Sulfonamide Inhibition Studies of the β-Class Carbonic Anhydrase CAS3 from the Filamentous Ascomycete Sordaria macrospora
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Construction of the cas3 Overexpression Vector
3.2. Heterologous Expression of the cas3 Gene in E. coli
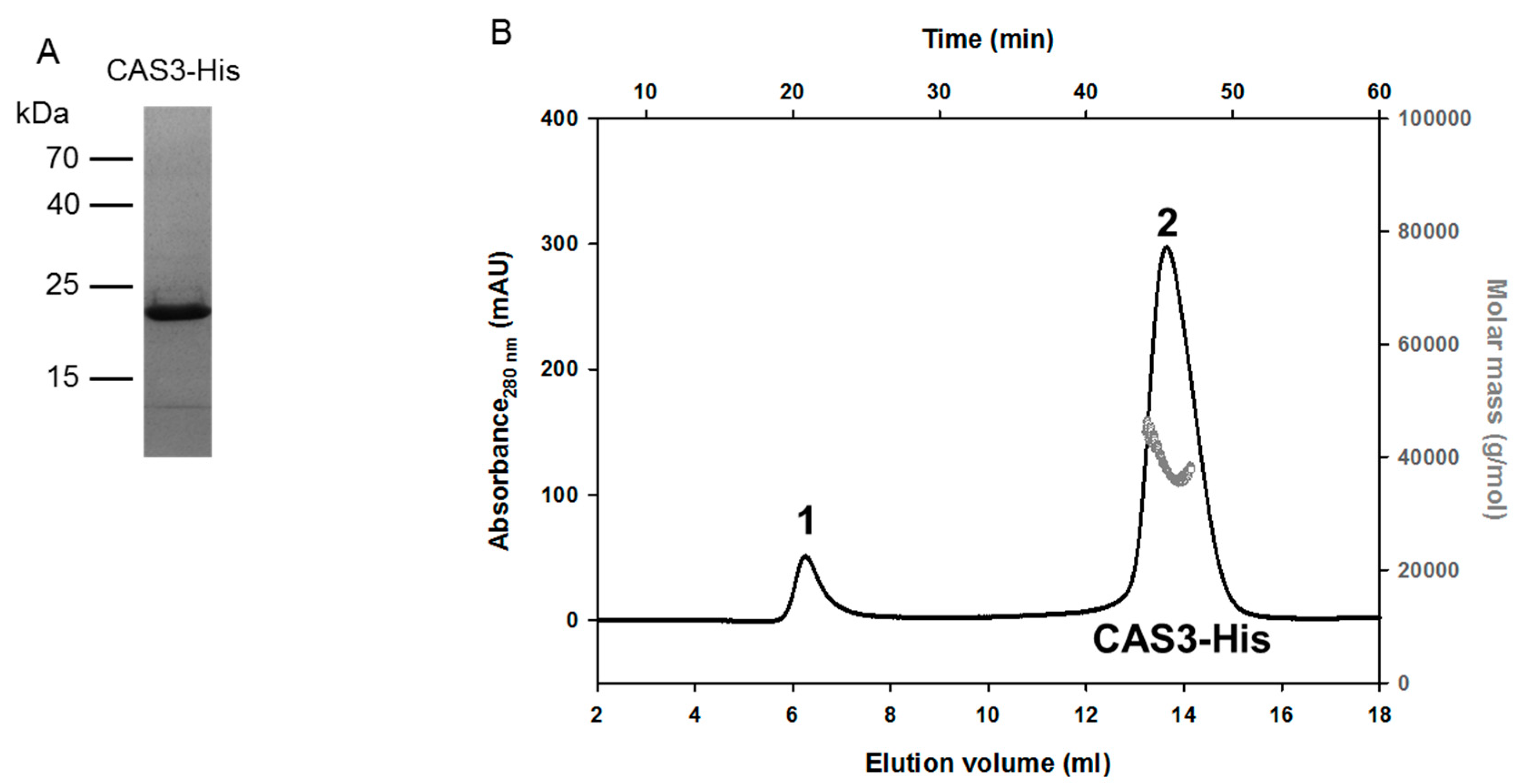
3.3. Purification of CAS3-His
3.4. Size-Exclusion Chromatography Coupled with Multi-Angle Laser Light Scattering
3.5. CA Inhibition Assay
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Teichert, I.; Nowrousian, M.; Pöggeler, S.; Kück, U. The filamentous fungus Sordaria macrospora as a genetic model to study fruiting body development. Adv. Genet. 2014, 87, 201–246. [Google Scholar]
- Elleuche, S.; Pöggeler, S. Evolution of carbonic anhydrases in fungi. Curr. Genet. 2009, 55, 211–222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elleuche, S.; Pöggeler, S. β-Carbonic anhydrases play a role in fruiting body development and ascospore germination in the filamentous fungus Sordaria macrospora. PLoS ONE 2009, 4, e5177. [Google Scholar] [CrossRef] [PubMed]
- Elleuche, S.; Pöggeler, S. Carbonic anhydrases in fungi. Microbiology 2010, 156, 23–29. [Google Scholar] [CrossRef]
- Lehneck, R.; Elleuche, S.; Pöggeler, S. The filamentous ascomycete Sordaria macrospora can survive in ambient air without carbonic anhydrases. Mol. Microbiol. 2014, 95, 931–944. [Google Scholar] [CrossRef]
- Innocenti, A.; Zimmerman, S.; Ferry, J.G.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the methanoarchaeon Methanobacterium thermoautotrophicum (cab) with anions. Bioorg. Med. Chem. Lett. 2004, 14, 4563–4567. [Google Scholar]
- Supuran, C.T. Structure and function of carbonic anhydrases. Biochem. J. 2016, 473, 2023–2032. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nature Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Zimmerman, S.A.; Ferry, J.G.; Supuran, C.T. Inhibition of the archaeal beta-class (Cab) and gamma-class (Cam) carbonic anhydrases. Curr. Top. Med. Chem. 2007, 7, 901–908. [Google Scholar] [CrossRef]
