Flow Synthesis of Biologically-Relevant Compound Libraries
Abstract
1. Introduction
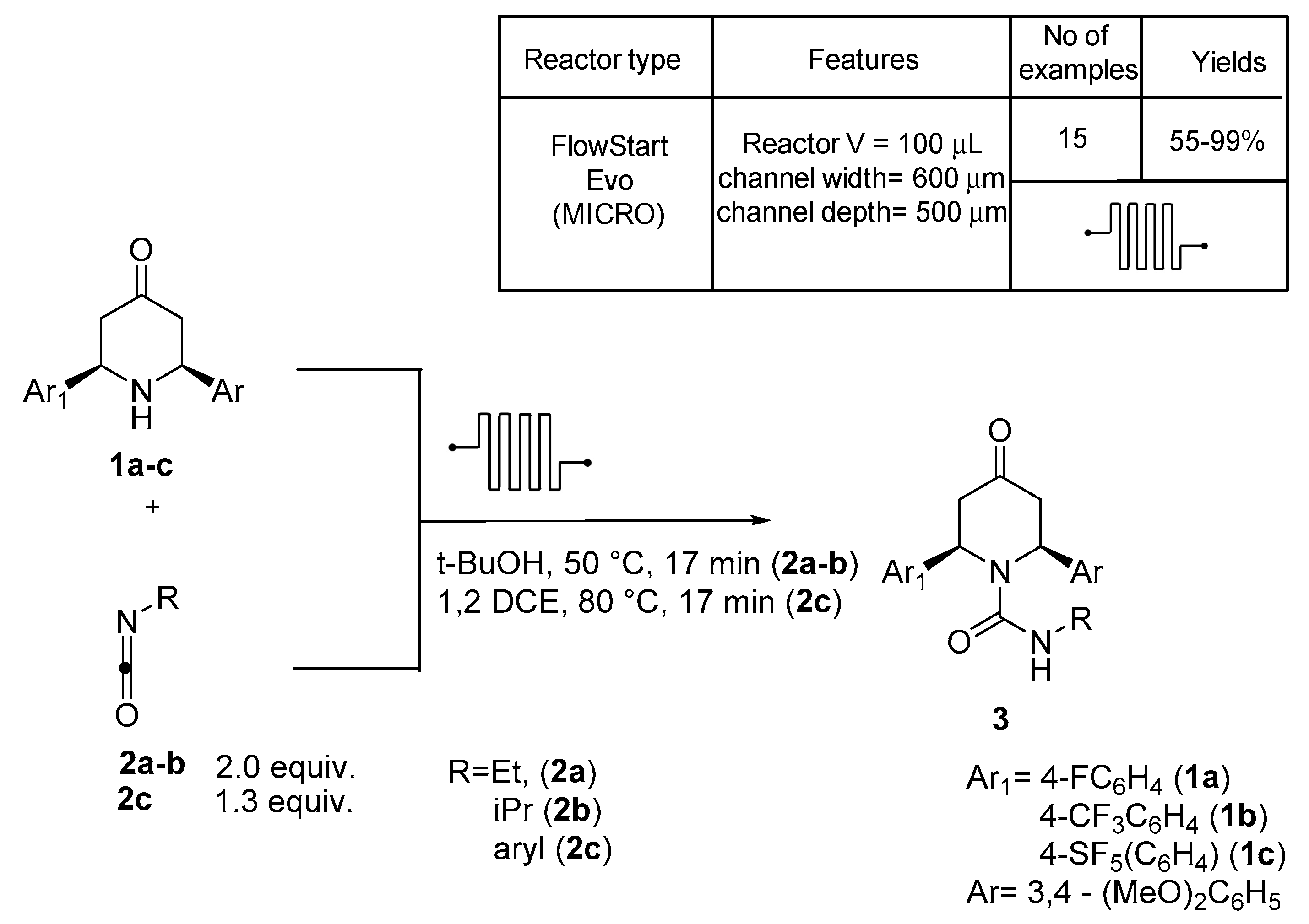
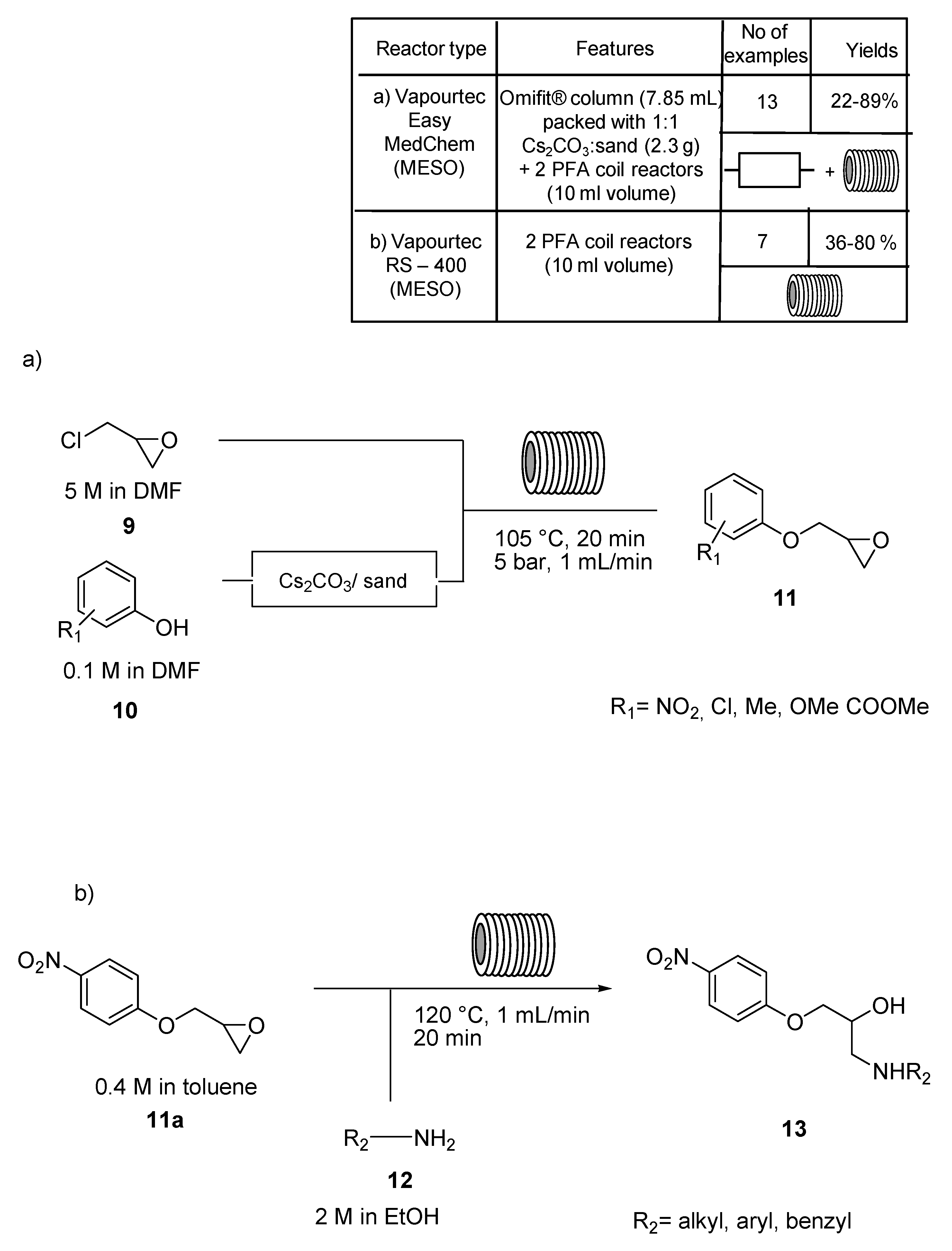
2. N,O-Based Compound Libraries
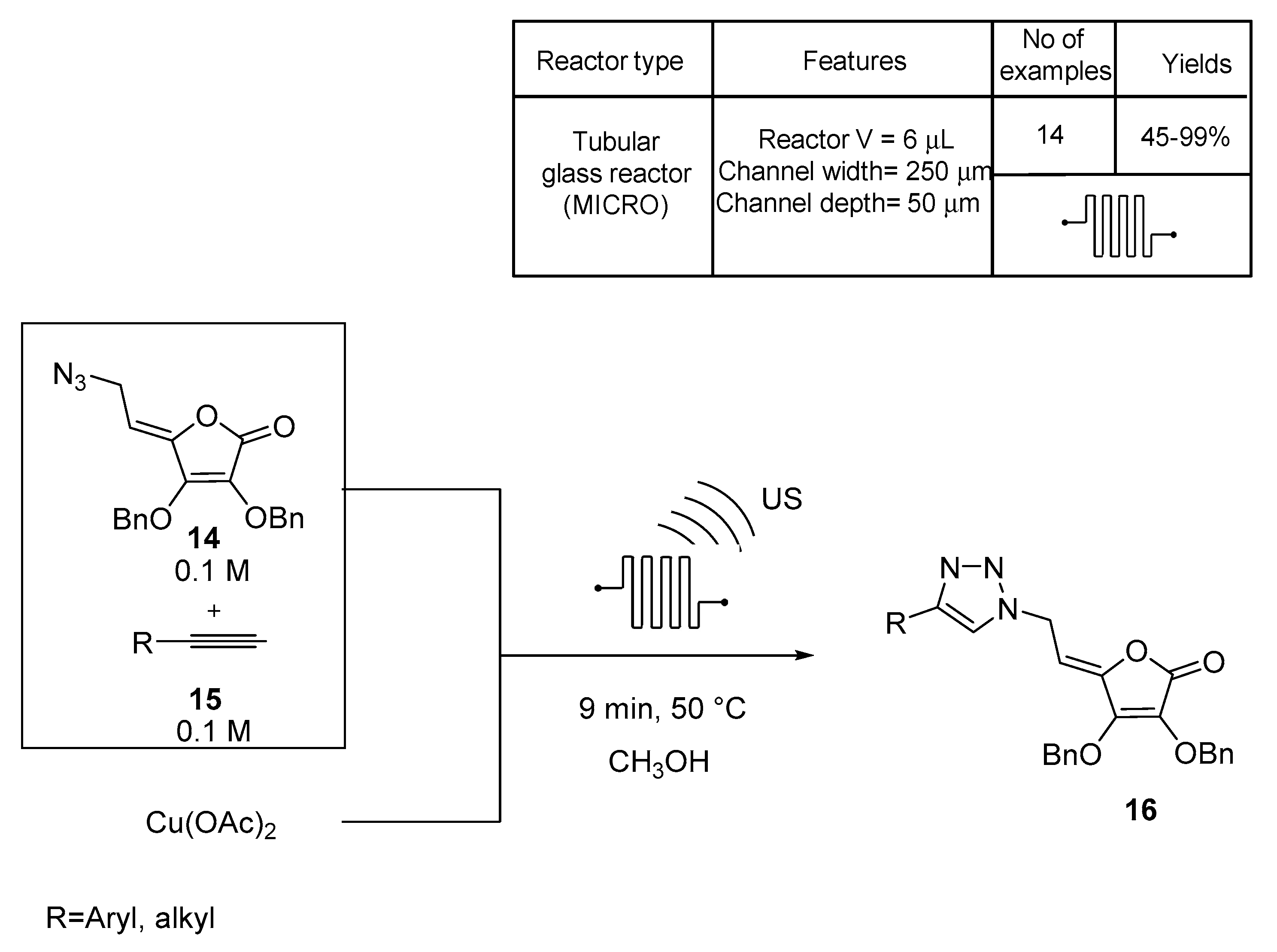
3. N-Based Compound Libraries
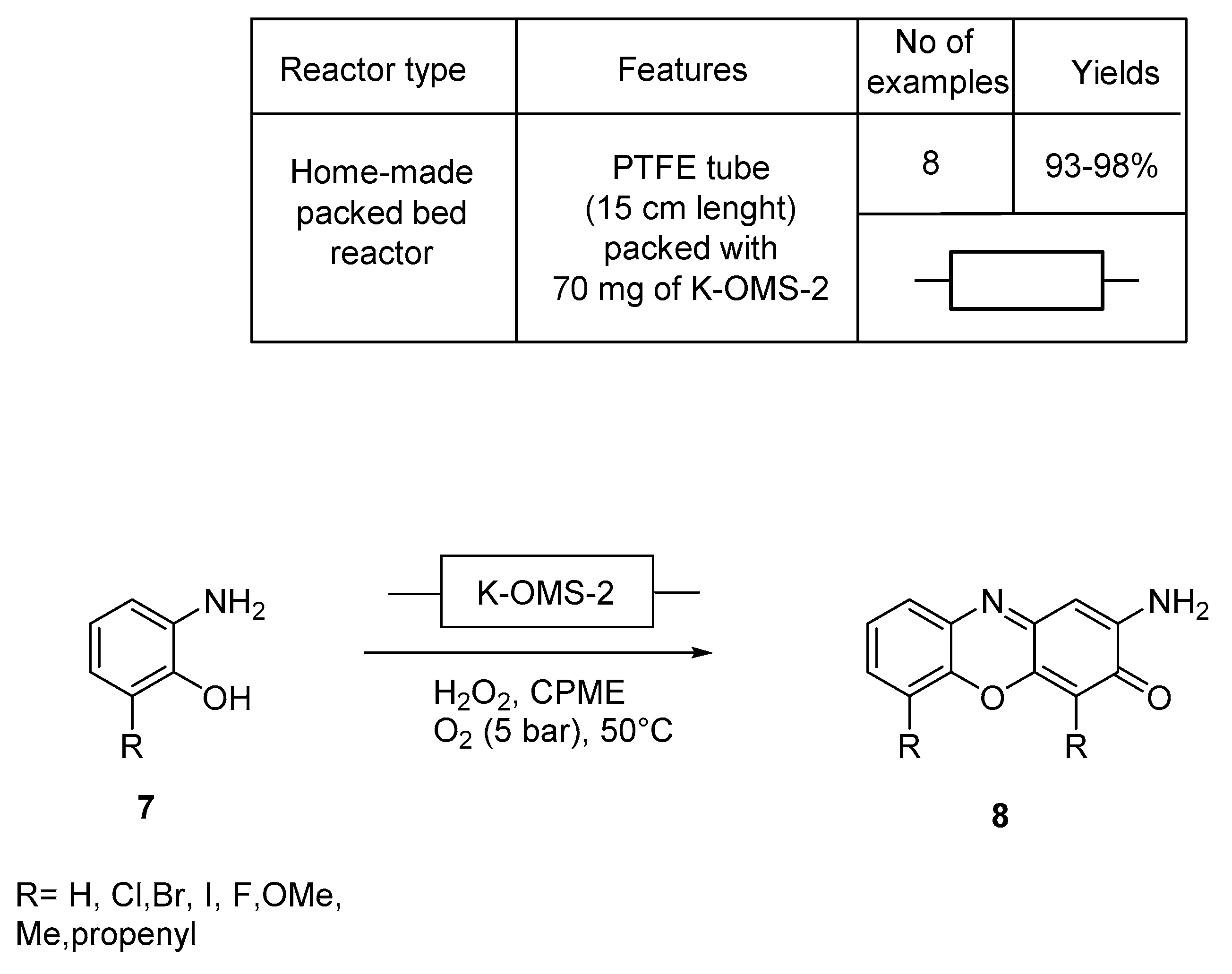
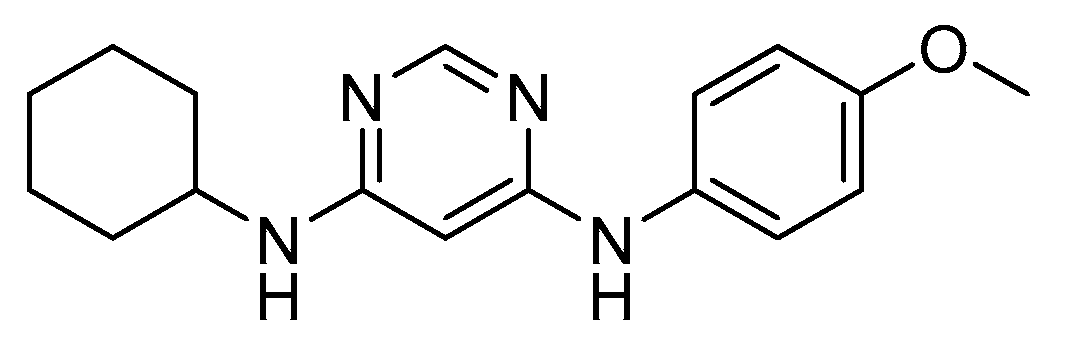
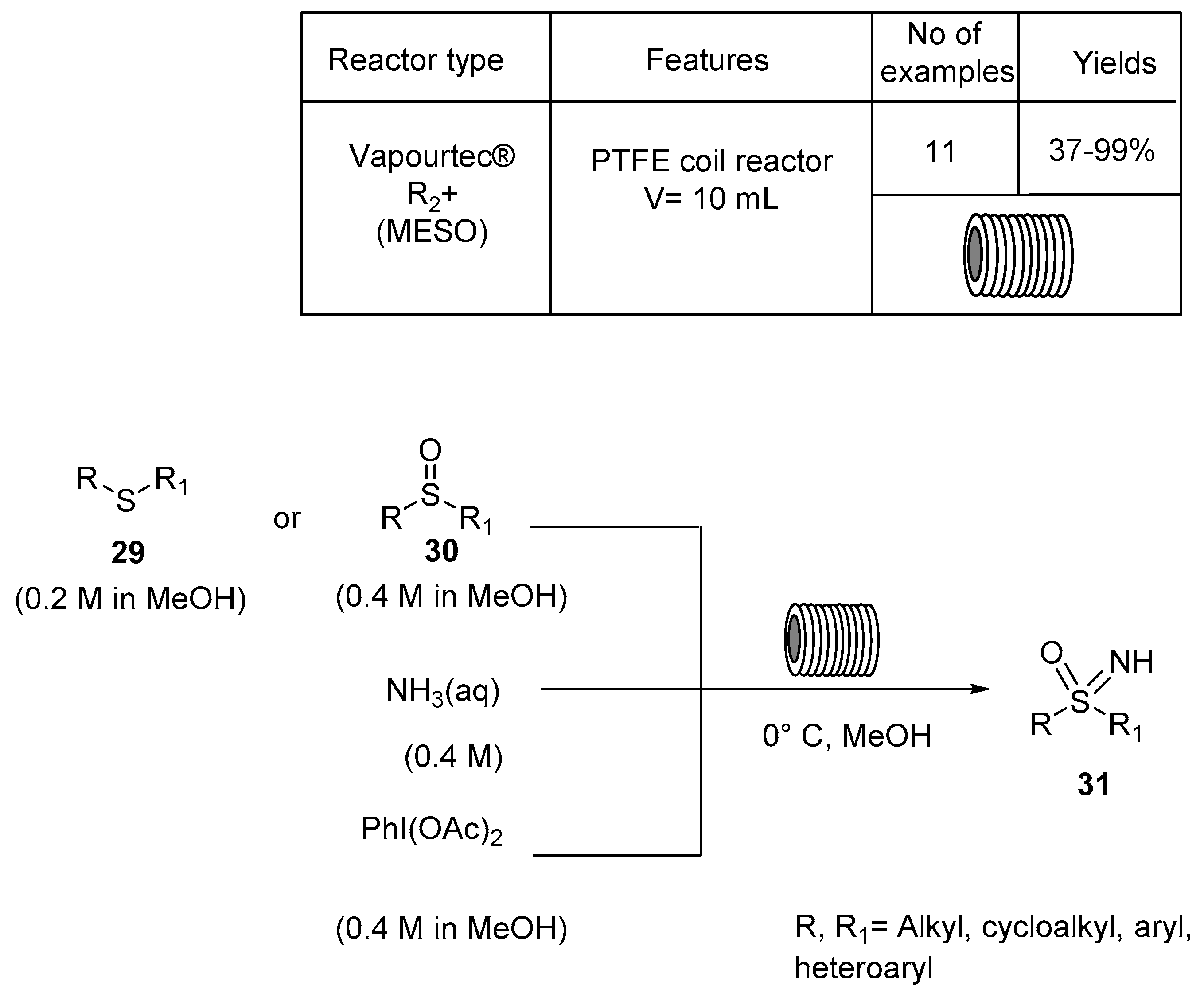
4. N,S-Based Compound Libraries
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirschning, A.; Solodenko, W.; Mennecke, K. Combining enabling techniques in organic synthesis: Continuous flow processes with heterogenized catalysts. Chem. Eur. J. 2006, 23, 5972–5990. [Google Scholar]
- Brien, M.O.; Denton, R.; Ley, S.V. Lesser-known enabling technologies for organic synthesis. Synthesis 2011, 8, 1157–1192. [Google Scholar]
- McNamara, C.A.; Dixon, M.J.; Bradley, M. Recoverable catalysts and reagents using recyclable polystyrene-based supports. Chem. Rev. 2002, 102, 3275–3300. [Google Scholar] [CrossRef] [PubMed]
- Kappe, C.O.; Dallinger, D. Controlled microwave heating in modern organic synthesis: Highlights from the 2004–2008 literature. Mol. Divers. 2009, 13, 71–193. [Google Scholar] [CrossRef]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef] [PubMed]
