Use of a Pleurotus ostreatus Complex Cell Wall Extract as Elicitor of Plant Defenses: From Greenhouse to Field Trial
Abstract
1. Introduction
2. Results and Discussion
2.1. Yields and Process Scale-Up
2.2. β-Glucan and Crude Protein Contents
2.3. Monosaccharide Composition
2.4. Steric Exclusion Chromatography/Multi-Angle Light-Scattering/Differential Refractive Index (SEC/MALS/DRI) Analysis
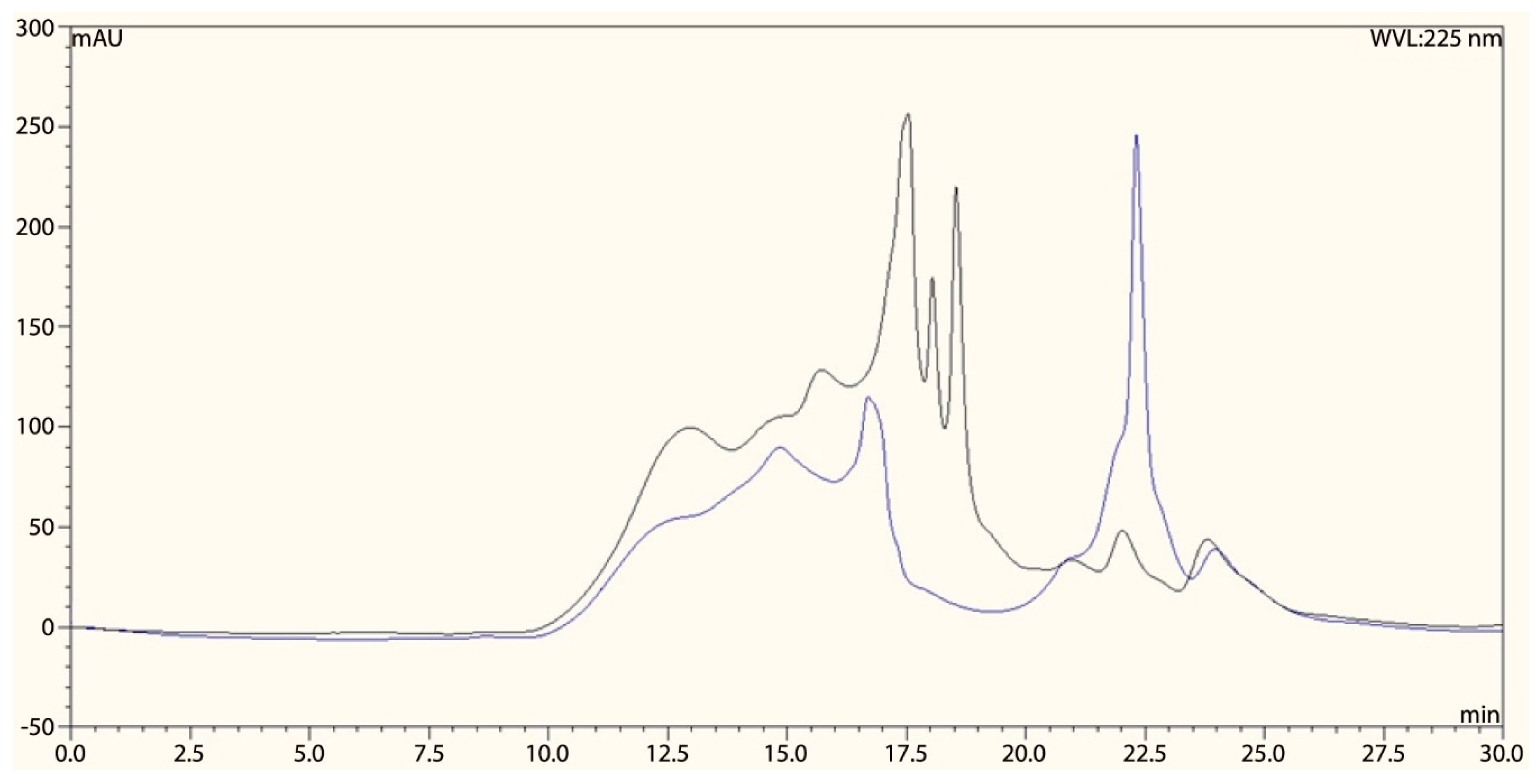
2.5. High-Performance Liquid Chromatography (HPLC)-SEC Analysis
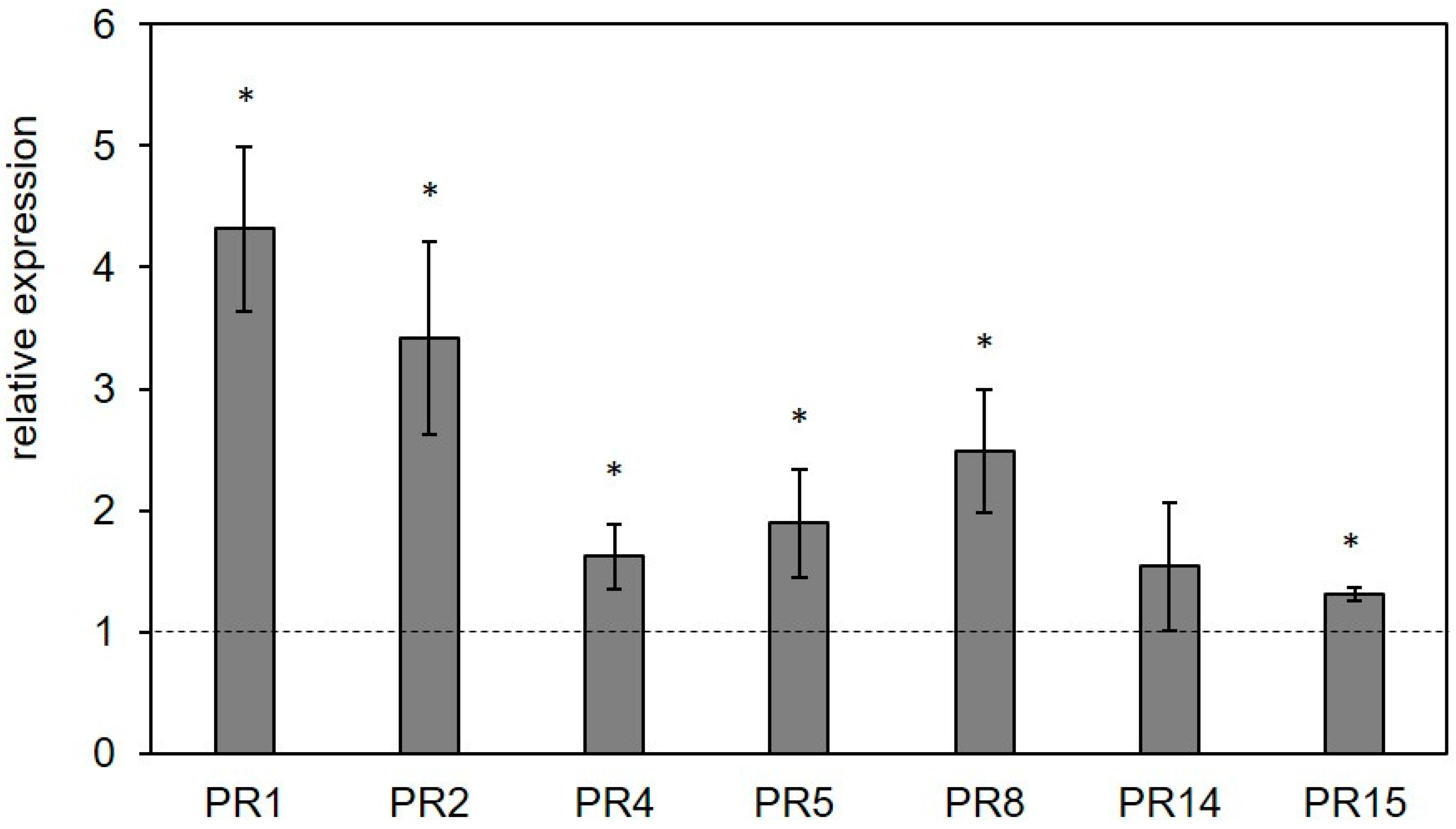
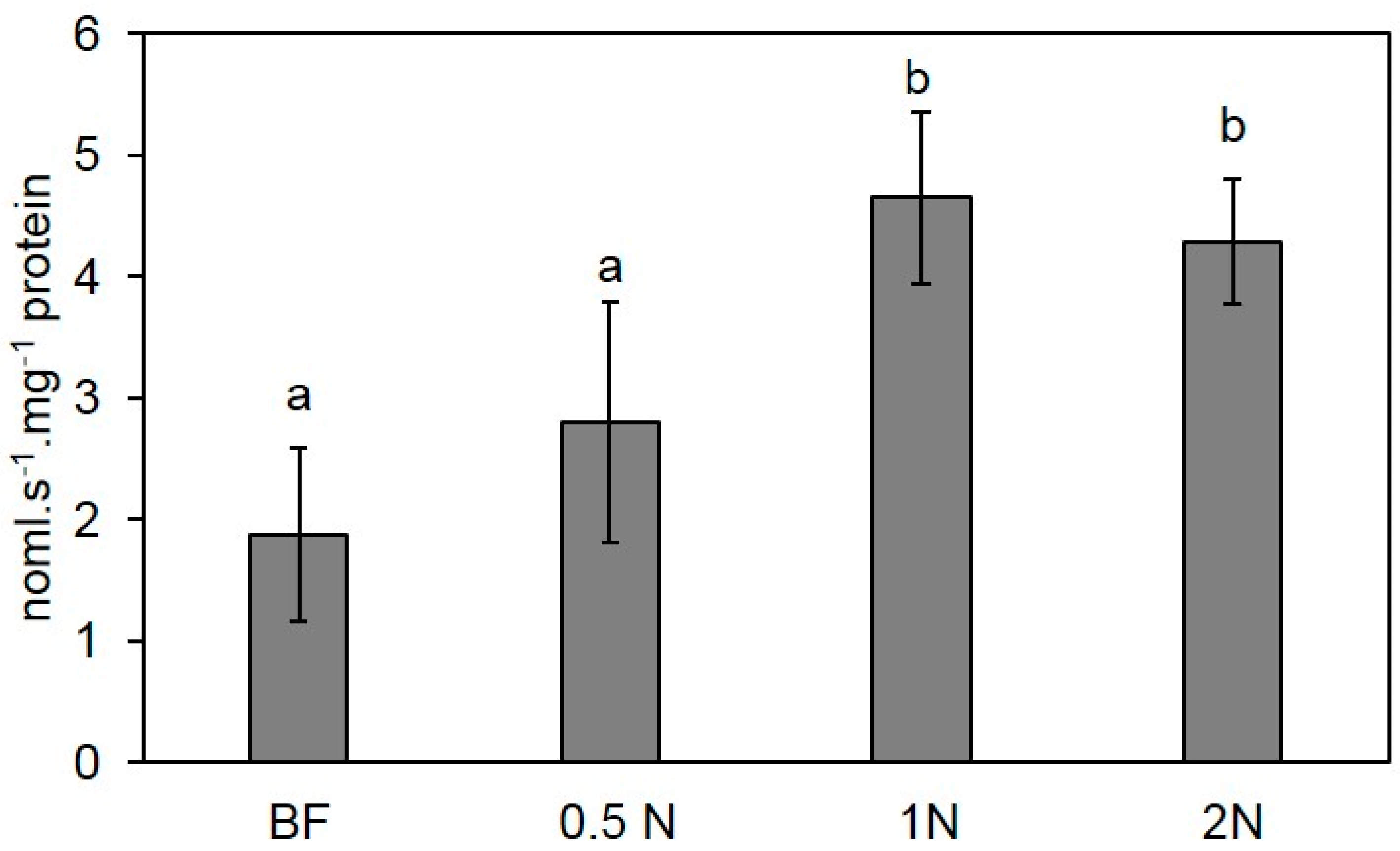
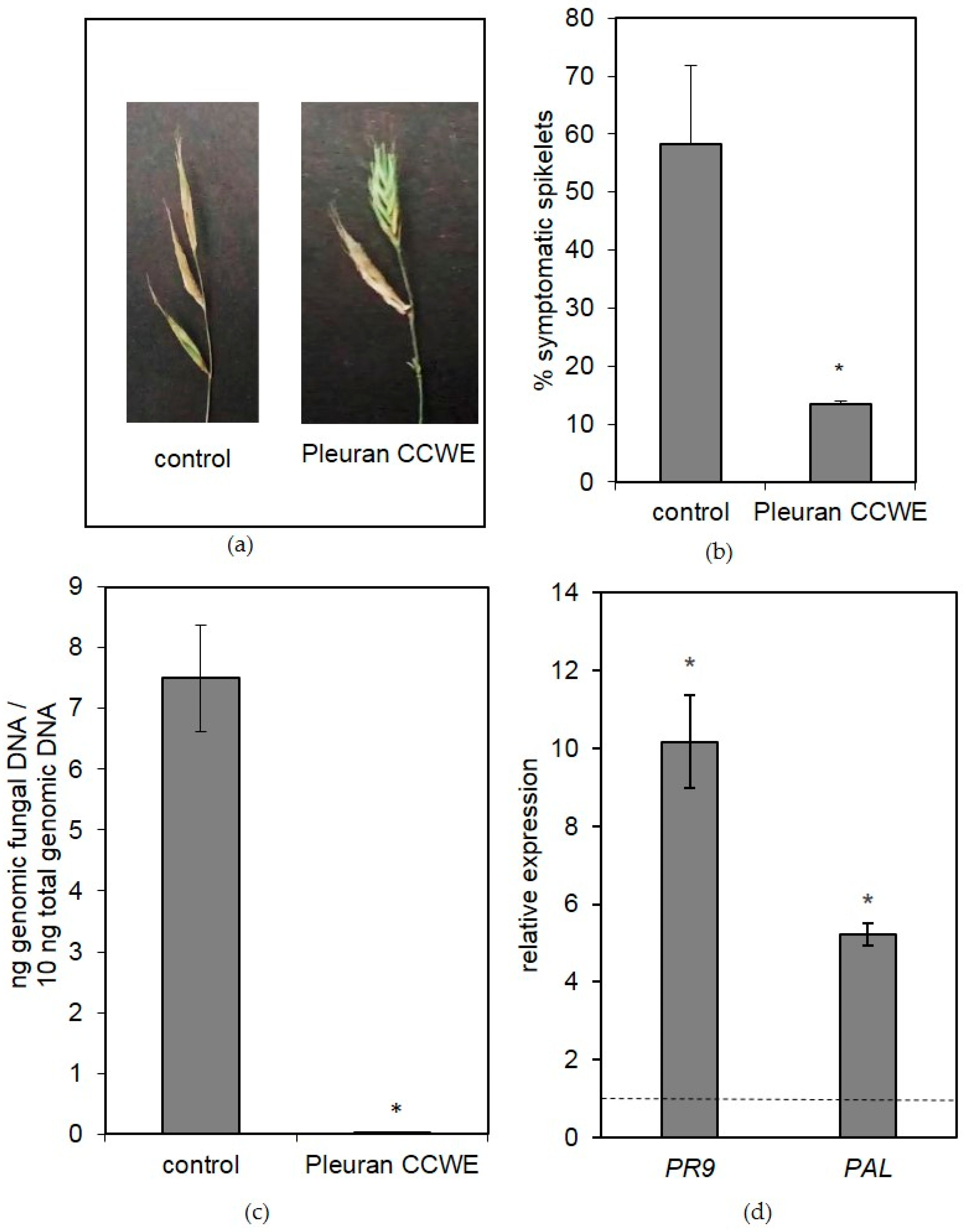
2.6. Biological Activities of Pleuran Complex Cell Wall Extract (CCWE)
2.6.1. Controlled Conditions
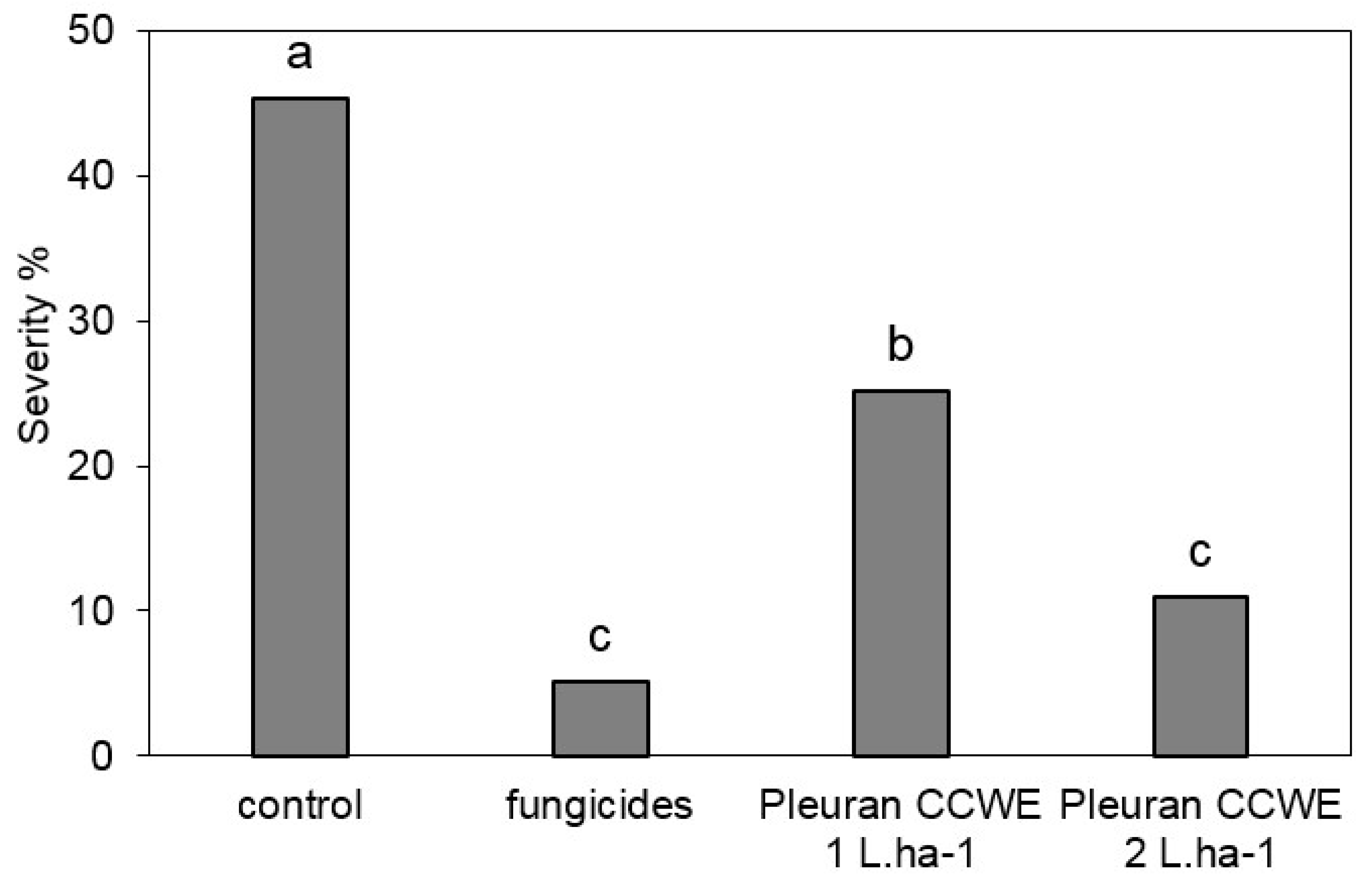
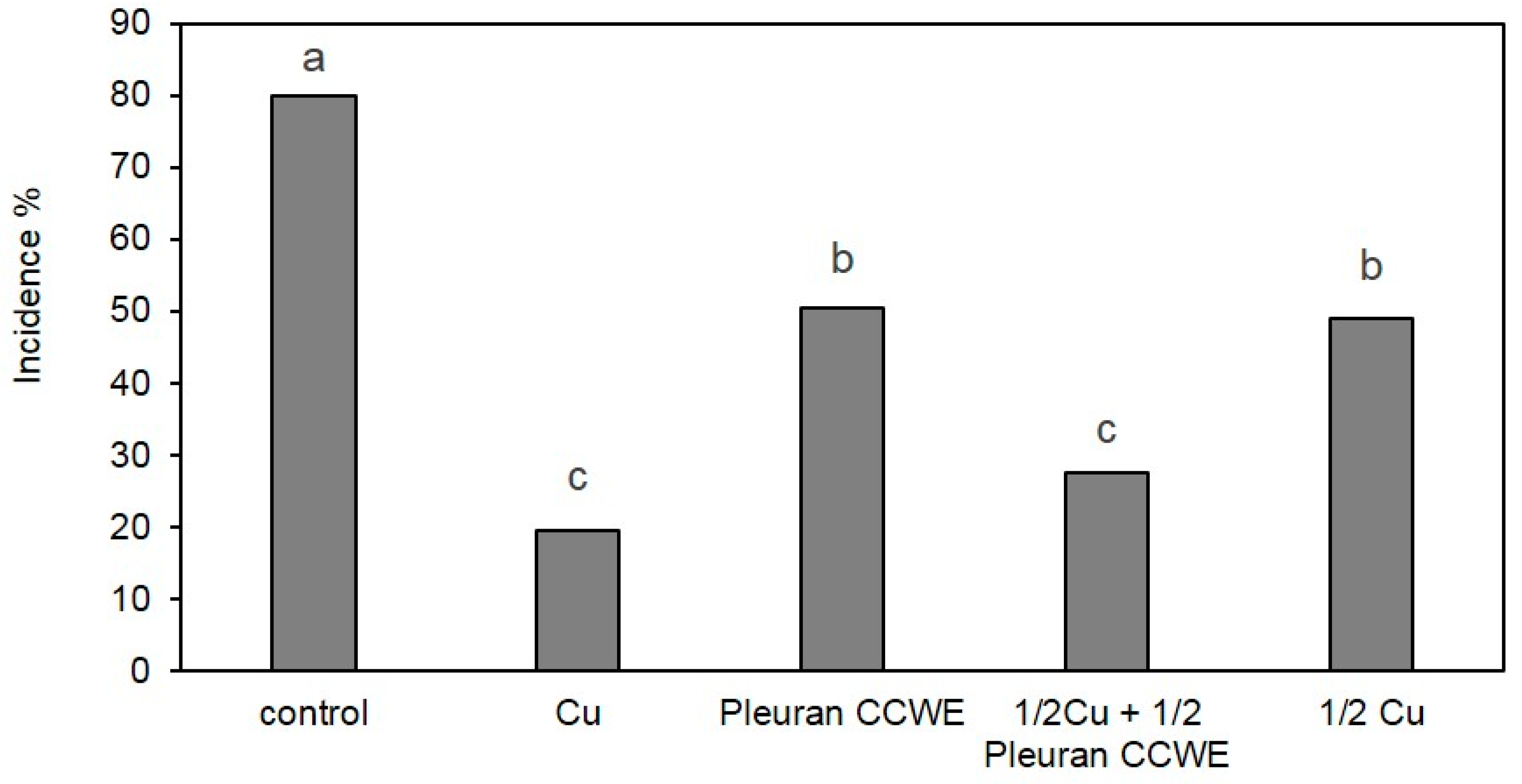
2.6.2. Field Trials
3. Materials and Methods
3.1. Mushroom Raw Material
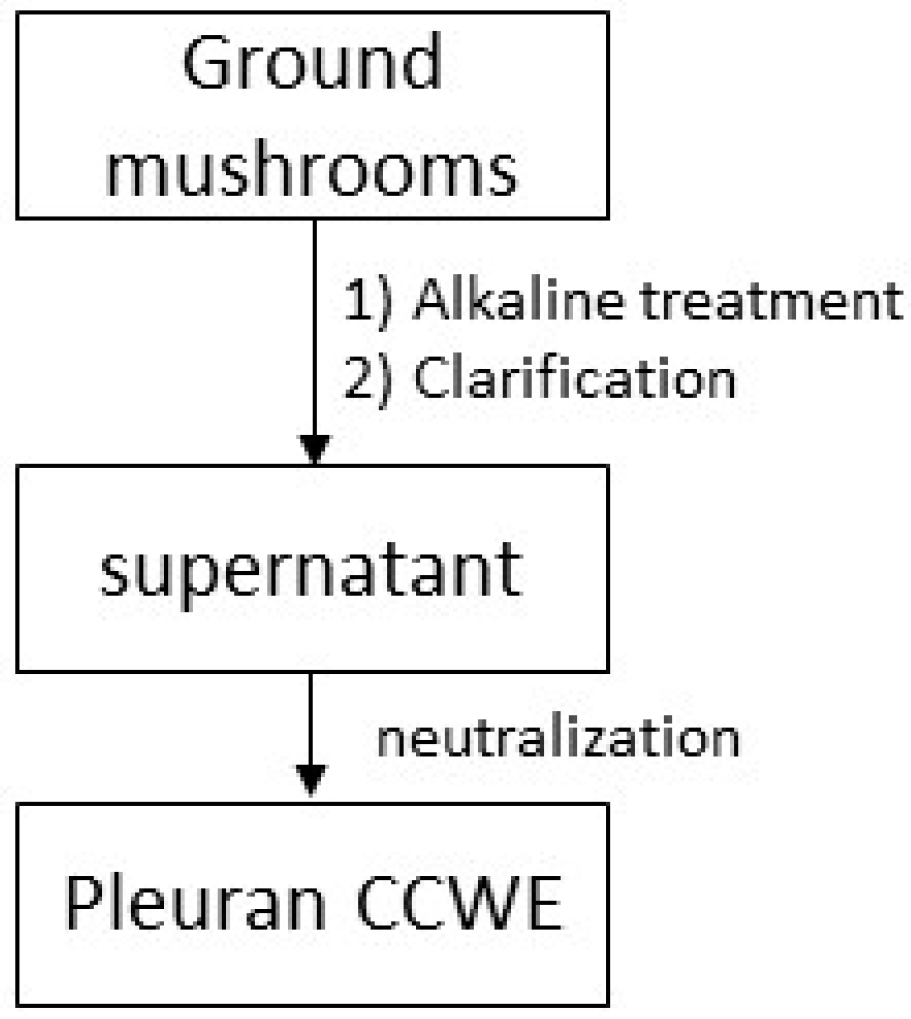
3.2. Extraction of Pleuran CCWE
3.3. β-Glucan Assay
3.4. Crude Proteins
3.5. Monosaccharide Composition
3.6. SEC/MALS/DRI Analysis
3.7. HPLC-SEC
3.8. Plant Culture and Treatments
3.8.1. Tomato Plants
3.8.2. Brachypodium Distachyon Plants
3.9. Peroxidase Activity Quantification
3.10. Gene Expression
3.10.1. Tomato Plantlets
3.10.2. Brachypodium Distachyon Plantlets
3.11. Field Trial
3.11.1. Field Trial on Wheat
3.11.2. Field Trial on Grapevine
3.12. Statistical Analysis
