Preparation of Nanocrystals for Insoluble Drugs by Top-Down Nanotechnology with Improved Solubility and Bioavailability
Abstract
1. Introduction
2. Results
2.1. Particle Size Distribution (PSD) of the Freshly Milled Product
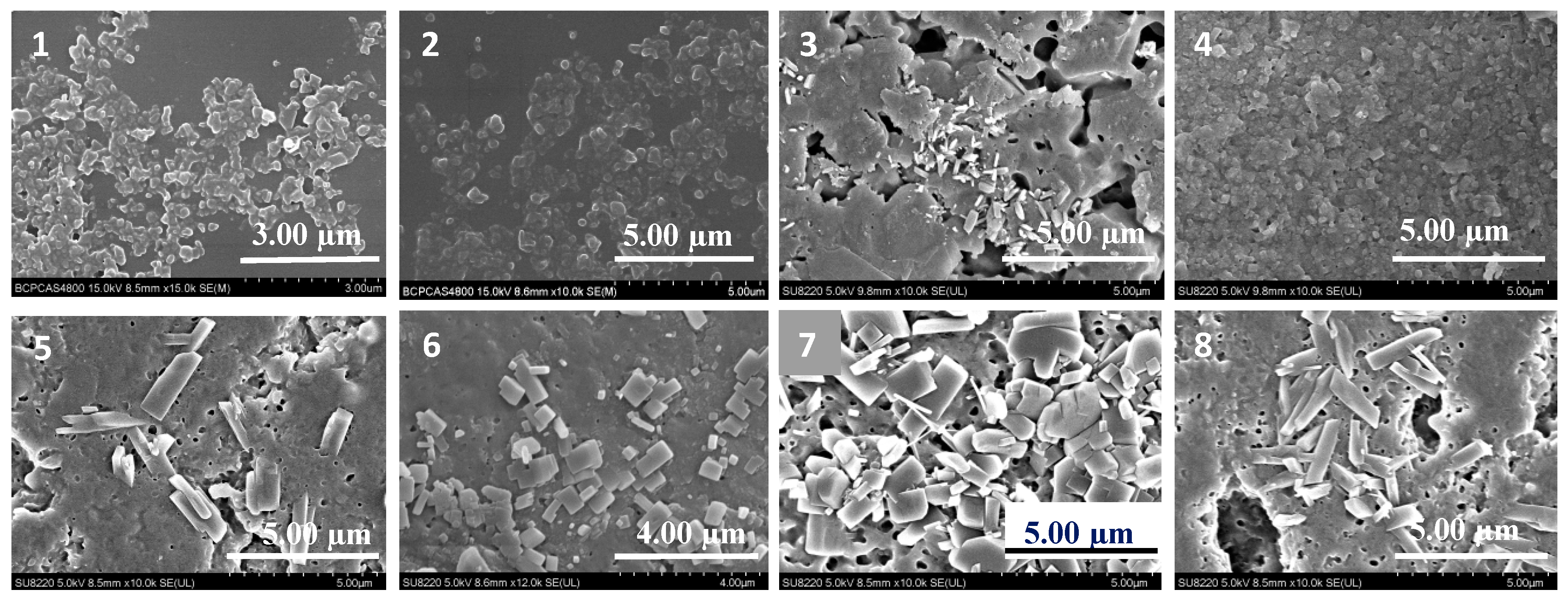
2.2. Effect of Morphology: SEM Studies
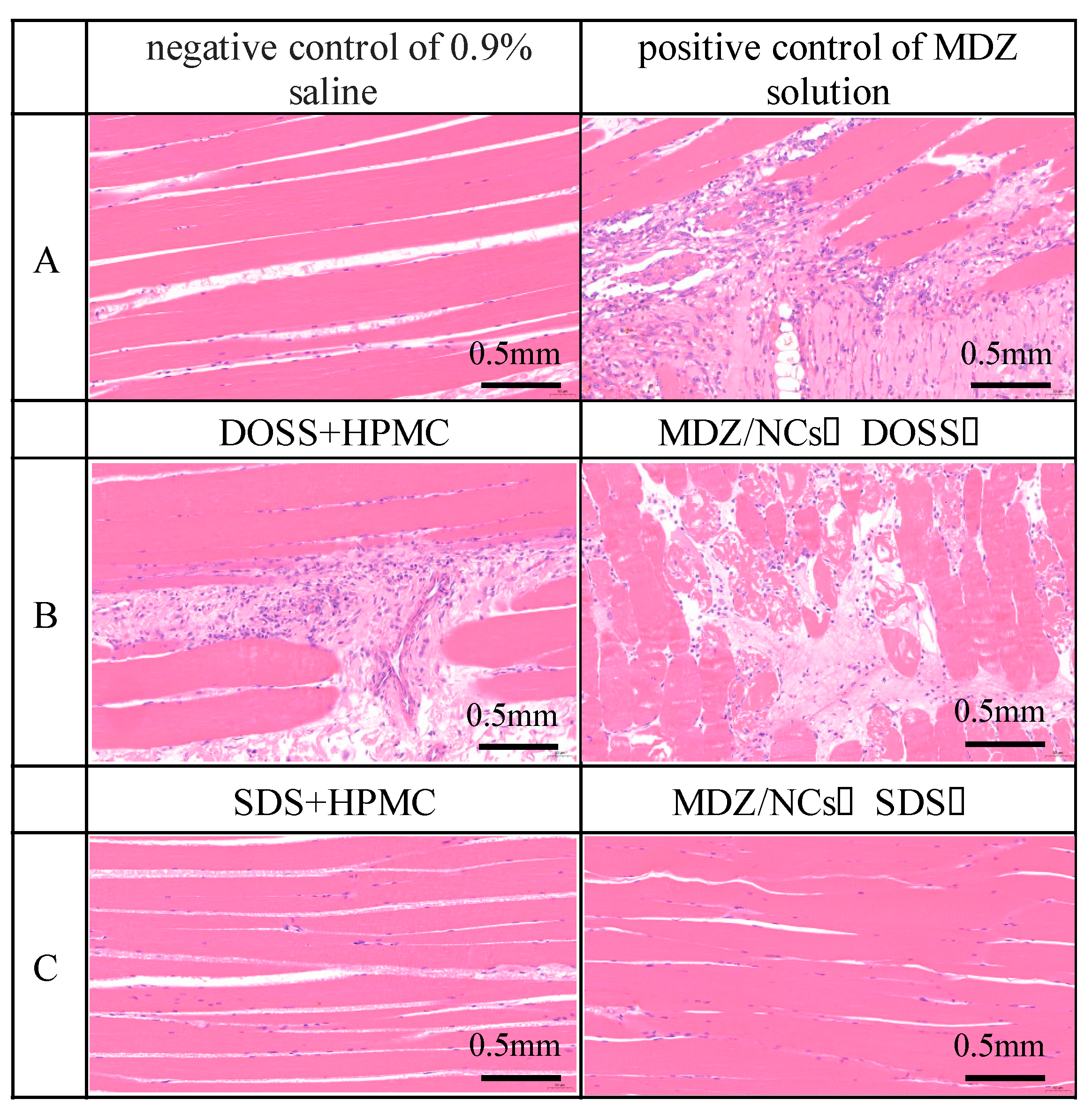
2.3. Muscle Irritation Test
2.4. Storage Stability
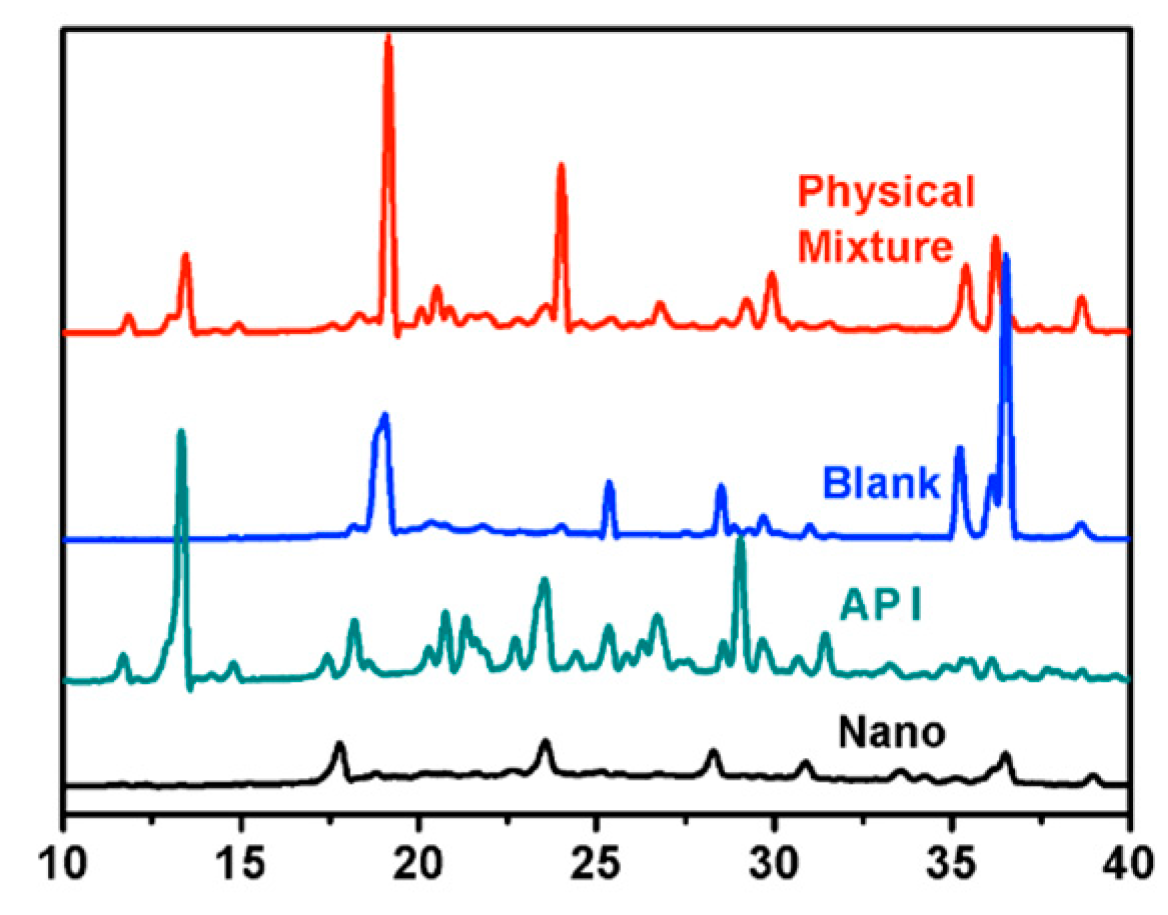
2.5. X-ray Powder Diffraction (XRPD)
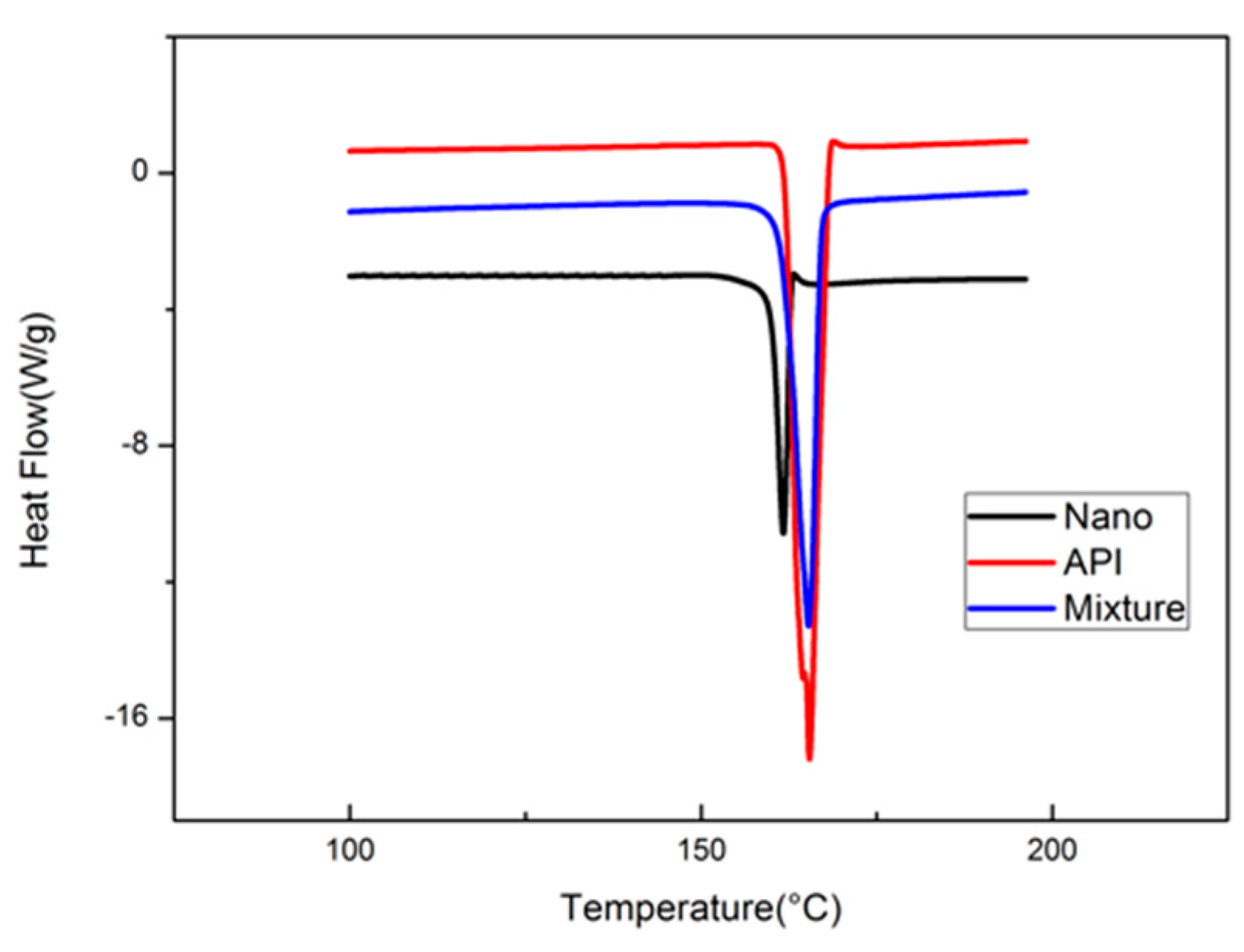
2.6. DSC
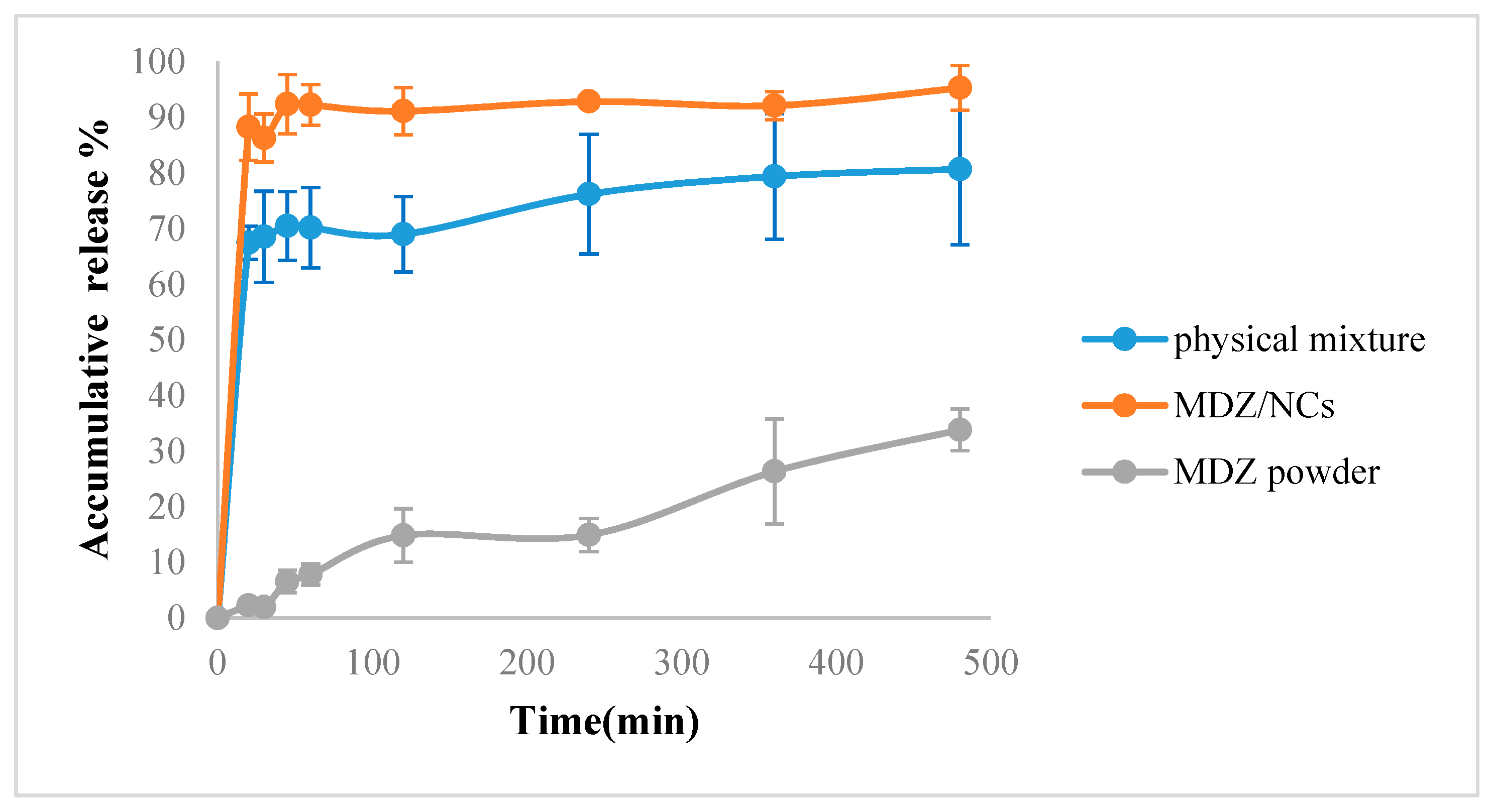
2.7. Dissolution Behavior of MDZ/NCs
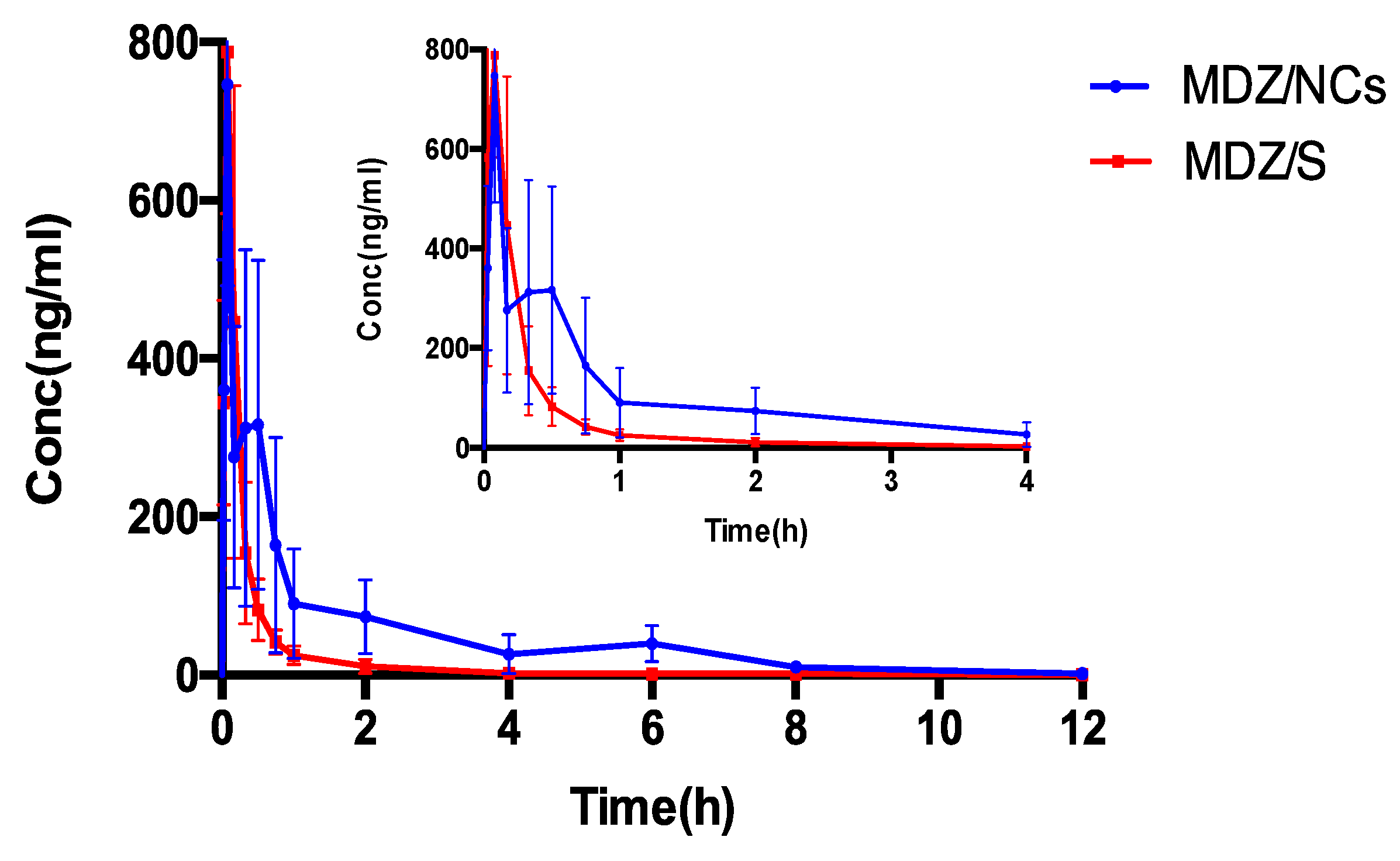
2.8. Pharmacokinetic Evaluation
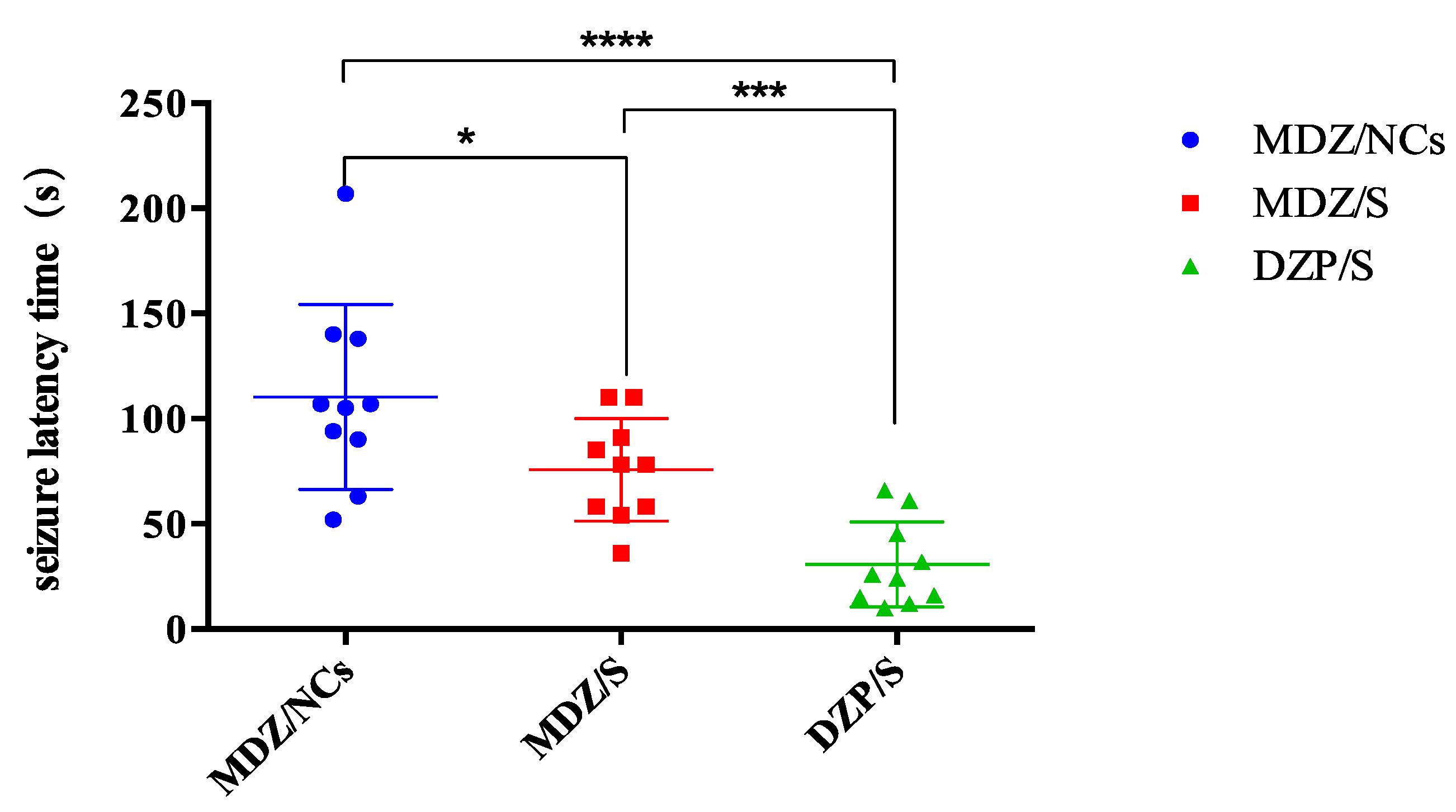
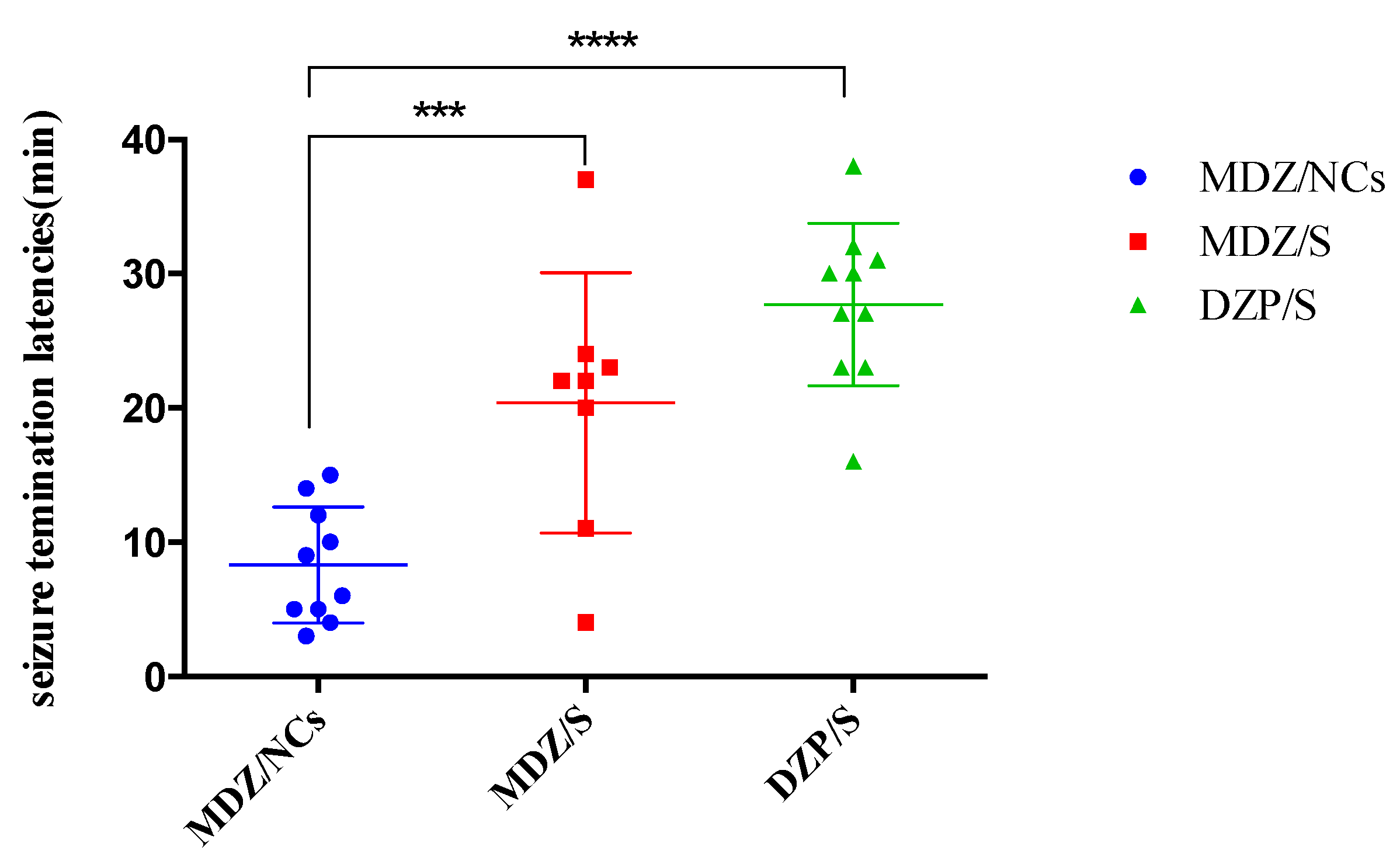
2.9. In vivo Anticonvulsant Effect
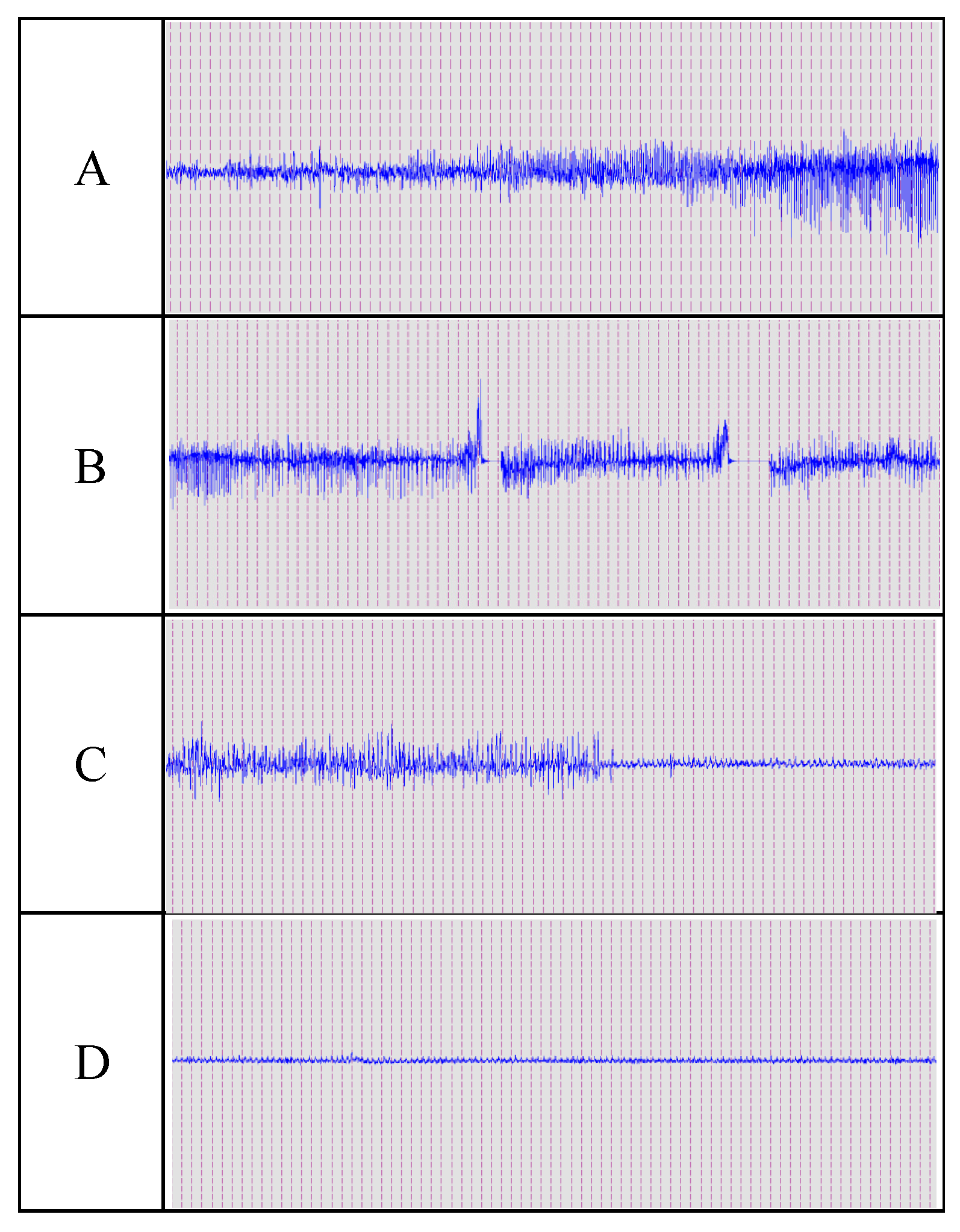
2.10. Histopathological Analysis of Brain
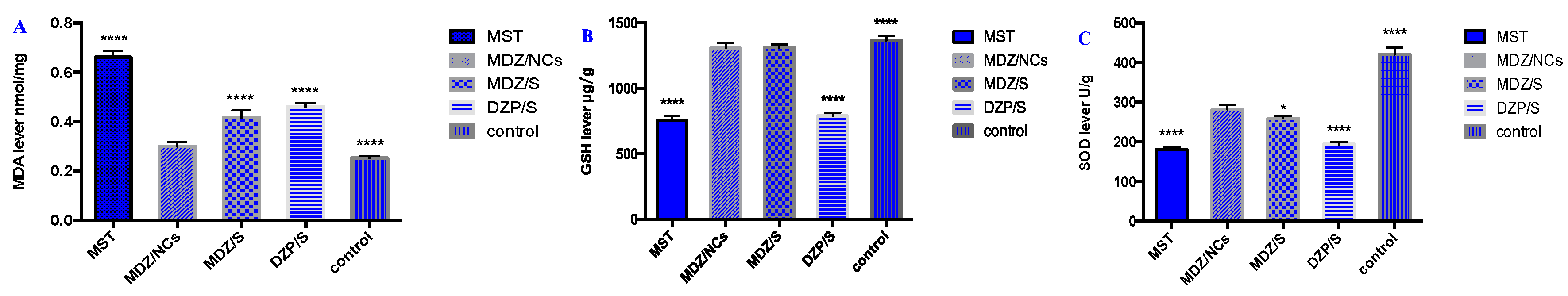
2.11. Oxidative Stress of Brain Tissue
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Materials
4.1.2. Animals
4.2. Preparation of MDZ/NCs
4.3. SEM
4.4. Muscle Irritation Test
4.5. Storage Stability
4.6. X-ray Powder Diffraction (XRPD) Study
4.7. DSC
4.8. Drug Release Behavior
4.9. Pharmacokinetic Studies
4.10. In vivo Anticonvulsant Effect
4.11. Histopathological Analysis of the Brain Sections
4.12. Oxidative Stress of the Brain Tissue
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 1–13. [Google Scholar] [CrossRef]
- Moshé, S.L.; Perucca, E.; Ryvlin, P.; Tomson, T. Epilepsy: New advances. Lancet 2015, 385, 884–898. [Google Scholar] [CrossRef]
- Schmidt, D.; Schachter, S.C. Drug treatment of epilepsy in adults. BMJ 2014, 348, g254. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Schachter, S.C.; Brodie, M.J. Drug-resistant epilepsy. NEJM Catal. 2011, 365, 919–926. [Google Scholar] [CrossRef]