- Wegner, J.; Ceylan, S.; Kirschning, A. Flow chemistry—Key enabling technology for (multistep) organic synthesis. Adv. Synth. Catal. 2012, 354, 17–57. [Google Scholar] [CrossRef]
- Bogdan, A.R.; Dombrowski, A.W. Emerging trends in flow chemistry and applications to the pharmaceutical industry. J. Med. Chem. 2019, 62, 6422–6468. [Google Scholar] [CrossRef]
- Akwi, F.M.; Watts, P. Continuous flow chemistry: Where are we now? Recent applications, challenges and limitations. Chem.Comm. 2018, 54, 13894–13928. [Google Scholar] [CrossRef]
- Plutschack, M.B.; Pieber, B.; Gilmore, K.; Seeberg, P.H. The hitchhiker’s guide to flow chemistry. Chem. Rev. 2017, 117, 11796–11893. [Google Scholar] [CrossRef]
- Brandao, P.; Pineiro, M. Flow chemistry: Towards a more sustainable heterocyclic synthesis. Eur. J. Org. Chem. 2019, 43, 7188–7217. [Google Scholar] [CrossRef]
- Vaccaro, L.; Lanari, D.; Marrocchi, A.; Strappaveccia, G. Flow approaches towards sustainability. Green Chem. 2014, 16, 3680–3704. [Google Scholar] [CrossRef]
- Fanelli, F.; Parisi, G.; Degennaro, L.; Luisi, R. Contribution of microreactor technology and flow chemistry to the development of green and sustainable synthesis. Beilstein J. Org. Chem. 2017, 13, 520–542. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.P.; James, K. Rapid access to compound libraries through flow technology: Fully automated synthesis of a 3-aminoindolizine library via orthogonal diversification. ACS Comb. Sci. 2012, 14, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Gallou, I. Unsymmetrical ureas. Synthetic methodologies and application in drug design. Org. Prep. Proced. Int. 2007, 39, 355–383. [Google Scholar] [CrossRef]
- Jagtap, A.D.; Kondekar, N.B.; Sadani, A.A.; Chern, J.W. Ureas: Applications in drug design. Curr. Med. Chem. 2017, 24, 622–651. [Google Scholar] [CrossRef]
- Riesco-Domínguez, A.; van der Zwaluw, N.; Blanco-Ania, F.D.; Rutjes, P.J.T. An enantio- and diastereoselective Mannich/Pictet–Spengler sequence to form spiro[piperidine-pyridoindoles] and application to library synthesis. Eur. J. Org. Chem. 2017, 662–670. [Google Scholar] [CrossRef]
- Riesco-Domínguez, A.; Blanco-Ania, F.D.; Rutjes, P.J.T. Continuous flow synthesis of urea-containing compound libraries based on the piperidin-4-one scaffold. Eur. J. Org. Chem. 2018, 11, 1312–1320. [Google Scholar] [CrossRef]
- De Matteis, V.; van Delft, F.L.; Floris, J.T.; Rutjes, P.J.T. A ring-closing metathesis pathway to fluorovinyl-containing nitrogen heterocyles. Eur. J. Org. Chem. 2006, 5, 1166–1176. [Google Scholar] [CrossRef]