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ruthes, A.C.; Ribeiro Smiderle, F.; Iacomini, M. D-Glucans from edible mushrooms: A review on the extraction, purification and chemical characterization approaches. Carbohyd. Polym. 2015, 117, 753–761. [Google Scholar] [CrossRef]
- Zhu, F.; Du, B.; Bian, Z.; Xu, B. Beta-glucans from edible and medicinal mushrooms: Characteristics, physicochemical and biological activities. J. Food Compos. Anal. 2015, 41, 165–173. [Google Scholar] [CrossRef]
- Baeva, E.; Bleha, R.; Lavrova, E.; Sushytskyi, L.; Čopíková, J.; Jablonsky, I.; Klouček, P.; Synytsya, A. Polysaccharides from basidiocarps of cultivating mushroom Pleurotus ostreatus: Isolation and structural characterization. Molecules 2019, 24, 2740. [Google Scholar] [CrossRef]
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Bobek, P.; Ozdín, Ĺ.; Kuniak, Ĺ. Effect of oyster mushroom and isolated β-glucan on lipid peroxidation and on the activities of antioxidative enzymes in rats fed the cholesterol diet. J. Nutr. Biochem. 1997, 8, 469–471. [Google Scholar] [CrossRef]
- Jesenak, M.; Majtan, J.; Rennerova, Z.; Kyselovic, J.; Banovcin, P.; Hrubisko, M. Immunomodulatory effect of pleuran (β-glucan from Pleurotus ostreatus) in children with recurrent respiratory tract infections. Int. Immunopharmacol. 2013, 15, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus): An effective nutritional supplement against upper respiratory tract infections? Med. Sport Sci. 2012, 59, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Bergendiova, K.; Tibenska, E.; Majtan, J. Pleuran (β-glucan from Pleurotus ostreatus) supplementation, cellular immune response and respiratory tract infections in athletes. Eur. J. Appl. Physiol. 