Synthesis and Cytotoxic Evaluation of Some Substituted 5-Pyrazolones and Their Urea Derivatives
Abstract
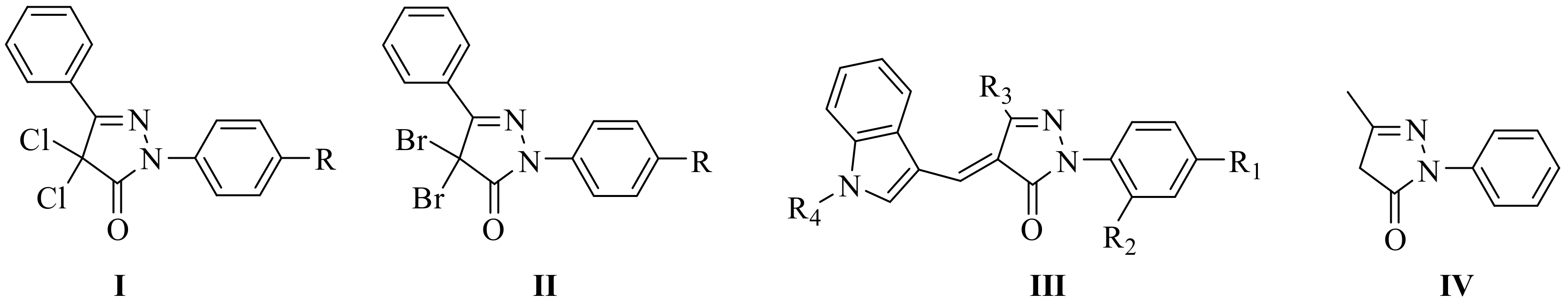
1. Introduction
2. Results and Discussion
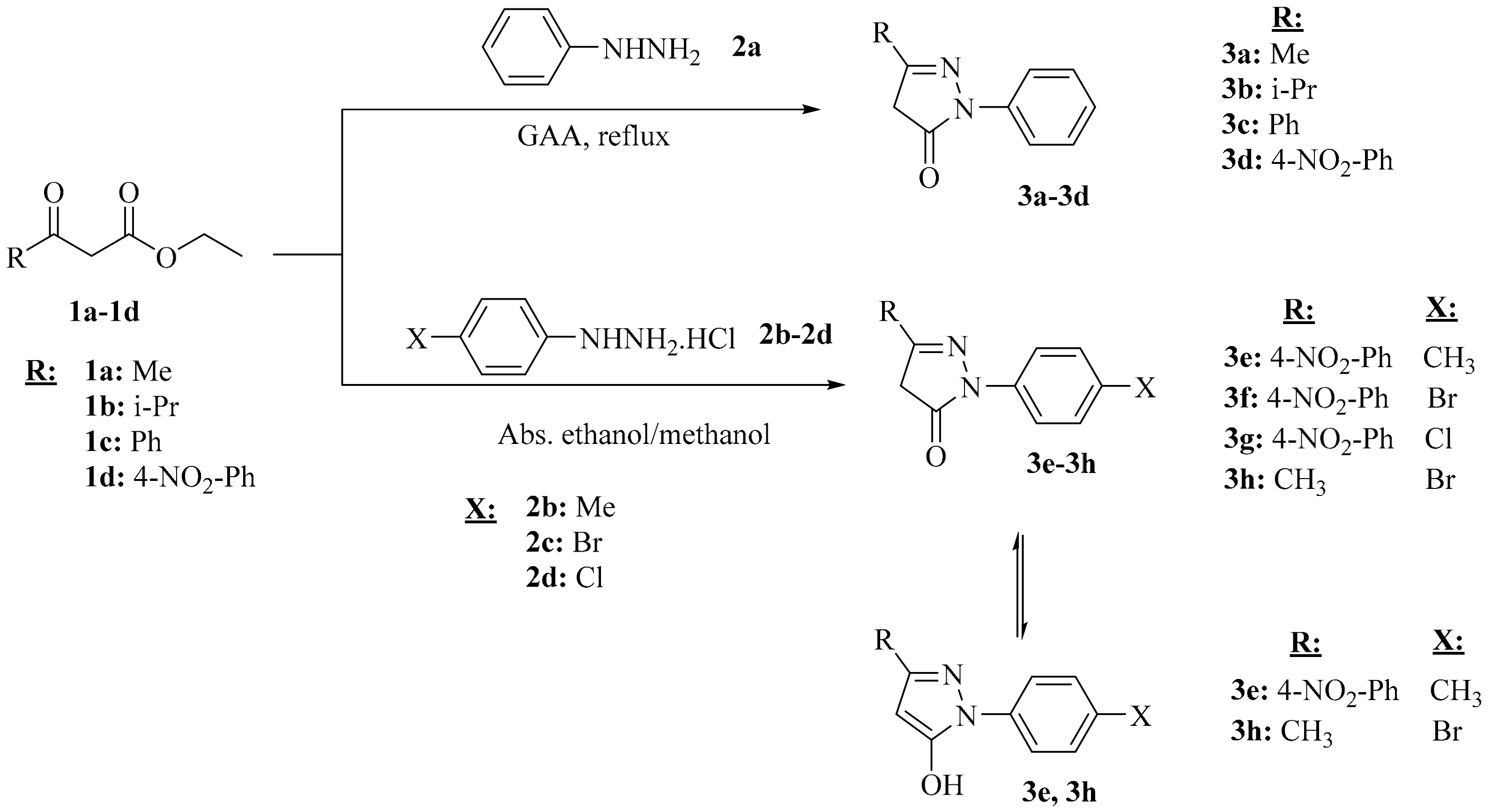
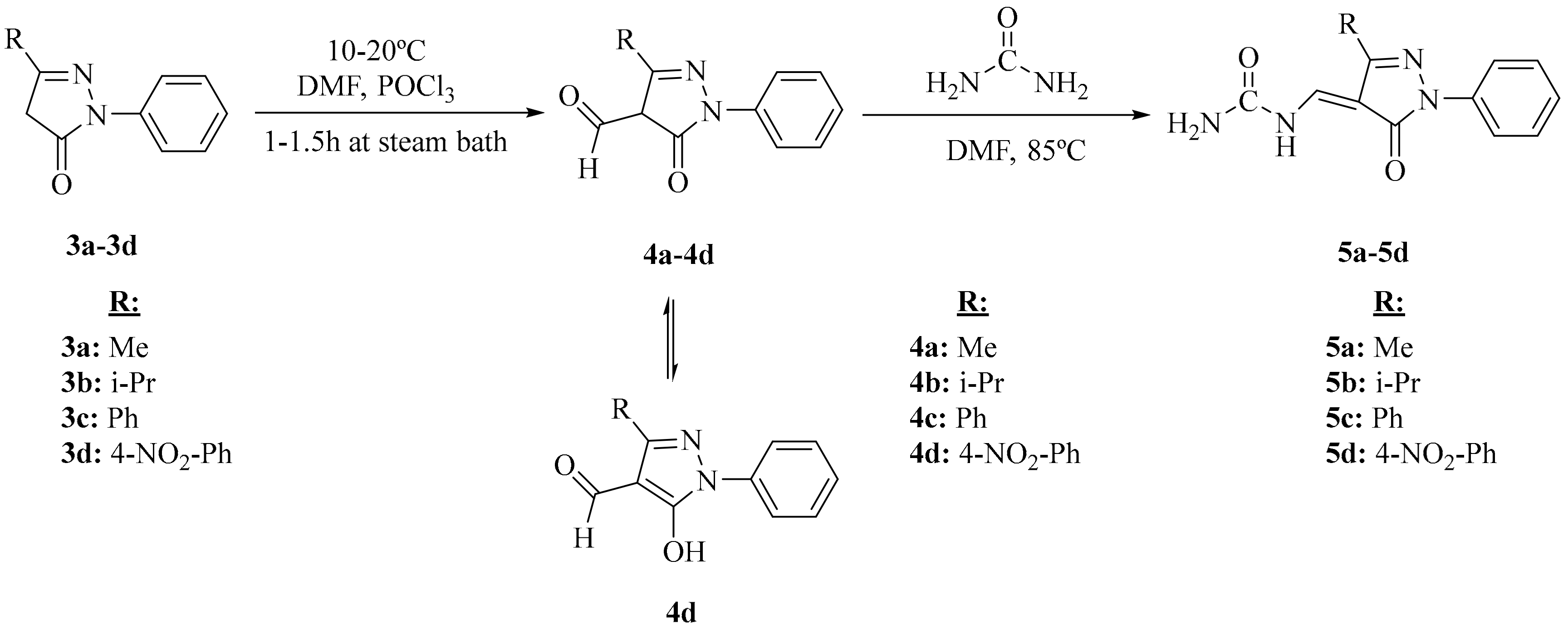
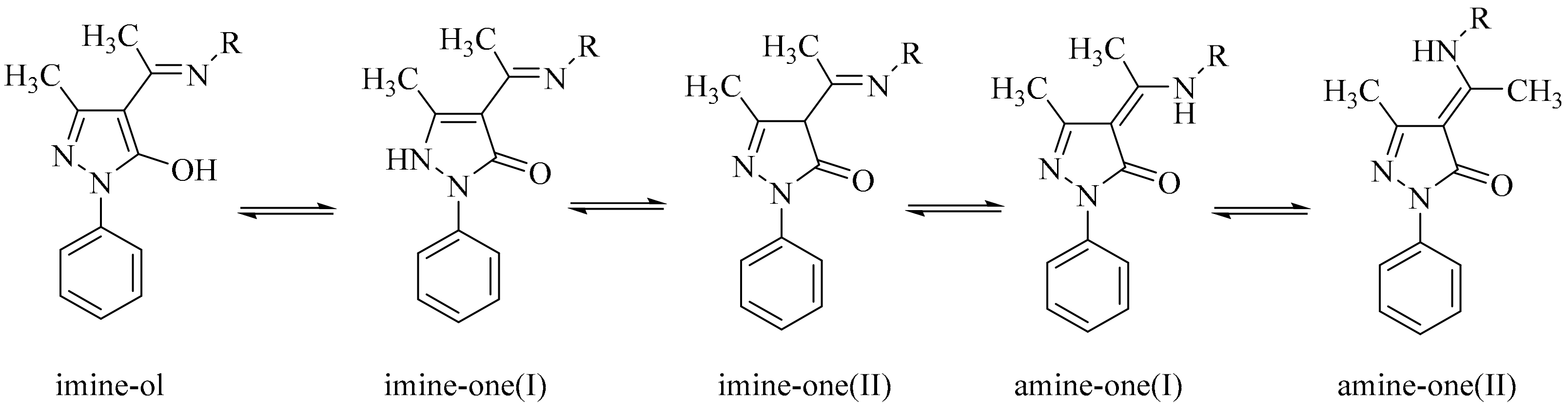
2.1. Chemistry
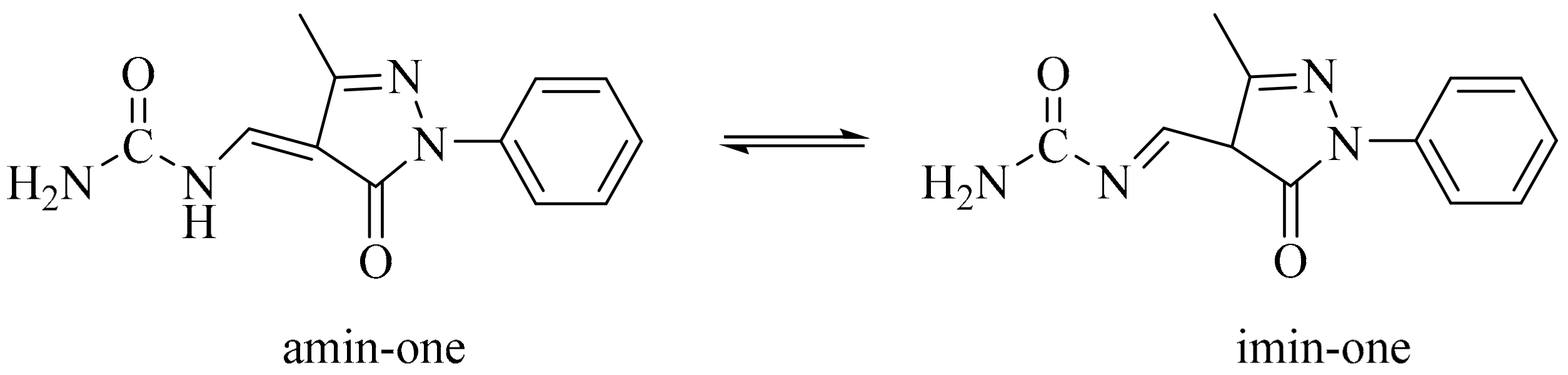
2.2. FTIR
2.3. NMR
2.4. Biological Activity of Compounds
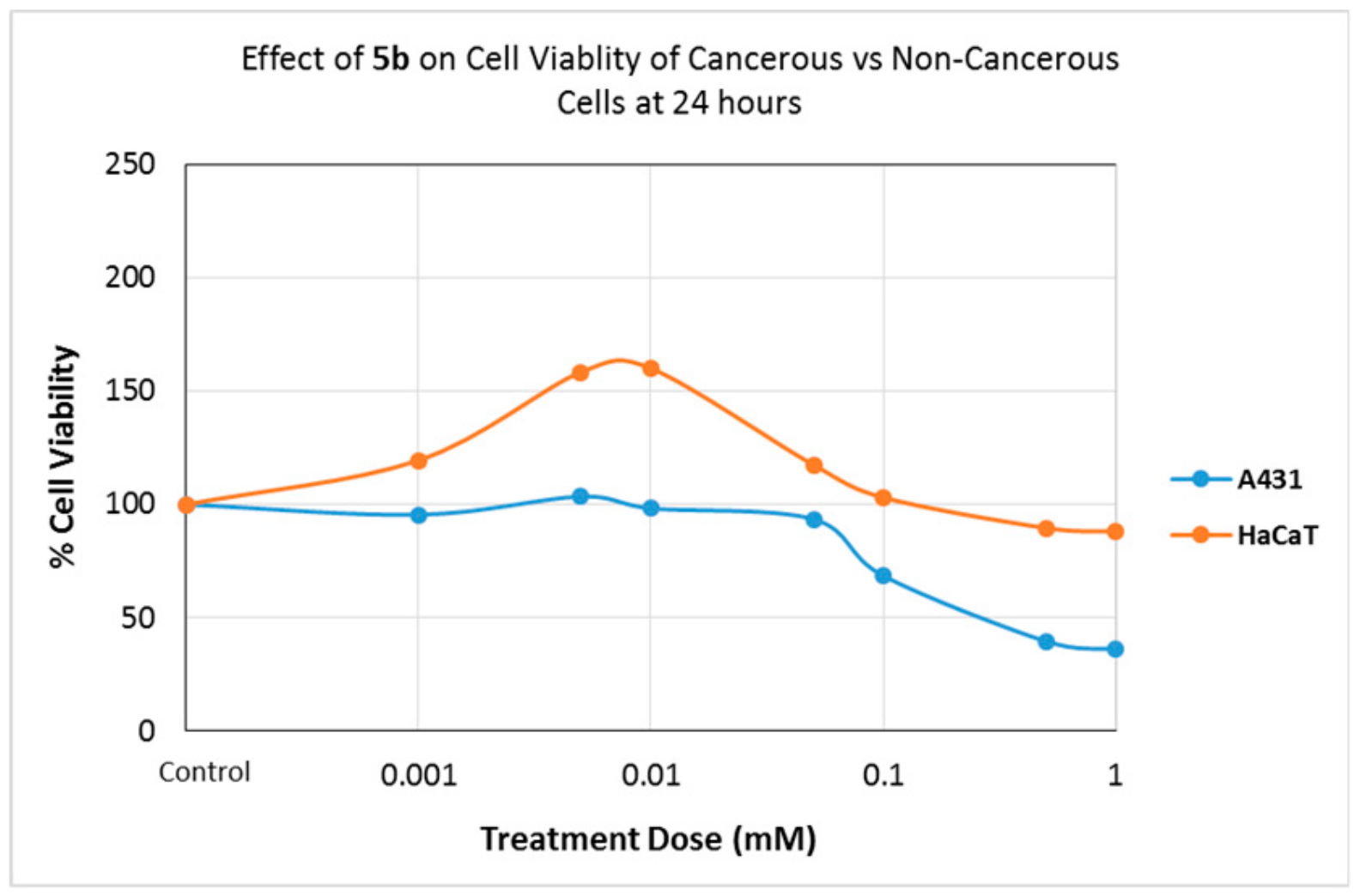
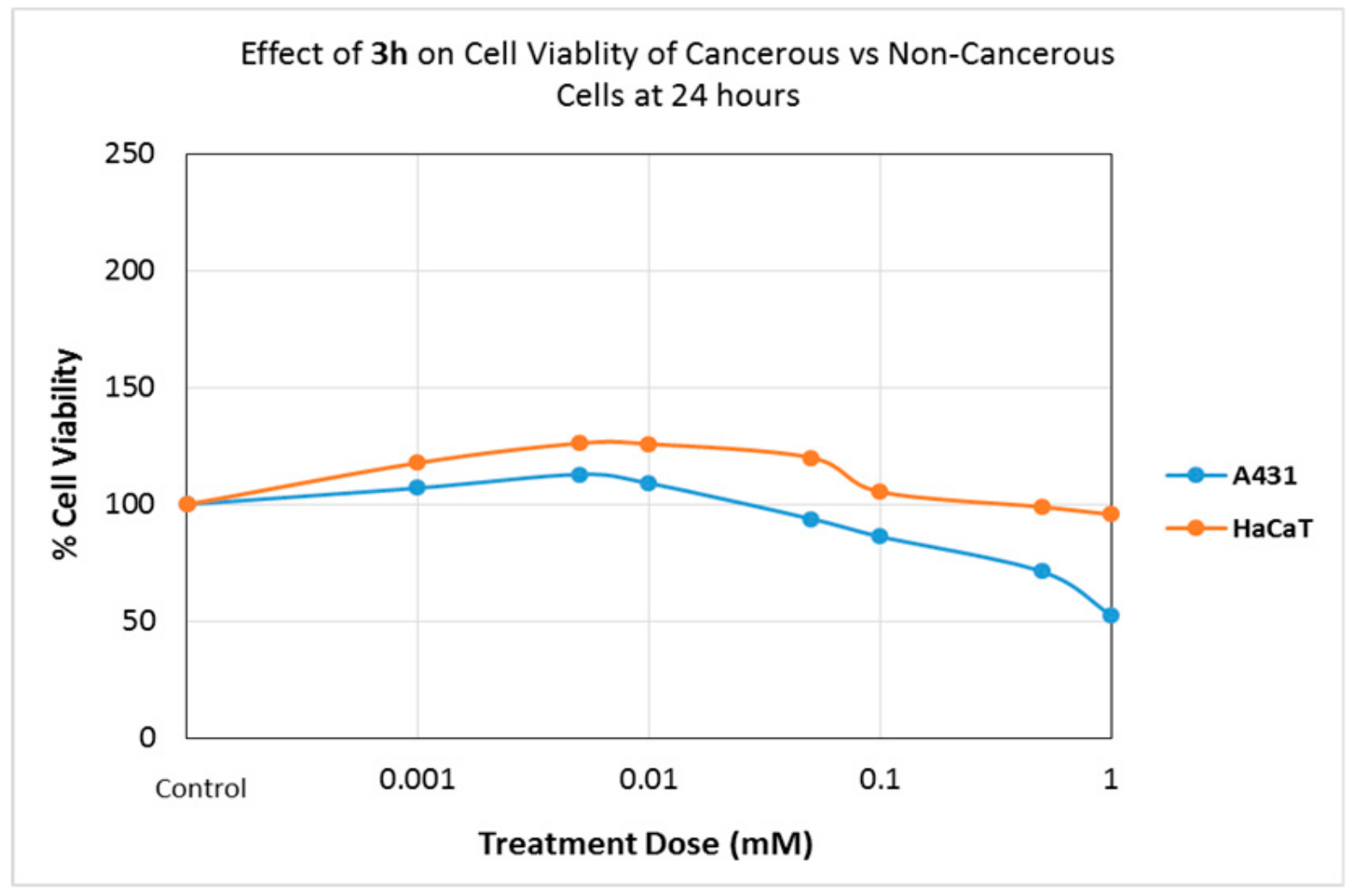
2.4.1. Effects of Compounds on Cell Viability
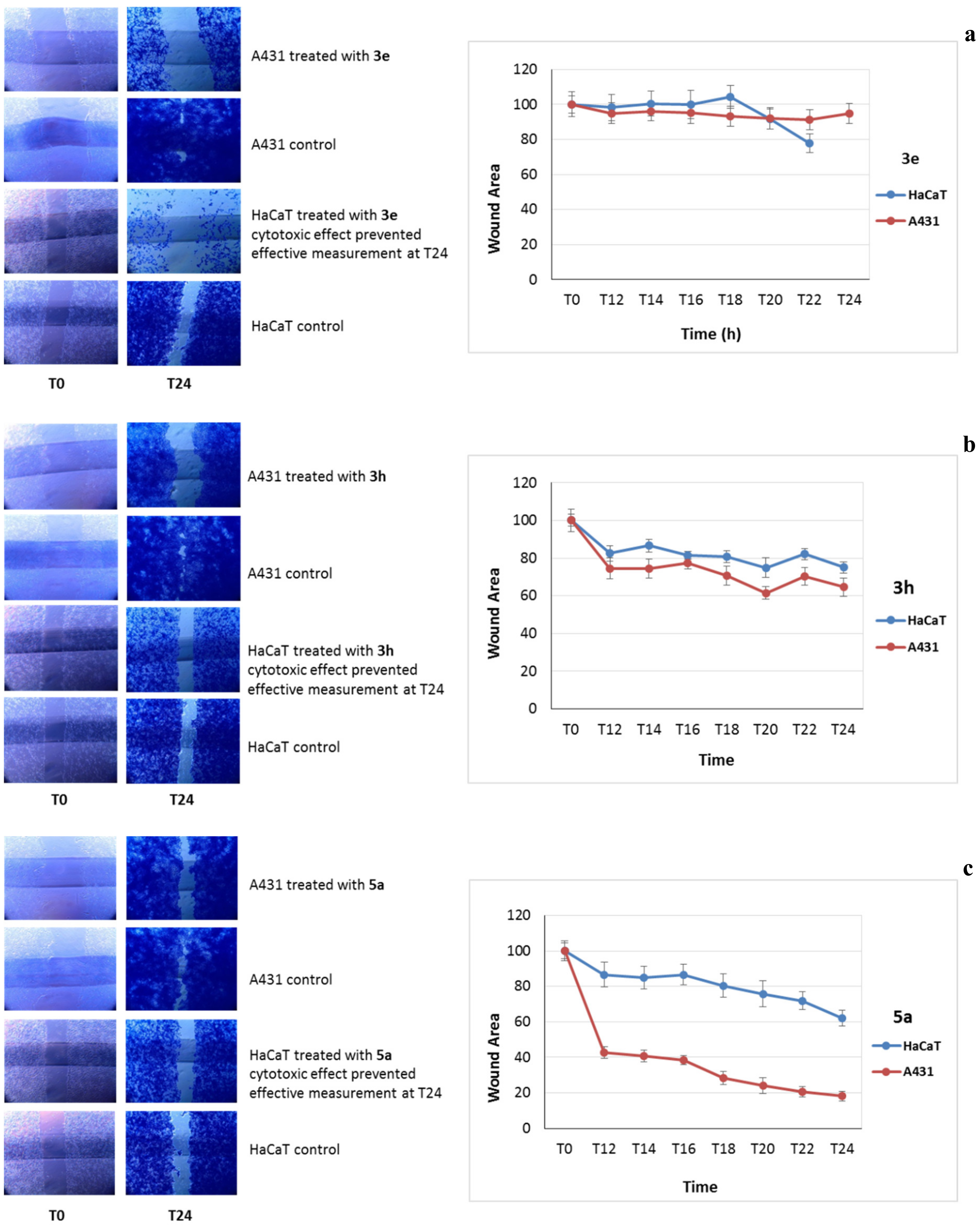
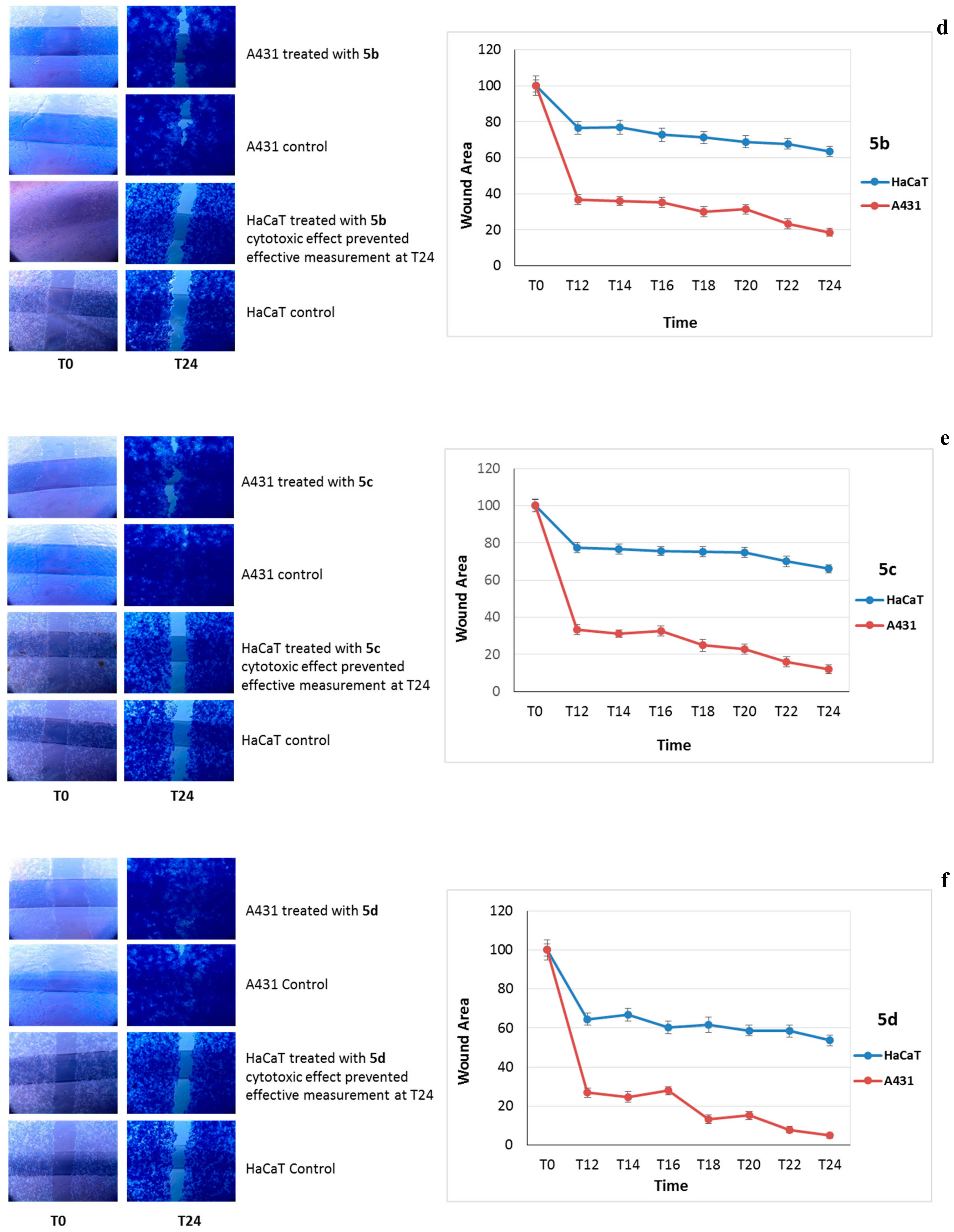
