DNA-Binding and Anticancer Activity of Binuclear Gold(I) Alkynyl Complexes with a Phenanthrenyl Bridging Ligand
Abstract
1. Introduction
2. Results and Discussion
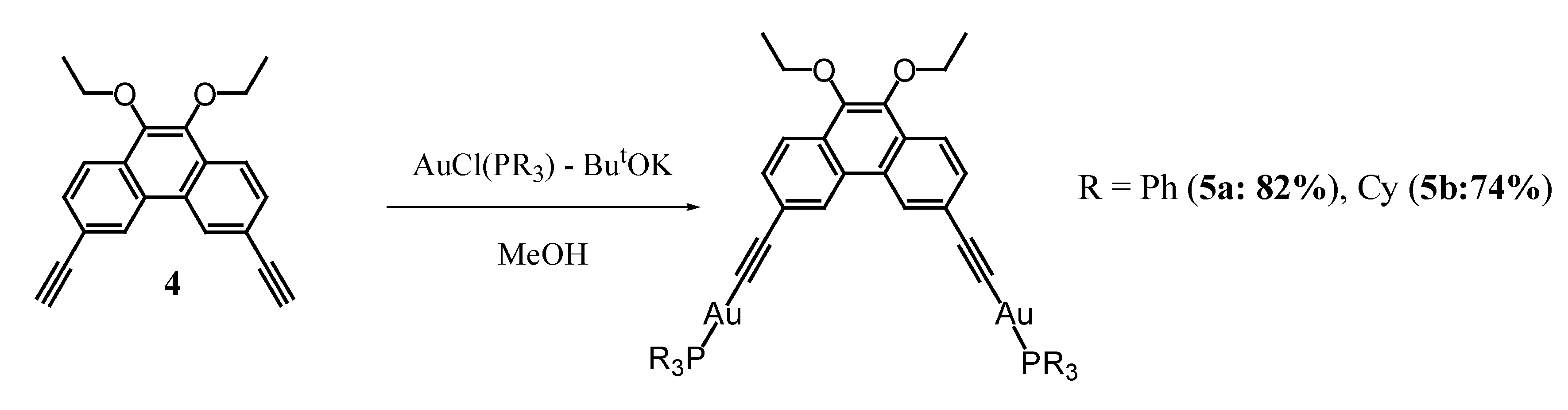
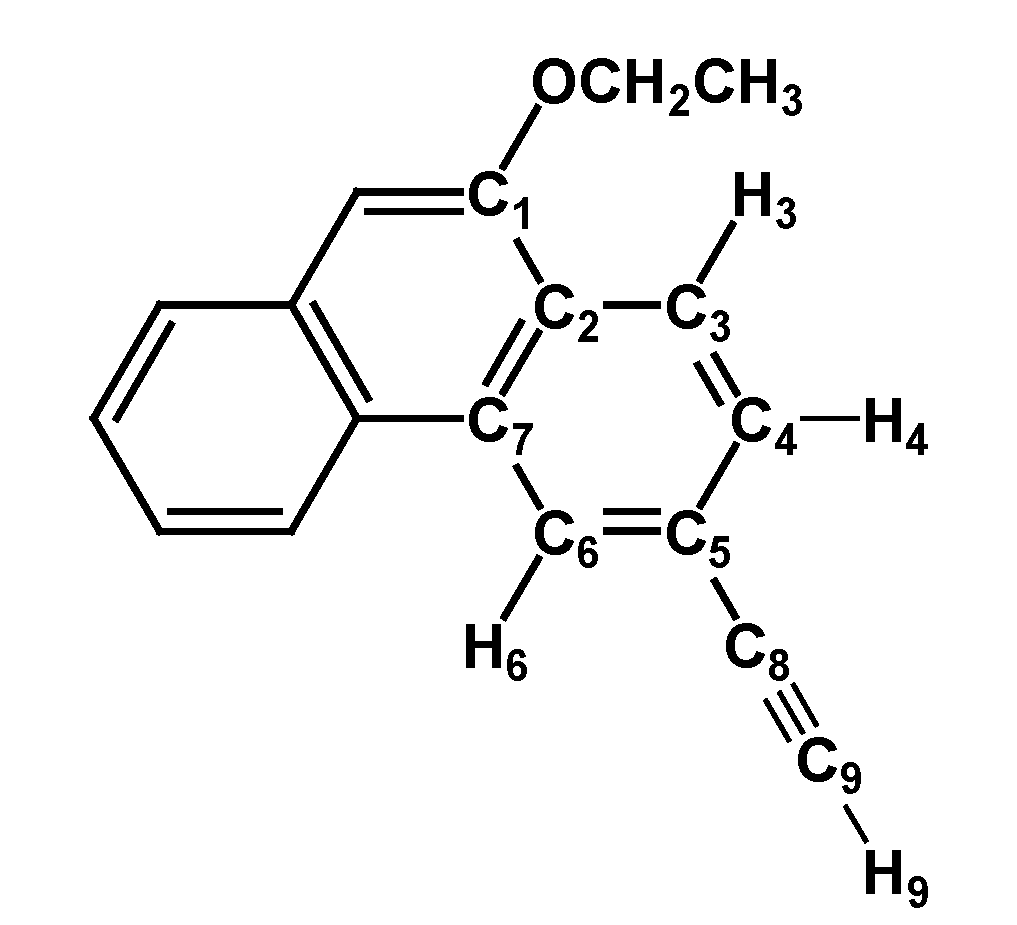
2.1. Synthesis and Characterization
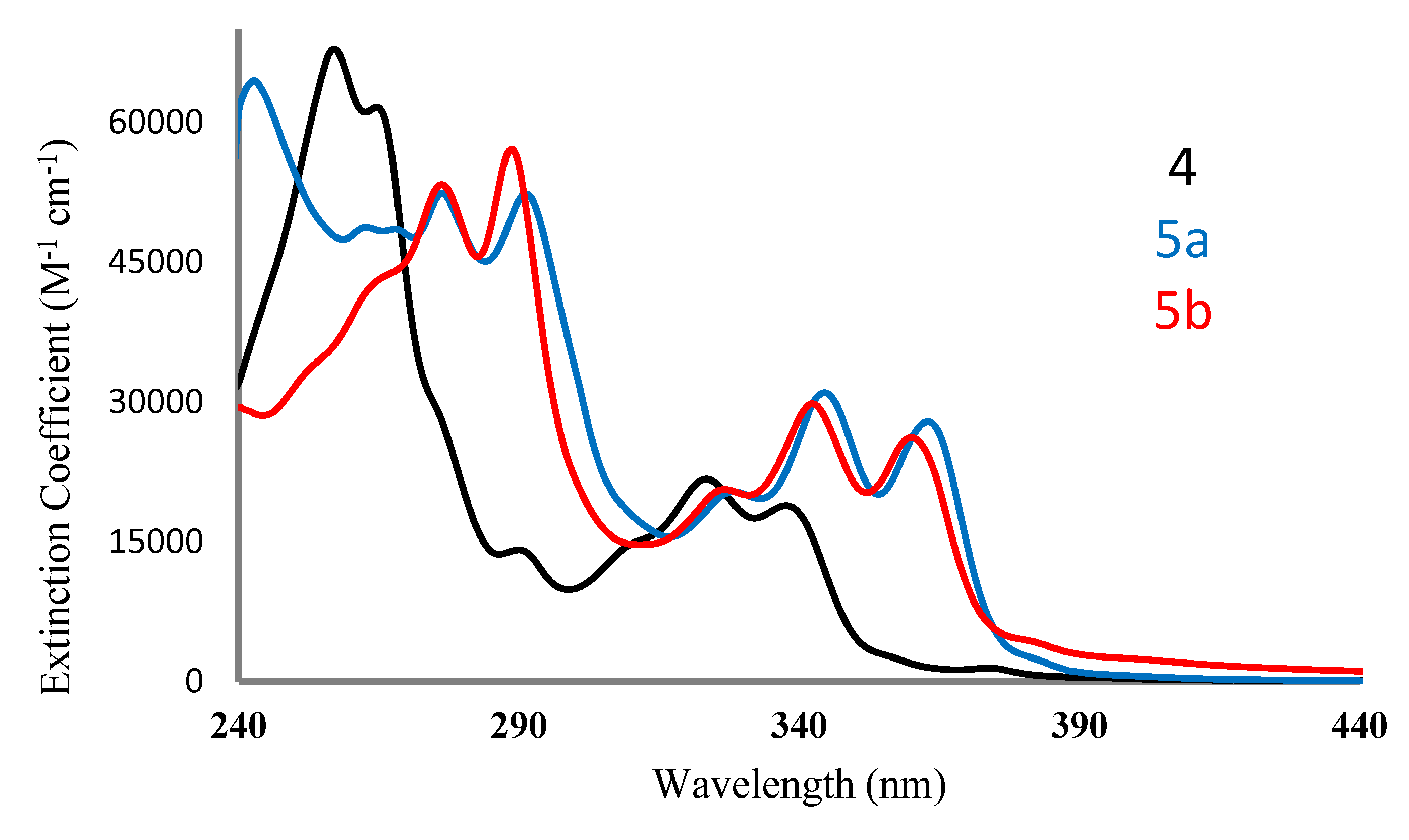
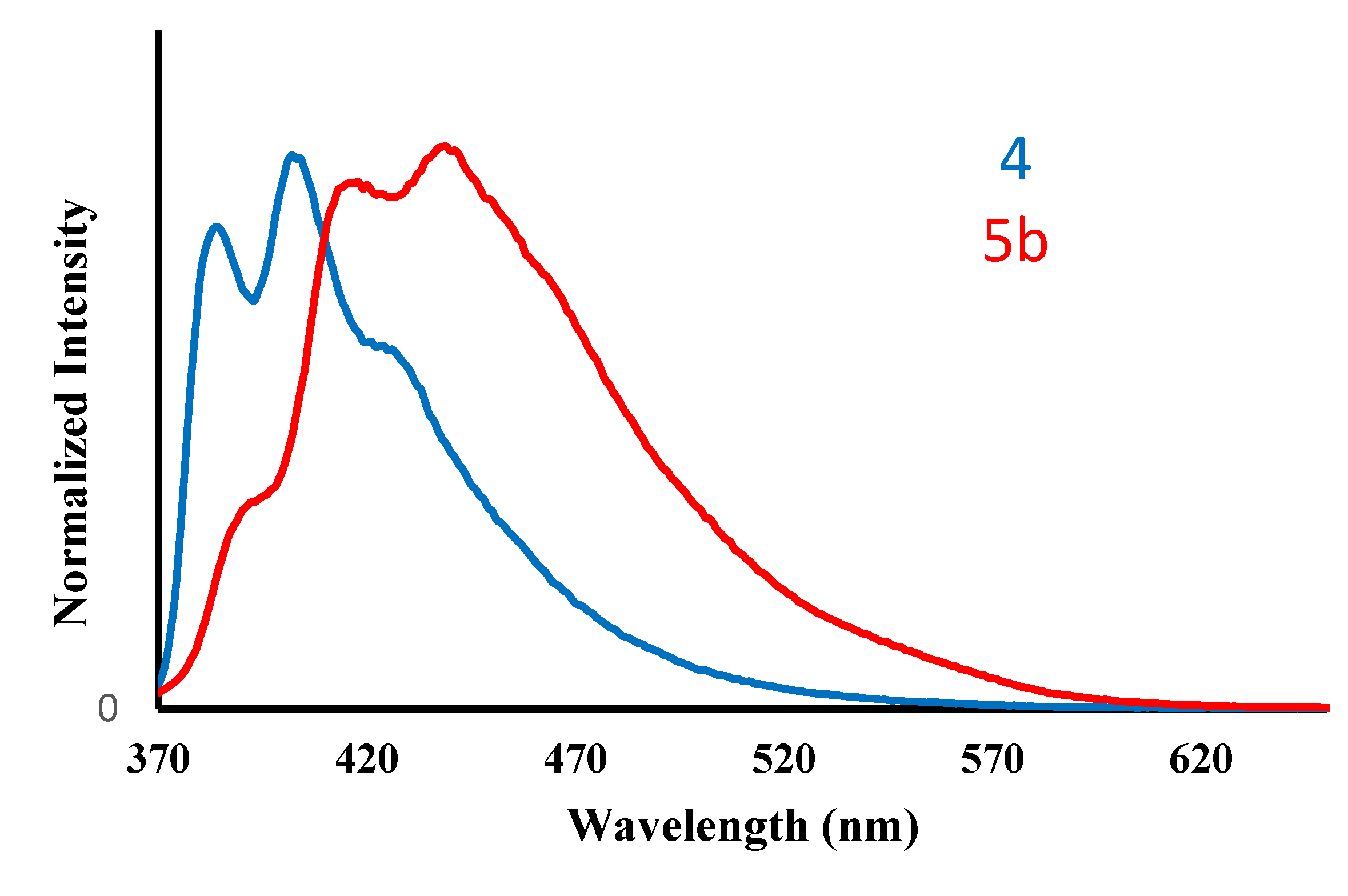
2.2. Absorption and Emission Spectra
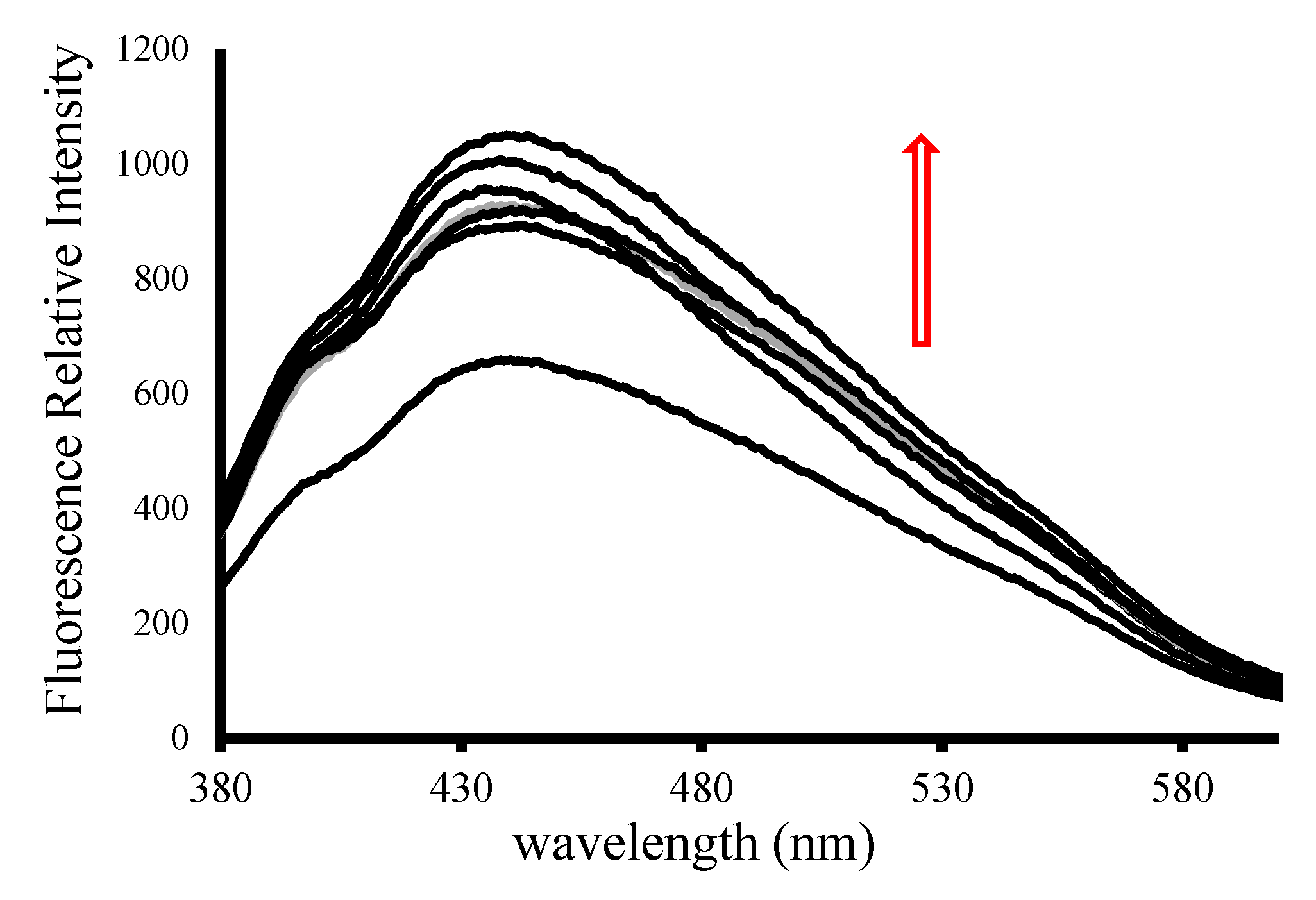
2.3. DNA-Binding Studies
2.4. Molecular Docking Studies
2.5. Anticancer Studies
3. Materials and Methods
3.1. Materials
3.2. Methods and Instrumentation
3.3. Synthesis and Characterization
3.4. DNA Binding Studies
3.4.1. Determination of the DNA Binding Constant using UV-Vis Absorption
3.4.2. Determining the Mode of Interaction by Fluorescence
3.5. Molecular Docking Studies
3.6. Anticancer Activity and Cytotoxcicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TG-CA | Thymine/guanine-cytosine/adenine. |
| Ct-DNA | calf-thymus DNA |
| MCF-7 | abbreviated from Michigan Cancer Foundation-7; a breast cancer cell line. |
| HEPG-2 | hepatic liver carcinoma cell line. |
| PC-3 | hhuman prostate cancer cell line. |
| MOLT-4 | Human acute lymphoblastic leukemia cell line. |
References
- Cram, D.J. The discovery of crown ethers. J. Incl. Phenom. Macro. 1988, 6, 397–413. [Google Scholar] [CrossRef]
- Cram, D.J. Molecular container compounds. Nature 1992, 356, 29–36. [Google Scholar] [CrossRef]
- Lehn, J.-M.J. Supramolecular chemistry- scope and perspectives: Molecules—supermolecules-molecular devices. Incl. Phenom. Macro 1988, 6, 351–396. [Google Scholar] [CrossRef]
- Lehn, J.-M. Constitutional dynamic chemistry: Bridge from supramolecular chemistry to adaptive chemistry. Top. Curr. Chem. 2012, 322, 1–32. [Google Scholar]
- Pedersen, C.J. Macrocyclic polyethers and their complexes. Angew. Chem. Int. Ed. Engl. 1972, 11, 16–25. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, G.; Huang, F. Supramolecular chemotherapy based on host–guest molecular recognition: A novel strategy in the battle against cancer with a bright future. Chem. Soc. Rev. 2017, 46, 7021–7053. [Google Scholar] [CrossRef]
- Chaires, J.B. Dissecting the free energy of drug binding to DNA. Anticancer Drug Des. 1996, 11, 569–680. [Google Scholar]