- Blattes, E.; Lockhart, B.; Lestage, P.; Schwendimann, L.; Gressens, P.; Fleury, M.-B.; Largeron, M. Novel 2-alkylamino-1,4-benzoxazine derivatives as potent neuroprotective agents: structure−activity relationship studies. J. Med. Chem. 2005, 48, 1282–1286. [Google Scholar] [CrossRef]
- Otsuka, H.; Hirai, Y.; Nagao, T.; Yamasaki, K. Anti-inflammatory activity of benzoxazinoids from roots of Coixlachryma-jobi var. Ma-yuen. J. Nat. Prod. 1988, 51, 74–79. [Google Scholar] [CrossRef]
- Wu, G.; Lv, T.; Mo, W.; Yang, X.; Gao, Y.; Chen, H. One-pot synthesis of tricyclo-1,4-benzoxazines via visible-lightphotoredox catalysis in continuous flow. Tet. Lett. 2017, 58, 1395–1398. [Google Scholar] [CrossRef]
- Wu, G.; Li, Y.; Yu, X.; Gao, Y.; Chen, H. Acetic acid accelerated visible-light photoredox catalyzed N-demethylation of N,N-dimethylaminophenyl derivatives. Adv. Synth. Catal. 2017, 359, 687–692. [Google Scholar] [CrossRef]
- Ferlin, F.; Navarro, L.P.M.; Gu, Y.; Lanari, D.; Vaccaro, L. Waste minimized synthesis of pharmaceutically active compounds via heterogeneous manganese catalysed C–H oxidation in flow. Green Chem 2020. [Google Scholar] [CrossRef]
- Kühlborn, J.; Konhäuser, M.; Groß, J.; Wich, P.R.; Opatz, T. Xylochemical synthesis of cytotoxic 2-aminophenoxazinone-type natural products through oxidative cross coupling. ACS Sustainable Chem. Eng. 2019, 7, 4414–4419. [Google Scholar] [CrossRef]