- Mohanraj, R.; Norrie, J.; Stephen, L.J.; Kelly, K.; Hitiris, N.; Brodie, M.J. Mortality in adults with newly diagnosed and chronic epilepsy: A retrospective comparative study. Lancet Neurol. 2006, 5, 481–487. [Google Scholar] [CrossRef]
- Wirrell, E.C. Epilepsy-related injuries. Epilepsia 2006, 47, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Marston, D.; Besag, F.; Binnie, C.; Fowler, M. Effects of transitory cognitive impairment on psychosocial functioning of children with epilepsy: A therapeutic trial. Dev. Med. Child Neurol. 1993, 35, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Arpita, T.; Preeti, S. A randomized controlled trial of intranasal-midazolam versus intravenous-diazepam for acute childhood seizures. J. Neurol. 2013, 260, 470–474. [Google Scholar]
- Klein, R.L.; Harris, R.A. Regulation of GABAA receptor structure and function by chronic drug treatments in vivo and with stably transfected cells. JPN J. Pharmacol. 1996, 70, 1–15. [Google Scholar] [CrossRef]
- Patravale, V.B.; Date AAKulkarni, R.M. Nanosuspensions: A promising drug delivery strategy. J. Pharm. Pharmacol. 2010, 56, 827–840. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G.; Cooper, E.R. Nanosizing: A formulation approach for poorly-water-soluble compounds. Eur. J. Pharm. Sci. 2003, 18, 113–120. [Google Scholar] [CrossRef]
- Chavhan, S.S.; Petkar, K.C.; Sawant, K.K. Nanosuspensions in drug delivery: Recent advances, patent scenarios, and commercialization aspects. Crit. Rev. Ther. Drug Carr. Syst. 2011, 28, 447. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, X.; Zhanga, D.; Song, S.; Wang, F.; Li, C.; Guo, H.; Liu, Y.; Zheng, D.; Zhang, Q. Studies on the preparation, characterization and pharmacokinetics of Amoitone B nanocrystals. Int. J. Pharm. 