2011, 111, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.-A.; Sundelin, T.; Nielsen, J.T.; Erbs, G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Front. Plant Sci. 2013, 4, 139. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Wang, G.; Zhou, J.M. Receptor kinases in plant-pathogen interactions: More than pattern recognition. Plant Cell 2017, 29, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Héloir, M.-C.; Adrian, M.; Brulé, D.; Claverie, J.; Cordelier, S.; Daire, X.; Dorey, S.; Gauthier, A.; Lemaître-Guillier, C.; Negrel, J.; et al. Recognition of elicitors in grapevine: From MAMP and DAMP perception to inducedresistance. Front. Plant Sci. 2019, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.-M.; Zhu, S.; Kachroo, P.; Kachroo, A. Signal regulators of systemic acquired resistance. Front. Plant Sci. 2015, 6, 228. [Google Scholar] [CrossRef] [PubMed]
- Trouvelot, S.; Héloir, M.C.; Poinssot, B.; Gauthier, A.; Paris, F.; Guillier, C.; Combier, M.; Trdà, L.; Daire, X.; Adrian, M. Carbohydrates in plant immunity and plant protection: Roles and potential application as foliar sprays. Front. Plant Sci. 2014, 5, 592. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bezier, A.; Lambert, B.; Joubert, J.M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. PlantMicrobe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef] [PubMed]
