloquine efficacy against falciparum malaria on the Cambodia-Thailand border. Emerg. Infect. Dis. 2008, 14, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Ashley, E.A.; Dhorda, M.; Fairhurst, R.M.; Amaratunga, C.; Lim, P.; Suon, S.; Sreng, S.; Anderson, J.M.; Mao, S.; Sam, B.; et al. Spread of artemisinin resistance in Plasmodium falciparum malaria. N. Engl. J. Med. 2014, 371, 411–423. [Google Scholar] [CrossRef]
- Miyata, Y.; Fujii, H.; Osa, Y.; Kobayashi, S.; Takeuchi, T.; Nagase, H. Opioid δ1 receptor antagonist 7-benzylidenenaltrexone as an effective resistance reverser for chloroquine-resistant Plasmodium chabaudi. Bioorg. Med. Chem. Lett. 2011, 21, 4710–4712. [Google Scholar] [CrossRef]
- Miyata, Y.; Fujii, H.; Uenohara, Y.; Kobayashi, S.; Takenouchi, T.; Nagase, H. Investigation of 7-benzylidenenaltrexone derivatives as resistance reverser for chloroquine-resistant Plasmodium chabaudi. Bioorg. Med. Chem. Lett. 2012, 22, 5174–5176. [Google Scholar] [CrossRef]
- Asahi, H.; Inoue, S.-I.; Niikura, M.; Kunigo, K.; Suzuki, Y.; Kobayashi, F.; Sendo, F. Profiling molecular factors associated with pyknosis and developmental arrest induced by an opioid receptor antagonist and dihydroarthemisinin in Plasmodium falciparum. PLoS ONE 2017, 12, e0184874. [Google Scholar] [CrossRef]
- Kutsumura, N.; Nakajima, R.; Koyama, Y.; Miyata, Y.; Saitoh, T.; Yamamoto, N.; Iwata, S.; Fujii, H.; Nagase, H. Investigation of 7-benzylidenenaltrexone derivatives as a novel structural antitrichomonal lead compound. Bioorg. Med. Chem. Lett. 2015, 25, 4890–4892. [Google Scholar] [CrossRef]
- Kutsumura, N.; Koyama, Y.; Nagumo, Y.; Nakajima, R.; Miyata, Y.; Yamamoto, N.; Saitoh, T.; Yoshida, N.; Iwata, S.; Nagase, H. Antitrichomonal activity of δ opioid receptor antagonists, 7-benzylidenenaltrexone derivatives. Bioorg. Med. Chem. 2017, 25, 4375–4383. [Google Scholar] [CrossRef]
- Dubois, V.L.; Platel, D.F.N.; Pauly, G.; Tribouleyduret, J. Plasmodium berghei: Implication of intracellular glutathione and its related enzyme in chloroquine resistance in vivo. Exp. Parasitol. 1995, 81, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.; Famin, O.; Zhang, J.; Krugliak, M. Inhibition of glutathione-dependent degradation of heme by chloroquine and amodiaquine as a possible basis for their antimalarial mode of action. Biochem. Pharmacol. 1998, 56, 1305–1313. [Google Scholar] [CrossRef]
- Famin, O.; Krugliak, M.; Ginsburg, H. Kinetics of inhibition of glutathione-mediated degradation of ferriprotoporphyrin IX by antimalarial drugs. Biochem. Pharmacol. 1999, 58, 59–68. [Google Scholar] [CrossRef]
- Ellis, J.E.; Yarlett, N.; Cole, D.; Humphreys, M.J.; Lloyd, D. Antioxidant defences in the microaerophilic protozoan Trichomonas vaginalis: Comparison of metronidazole-resistant and sensitive strains. Microbiology 1994, 140, 2489–2494. [Google Scholar] [CrossRef]
- Coombs, G.H.; Westrop, G.D.; Suchan, P.; Puzova, G.; Hirt, R.P.; Embley, T.M.; Mottram, J.C.; Müller, S. The amitochondriate eukaryote Trichomonas vaginalis contains a divergent thioredoxin-linked peroxiredoxin antioxidant system. J. Biol. Chem. 2004, 279, 5249–5256. [Google Scholar] [CrossRef]
