Evaluation of the Textural Parameters of Zeolite Beta in LDPE Catalytic Degradation: Thermogravimetric Analysis Coupled with FTIR Operando Studies
Abstract
:1. Introduction
2. Results
2.1. Physicochemical Properties of the Catalysts
2.2. Acidic Properties: Pyridine and Carbon Monoxide Sorption Studies
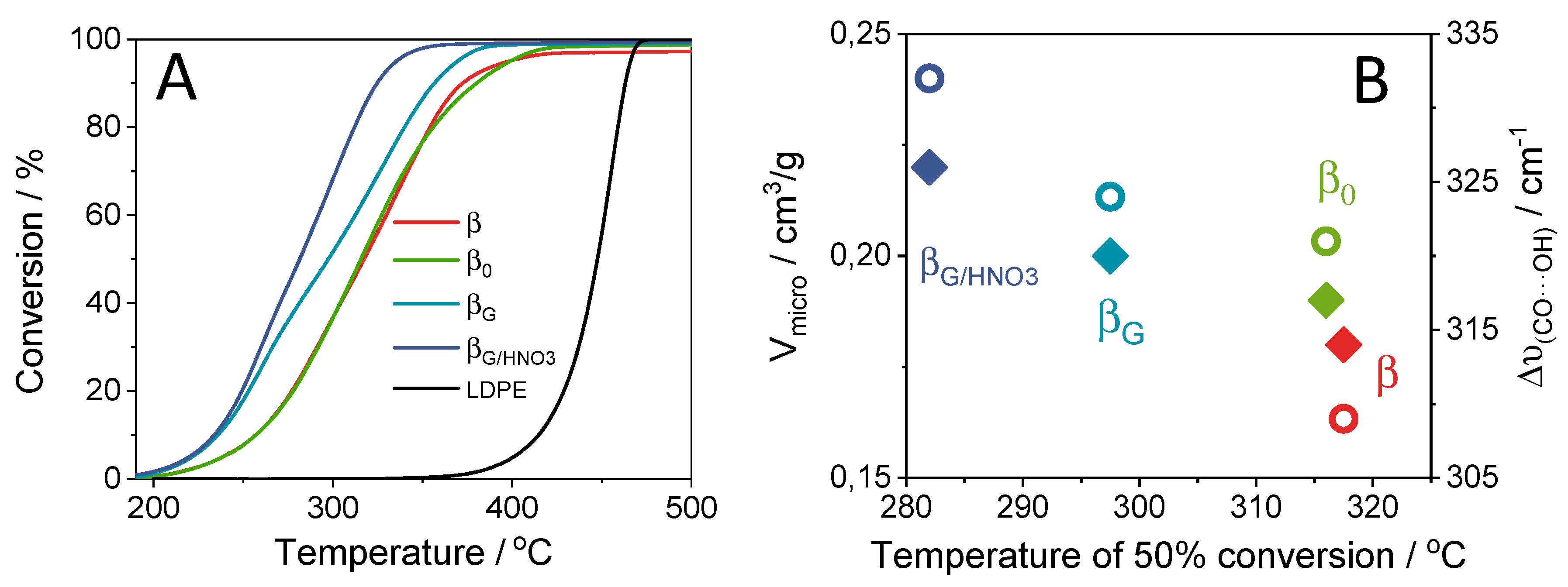
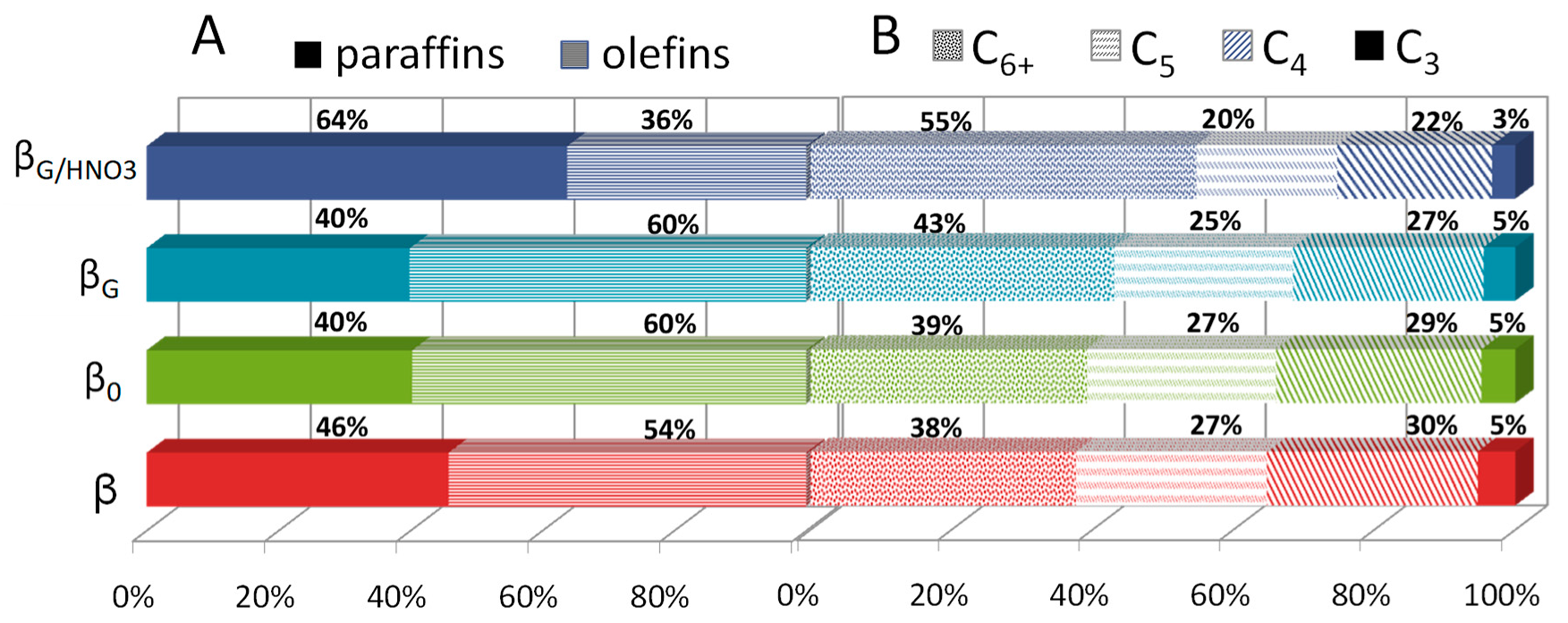
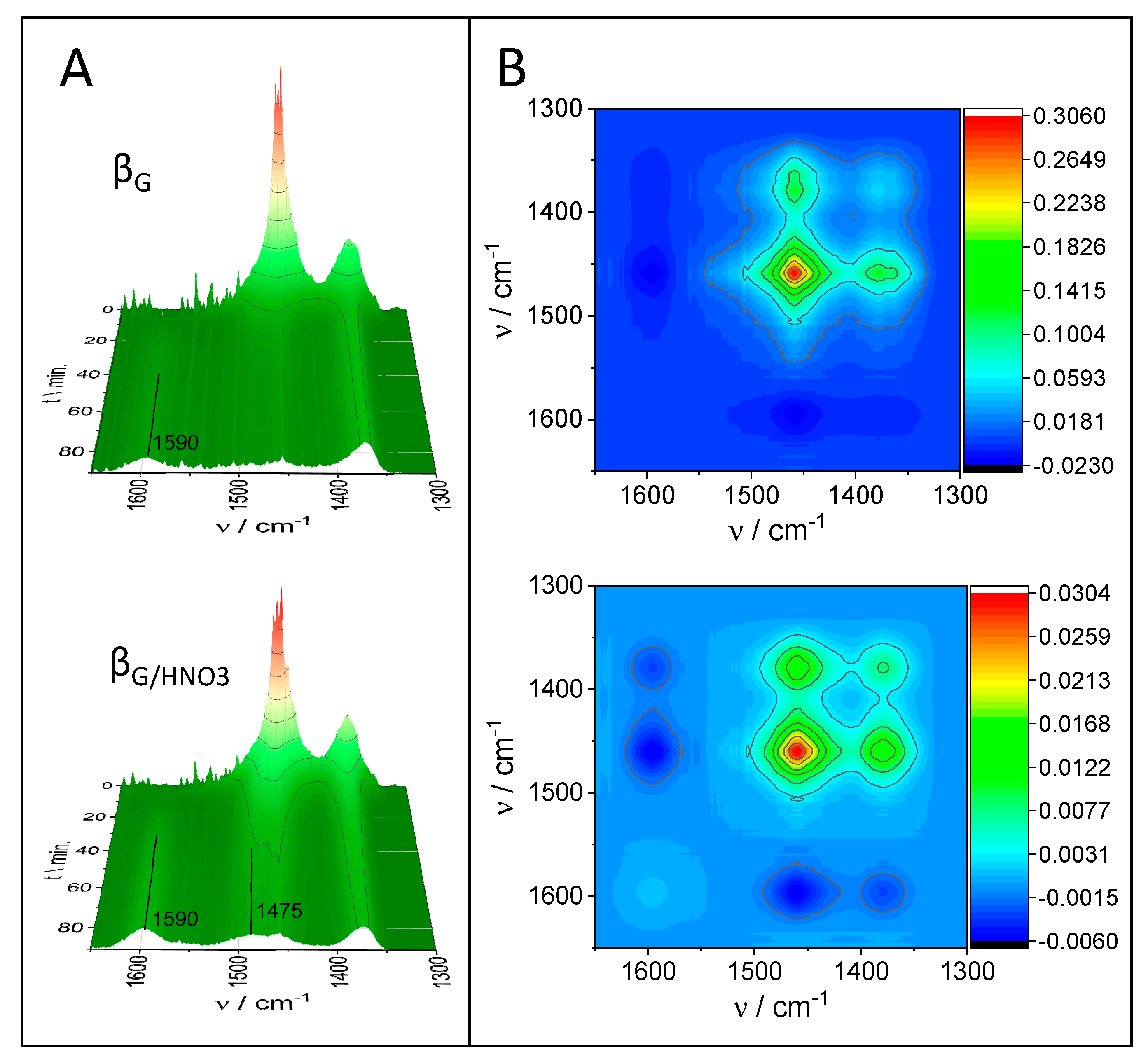
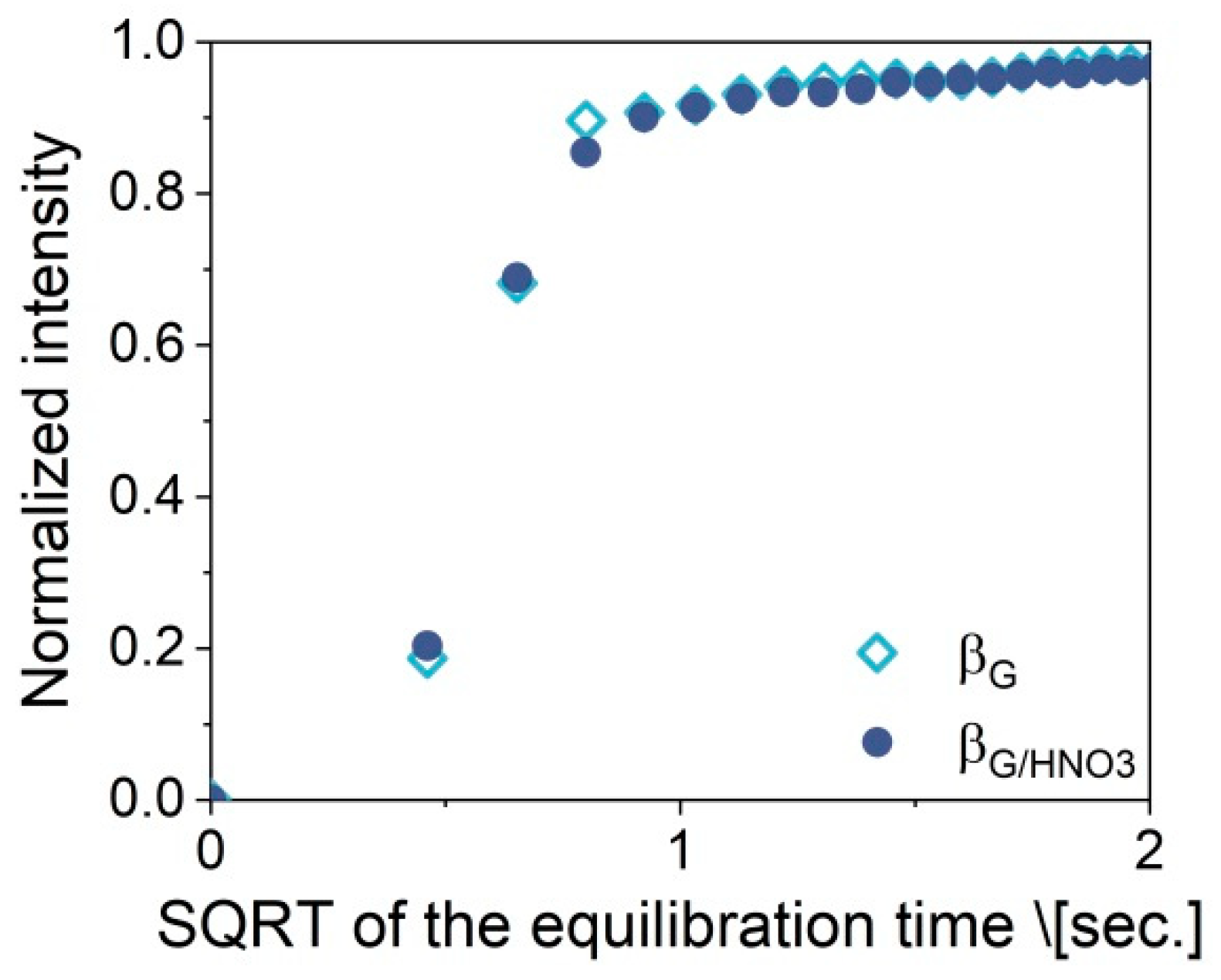
2.3. Catalytic Performance: Thermogravimetric and Operando IR Studies
3. Materials and Methods
3.1. Materials
3.1.1. Preparation of Beta Seeds
3.1.2. Functionalization: Zeolite βG
3.2. Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Camblor, M.A.; Corma, A.; Valencia, S. Spontaneous nucleation and growth of pure silica zeolite-β free of connectivity defects. Chem. Commun. 1996, 2365–2366. [Google Scholar] [CrossRef]
- Borade, R.B.; Clearfield, A. Preparation of aluminum-rich Beta zeolite. Microporous Mater. 1996, 5, 289–297. [Google Scholar] [CrossRef]
- Vaudry, F.; di Renzo, F.; Espiau, P.; Fajula, F.; Schulz, P. Aluminum-rich zeolite beta. Zeolites 1997, 19, 253–258. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Newsam, J.M. Two new three-dimensional twelve-ring zeolite frameworks of which zeolite beta is a disordered intergrowth. Nature 1988, 332, 249–251. [Google Scholar] [CrossRef]
- Tarach, K.; Góra-Marek, K.; Tekla, J.; Brylewska, K.; Datka, J.; Mlekodaj, K.; Makowski, W.; López, M.C.I.; Triguero, J.M.; Rey, F. Catalytic cracking performance of alkaline-treated zeolite Beta in the terms of acid sites properties and their accessibility. J. Catal. 2014, 312, 46–57. [Google Scholar] [CrossRef] [Green Version]