2.4.2. Cell Migration Assay
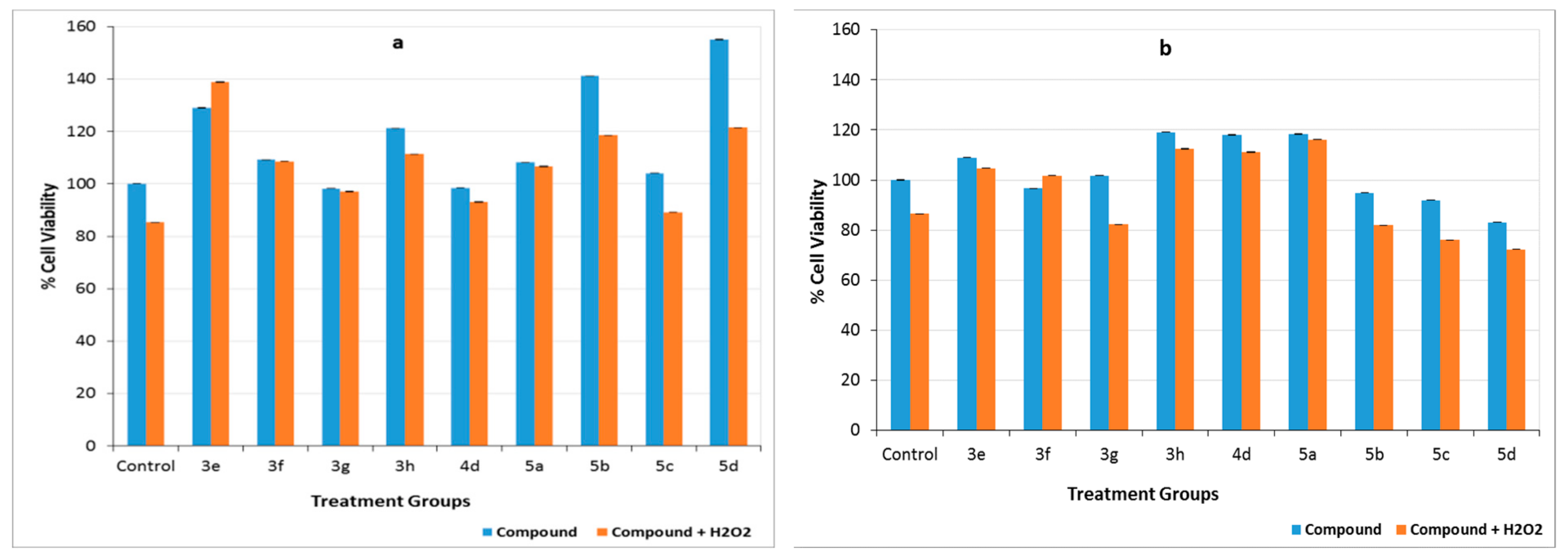
2.4.3. Preventing Cell Death Caused by Hydrogen Peroxide
3. Materials and Methods
3.1. Materials and Physical Measurements
3.2. Synthesis of Compounds
3.2.1. Synthesis of 5-Pyrazolone Compounds (3a–c) Obtained from Phenylhydrazine
3.2.2. Synthesis of 5-Pyrazolone Compounds (3e–g) Derived from Phenylhydrazine Hydrochloride
3.2.3. General Procedure for the Preparation of 4-Formyl-2-Pyrazoline-5-One (4a–d)
3.2.4. General Procedure for the Preparation of Urea-5-Pyrazolones (5a–d)
3.3. Biological Assays
3.3.1. Cell Cultures
3.3.2. Cell Viability Assay