- Hambley, T.W. Platinum binding to DNA: Structural controls and consequences. J. Chem. Soc. Dalton Trans. 2001, 19, 2711–2718. [Google Scholar] [CrossRef]
- Brabec, V.; Novakova, O. DNA binding mode of ruthenium complexes and relationship to tumor cell toxicity. Drug Resist. Updates 2006, 9, 111–122. [Google Scholar] [CrossRef]
- Xiong, Y.; Ji, L.-N. Synthesis, DNA-binding and DNA-mediated luminescence quenching of Ru(II) polypyridine complexes. Coord. Chem. Rev. 1999, 185–186, 711–733. [Google Scholar] [CrossRef]
- Brissos, R.F.; Caubet, A.; Gamez, P. Possible DNA-Interacting Pathways for Metal-Based Compounds Exemplified with Copper Coordination Compounds. Eur. J. Inorg. Chem. 2015, 6, 2633–2645. [Google Scholar] [CrossRef]
- Barone, G.; Terenzi, A.; Lauria, A.; Almerico, A.M.; Leal, J.M.; Busto, N.; Garcia, B. DNA-binding of nickel(II), copper(II) and zinc(II) complexes: Structure–affinity relationships. Coord. Chem. Rev. 2013, 257, 2848–2862. [Google Scholar] [CrossRef]
- Amir, M.K.; Khan, S.Z.; Hayat, F.; Hassan, A.; Butler, I.S. Zia-ur-Rahman, Anticancer Activity, DNA-binding and DNA-denaturation Aptitude of Palladium(II) Dithiocarbamates. Inorg. Chim. Acta 2016, 451, 31–40. [Google Scholar] [CrossRef]
- Zaki, M.; Arjmand, F.; Tabassum, S. Current and future potential of metallo drugs: Revisiting DNA-binding of metal containing molecules and their diverse mechanism of action. Inorg. Chim. Acta 2016, 444, 1–22. [Google Scholar] [CrossRef]
- Wilson, W.D.; Jones, R.L. Intercalating drugs: DNA binding and molecular pharmacology. In Advances in Pharmacology; Silvio, A.G., Ed.; Academic Press: London, UK, 1981; Volume 13, pp. 177–222. [Google Scholar]
- Rescifina, A.; Zagni, C.; Verrica, M.G.; Pistara, V.; Corsaro, A. Recent advances in small organic molecules as DNA intercalating agents: Synthesis, activity, and modeling. Eur. J. Med. Chem. 2014, 74, 95–115. [Google Scholar] [CrossRef]
- Galind-Murillo, R.; Garcia-Ramos, J.C.; Ruiz-Azurara, L.; Cheatham, T.E.; Cortes-Guzman, F. Intercalation processes of copper complexes in DNA. Nucleic Acids Res. 2015, 43, 5364–5376. [Google Scholar] [CrossRef]