- Philippou, A.; Anderson, M.W. Solid-state NMR investigation of n-heptane cracking over zeolite beta. J. Catal. 1996, 158, 385–401. [Google Scholar] [CrossRef]
- Taufiqurrahmi, N.; Mohamed, A.R.; Bhatia, S. Deactivation and coke combustion studies of nanocrystalline zeolite beta in catalytic cracking of used palm oil. Chem. Eng. J. 2010, 163, 413–421. [Google Scholar] [CrossRef]
- Ramos, M.J.; de Lucas, A.; Jiménez, V.; Sánchez, P.; Valverde, J.L. Hydroisomerization of different refinery naphtha streams by using a beta zeolite catalyst. Fuel Process. Technol. 2008, 89, 721–727. [Google Scholar] [CrossRef]
- Chica, A.; Corma, A. Comparison of large pore zeolites for n-octane hydroisomerization: Activity, selectivity and kinetic features. Chem. -Ing. -Tech. 2007, 79, 857–870. [Google Scholar] [CrossRef]
- Sridevi, U.; Bokade, V.V.; Satyanarayana, C.V.V.; Rao, B.S.; Pradhan, N.C.; Rao, B.K.B. Kinetics of propylation of benzene over H-beta and SAPO-5 catalysts: A comparison. J. Mol. Catal. A Chem. 2002, 181, 257–262. [Google Scholar] [CrossRef]
- Zhang, H.; Dai, B.; Wang, X.; Xu, L.; Zhu, M. Hydrochlorination of acetylene to vinyl chloride monomer over bimetallic Au-La/SAC catalysts. J. Ind. Eng. Chem. 2012, 18, 49–54. [Google Scholar] [CrossRef]
- Siffert, S.; Gaillard, L.; Su, B.L. Alkylation of benzene by propene on a series of Beta zeolites: Toward a better understanding of the mechanisms. J. Mol. Catal. A Chem. 2000, 153, 267–279. [Google Scholar] [CrossRef]