3.3.3. Cell Migration Assay
3.3.4. Effects of Compounds on H2O2-Induced Cytotoxicity
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chmutova, G.A.; Kataeva, O.N.; Ahlbrecht, H.; Kurbangalieva, A.R.; Movchan, A.I.; Lenstra, A.T.H.; Geise, H.J.; Litvinov, I.A. Derivatives of 1-phenyl-3-methylpyrazol-2-in-5-thione and their oxygen analogues in the crystalline phase and their tautomeric transformations in solutions and in the gas phase. J. Mol. Struct. 2001, 570, 215–223. [Google Scholar]
- Al-Mutairi, A.A.; El-Baih, F.E.M.; Al-Hazimi, H. Microwave versus ultrasound assisted synthesis of some new heterocycles based on pyrazolone moiety. J. Saudi Chem. Soc. 2010, 14, 287–299. [Google Scholar] [CrossRef]
- Mojtahedi, M.M.; Javadpour, M.; Abaee, M.S. Convenient ultrasound mediated synthesis of substituted pyrazolones under solvent-free conditions. Ultrason. Sonochem. 2008, 15, 828–832. [Google Scholar] [CrossRef] [PubMed]
- Heise, H.; Hintzmann, M. Pyrazolone derivatives. In Ullmann’s Encycloprdia of Industrial Chemistry; Wiley-VCH, Ed.; Wiley-VCH: Weinheim, Germany, 2010; Volume 30, p. 547. [Google Scholar]
- Pal, S.; Mareddy, J.; Devi, N.S. High speed synthesis of pyrazolones using microwave-assisted neat reaction technology. J. Braz. Chem. Soc. 2008, 19, 1207–1214. [Google Scholar] [CrossRef]