- Ménard, R.; de Ruffray, P.; Fritig, B.; Yvin, J.C.; Kauffmann, S. Defense and resistance-inducing activities in tobacco of the sulfated β-1, 3 glucan PS3 and its synergistic activities with the unsulfated molecule. Plant Cell Physiol. 2005, 46, 1964–1972. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, J.-C.; Boland, A.; Cambier, P.; Frettinger, P.; Van Cutsem, P. Chitosan oligosaccharides modulate the supramolecular conformation and the biological activity of oligogalacturonides in Arabidopsis. Glycobiology 2010, 20, 775–786. [Google Scholar] [CrossRef] [PubMed]
- van Aubel, G.; Buonatesta, R.; Van Cutsem, P. COS-OGA: A novel oligosaccharidic elicitor that protects grapes and cucumbers against powdery mildew. Crop. Prot. 2014, 65, 129–137. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef]
- Palacios, I.; García-Lafuente, A.; Guillamón, E.; Villares, A. Novel isolation of water-soluble polysaccharides from the fruiting bodies of Pleurotus ostreatus mushrooms. Carbohyd. Res. 2012, 358, 72–77. [Google Scholar] [CrossRef]
- Karácsonyi, Š.; Kuniak, Ľ. Polysaccharides of Pleurotus ostreatus: Isolation and structure of pleuran, an alkali-insoluble β-D-glucan. Carbohyd. Polym. 1994, 24, 107–111. [Google Scholar] [CrossRef]
- Synytsya, A.; Míčková, K.; Synytsya, A.; Jablonský, I.; Spěváček, J.; Erban, V.; Kováříková, E.; Čopíková, J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: Structure and potential prebiotic activity. Carbohyd. Polym. 2009, 76, 548–556. [Google Scholar] [CrossRef]