- Aguado, J.; Serrano, D.P.; Escola, J.M.; Garagorri, E.; Fernández, J.A. Catalytic conversion of polyolefins into fuels over zeolite beta. Polym. Degrad. Stab. 2000, 69, 11–16. [Google Scholar] [CrossRef]
- Manos, G.; Garforth, A.; Dwyer, J. Catalytic degradation of high-density polyethylene over different zeolitic structures. Ind. Eng. Chem. Res. 2000, 39, 1198–1202. [Google Scholar] [CrossRef]
- Liu, C.; Tranca, I.; van Santen, R.A.; Hensen, E.J.M.; Pidko, E.A. Scaling Relations for Acidity and Reactivity of Zeolites. J. Phys. Chem. C 2017, 121, 23520–23530. [Google Scholar] [CrossRef]
- Van Oers, C.J.; Kurttepeli, M.; Mertens, M.; Bals, S.; Meynen, V.; Coo, P. Zeolite β nanoparticles based bimodal structures: Mechanism and tuning of the porosity and zeolitic properties. Microporous Mesoporous Mater. 2014, 185, 204–212. [Google Scholar] [CrossRef]
- Grecco, S.d.F.; de Carvalho, D.R.; Zandonai, C.H.; Fernandes-Machado, N.R.C.; Lião, L.M.; Urquieta-González, E.A.; Rangel, M.d.C. Catalytic cracking of crude soybean oil on Beta nanozeolites. J. Mol. Catal. A Chem. 2016, 422, 89–102. [Google Scholar] [CrossRef]
- Huang, J.; Li, G.; Wu, S.; Wang, H.; Xing, L.; Song, K.; Wu, T.; Kan, Q. Synthesis, characterization and catalytic activity of cubic Ia3d and hexagonal p6mm mesoporous aluminosilicates with enhanced acidity. J. Mater. Chem. 2005, 15, 1055–1060. [Google Scholar] [CrossRef]