- Sundaravadivel, E.; Reddy, G.R.; Manoj, D.; Rajendran, S.; Kandaswamy, M.; Janakiraman, M. DNA binding and cleavage studies of copper(II) complex containing N2O2 Schiff base ligand. Inorg. Chim. Acta 2018, 482, 170–178. [Google Scholar] [CrossRef]
- Farhangian, H.; Moghadam, M.E.; Divsalar, A.; Rahiminezhad, A. Anticancer activity of novel amino acid derivative of palladium complex with phendione ligand against of human colon cancer cell line. J. Biol. Inorg. Chem. 2017, 22, 1055–1064. [Google Scholar] [CrossRef]
- Nikolic, S.; Rangasamy, L.; Gligorijevic, N.; Arandelovic, S.; Radulovic, S.; Gasser, G.; Grguric-Sipka, S. Synthesis, Characterization and Biological Evaluation of Novel Ru(II)-Arene Complexes Containing Intercalating Ligands. J. Inorg. Biochem. 2016, 160, 156–165. [Google Scholar] [CrossRef]
- Valladolid, J.; Hortiguela, C.; Busto, N.; Espino, G.; Rodriguez, A.M.; Leal, J.M.; Jolan, F.A.; Manzano, B.R.; Carbayo, A.; Garcia, B. Phenanthroline ligands are biologically more active than their corresponding ruthenium(II) arene complexes. Dalton Trans. 2014, 43, 2629–2645. [Google Scholar] [CrossRef]
- Busto, N.; Martinez-Alonso, M.; Leal, J.M.; Rodriguez, A.M.; Domingueze, F.; Acuna, M.I.; Espino, G.; Garcia, B. Monomer-Dimer Divergent Behavior toward DNA in a Half-Sandwich Ruthenium(II) Aqua Complex. Antiproliferative Biphasic Activity. Organometallics 2015, 34, 319–327. [Google Scholar] [CrossRef]
- Ljubijankic, N.; Zahirovic, A.; Turkusic, E.; Kahrovic, E. DNA Binding Properties of Two Ruthenium(III) Complexes Containing Schiff Bases Derived from Salicylaldehyde: Spectroscopic and Electrochemical Evidence of CT DNA Intercalation. Croat. Chem. Acta 2013, 86, 215–222. [Google Scholar] [CrossRef]
- Kocak, A.; Yilmaz, H.; Faiz, O.; Andac, O. Experimental and theoretical studies on Cu(II) complex of N,N′-disalicylidene-2,3-diaminopyridine ligand reveal indirect evidence for DNA intercalation. Polyhedron 2016, 104, 106–115. [Google Scholar] [CrossRef]