- Almagro, L.; Gómez Ros, L.V.; Belchi-Navarro, S.; Bru, R.; Ros Barceló, A.; Pedreño, M.A. Class III peroxidases in plant defence reactions. J. Exp. Bot. 2009, 60, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Fraser, C.M.; Chapple, C. The phenylpropanoid pathway in Arabidopsis. Arabidopsis Book 2011, 9, e0152. [Google Scholar] [CrossRef]
- Mackie, K.A.; Müller, T.; Kandeler, E. Remediation of copper in vineyards – A mini review. Environ. Pollut. 2012, 167, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Rickert, D.A.; Deak, N.A.; Aldin, E.D.; Recknor, J.; Johnson, L.A.; Murphy, P.A. Comparison of Kjeldahl and Dumas methods for determining protein contents of soybean products. JAOCS 2003, 80, 1169. [Google Scholar] [CrossRef]
- Wong, K.H.; Cheung, P.C.; Wu, J.Z. Biochemical and microstrucural characteristics of insoluble and soluble dietary fiber prepared from mushroom sclerotia of Pleurotus tuber-regium, Polyporus rhinocerus, and Wolfiporia cocos. J. Agric. Food Chem. 2003, 51, 7197–7202. [Google Scholar] [CrossRef]
- Yildiz, Y.; Yeşil, Ö.F.; Karakaplan, M. Organic elements and protein in some macrofungi of south east Anatolia in Turkey. Food Chem. 2005, 89, 605–609. [Google Scholar] [CrossRef]
- Zhong, L.; Ma, N.; Wu, Y.; Zhao, L.; Ma, G.; Pei, F.; Hu, Q. Characterization and functional evaluation of oat protein isolate-Pleurotus ostreatus β-glucan conjugates formed via Maillard reaction. Food hydrocolloid. 2019, 87, 459–469. [Google Scholar] [CrossRef]