- Campelo, J.M.; Luna, D.; Luque, R.; Marinas, J.M.; Romero, A.A.; Calvino, J.J.; Rodríguez-Luque, M.P. Synthesis of acidic Al-MCM-48: Influence of the Si/Al ratio, degree of the surfactant hydroxyl exchange, and post-treatment in NH4F solution. J. Catal. 2005, 230, 327–338. [Google Scholar] [CrossRef]
- Rouquerol, J.; Llewellyn, P.; Rouquerol, F. Is the bet equation applicable to microporous adsorbents? Stud. Surf. Sci. Catal. 2007, 2991, 49–56. [Google Scholar] [CrossRef]
- Maache, M.; Janin, A.; Lavalley, J.C.; Benazzi, E. FT infrared study of Brønsted acidity of H-mordenites: Heterogeneity and effect of dealumination. Zeolites 1995, 15, 507–516. [Google Scholar] [CrossRef]
- Zecchina, A.; Spoto, G.; Ricchiardi, G.; Bordiga, S.; Bonino, F.; Prestipino, C.; Lamberti, C. Zeolite characterization with spectroscopic methods. Stud. Surf. Sci. Catal. 2002, 142, 3–14. [Google Scholar] [CrossRef]
- Pazé, C.; Bordiga, S.; Lamberti, C.; Salvalaggio, M.; Zecchina, A.; Bellussi, G. Acidic properties of H-β zeolite as probed by bases with proton affinity in the 118-204 kcal mol-1 range: A FTIR investigation. J. Phys. Chem. B 1997, 101, 4740–4751. [Google Scholar] [CrossRef]
- Van Bokhoven, J.A.; van der Eerden, A.M.J.; Koningsberger, D.C. Three-coordinate aluminum in zeolites observed with in situ x-ray absorption near-edge spectroscopy at the Al K-edge: Flexibility of aluminum coordinations in zeolites. J. Am. Chem. Soc. 2003, 125, 7435–7442. [Google Scholar] [CrossRef] [PubMed]
- Janda, A.; Bell, A.T. Effects of Si/Al ratio on the distribution of framework Al and on the rates of alkane monomolecular cracking and dehydrogenation in H-MFI. J. Am. Chem. Soc. 2013, 135, 19193–19207. [Google Scholar] [CrossRef] [Green Version]