2012, 433, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Zhang, Y.; Liu, Q.; Zhang, X.; Chokshi, R.; Mao, S. Exploration of alginates as potential stabilizers of nanosuspension. AAPS PharmSciTech 2017, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jodar, K.S.P.; Balcao, V.M.; Chaud, M.V.; Tubino, M.; Vila, M.M.D.C. Development and Characterization of a Hydrogel Containing Silver Sulfadiazine for Antimicrobial Topical Applications. J. Pharm. Sci. 2015, 104, 2241–2254. [Google Scholar] [CrossRef] [PubMed]
- Yadollahi, R.; Vasilev, K.; Simovic, S. Nanosuspension technologies for delivery of poorly soluble drugs. J. Nanomater. 2015, 2015, 1–13. [Google Scholar] [CrossRef]
- Owen, H.; Graham, S.; Werling, J.O.; Carter, P.W. Anion effects on electrostatic charging of sterically stabilized, water insoluble drug particles. Int. J. Pharm. 2009, 368, 154–159. [Google Scholar] [CrossRef]
- Yue, P.F.; Li, Y.; Wan, J.; Yang, M.; Zhu, W.F.; Wang, C.H. Study on formability of solid nanosuspensions during nanodispersion and solidification: I. Novel role of stabilizer/drug property. Int. J. Pharm. 2013, 454, 269–277. [Google Scholar] [CrossRef]
- Han, S.W.; Song, H.Y.; Moon, T.W.; Choi, S.J. Influence of emulsion interfacial membrane characteristics on Ostwald ripening in a model emulsion. Food Chem. 2018, 242, 91–97. [Google Scholar] [CrossRef]
- Mauludin, R.; Müller, R.H.; Keck, C.M. Kinetic solubility and dissolution velocity of rutin nanocrystals. Eur. J. Pharm. Biopharm. 2009, 36, 502–510. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug Deliv. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Malamatari, M.; Taylor, K.M.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Detroja, C.; Chavhan, S.; Sawant, K. Enhanced antihypertensive activity of candesartan cilexetil nanosuspension: Formulation, characterization and pharmacodynamic study. Sci. Pharm. 2011, 79, 635–652. [Google Scholar] [CrossRef] [PubMed]