- Sharma, S.; Panjamurthy, K.; Choudhary, B.; Srivastava, M.; Shahabuddin, M.S.; Giri, R.; Advirao, G.M.; Roghavan, S.C. A novel DNA intercalator, 8-methoxy pyrimido[4′,5′:4,5]thieno (2,3-b)quinoline-4(3H)-one induces apoptosis in cancer cells, inhibits the tumor progression and enhances lifespan in mice with tumor. Mol. Carcinog. 2013, 52, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Banik, B.K.; Becker, F.F. Synthesis, electrophilic substitution and structure-activity relationship studies of polycyclic aromatic compounds towards the development of anticancer agents. Curr. Med. Chem. 2001, 8, 1513–1533. [Google Scholar] [CrossRef] [PubMed]
- Holmlin, R.E.; Dendliker, P.J.; Barton, J.K. Synthesis of metallointercalator-DNA conjugates on a solid support. Bioconj. Chem. 1999, 10, 1122–1130. [Google Scholar] [CrossRef][Green Version]
- Notaro, A.; Gasser, G. Monomeric and dimeric coordinatively saturated and substitutionally inert Ru(ii) polypyridyl complexes as anticancer drug candidates. Chem. Soc. Rev. 2017, 46, 7317–7337. [Google Scholar] [CrossRef]
- Zhao, C.Q.; Sun, Y.H.; Ren, J.S.; Qu, X.G. Recent progress in lanthanide complexes for DNA sensing and targeting specific DNA structures. Inorg. Chim. Acta 2016, 452, 50–61. [Google Scholar] [CrossRef]
- Esmail, S.A.A.; Shamsi, M.; Chen, T.; Al-asbahy, W.M. Design, synthesis and characterization of Tin–based cancer chemotherapy drug entity; in vitro DNA binding, cleavage studies, induce cancer cell apoptosis by triggering DNA damage–mediated p53 phosphorylation and Molecular docking studies. Appl. Organomet. Chem. 2018, 33, e4651. [Google Scholar] [CrossRef]
- Wong, E.; Giandomenico, C.M. Current status of platinum-based antitumor drugs. Chem. Rev. 1999, 99, 2451–2466. [Google Scholar] [CrossRef]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Guo, Z.J. Towards the rational design of platinum(ii) and gold(iii) complexes as antitumour agents. Dalton Trans. 2008, 12, 1521–1532. [Google Scholar] [CrossRef] [PubMed]
- Rabik, C.A.; Dolan, M.E. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat. Rev. 2007, 33, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Heffeter, P.; Jungwirth, U.; Jakupec, M.; Hartinger, C.; Galanski, M.; Elbling, L.; Micksche, M.; Keppler, B.; Berger, W. Resistance against novel anticancer metal compounds: Differences and similarities. Drug Resist. Updates 2008, 11, 1–16. [Google Scholar] [CrossRef]