- Kunkeler, P.J.; Zuurdeeg, B.J.; Van Der Waal, J.C.; van Bokhoven, J.A.; Koningsberger, D.C.; Van Bekkum, H. Zeolite Beta the Relationship Between Calcination Procedure, Aluminum Configuration, and Lewis Acidity. J. Catal. 1998, 180, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Woolery, G.L.; Kuehl, G.H.; Timken, H.C.; Chester, A.W.; Vartuli, J.C. On the nature of framework Brønsted and Lewis acid sites in ZSM-5. Zeolites 1997, 19, 288–296. [Google Scholar] [CrossRef]
- BWouters, H.; Chen, T.H.; Grobet, P.J. Reversible tetrahedral-octahedral framework aluminum transformation in zeolite Y. J. Am. Chem. Soc. 1998, 120, 11419–11425. [Google Scholar] [CrossRef]
- Ravi, M.; Sushkevich, V.L.; van Bokhoven, J.A. Lewis Acidity Inherent to the Framework of Zeolite Mordenite. J. Phys. Chem. C 2019, 123, 15139–15144. [Google Scholar] [CrossRef]
- Miyaji, A.; Sakamoto, Y.; Iwase, Y.; Yashima, T.; Koide, R.; Motokura, K.; Baba, T. Selective production of ethylene and propylene via monomolecular cracking of pentene over proton-exchanged zeolites: Pentene cracking mechanism determined by spatial volume of zeolite cavity. J. Catal. 2013, 302, 101–114. [Google Scholar] [CrossRef]
- Gounder, R.; Iglesia, E. Catalytic consequences of spatial constraints and acid site location for monomolecular alkane activation on zeolites. J. Am. Chem. Soc. 2009, 131, 1958–1971. [Google Scholar] [CrossRef] [PubMed]
- Pyra, K.; Tarach, K.A.; Majda, D.; Góra-Marek, K. Desilicated zeolite BEA for the catalytic cracking of LDPE: The interplay between acidic sites’ strength and accessibility. Catal. Sci. Technol. 2019, 9, 1794–1801. [Google Scholar] [CrossRef]