- George, M.; Ghosh, I. Identifying the correlation between drug/stabilizer properties and critical quality attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur. J. Pharm. Sci. 2013, 48, 142–152. [Google Scholar] [CrossRef]
- Salazar, J.; Ghanem, A.; Müller, R.H.; Möschwitzer, J.P. Nanocrystals: Comparison of the size reduction effectiveness of a novel combinative method with conventional top-down approaches. Eur. J. Pharm. Biopharm. 2012, 81, 82–90. [Google Scholar] [CrossRef]
- Singare, D.S.; Marella, S.; Gowthamrajan, K.; Kulkarni, G.T.; Vooturi, R.; Rao, P.S. Optimization of formulation and process variable of nanosuspension: An industrial perspective. Int. J. Pharm. 2010, 402, 213–220. [Google Scholar] [CrossRef]
- Salazar, J.; Heinzerling, O.; Müller, R.H.; Möschwitzer, J.P. Process optimization of a novel production method for nanosuspensions using design of experiments (DoE). Int. J. Pharm. 2011, 420, 395–403. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, S.; Gokhale, R.; Burgess, D.J. Physical stability of nanosuspensions: Investigation of the role of stabilizers on Ostwald ripening. Int. J. Pharm. 2011, 406, 145–152. [Google Scholar] [CrossRef]
- Verma, S.; Lan, Y.; Gokhale, R.; Burgess, D.J. Quality by design approach to understand the process of nanosuspension preparation. Int. J. Pharm. 2009, 377, 185–198. [Google Scholar] [CrossRef]
- Cerdeira, A.M.; Mazzotti, M.; Gander, B. Miconazole nanosuspensions: Influence of formulation variables on particle size reduction and physical stability. Int. J. Pharm. 2010, 396, 210–218. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Zhang, L.; Wang, Q.; Zhang, D. Stability of nanosuspensions in drug delivery. J. Control Release 2013, 172, 1126–1141. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.; Jacobs, C. Buparvaquone mucoadhesive nanosuspension: Preparation, optimisation and long-term stability. Int. J. Pharm. 2002, 237, 151–161. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Watanabe, W. Physical and chemical stability of drug nanoparticles. Adv. Drug Deliv. Rev. 2011, 63, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Huang, L.; Liu, F. Understanding the structure and stability of paclitaxel nanocrystals. Int. J. Pharm. 2010, 390, 242–249. [Google Scholar] [CrossRef]
- Bürger, R.; Karlsen, K.H.; Tory, E.M. Model Equations and Instability Regions for the Sedimentation of Polydisperse Suspensions of Spheres. ZAMM 2002, 82, 699–722. [Google Scholar] [CrossRef]
- Adamczyk, Z.; Weroński, P. Application of the DLVO theory for particle deposition problems. Adv. Colloid Interface Sci. 1999, 83, 137–226. [Google Scholar] [CrossRef]
- El Badawy, A.M.; Scheckel, K.G.; Suidan, M.; Tolaymat, T. The impact of stabilization mechanism on the aggregation kinetics of silver nanoparticles. Sci. Total Environ. 2012, 429, 325–331. [Google Scholar] [CrossRef]
- Heng, D.; Cutler, D.J.; Chan, H.-K.; Yun, J.; Raper, J.A. What is a suitable dissolution method for drug nanoparticles? Pharm. Res.-Dordr. 2008, 25, 1696–1701. [Google Scholar] [CrossRef]
- Liversidge, G.G.; Cundy, K.C. Particle size reduction for improvement of oral bioavailability of hydrophobic drugs: I. Absolute oral bioavailability of nanocrystalline danazol in beagle dogs. Int. J. Pharm. 1995, 125, 91–97. [Google Scholar] [CrossRef]
- Cheolsu Shin, M.D.; James O. McNamara, M. Mechanism of epilepsy. Annu. Rev. Med. 1994, 45, 379–389. [Google Scholar] [CrossRef]
- Traub, R.D.; Wong, R. Cellular mechanism of neuronal synchronization in epilepsy. Science 1982, 216, 745–747. [Google Scholar] [CrossRef]
- Wei, F.; Yan, L.-M.; Su, T.; He, N.; Lin, Z.-J.; Wang, J.; Shi, Y.-W.; Yi, Y.-H.; Liao, W.-P. Ion channel genes and epilepsy: Functional alteration, pathogenic potential, and mechanism of epilepsy. Neurosci. Bull. 2017, 33, 455–477. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Kettenmann, H. Calcium signalling in glial cells. Trends Neurosci. 1996, 19, 346–352. [Google Scholar] [CrossRef]