- Meyer, A.; Gutierrez, A.; Ott, I.; Rodriguez, L. Phosphine-bridged dinuclear gold(I) alkynyl complexes: Thioredoxin reductase inhibition and cytotoxicity. Inorg. Chim. Acta 2013, 398, 72–76. [Google Scholar] [CrossRef]
- Andermark, V.; Goke, K.; Kokoschka, M.; Abu el Maaty, M.A.; Lum, C.T.; Zou, T.; Sun, R.W.-Y.; Aguilo, E.; Oehninger, L.; Rodriguez, L.; et al. Alkynyl gold(I) phosphane complexes: Evaluation of structure-activity-relationships for the phosphane ligands, effects on key signaling proteins and preliminary in-vivo studies with a nanoformulated complex. J. Inorg. Biochem. 2016, 160, 140–148. [Google Scholar] [CrossRef]
- Marmol, I.; Virumbrales-Munoz, M.; Quero, J.; Sánchez-de-Diego, C.; Fernández, L.; Ochoa, I.; Cerrada, E.; Yoldi, M.J.R. Alkynyl gold(I) complex triggers necroptosis via ROS generation in colorectal carcinoma cells. J. Inorg. Biochem. 2017, 176, 123–133. [Google Scholar] [CrossRef]
- Shaw, C.F. Gold-Based Therapeutic Agents. Chem. Rev. 1999, 99, 2589–2600. [Google Scholar] [CrossRef]
- Bagowski, C.P.; You, Y.; Scheffler, H.; Velcken, D.H.; Schmitz, D.J.; Ott, I. Naphthalimide gold(I) phosphine complexes as anticancer metallodrugs. Dalton Trans. 2009, 48, 10799–10805. [Google Scholar] [CrossRef]
- Abas, E.; Espallargas, N.; Burbello, G.; Mesonero, J.E.; Rodriguez-Dieguez, A.; Grasa, L.; Laguna, M. Anticancer Activity of Alkynylgold(I) with P(NMe2)3 Phosphane in Mouse Colon Tumors and Human Colon Carcinoma Caco-2 Cell Line. Inorg. Chem. 2019, 58, 15536–15551. [Google Scholar] [CrossRef]
- Marmol, I.; Castellnou, P.; Alvarez, R.; Gimeno, M.C.; Rodríguez-Yoldi, M.J.; Cerrada, E. Alkynyl gold(I) complexes derived from 3-hydroxyflavones as multi-targeted drugs against colon cancer. Eur. J. Med. Chem. 2019, 183, 111661. [Google Scholar] [CrossRef] [PubMed]
- Caikovskii, V.K.; Filimonov, V.D.; Skorokhodov, V.I.; Ogorodnikov, V.D. Superactivity and dual reactivity of the system N-Iodosuccinimide-H2SO4 in the lodination of deactivated arenes. Russ. J. Org. Chem. 2007, 43, 1278–1281. [Google Scholar] [CrossRef]
- Suh, Y.-G.; Lee, Y.-S.; Min, K.-H.; Park, O.-H.; Kim, J.-K.; Seung, H.-S.; Seo, S.-Y.; Lee, B.-Y.; Nam, Y.-H.; Lee, K.-O.; et al. Novel potent antagonists of transient receptor potential channel, vanilloid subfamily member 1: Structure-activity relationship of 1,3-diarylalkyl thioureas possessing new vanilloid equivalents. J. Med. Chem. 2005, 48, 5823–5836. [Google Scholar] [CrossRef] [PubMed]