- Dube, P.N.; Bule, S.S.; Mokale, S.N.; Kumbhare, M.R.; Dighe, P.R.; Ushir, Y.V. Synthesis and biologic evaluation of substituted 5-methyl-2-phenyl-1H-pyrazol-3(2H)-one derivatives as selective COX-2 inhibitors: Molecular docking study. Chem. Biol. Drug Des. 2014, 84, 409–419. [Google Scholar] [CrossRef]
- Khan, R.; Md. Uddin, I.; Md. Alam, S.; Hossain, M.M.; Md. Islam, R. Synthesis and preliminary evaluation of brominated 5-methyl-2,4-dihydropyrazol-3-one and its derivatives as cytotoxic agents. Bangladesh J Pharmacol. 2008, 3, 27–35. [Google Scholar]
- Ragab, F.A.F.; Abdel-Gawad, N.M.; Georgey, H.H.; Said, M.F. Pyrazolone derivatives: Synthesis, anti-inflammatory, analgesic, quantitative structure–activity relationship and in vitro studies. Chem. Pharm. Bull. 2013, 61, 834–845. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Lin, H.C.; Cheng, K.M.; Su, W.N.; Sung, K.C.; Lin, T.P.; Huang, J.J.; Lin, S.K.; Wong, F.F. Efficient di-bromination of 5-pyrazolones and 5-hydroxypyrazoles by N-bromobenzamide. Tetrahedron 2009, 65, 9592–9597. [Google Scholar] [CrossRef]
- Jing, L.; Wang, L.; Zhao, Y.; Tan, R.; Xing, X.; Liu, T.; Huang, W.; Luo, Y.; Li, Z. Synthesis, crystal structure and evaluation of