- Bringmann, A.; Pannicke, T.; Biedermann, B.; Francke, M.; Iandiev, I.; Grosche, J.; Wiedemann, P.; Albrecht, J.; Reichenbach, A. Role of retinal glial cells in neurotransmitter uptake and metabolism. Neurochem. Int. 2009, 54, 143–160. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef]
- Buck, M. Intranasal Administration of Benzodiazepines for the Treatment of Acute Repetitive Seizures in Children. Pediatr. Pharm. 2013, 19. [Google Scholar]
- Marini, H.; Costa, C.; Passaniti, M.; Esposito, M.; Campo, G.M.; Ientile, R.; Adamo, E.B.; Marini, R.; Calabresi, P.; Altavilla, D. Levetiracetam protects against kainic acid-induced toxicity. Life Sci. 2004, 74, 1253–1264. [Google Scholar] [CrossRef]
- Pahuja, M.; Mehla, J.; Gupta, Y.K. Anticonvulsant and antioxidative activity of hydroalcoholic extract of tuber of Orchis mascula in pentylenetetrazole and maximal electroshock induced seizures in rats. J. Ethnopharmacol. 2012, 142, 23–27. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds midazolam are available from the authors. |



| Properties | |||||||
|---|---|---|---|---|---|---|---|
| M.P./°C | Solubility (mg/mL) | pKa | log P | M.W. | Enthalpy (kJ/g) | Morphology | |
| MDZ | 159 | 0.024 | 5.5 | 4.33 | 325.771 | 15.7 | White to light yellow crystalline |
| Drug | Properties | |||||
|---|---|---|---|---|---|---|
| M.P./Tg * | CMC (%) | HLB | M.W. | ST (mN/m) | Contact Angle | |
| HPMC | 106 | - | - | 104100 | 32.16874 | 62.8 |
| DOSS | 153–157 | 0.29 | 10.2 | 444.56 | 24.50028 | 50.5 |
| SDS | 204–207 | 0.25 | 40 | 290–310 | 30.09747 | 26.8 |
| CMC-Na | 300 | - | 43.9 | 240 | 41.98717 | 63.2 |
| PVPK30 | 130 | - | 14 | 3.8 *104 | 32.82852 | 74.1 |
| P188 | 52–57 | 0.017 | 29 | 102.13 | 32.60333 | 86.0 |