- Lehneck, R.; Pöggeler, S. Fungal carbonic anhydrases and their inhibition. In Zinc Enzyme Inhibitor -Volume 1: Enzymes from microorganisms; Supuran, C.T., Capasso, C., Eds.; Topics in Medicinal Chemistry; Springer International Publishing AG: Cham, Swizerland, 2017; Volume 22, pp. 95–110. [Google Scholar]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert Opin. Drug Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef]
- Supuran, C.T.; Capasso, C. Biomedical applications of prokaryotic carbonic anhydrases. Expert Opin. Ther. Pat. 2018, 28, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Supuran, C.T. Advances in the structural annotation of human carbonic anhydrases and impact on future drug discovery. Expert Opin. Drug Discov. 2019, 14, 1175–1197. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, G.; Giovati, L.; Angeli, A.; Pavone, M.; Del Prete, S.; Pieroni, M.; Capasso, C.; Bruno, A.; Conti, S.; Magliani, W.; et al. Discovering a new class of antifungal agents that selectively inhibits microbial carbonic anhydrases. J. Enzyme Inhib. Med. Chem. 2018, 33, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Nocentini, A.; Vullo, D.; Del Prete, S.; Osman, S.M.; Alasmary, F.A.S.; AlOthman, Z.; Capasso, C.; Carta, F.; Gratteri, P.; Supuran, C.T. Inhibition of the beta-carbonic anhydrase from the dandruff-producing fungus Malassezia globosa with monothiocarbamates. J. Enzyme Inhib. Med. Chem. 2017, 32, 1064–1070. [Google Scholar] [CrossRef]
- Del Prete, S.; Angeli, A.; Ghobril, C.; Hitce, J.; Clavaud, C.; Marat, X.; Supuran, C.T.; Capasso, C. Sulfonamide Inhibition Profile of the β-Carbonic Anhydrase from Malassezia restricta, An Opportunistic Pathogen Triggering Scalp Conditions. Metabolites 2020, 10, E39. [Google Scholar] [CrossRef]
- Lehneck, R.; Neumann, P.; Vullo, D.; Elleuche, S.; Supuran, C.T.; Ficner, R.; Pöggeler, S. Crystal structures of two tetrameric β-carbonic anhydrases from the filamentous ascomycete Sordaria macrospora. FEBS J. 2014, 281, 1759–1772. [Google Scholar] [CrossRef]