- Iskra, J.; Stavber, S.; Zupan, M. Nonmetal-Catalyzed Iodination of Arenes with Iodide and Hydrogen Peroxide. Synthesis 2004, 11, 1869–1873. [Google Scholar] [CrossRef]
- Balasingham, R.G.; Williams, C.F.; Mottram, H.J.; Coogan, M.P.; Pope, S.J.A. Gold(I) Complexes Derived from Alkynyloxy-Substituted Anthraquinones: Syntheses, Luminescence, Preliminary Cytotoxicity, and Cell Imaging Studies. Organometallics. Organometallics 2012, 31, 5835–5843. [Google Scholar] [CrossRef]
- Long, N.J.; Williams, C.H. Metal alkynyl σ complexes: Synthesis and materials. Angew. Chem. Int. Ed. 2003, 42, 2586–2617. [Google Scholar] [CrossRef]
- Karver, M.R.; Krishnamurthy, D.; Kulkarni, R.A.; Bottini, N.; Barrios, A.M. Identifying Potent, Selective protein tyrosine phosphatase inhibitors from a library of Au(I) complexes. J. Med. Chem. 2009, 52, 6912–6918. [Google Scholar] [CrossRef]
- Barlow, A.; Babgi, B.; Samoc, M.; Corkery, T.C.; van Cleuvenbergen, S.; Asselberghs, I.; Clays, K.; Cifuentes, M.P.; Humphrey, M.G. Organometallic complexes for non-linear optics. 51. Second- and third-order non-linear optical properties of alkynylgold complexes. Aust. J. Chem. 2012, 65, 834–841. [Google Scholar] [CrossRef]
- Chao, H.-Y.; Lu, W.; Li, Y.; Chan, M.C.W.; Che, C.-M.; Cheung, K.-K.; Zhu, N. Organic Triplet Emissions of Arylacetylide Moieties Harnessed through Coordination to [Au(PCy3)]+. Effect of Molecular Structure upon Photoluminescent Properties. J. Am. Chem. Soc. 2002, 124, 14696–14706. [Google Scholar] [CrossRef]
- Chui, C.-H.; Wong, R.S.-M.; Gambari, R.; Cheng, G.Y.-M.; Yuen, M.C.-W.; Chan, K.-W.; Tong, S.-W.; Lau, F.-Y.; Lai, P.B.-S.; Lam, K.-H.; et al. Antitumor activity of diethynylfluorene derivatives of gold(I). Bioorg. Med. Chem. 2009, 17, 7872. [Google Scholar] [CrossRef]
- Ali, R.; Razi, S.S.; Shahid, M.; Srivastava, P.; Misra, A. Off-On-Off fluorescence behavior of an intramolecular charge transfer probe toward anions and CO2, Spectrochim. Acta A Mol. Biomol. Spec. 2016, 168, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.I.; Nicholsan, B.K.; Bin Shawkataly, O.; Shapley, J.R.; Henly, T. Synthesis of Gold-Containing Mixed-Metal Cluster Complexes. Inorg. Synth. 1989, 26, 324–328. [Google Scholar]