| P407 | 56 | 0.02 | 18–23 | 12600 | 36.72650 | 86.0 |
| T-80 | −21 | 0.014 | 15 | 1310 | 34.50074 | 58.6 |
| T-20 | −21 | 0.031 | 16.7 | 1127.48 | 34.50074 | 34.9 |
| Drug (%) | Excipient | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HPMC E5 (%) | DOSS (%) | SDS (%) | CMC-Na (%) | PVP K30 (%) | P188 (%) | P407 (%) | TW80 (%) | TW20 (%) | ||
| 1 | 5 | 2.5 | 1 | |||||||
| 2 | 5 | 2.5 | 1 | |||||||
| 3 | 5 | 2.5 | 1 | |||||||
| 4 | 5 | 2.5 | 1 | |||||||
| 5 | 5 | 2.5 | 1 | |||||||
| 6 | 5 | 2.5 | 1 | |||||||
| 7 | 5 | 2.5 | 1 | |||||||
| 8 | 5 | 2.5 | 1 | |||||||
| # | Excipient | Results | ||||
|---|---|---|---|---|---|---|
| Speed (rpm) | Time (h) | Size (nm) | PDI | Zeta Potential (mV) | ||
| 1 | HPMC\DOSS | 3000 | 1 | 285.9 | 0.167 | 11.1 |
| 2 | HPMC\SDS | 3000 | 1 | 286.6 | 0.124 | 23.4 |
| 3 | HPMC\CMC-Na | 3000 | 1 | 370.8 | 0.315 | 55.5 |
| 4 | HPMC\PVPK30 | 3000 | 1 | 381.2 | 0.21 | 4.34 |
| 5 | HPMC\P188 | 3000 | 1 | 412 | 0.273 | 11.9 |
| 6 | HPMC\P407 | 3000 | 1 | 275.9 | 0.187 | 13.8 |
| 7 | HPMC\TW-80 | 3000 | 1 | 681.6 | 0.238 | 8.99 |
| 8 | HPMC\TW-20 | 3000 | 1 | 315.8 | 0.208 | 12.6 |
| Parameters | Unit | MDZ/NCs | MDZ/S |
|---|---|---|---|
| T1/2 | h | 1.67 ± 0.46 | 1.48 ± 0.60 |
| Tmax | h | 0.15 ± 0.17 | 0.09 ± 0.04 |
| Cmax | ng/mL | 797.20 ± 236.88 | 805.62 ± 204.71 |
| AUC(0-t) | h·ng/mL | 581.69 ± 225.05 ** | 217.01 ± 79.12 |
| AUC(0-∞) | h·ng/mL | 591.46 ± 219.16 ** | 219.82 ± 78.59 |
| V | mL | 1392.24 ± 656.24 | 3279.63 ± 1708.20 |
| CL | mL/h | 563.02 ± 213.52 ** | 1545.07 ± 662.59 |
| MRT(0-t) | h | 2.16 ± 0.74 ** | 0.69 ± 0.19 |
| MRT(0-∞) | h | 2.50 ± 0.72 ** | 0.89 ± 0.34 |
| Route | Drug | ED50 (mg/kg) | 95% Confidence Limits (mg/kg) |
|---|---|---|---|
| Intramuscular | MDZ/NCs | 0.017 mg/kg | 0.001–0.043 |
| MDZ/S | 0.043 mg/kg | 0.001–0.098 | |
| DZP/S | 0.212 mg/kg | 0.139–0.341 |