- Pal, C.; Bandyopadhyay, U. Redox-active antiparasitic drugs. Antiox. Redox Signal. 2012, 17, 555–587. [Google Scholar] [CrossRef]
- Beltrán, N.C.; Horváthová, L.; Jedelský, P.L.; Šedinová, M.; Rada, P.; Marcinčiková, M.; Hrdý, I.; Tachezy, J. Iron-induced changes in the proteome of Trichomonas vaginalis hydrogenosomes. PLoS ONE 2013, 8, e65148. [Google Scholar] [CrossRef]
- Puente-Rivera, J.; Ramón-Luing, L.Á.; Figueroa-Angulo, E.E.; Ortega-López, J.; Arroyo, R. Trichocystatin-2(TC-2): An endogenous inhibitor of cysteine proteinases in Trichomonas vaginalis is associated with TvCP39. Int. J. Biochem. Cell Biol. 2014, 54, 255–265. [Google Scholar] [CrossRef]
- Nagase, H.; Hayakawa, J.; Kawamura, K.; Kawai, K.; Takezawa, Y.; Matsuura, H.; Tajima, C.; Endo, T. Discovery of a structurally novel opioid κ-agonist derived from 4,5-epoxymorphinan. Chem. Pharm. Bull. 1998, 46, 366–369. [Google Scholar] [CrossRef]
- Otoguro, K.; Kohana, A.; Manabe, C.; Ishiyama, A.; Ui, H.; Shiomi, K.; Yamada, H.; Omura, S. Potent antimalarial activities of polyether antibiotic, X-206. J. Antibiot. 2001, 54, 658–663. [Google Scholar] [CrossRef]
- Van’t Erve, T.J.; Wagner, B.A.; Ryckman, K.K.; Raife, T.J.; Buettner, G.R. The concentration of glutathione in human erythrocytes is a heritable trait. Free Radic. Biol. Med. 2013, 65, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Ginsburg, H. The malaria parasite supplies glutathione to its host cell–Investigation of glutathione transport and metabolism in human erythrocytes infected with Plasmodium falciparum. Eur. J. Biochem. 1997, 250, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Müller, T.; Johann, L.; Jannack, B.; Brückner, M.; Lanfranchi, D.A.; Bauer, H.; Sanchez, C.; Yardley, V.; Deregnaucourt, C.; Schrével, J.; et al. Glutathione reductase-catalyzed cascade of redox reactions to bioactive potent antimalarial 1,4-naphthoquinones–A new strategy to combat malarial parasites. J. Am. Chem. Soc. 2011, 133, 11557–11571. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sabnis, Y.; Zhao, Z.; Zhang, T.; Buhrlage, S.J.; Jones, L.H.; Gray, N.S. Developing irreversible inhibitors of the protein kinase cysteinome. Chem. Biol. 2013, 20, 146–159. [Google Scholar] [CrossRef] [PubMed]
- Lanning, B.R.; Whitby, L.R.; Dix, M.M.; Douhan, J.; Gilbert, A.M.; Hett, E.C.; Johnson, T.O.; Joslyn, C.; Kath, J.C.; Niessen, S.; et al. A road map to evaluate the proteome-wide selectivity of covalent kinase inhibitors. Nat. Chem. Biol. 2014, 10, 760–767. [Google Scholar] [CrossRef] [PubMed]
- Goedken, E.R.; Argiriadi, M.A.; Banach, D.L.; Fiamengo, B.A.; Foley, S.E.; Frank, K.E.; George, J.S.; Harris, C.M.; Hobson, A.D.; Ihle, D.C.; et al. Tricyclic covalent inhibitors selectively target Jak3 through an active site thiol. J. Biol. Chem. 2015, 290, 4573–4589. [Google Scholar] [CrossRef]