- Ferlin, F.; Marini, A.; Ascani, N.; Ackermann, L.; Lanari, D.; Vaccaro, L. Heterogeneous manganese-catalyzed oxidase C−H/C−O cyclization to access pharmaceutically active compounds. Chem.Cat.Chem. 2020, 2, 449–454. [Google Scholar] [CrossRef]
- Shen, Y.F.; Zerger, R.P.; DeGuzman, R.N.; Suib, S.L.; McCurdy, L.; Potter, D.I.; O’Young, C.L. Manganese oxide octahedral molecular sieves: Preparation, characterization, and applications. Science. Science 260 1993, 60, 511–515. [Google Scholar] [CrossRef]
- Cossar, P.J.; Baker, J.R.; Cain, N.; Mc Cluskey, A. In situ epoxide generation by dimethyldioxirane oxidation and the use of epichlorohydrin in the flow synthesis of a library of β-amino alcohols. R. Soc. Open Sci. 2018, 5, 171190. [Google Scholar] [CrossRef] [PubMed]
- Meščić, A.; Šalić, A.; Gregorić, T.; Zelić, T.; Raić-Malić, S. Continuous flow-ultrasonic synergy in click reactions for the synthesis of novel 1,2,3-triazolyl appended 4,5-unsaturated l-ascorbic acid derivatives. RSC Adv. 2017, 7, 791–800. [Google Scholar] [CrossRef]
- Gutmann, B.; Cantillo, D.; Kappe, C.O. Continuous-flow technology—A tool for the safe manufacturing of active pharmaceutical ingredients. Angew. Chem. Int. Ed. 2015, 54, 6688–6728. [Google Scholar] [CrossRef]