| Group | Convulsion Latency Time (s) | Convulsion Termination Latencies (min) |
|---|---|---|
| MDZ/NCs | 110.30 ± 13.89 | 8.30 ± 1.37 |
| MDZ/S | 75.80 ± 7.72 | 20.38 ± 3.43 |
| DZP/S | 30.70 ± 6.40 | 27.70 ± 1.92 |
| Group | Concentration | ||
|---|---|---|---|
| MDA (nmol/mg) | GSH (μg/g) | SOD (U/g) | |
| MST | 0.6615 ± 0.0244 **** | 1311.394 ± 25.100 **** | 181.48 ± 4.875 **** |
| MDZ/NCs | 0.4156 ± 0.1493 | 791.671 ± 21.152 | 276.276 ± 8.630 |
| MDZ/S | 0.4603 ± 0.1544 **** | 753.148 ± 36.238 | 268.810 ± 6.432 * |
| DZP/S | 0.2993 ± 0.1641 **** | 1308.508 ± 37.736 **** | 193.924 ± 5.183 **** |
| Control | 0.2527 ± 0.0778 **** | 1367.027 ± 33.084 **** | 420.014 ± 17.790 **** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, Z.; Gao, J.; Wang, Z.; Gao, X.; Liu, N.; Li, M.; Zhang, H.; Zheng, A. Preparation of Nanocrystals for Insoluble Drugs by Top-Down Nanotechnology with Improved Solubility and Bioavailability. Molecules 2020, 25, 1080. https://doi.org/10.3390/molecules25051080
Zhang X, Li Z, Gao J, Wang Z, Gao X, Liu N, Li M, Zhang H, Zheng A. Preparation of Nanocrystals for Insoluble Drugs by Top-Down Nanotechnology with Improved Solubility and Bioavailability. Molecules. 2020; 25(5):1080. https://doi.org/10.3390/molecules25051080
Chicago/Turabian StyleZhang, Xun, Zhiguo Li, Jing Gao, Zengming Wang, Xiang Gao, Nan Liu, Meng Li, Hui Zhang, and Aiping Zheng. 2020. "Preparation of Nanocrystals for Insoluble Drugs by Top-Down Nanotechnology with Improved Solubility and Bioavailability" Molecules 25, no. 5: 1080. https://doi.org/10.3390/molecules25051080
APA StyleZhang, X., Li, Z., Gao, J., Wang, Z., Gao, X., Liu, N., Li, M., Zhang, H., & Zheng, A. (2020). Preparation of Nanocrystals for Insoluble Drugs by Top-Down Nanotechnology with Improved Solubility and Bioavailability. Molecules, 25(5), 1080. https://doi.org/10.3390/molecules25051080