- Jackson, P.A.; Widen, J.C.; Harki, D.A.; Brummond, K.M. Covalent modifiers: A chemical perspective on the reactivity of α,β-unsaturated carbonyls with thiols via hetero-Michael addition reactions. J. Med. Chem. 2017, 60, 839–885. [Google Scholar] [CrossRef]
- Shindo, N.; Fuchida, H.; Sato, M.; Watari, K.; Shibata, T.; Kuwata, K.; Miura, C.; Okamoto, K.; Hatsuyama, Y.; Tokunaga, K.; et al. Selective and reversible modification of kinase cysteines with chlorofluoroacetamides. Nat. Chem. Biol. 2019, 15, 250–258. [Google Scholar] [CrossRef]
- Ohkawa, S.; Portoghese, P.S. 7-Arylidenenaltrexones as selective δ1 opioid receptor antagonists. J. Med. Chem. 1998, 41, 4177–4180. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |

| Compound | IC50 (μM) | Compound | IC50 (μM) | ||
|---|---|---|---|---|---|
| K1 a | FCR3 b | K1a | FCR3 b | ||
| Artemisinin | 0.03 | 0.04 | 10d | 7.33 | 6.38 |
| Chloroquine | 0.57 | 0.07 | 11c | 2.60 | 2.50 |
| BNTX (1) c | 4.08 | 3.26 | 12d | 3.93 | 4.66 |
| 2d | 4.31 | 3.94 | 13d | 7.89 | 9.43 |
| 3d | 3.60 | 2.86 | 14d | 2.08 | 1.95 |
| 4d | 2.82 | 2.67 | 15d | 7.37 | 7.16 |
| 5d | 3.07 | 2.76 | 16d | 7.58 | 6.61 |
| 6d | 2.78 | 2.24 | 17d | >20.1 | N/A |
| 7d | 3.16 | 2.38 | 18d | 18.3 | 15.0 |
| 8d | 3.04 | 2.24 | 19d | 15.6 | 10.3 |
| 9d | 2.99 | 2.13 | 20d | 19.8 | >21.5 |
| Compound | Residual Rate (%) | Compound | Residual Rate (%) | ||
|---|---|---|---|---|---|
| 1 day | 2 days | 1 day | 2 days | ||
| 1c | 40.2 | 38.7 b | 11a | 35.1 | 14.0 |
| 2c | 36.0 | 34.1 b | 12c | 54.9 | 53.2 |
| 3c | 70.0 | 56.0 | 13c | 45.8 | 38.6 |
| 4c | 46.4 | 36.3 | 14c | 25.3 | 16.2 |
| 5c | 41.8 | 31.2 | 15c | 6.8 | 6.0 |
| 6c | 51.3 | 40.1 | 16c | 79.0 | 66.8 |
| 7c | 24.6 | 17.7 | 17c | 90.0 | 88.3 |
| 8c | 15.6 | 10.3 | 18c | 82.6 | 80.2 |
| 9c | 6.7 | 6.3 | 19c | 90.9 | 83.8 |
| 10c | 36.6 | 24.8 | 20c | 97.9 | 89.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutsumura, N.; Koyama, Y.; Saitoh, T.; Yamamoto, N.; Nagumo, Y.; Miyata, Y.; Hokari, R.; Ishiyama, A.; Iwatsuki, M.; Otoguro, K.; et al. Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities. Molecules 2020, 25, 1112. https://doi.org/10.3390/molecules25051112
Kutsumura N, Koyama Y, Saitoh T, Yamamoto N, Nagumo Y, Miyata Y, Hokari R, Ishiyama A, Iwatsuki M, Otoguro K, et al. Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities. Molecules. 2020; 25(5):1112. https://doi.org/10.3390/molecules25051112
Chicago/Turabian StyleKutsumura, Noriki, Yasuaki Koyama, Tsuyoshi Saitoh, Naoshi Yamamoto, Yasuyuki Nagumo, Yoshiyuki Miyata, Rei Hokari, Aki Ishiyama, Masato Iwatsuki, Kazuhiko Otoguro, and et al. 2020. "Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities" Molecules 25, no. 5: 1112. https://doi.org/10.3390/molecules25051112
APA StyleKutsumura, N., Koyama, Y., Saitoh, T., Yamamoto, N., Nagumo, Y., Miyata, Y., Hokari, R., Ishiyama, A., Iwatsuki, M., Otoguro, K., Ōmura, S., & Nagase, H. (2020). Structure-Activity Relationship between Thiol Group-Trapping Ability of Morphinan Compounds with a Michael Acceptor and Anti-Plasmodium falciparum Activities. Molecules, 25(5), 1112. https://doi.org/10.3390/molecules25051112