- Isab, A.A.; Fettouhi, M.; Ahmad, S.; Ouahab, L. Mixed ligand gold (I) complexes of phosphines and thiourea and X-ray structure of (thiourea-κS)(tricyclohexylphosphine) gold (I) chloride. Polyhedron 2003, 22, 1349–1354. [Google Scholar] [CrossRef]
- Pages, B.J.; Ang, D.L.; Wright, E.P.; Aldrich-Wright, J.R. Metal complex interactions with DNA. Dalton Trans. 2015, 44, 3505–3526. [Google Scholar] [CrossRef]
- Wolfe, A.; Shimer, G.H.; Meehan, T. Polycyclic aromatic hydrocarbons physically intercalate into duplex regions of denatured DNA. Biochemistry 1987, 26, 6392–6396. [Google Scholar] [CrossRef]
- Srishailam, A.; Kumar, Y.P.; Reddy, P.V.; Nambigari, N.; Vuruputuri, U.; Singh, S.S.; Satyanarayana, S. Cellular uptake, cytotoxicity, apoptosis, DNA-binding, photocleavage and molecular docking studies of ruthenium (II) polypyridyl complexes. J. Photochem. Photobiol. B Biol. 2014, 132, 111–123. [Google Scholar] [CrossRef]
- Muanza, D.; Kim, B.; Euler, K.; Williams, L. Antibacterial and antifungal activities of nine medicinal plants from Zaire. Pharm. Biol. 1994, 32, 337–345. [Google Scholar] [CrossRef]
- Pezzuto, J.; Che, C.; McPherson, D.; Zhu, P.; Topcu, G.; Erdelmeier, C.; Cordell, G. DNA as an Affinity Probe Useful in the Detection and Isolation of Biologically Active Natural Products. J. Nat. Prod. 1991, 54, 1522–1597. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Nat. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]

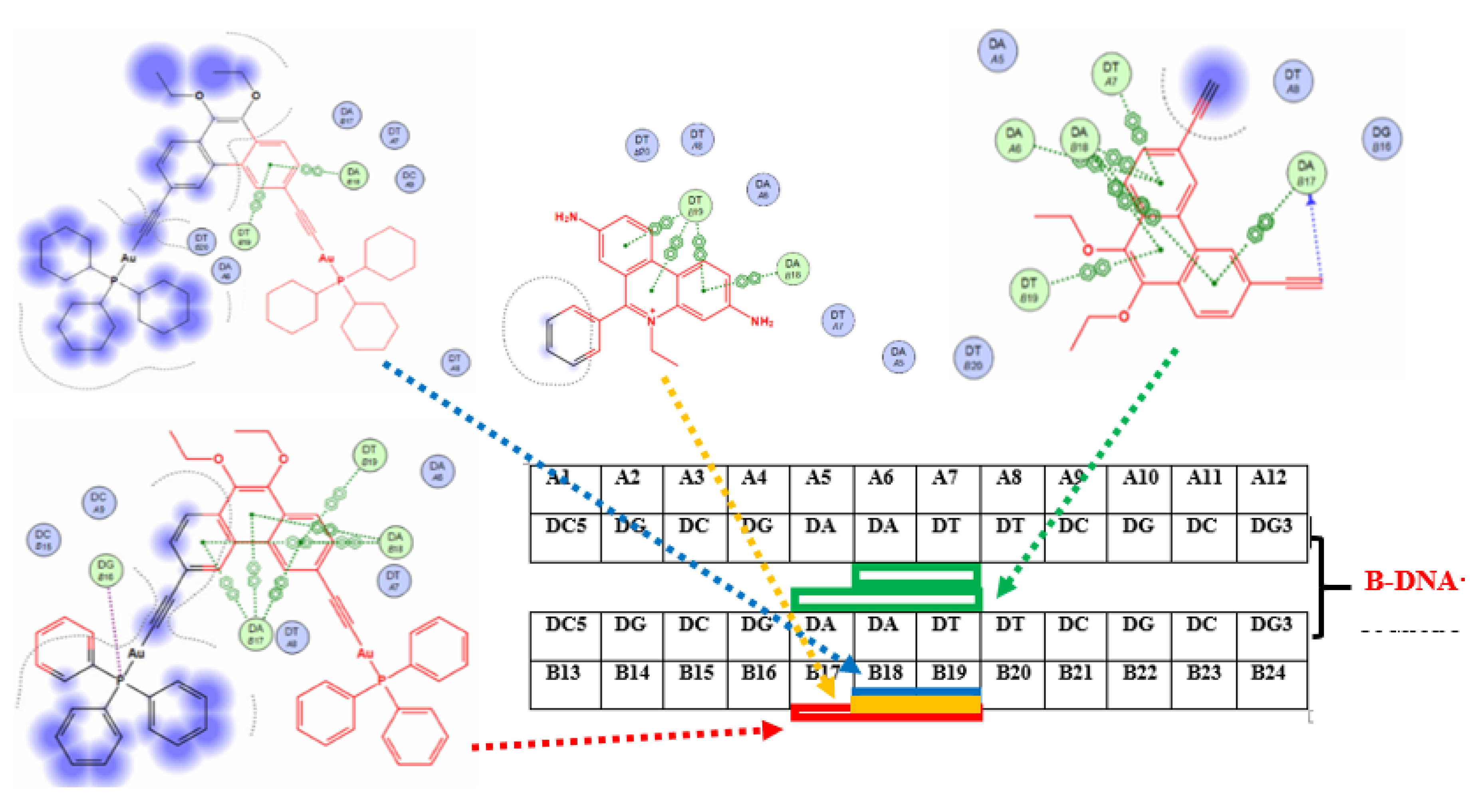
| Compound | Kb (DNA Binding Constant) |
|---|---|
| Ethidium Bromide | 5.00 × 105 |
| 4 | 7.40 × 105 |
| 5a | 8.71 × 105 |
| 5b | 7.00 × 105 |
| Compound | Docking Score | Ligand | Receptor | Interaction | Distance |
|---|---|---|---|---|---|
| Ethidium Bromide | −4.6372 | Phenanthridine | 5-ring DA 18 (B) | pi–pi | 3.80 |
| Phenanthridine | 6-ring DT 19 (B) | pi–pi | 3.83 | ||
| 4 | −5.1630 | C≡C | OP2 DA 17 (B) | H-donor | 3.11 |
| Phenanthrene | 6-ring DA 17 (B) | pi–pi | 3.87 | ||
| Phenanthrene | 5-ring DA 17 (B) | pi–pi | 3.56 | ||