- Derouane, E.G.; André, J.M.; Lucas, A.A. A simple van der waals model for molecule-curved surface interactions in molecular-sized microporous solids. Chem. Phys. Lett. 1987, 137, 336–340. [Google Scholar] [CrossRef]
- Jones, A.J.; Iglesia, E. The Strength of Brønsted Acid Sites in Microporous Aluminosilicates. ACS Catal. 2015, 5, 5741–5755. [Google Scholar] [CrossRef]
- Demuth, T.; Hafner, J.; Benco, L.; Toulhoat, H. Structural and Acidic Properties of Mordenite. An ab Initio Density-Functional Study. J. Phys. Chem. B 2000, 104, 4593–4607. [Google Scholar] [CrossRef]
- Song, C.; Chu, Y.; Wang, M.; Shi, H.; Zhao, L.; Guo, X.; Yang, W.; Shen, J.; Xue, N.; Peng, L.; et al. Cooperativity of adjacent BrØnsted acid sites in MFI zeolite channel leads to enhanced polarization and cracking of alkanes. J. Catal. 2017, 349, 163–174. [Google Scholar] [CrossRef]
- Yang, C.T.; Janda, A.; Bell, A.T.; Lin, L.C. Atomistic Investigations of the Effects of Si/Al Ratio and Al Distribution on the Adsorption Selectivity of n-Alkanes in Brønsted-Acid Zeolites. J. Phys. Chem. C 2018, 122, 9397–9410. [Google Scholar] [CrossRef] [Green Version]
- Čapek, L.; Dědeček, J.; Sazama, P.; Wichterlová, B. The decisive role of the distribution of Al in the framework of beta zeolites on the structure and activity of Co ion species in propane-SCR-NO x in the presence of water vapour. J. Catal. 2010, 272, 44–54. [Google Scholar] [CrossRef]