- Picton, L.; Bataille, I.; Muller, G. Analysis of a complex polysaccharide (gum arabic) by multi-angle laser light scattering coupled on-line to size exclusion chromatography and flow field flow fractionation. Carbohyd. Polym. 2000, 42, 23–31. [Google Scholar] [CrossRef]
- Simon, S.; Le Cerf, D.; Picton, L.; Muller, G. Adsorption of cellulose derivatives onto montmorillonite: A SEC–MALLS study of molar masses influence. Colloid. Surf. A 2002, 203, 77–86. [Google Scholar] [CrossRef]
- Peng, Y.; Zhang, L. Characterization of a polysaccharide-protein complex from Ganoderma tsugae mycelium by size-exclusion chromatography combined with laser light scattering. J. Biochem. Biophys. Methods 2003, 56, 243–252. [Google Scholar] [CrossRef]
- Pasquet, J.C.; Chaouch, S.; Macadré, C.; Balzergue, S.; Huguet, S.; Martin-Magniette, M.L.; Bellvert, F.; Deguercy, X.; Thareau, V.; Heintz, D.; et al. Differential gene expression and metabolomic analyses of Brachypodium distachyon infected by deoxynivalenol producing and non-producing strains of Fusarium graminearum. BMC Genom. 2014, 15, 629. [Google Scholar] [CrossRef] [PubMed]
- Shindler, J.S.; Childs, R.E.; Bardsley, W.G. Peroxidase from human cervical mucus: The isolation and characterization. Eur. J. Biochem. 1976, 65, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Brisset, M.N.; Duge De Bernonville, T. Device for Determining or Studying the State of Stimulation of the Natural Defences of Plants or Portions of Plants. Patent INRA WO 2011/161388 A1, 29 December 2011. [Google Scholar]