- Vullo, D.; Lehneck, R.; Pöggeler, S.; Supuran, C.T. Sulfonamide inhibition studies of two β-carbonic anhydrases from the ascomycete fungus Sordaria macrospora, CAS1 and CAS2. J. Enzyme Inhib. Med. Chem. 2018, 33, 390–396. [Google Scholar] [CrossRef]
- Del Prete, S.; De Luca, V.; Vullo, D.; Osman, S.M.; AlOthman, Z.; Carginale, V.; Supuran, C.T.; Capasso, C. A new procedure for the cloning, expression and purification of the beta-carbonic anhydrase from the pathogenic yeast Malassezia globosa, an anti-dandruff drug target. J. Enzyme Inhib. Med. Chem. 2016, 31, 1156–1161. [Google Scholar] [CrossRef]
- Ferraroni, M.; Del Prete, S.; Vullo, D.; Capasso, C.; Supuran, C.T. Crystal structure and kinetic studies of a tetrameric type II beta-carbonic anhydrase from the pathogenic bacterium Vibrio cholerae. Acta Crystallogr. D Biol. Crystallogr. 2015, 71, 2449–2456. [Google Scholar] [CrossRef]
- Nishimori, I.; Minakuchi, T.; Vullo, D.; Scozzafava, A.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Cloning, characterization, and inhibition studies of a new beta-carbonic anhydrase from Mycobacterium tuberculosis. J. Med. Chem. 2009, 52, 3116–3120. [Google Scholar] [CrossRef]
- Schlicker, C.; Hall, R.A.; Vullo, D.; Middelhaufe, S.; Gertz, M.; Supuran, C.T.; Muhlschlegel, F.A.; Steegborn, C. Structure and inhibition of the CO2-sensing carbonic anhydrase Can2 from the pathogenic fungus Cryptococcus neoformans. J. Mol. Biol. 2009, 385, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T.; Capasso, C. An Overview of the Bacterial Carbonic Anhydrases. Metabolites. 2017, 7, E56. [Google Scholar] [CrossRef]
- Khalifah, R.G. The carbon dioxide hydration activity of carbonic anhydrase. I. Stop-flow kinetic studies on the native human isoenzymes B and C. J. Biol. Chem. 1971, 246, 2561–2573. [Google Scholar] [PubMed]
- Innocenti, A.; Hall, R.A.; Schlicker, C.; Scozzafava, A.; Steegborn, C.; Mühlschlegel, F.A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition and homology modeling studies of the fungal beta-carbonic anhydrase from Candida albicans with sulfonamides. Bioorg. Med. Chem. 2009, 17, 4503–4509. [Google Scholar] [PubMed]