- Koley, M.; Mike, A.K.; Heher, P.; Koenig, X.; Schön, M.; Schnürch, M.; Hilber, K.; Weitzer, G.; Mihovilovic, M.D. VUT-MK142: A new cardiomyogenic small molecule promoting the differentiation of pre-cardiac mesoderm into cardiomyocytes. Med. Chem. Commun. 2013, 4, 1189–1195. [Google Scholar] [CrossRef][Green Version]
- Schön, M.; Dreier, D.; Schnürch, M.; Mihovilovic, M.D. Library synthesis of cardiomyogenesis inducing compounds using an efficient two-step-one-flow process. Monatsh Chem. 2016, 147, 523–532. [Google Scholar] [CrossRef]
- Sridharan, V.; SuryavanshiJ, P.A.; Menéndez, C. Advances in the chemistry of tetrahydroquinolines. Chem. Rev. 2011, 111, 7157–7259. [Google Scholar] [CrossRef] [PubMed]
- Cerra, B.; Mostarda, S.; Custodi, C.; Macchiarulo, A.; Gioiello, A. Integrating multicomponent flow synthesis and computational approaches for the generation of atetrahydroquinoline compound based library. Med. Chem. Commun. 2016, 7, 439–446. [Google Scholar]
- Cerra, B.; Carotti, A.; Passeri, D.; Sardella, R.; Moroni, G.; Di Michele, A.; Macchiarulo, A.; Pellicciari, R.; Gioiello, A. Exploiting chemical toolboxes for the expedited generation of tetracyclic quinolines as a novel class of PXR agonists. ACS Med. Chem. Lett. 2019, 10, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Alam, P.M.; Bilousova, T.; Spilman, P.; Vadivel, K.; Bai, D.; Elias, C.J.; Evseenko, D.; John, V. A small molecule mimetic of the humanin peptide as a candidate for modulating NMDA-induced neurotoxicity. ACS Chem. Neurosci. 2018, 9, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Sonkusare, S.K.; Kaul, C.L.; Ramarao, P. Dementia of Alzheimer’s disease and other neurodegenerative disorders−memantine, a new hope. Pharmacol. Res. 2005, 51, 1–17. [Google Scholar] [CrossRef]
- Langner, M.; Bolm, C. C1-symmetric sulfoximines as ligands in copper-catalyzed asymmetric Mukaiyama-type aldol reactions. Angew. Chem. Int. Ed. 2004, 43, 5984–5987. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Q.; Zhang, W.; Hu, J. Stereoselective synthesis of (Sulfonimidoyl)cyclopropanes with (R)-PhSO(NTs)CH2Cl and α,β-unsaturated Weinreb amides: Tuning the of selectivity between C–Cl and C–S bond cleavage. Eur. J. Org. Chem. 2016, 5, 906–909. [Google Scholar] [CrossRef]
- Lücking, U. Sulfoximines: A neglected opportunity in medicinal chemistry. Angew. Chem. Int. Ed. 2013, 52, 9399–9408. [Google Scholar] [CrossRef]
- Sirvent, J.A.; Lücking, U. Novel pieces for the emerging picture of sulfoximines in drug discovery: Synthesis and evaluation of sulfoximine analogues of marketed drugs and advanced clinical candidates. Chem. Med. Chem. 2017, 12, 487–501. [Google Scholar] [CrossRef]