- Dugé De Bernonville, T.; Marolleau, B.; Staub, J.; Gaucher, M.; Brisset, M.-N. Using molecular tools to decipher the complex world of plant resistance inducers: An apple case study. J. Agric. Food Chem. 2014, 62, 11403–11411. [Google Scholar] [CrossRef]
- Van Wordragen, M.F.; De Maagd, R.A.; Mes Jurriaan, J.; Balk, P.A. Quality Control of Agricultural Products Based on Gene Expression. Patent WO 2008018790, 3 April 2008. [Google Scholar]
- Gatti, M.; Choulet, F.; Macadré, C.; Guérard, F.; Seng, J.-M.; Langin, T.; Dufresne, M. Identification, molecular cloning, and functional characterization of a wheat UDP-glucosyltransferase involved in resistance to fusarium head blight and to mycotoxin accumulation. Front. Plant. Sci. 2018. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Laboratory Process | Pilot Process | |
|---|---|---|
| Pleurotus ostreatus (kg) | 0.2 | 2.8 |
| Volume produced (L) | 1.7 +/− 0.2 | 22.3 +/− 1.6 |
| Pleuran CCWE concentration (g/L) | 32.9 +/− 5.0 | 40.7 +/− 2.9 |
| Yield (% of dry raw material) | 30.1 +/− 4.7 | 32.0 +/− 4.3 |
| % (w/w Dry Extract) | ||
|---|---|---|
| Laboratory | Pilot | |
| β-glucans | 15.5 +/− 4.1 | 17.3 +/− 4.5 |
| α-glucans | 1.4 +/− 1.0 | 1.5 +/− 0.8 |
| Crude proteins (N × 6.25) | 22.6 +/− 2.5 | 23.4 +/− 1.1 |
| % Molar Ratio | ||
| Laboratory | Pilot | |
| Glucose | 93.3 +/− 1.8 | 92.6 |
| Mannose | 3.9 +/− 0.8 | 4.1 |
| Galactose | 2.8 +/− 1.1 | 3.3 |
| Dates | Development Stage1 | Treatments | ||||
|---|---|---|---|---|---|---|
| Control | Cu | Pleuran CCWE | 1/2Cu + 1/2Pleuran CCWE | ½ Cu | ||