- Isik, S.; Kockar, F.; Arslan, O.; Guler, O.O.; Innocenti, A.; Supuran, C.T. Carbonic anhydrase inhibitors. Inhibition of the beta-class enzyme from the yeast Saccharomyces cerevisiae with anions. Bioorg. Med. Chem. Lett. 2008, 18, 6327–6331. [Google Scholar] [CrossRef]
- Durdagi, S.; Vullo, D.; Pan, P.; Kähkönen, N.; Määttä, J.A.; Hytönen, V.P.; Scozzafava, A.; Parkkila, S.; Supuran, C.T. Protein-protein interactions: Inhibition of mammalian carbonic anhydrases I-XV by the murine inhibitor of carbonic anhydrase and other members of the transferrin family. J. Med. Chem. 2012, 55, 5529–5535. [Google Scholar] [CrossRef]
- Carta, F.; Scozzafava, A.; Supuran, C.T. Sulfonamides: A patent review (2008–2012). Expert Opin. Ther. Pat. 2012, 22, 747–758. [Google Scholar] [CrossRef]
- Supuran, C.T. Applications of carbonic anhydrases inhibitors in renal and central nervous system diseases. Expert Opin. Ther. Pat. 2018, 28, 713–721. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrase inhibitors and their potential in a range of therapeutic areas. Expert Opin. Ther. Pat. 2018, 28, 709–712. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin. Emerg. Drugs 2012, 17, 11–15. [Google Scholar] [CrossRef]
- Supuran, C.T.; Altamimi, A.S.A.; Carta, F. Carbonic anhydrase inhibition and the management of glaucoma: A literature and patent review 2013–2019. Expert Opin. Ther. Pat. 2019, 29, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors as emerging agents for the treatment and imaging of hypoxic tumors. Expert Opin. Investig. Drugs 2018, 27, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. The management of glaucoma and macular degeneration. Expert Opin. Ther. Pat. 2019, 29, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T.H.; Tran, T.N.; Podgorski, M.N.; Bell, S.G.; Supuran, C.T.; Donald, W.A. Nanoscale Ion Emitters in Native Mass Spectrometry for Measuring Ligand-Protein Binding Affinities. ACS. Cent. Sci. 2019, 5, 308–318. [Google Scholar] [CrossRef]