- Xu, B.; Bordiga, S.; Prins, R.; van Bokhoven, J.A. Effect of framework Si/Al ratio and extra-framework aluminum on the catalytic activity of Y zeolite. Appl. Catal. A Gen. 2007, 333, 245–253. [Google Scholar] [CrossRef]
- Arsenova, N.; Bludau, H.; Haag, W.O.; Karge, H.G. In situ IR spectroscopic study of the adsorption behaviour of ethylbenzene and diethylbenzenes related to ethylbenzene disproportionation over HY zeolite. Microporous Mesoporous Mater. 1998, 23, 1–10. [Google Scholar] [CrossRef]
- Castaño, P.; Elordi, G.; Olazar, M.; Aguayo, A.T.; Pawelec, B.; Bilbao, J. Insights into the coke deposited on HZSM-5, Hβ and HY zeolites during the cracking of polyethylene. Appl. Catal. B Environ. 2011, 104, 91–100. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Sadowska, K.; Góra-Marek, K.; Datka, J. Hierarchic zeolites studied by IR spectroscopy: Acid properties of zeolite ZSM-5 desilicated with NaOH and NaOH/tetrabutylamine hydroxide. Vib. Spectrosc. 2012, 63, 418–425. [Google Scholar] [CrossRef]
- Góra-Marek, K.; Derewiński, M.; Sarv, P.; Datka, J. IR and NMR studies of mesoporous alumina and related aluminosilicates. Catal. Today 2005, 101, 131–138. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
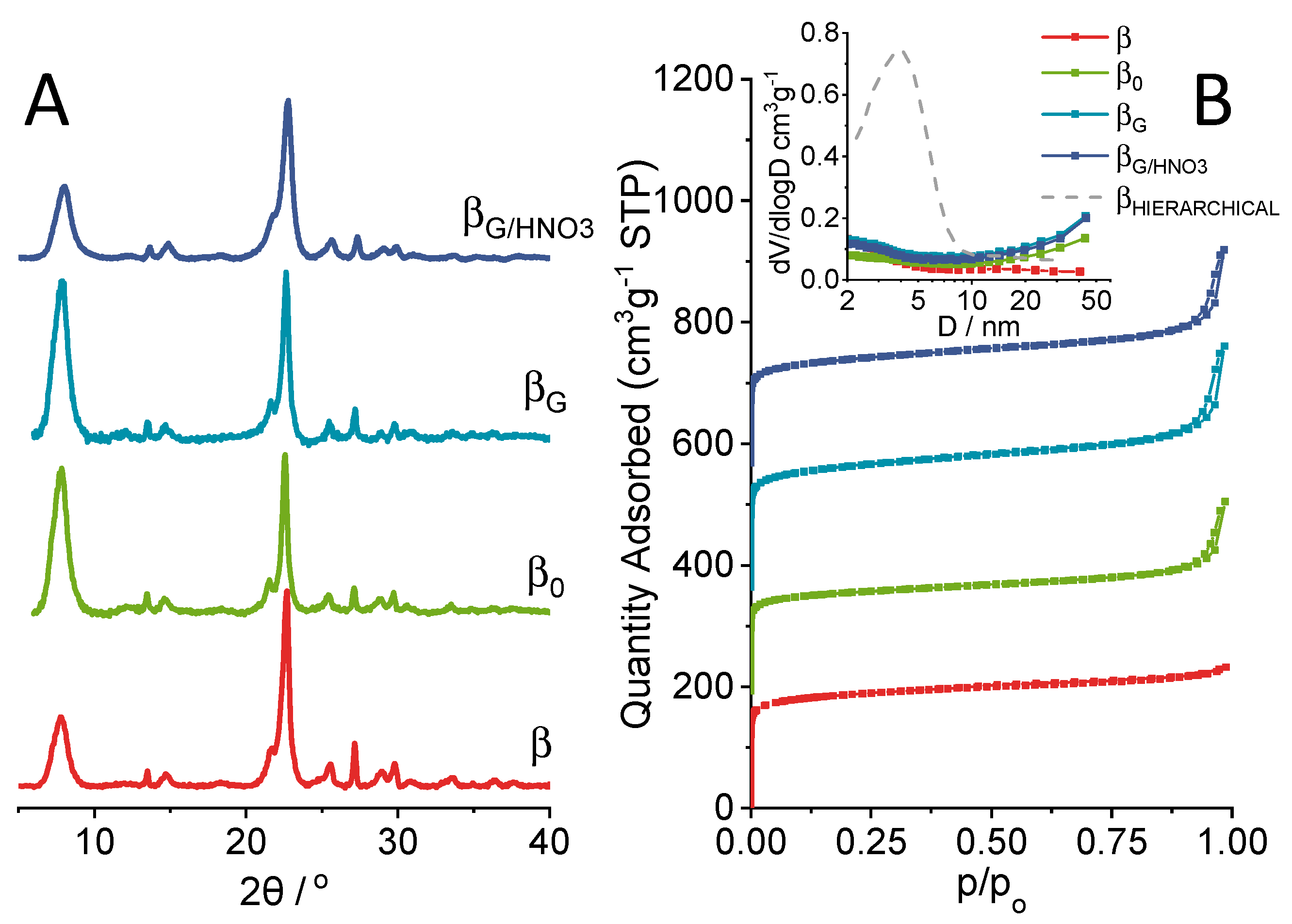
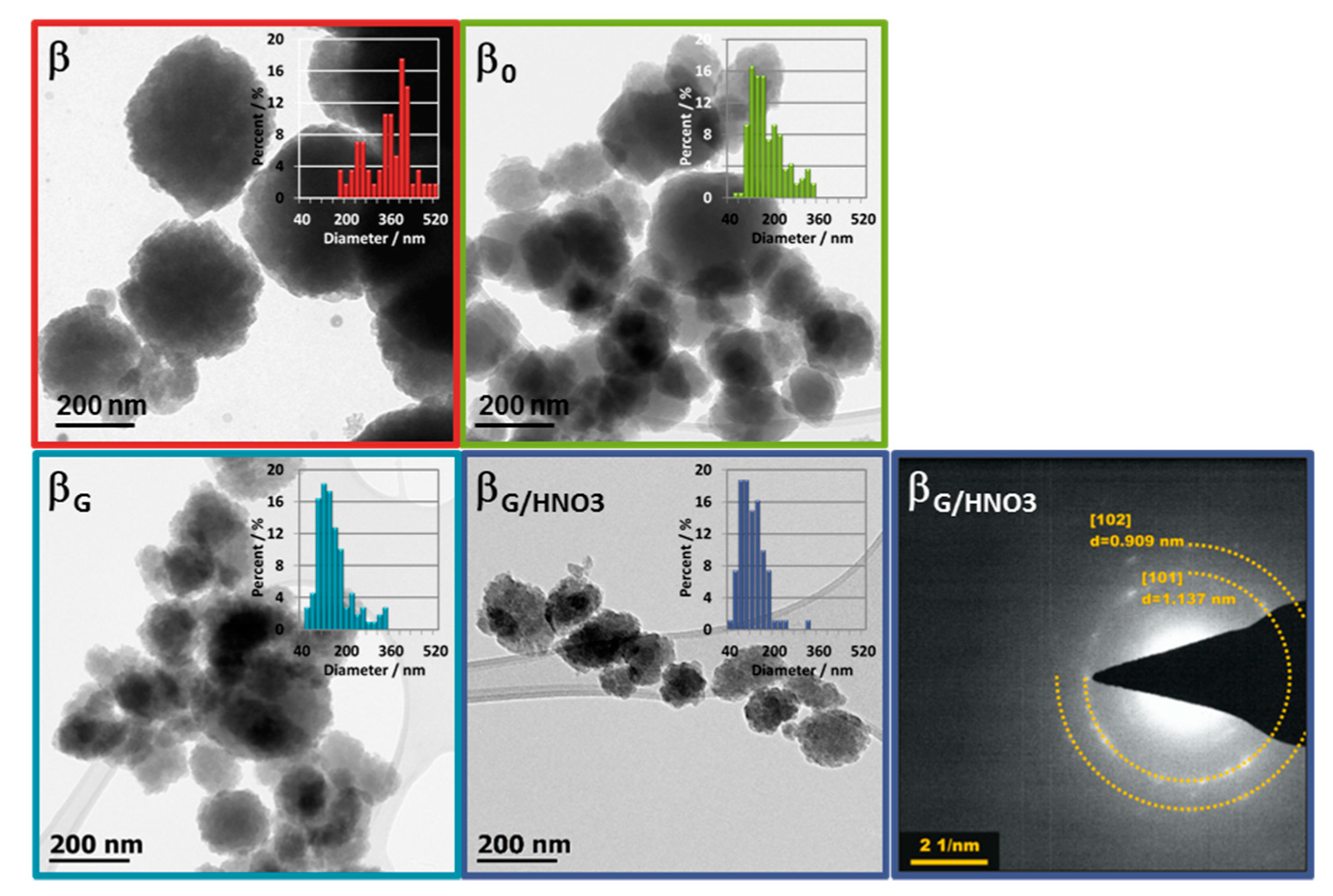
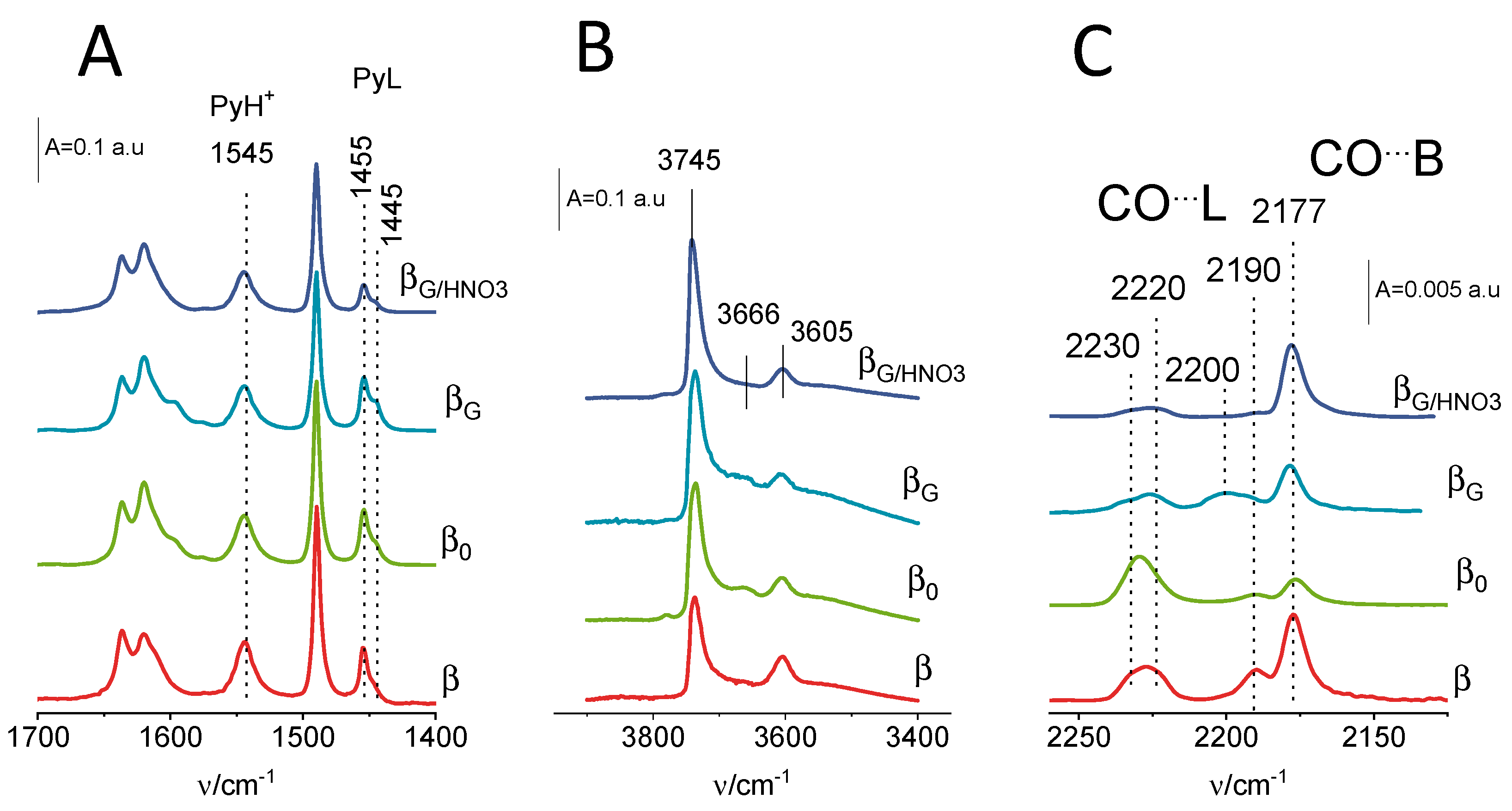
| material | Si/Al a | SBET b | Smicro c | SEXT d | Vmicro a | VEXT e |
|---|---|---|---|---|---|---|
| (m2·g−1) | (cm3·g−1) | |||||
| β | 22 | 567 | 523 | 44 | 0.18 | 0.06 |
| β0 | 19 | 667 | 535 | 134 | 0.19 | 0.11 |
| βG | 20 | 690 | 537 | 153 | 0.20 | 0.15 |
| βG/HNO3 | 22 | 740 | 586 | 154 | 0.22 | 0.18 |