| 12 May | BBCH55 | - | Cu | Pleuran CCWE | Cu | Cu |
| 18 May | BBCH57 | - | Cu | Pleuran CCWE | Pleuran CCWE | - |
| 28 May | BBCH63 | - | Cu | Pleuran CCWE | Cu | Cu |
| 5 June | BBCH73 | - | Cu | Pleuran CCWE | Pleuran CCWE | - |
| 16 June | BBCH75 | - | Cu | Pleuran CCWE | Pleuran CCWE | - |
| 26 June | BBCH79 | - | Cu | Pleuran CCWE | Cu | Cu |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faugeron-Girard, C.; Gloaguen, V.; Koçi, R.; Célérier, J.; Raynaud, A.; Moine, C. Use of a Pleurotus ostreatus Complex Cell Wall Extract as Elicitor of Plant Defenses: From Greenhouse to Field Trial. Molecules 2020, 25, 1094. https://doi.org/10.3390/molecules25051094
Faugeron-Girard C, Gloaguen V, Koçi R, Célérier J, Raynaud A, Moine C. Use of a Pleurotus ostreatus Complex Cell Wall Extract as Elicitor of Plant Defenses: From Greenhouse to Field Trial. Molecules. 2020; 25(5):1094. https://doi.org/10.3390/molecules25051094
Chicago/Turabian StyleFaugeron-Girard, Céline, Vincent Gloaguen, Rromir Koçi, Julien Célérier, Anaïs Raynaud, and Charlotte Moine. 2020. "Use of a Pleurotus ostreatus Complex Cell Wall Extract as Elicitor of Plant Defenses: From Greenhouse to Field Trial" Molecules 25, no. 5: 1094. https://doi.org/10.3390/molecules25051094
APA StyleFaugeron-Girard, C., Gloaguen, V., Koçi, R., Célérier, J., Raynaud, A., & Moine, C. (2020). Use of a Pleurotus ostreatus Complex Cell Wall Extract as Elicitor of Plant Defenses: From Greenhouse to Field Trial. Molecules, 25(5), 1094. https://doi.org/10.3390/molecules25051094