| Phenanthrene | 6-ring DT 19 (B) | pi–pi | 3.36 | ||
| Phenanthrene | 6-ring DA 18 (B) | pi–pi | 3.21 | ||
| Phenanthrene | 6-ring DA 6 (A) | pi–pi | 3.95 | ||
| 5a | −5.4070 | Phenanthrene | 5-ring DA 17 (B) | pi–pi | 3.28 |
| Phenanthrene | 5-ring DA 18 (B) | pi–pi | 3.77 | ||
| Phenanthrene | 5-ring DA 17 (B) | pi–p | 3.65 | ||
| Phenanthrene | 6-ring DA 18 (B) | pi–pi | 3.63 | ||
| Phenanthrene | 6-ring DA 17 (B) | pi–pi | 3.09 | ||
| Phenanthrene | 5-ring DA 17 (B) | pi–pi | 3.75 | ||
| Phenanthrene | 6-ring DT 19 (B) | pi–pi | 3.99 | ||
| 5b | −4.1090 | Phenanthrene | 5-ring DA 18 (B) | pi–pi | 3.41 |
| Phenanthrene | 6-ring DT 19 (B) | pi–pi | 3.50 |
| Complex | IC50 ± SD (μM) | |||
|---|---|---|---|---|
| MCF-7 | HEPG-2 | PC-3 | MOLT-4 | |
| 3 | 193.37 ± 0.20 | 164.56 ± 0.20 | 149.28 ± 0.22 | 177.87 ± 0.16 |
| 4 | 283.48 ± 0.13 | 245.78 ± 0.15 | 227.63 ± 0.06 | 269.02 ± 0.10 |
| 5a | 22.58 ± 0.03 | 26.01 ± 0.03 | 27.46 ± 0.01 | 25.91 ± 0.03 |
| 5b | 18.63 ± 0.03 | 27.94 ± 0.03 | 27.31 ± 0.01 | 20.28 ± 0.03 |
| Cisplatin | 16.00 ± 0.06 | - | 39.99 ± 0.05 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaeedi, M.S.; Babgi, B.A.; Hussien, M.A.; Abdellattif, M.H.; Humphrey, M.G. DNA-Binding and Anticancer Activity of Binuclear Gold(I) Alkynyl Complexes with a Phenanthrenyl Bridging Ligand. Molecules 2020, 25, 1033. https://doi.org/10.3390/molecules25051033
Alsaeedi MS, Babgi BA, Hussien MA, Abdellattif MH, Humphrey MG. DNA-Binding and Anticancer Activity of Binuclear Gold(I) Alkynyl Complexes with a Phenanthrenyl Bridging Ligand. Molecules. 2020; 25(5):1033. https://doi.org/10.3390/molecules25051033
Chicago/Turabian StyleAlsaeedi, Mona S., Bandar A. Babgi, Mostafa A. Hussien, Magda H. Abdellattif, and Mark G. Humphrey. 2020. "DNA-Binding and Anticancer Activity of Binuclear Gold(I) Alkynyl Complexes with a Phenanthrenyl Bridging Ligand" Molecules 25, no. 5: 1033. https://doi.org/10.3390/molecules25051033
APA StyleAlsaeedi, M. S., Babgi, B. A., Hussien, M. A., Abdellattif, M. H., & Humphrey, M. G. (2020). DNA-Binding and Anticancer Activity of Binuclear Gold(I) Alkynyl Complexes with a Phenanthrenyl Bridging Ligand. Molecules, 25(5), 1033. https://doi.org/10.3390/molecules25051033