- Degennaro, L.; Tota, A.; De Angelis, S.; Andresini, M.; Cardellicchio, C.; Capozzi, M.A.; Romanazzi, G.; Luisi, R. A convenient, mild, and green synthesis of NH-sulfoximines in flow reactors. Eur. J. Org. Chem. 2017, 44, 6486–6490. [Google Scholar] [CrossRef]
- Bull, J.A.; Degennaro, L.; Luisi, R. Straightforward strategies for the preparation of NH-sulfox-imines: A serendipitous story. Synlett 2017, 28, 2525–2538. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luque Navarro, P.M.; Lanari, D. Flow Synthesis of Biologically-Relevant Compound Libraries. Molecules 2020, 25, 909. https://doi.org/10.3390/molecules25040909
Luque Navarro PM, Lanari D. Flow Synthesis of Biologically-Relevant Compound Libraries. Molecules. 2020; 25(4):909. https://doi.org/10.3390/molecules25040909
Chicago/Turabian StyleLuque Navarro, Pilar María, and Daniela Lanari. 2020. "Flow Synthesis of Biologically-Relevant Compound Libraries" Molecules 25, no. 4: 909. https://doi.org/10.3390/molecules25040909
APA StyleLuque Navarro, P. M., & Lanari, D. (2020). Flow Synthesis of Biologically-Relevant Compound Libraries. Molecules, 25(4), 909. https://doi.org/10.3390/molecules25040909