- Nocentini, A.; Donald, W.A.; Supuran, C.T. Human carbonic anhydrases: Tissue distribution, physiological role, and druggability. In Carbonic Anhydrases–Biochemistry and Pharmacology of an Evergreen Pharmaceutical Target; Supuran, C.T., Nocentini, A., Eds.; Elsevier–Academic Press: London, UK, 2019; pp. 151–185. [Google Scholar]
Sample Availability: Samples of the inhibitors are potentially available upon request from the authors. |
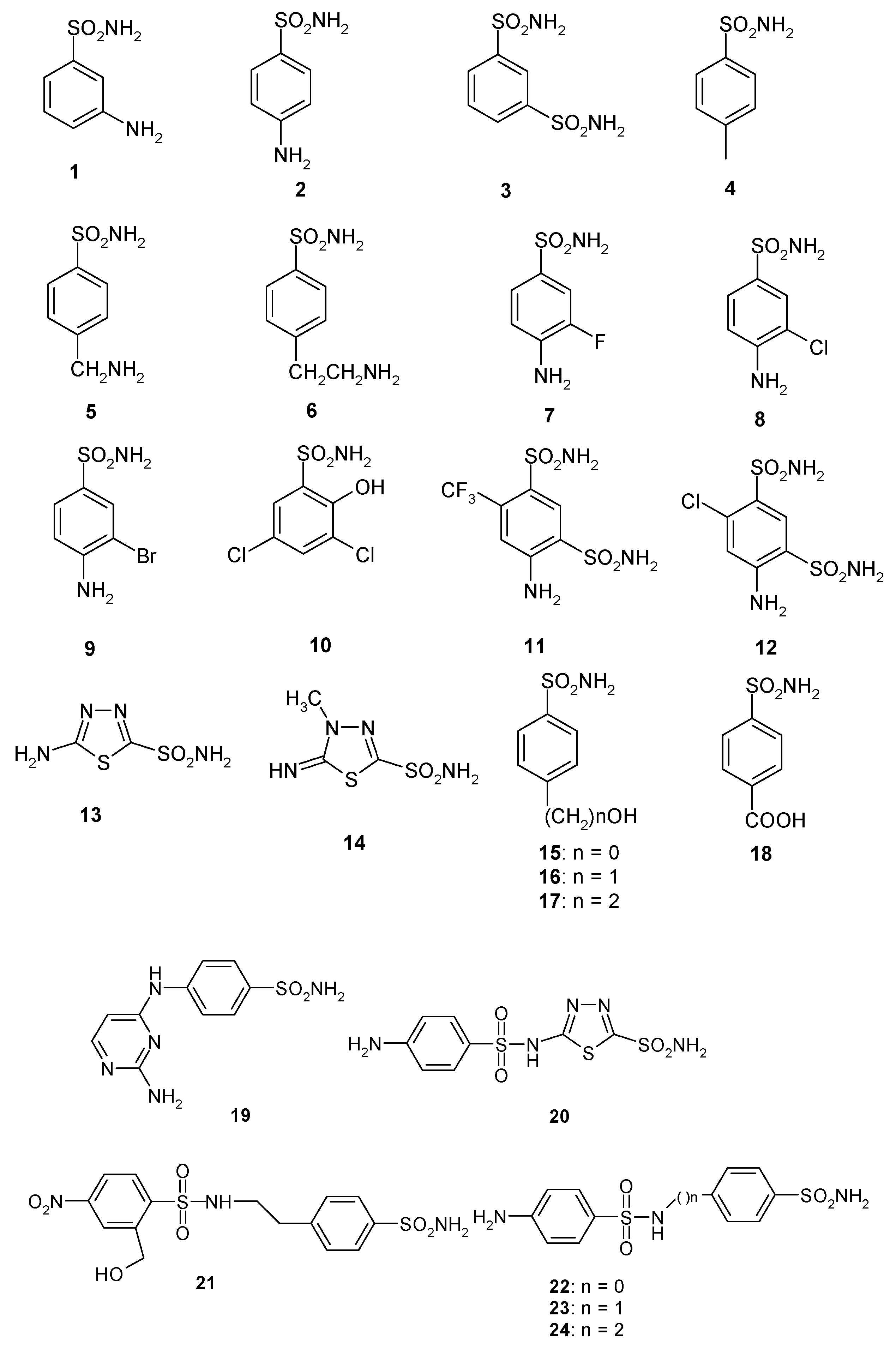

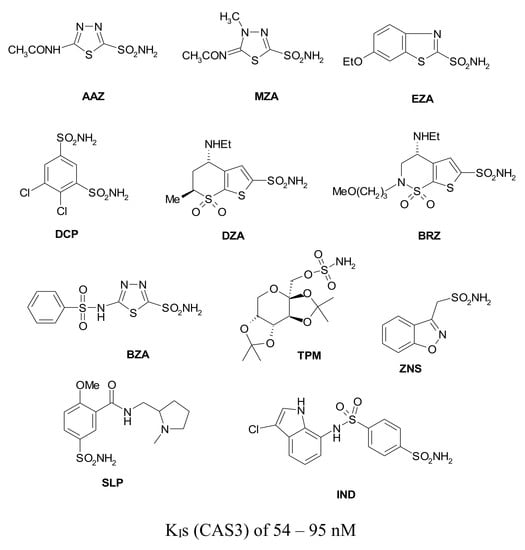
| Isozyme | Activity Level | Kcat (s−1) | Kcat/Km (M−1∙s−1) | KI (Acetazolamide) (nM) |
|---|---|---|---|---|
| hCA I a | Moderate | 2.0 × 105 | 5.0 × 107 | 250 |
| hCA II a | Very high | 1.4 × 106 | 1.5 × 108 | 12 |
| Can2 b | Moderate | 3.9 × 105 | 4.3 × 107 | 10.5 |
| CalCA c | High | 8.0 × 105 | 9.7 × 107 | 132 |
| SceCA c | High | 9.4 × 105 | 9.8 × 107 | 82 |
| CAS1 d | Low | 1.2 × 104 | 1.30 × 106 | 445 |
| CAS2 d | Low | 1.3 × 104 | 1.21 × 106 | 816 |
| CAS3 e | High | (7.9 ± 0.2) × 105 | (9.5 ± 0.12) × 107 | 94 ± 3 |
| Inhibitor/ Enzyme Class | KI* (nM) | ||||
|---|---|---|---|---|---|
| hCA Ia | hCA II a | CAS1b | CAS2 b | CAS3 c | |
| α | α | β | β | β | |
| 1 | 28,000 | 300 | 361 | 386 | 90 ± 7.1 |
| 2 | 25,000 | 240 | 144 | 3480 | 84 ± 5.6 |
| 3 | 79 c | 8 | 225 | 3630 | 83 ± 6.0 |
| 4 | 78,500 | 320 | 47.1 | 6900 | 560 ± 21 |
| 5 | 25,000 | 170 | 323 | 8720 | 726 ± 30 |
| 6 | 21,000 | 160 | 241 | 7650 | 441 ± 14 |
| 7 | 8300 | 60 | 43.2 | 7360 | 585 ± 23 |
| 8 | 9800 | 110 | 79.6 | 9120 | 2078 ± 61 |
| 9 | 6500 | 40 | 580 | 12,000 | 712 ± 28 |
| 10 | 7300 | 54 | >50,000 | 23,500 | 350 ± 13 |
| 11 | 5800 | 63 | 890 | 18,700 | 235 ± 16 |
| 12 | 8400 | 75 | 3350 | >50,000 | 90 ± 5.7 |
| 13 | 8600 | 60 | 8650 | 48.1 | 88 ± 2.9 |
| 14 | 9300 | 19 | 7215 | 280 | 94 ± 4.1 |
| 15 | 5500 | 80 | 3160 | 143 | 605 ± 23 |
| 16 | 9500 | 94 | 4520 | 92.5 | 82 ± 3.7 |
| 17 | 21,000 | 125 | >50,000 | 390 | 507 ± 26 |
| 18 | 164 | 46 | 4435 | 3250 | 226 ± 13 |
| 19 | 109 | 33 | 475 | 6760 | 91 ± 4.4 |