| Material | Ala | CB | CL | CB + CL | Py450/Py170 | ∆υ(CO•••OH) |
|---|---|---|---|---|---|---|
| (µmol·g−1) | (µmol·g−1) | (cm−1) | ||||
| β | 675 | 395 | 220 | 615 | 0.80 | 309 |
| β0 | 762 | 405 | 236 | 641 | 0.84 | 321 |
| βG | 756 | 398 | 230 | 628 | 0.86 | 324 |
| βG/HNO3 | 670 | 390 | 124 | 534 | 0.90 | 330 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pyra, K.; Tarach, K.A.; Janiszewska, E.; Majda, D.; Góra-Marek, K. Evaluation of the Textural Parameters of Zeolite Beta in LDPE Catalytic Degradation: Thermogravimetric Analysis Coupled with FTIR Operando Studies. Molecules 2020, 25, 926. https://doi.org/10.3390/molecules25040926
Pyra K, Tarach KA, Janiszewska E, Majda D, Góra-Marek K. Evaluation of the Textural Parameters of Zeolite Beta in LDPE Catalytic Degradation: Thermogravimetric Analysis Coupled with FTIR Operando Studies. Molecules. 2020; 25(4):926. https://doi.org/10.3390/molecules25040926
Chicago/Turabian StylePyra, Kamila, Karolina A. Tarach, Ewa Janiszewska, Dorota Majda, and Kinga Góra-Marek. 2020. "Evaluation of the Textural Parameters of Zeolite Beta in LDPE Catalytic Degradation: Thermogravimetric Analysis Coupled with FTIR Operando Studies" Molecules 25, no. 4: 926. https://doi.org/10.3390/molecules25040926
APA StylePyra, K., Tarach, K. A., Janiszewska, E., Majda, D., & Góra-Marek, K. (2020). Evaluation of the Textural Parameters of Zeolite Beta in LDPE Catalytic Degradation: Thermogravimetric Analysis Coupled with FTIR Operando Studies. Molecules, 25(4), 926. https://doi.org/10.3390/molecules25040926