| 20 | 6 | 2 | 363 | 9880 | 85 ± 2.7 |
| 21 | 69 | 11 | 4550 | 4060 | 95 ± 3.9 |
| 22 | 164 | 46 | 1985 | 25,200 | 85 ± 2.6 |
| 23 | 109 | 33 | 282 | >50,000 | 89 ± 3.4 |
| 24 | 95 | 30 | 294 | >50,000 | 84 ± 1.5 |
| AAZ | 250 | 12 | 445 | 816 | 94 ± 3.0 |
| MZA | 50 | 14 | 421 | 8140 | 91 ± 6.2 |
| EZA | 25 | 8 | 440 | 3170 | 95 ± 2.8 |
| DCP | 1200 | 38 | 1220 | 5790 | 73 ± 3.0 |
| DZA | 50,000 | 9 | 360 | 742 | 274 ± 14 |
| BRZ | 45,000 | 3 | 451 | 739 | 61 ± 1.4 |
| BZA | 15 | 9 | 2115 | 410 | 54 ± 0.9 |
| TPM | 250 | 10 | 414 | 673 | 363 ± 23 |
| ZNS | 56 | 35 | 1820 | 1885 | 710 ± 40 |
| SLP | 1200 | 40 | 1715 | 670 | 493 ± 27 |
| IND | 31 | 15 | 4240 | 216 | 94 ± 4.6 |
| VLX | 54,000 | 43 | 4425 | 3730 | 831 ± 38 |
| CLX | 50,000 | 21 | 2513 | 857 | 669 ± 29 |
| SLT | 374 | 9 | 3210 | 496 | 4838 ± 257 |
| SAC | 18,540 | 5959 | 5280 | 7075 | 191 ± 12 |
| HCT | 328 | 290 | 3350 | 6680 | 545 ± 41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vullo, D.; Lehneck, R.; Donald, W.A.; Pöggeler, S.; Supuran, C.T. Sulfonamide Inhibition Studies of the β-Class Carbonic Anhydrase CAS3 from the Filamentous Ascomycete Sordaria macrospora. Molecules 2020, 25, 1036. https://doi.org/10.3390/molecules25051036
Vullo D, Lehneck R, Donald WA, Pöggeler S, Supuran CT. Sulfonamide Inhibition Studies of the β-Class Carbonic Anhydrase CAS3 from the Filamentous Ascomycete Sordaria macrospora. Molecules. 2020; 25(5):1036. https://doi.org/10.3390/molecules25051036
Chicago/Turabian StyleVullo, Daniela, Ronny Lehneck, William A. Donald, Stefanie Pöggeler, and Claudiu T. Supuran. 2020. "Sulfonamide Inhibition Studies of the β-Class Carbonic Anhydrase CAS3 from the Filamentous Ascomycete Sordaria macrospora" Molecules 25, no. 5: 1036. https://doi.org/10.3390/molecules25051036
APA StyleVullo, D., Lehneck, R., Donald, W. A., Pöggeler, S., & Supuran, C. T. (2020). Sulfonamide Inhibition Studies of the β-Class Carbonic Anhydrase CAS3 from the Filamentous Ascomycete Sordaria macrospora. Molecules, 25(5), 1036. https://doi.org/10.3390/molecules25051036