cancer inhibitory activity of 4-[indol-3-yl-methylene]-1H-pyrazol-5(4H)-one derivatives. J. Chem. Res. 2012, 36, 691–696. [Google Scholar] [CrossRef]
- Sheng, X.; Hua, K.; Yang, C.; Wang, X.; Ji, H.; Xu, J.; Huang, Z.; Zhang, Y. Novel hybrids of 3-n-butylphthalide and edaravone: Design, synthesis and evaluations as potential anti-ischemic stroke agents. Bioorg. Med. Chem. Lett. 2015, 25, 3535–3540. [Google Scholar] [CrossRef]
- Nakagawa, H.; Ohyama, R.; Kimata, A.; Suzuki, T.; Miyata, N. Hydroxyl radical scavenging by edaravone derivatives: Efficient scavenging by 3-methyl-1-(pyridin-2-yl)-5-pyrazolone with an intramolecular base. Bioorg. Med. Chem. Lett. 2006, 16, 5939–5942. [Google Scholar] [CrossRef] [PubMed]
- Štefane, B.; Polanc, S. Aminolysis of 2,2-difluoro-4-alkoxy-1,3,2-dioxaborinanes: Route to β-keto amides and β-enamino carboxamides. Tetrahedron 2007, 63, 10902–10913. [Google Scholar] [CrossRef]
- Porai-Koshits, B.A.; Kvitko, I.Y.; Shutkotva, E.A.; Galkina, T.M. Aminomethylene derivatives of azoles. VI. Hydrolysis product of aminomethylene derivatives of 1-phenyl-3-methyl-5-pyrazolone. Zh. Obshch. Khim. 1966, 2, 174–177. [Google Scholar]
- Hawkes, G.E.; Randall, E.W.; Elguero, J.; Marzin, C.J. Proton, carbon-13, and nitrogen-15 nuclear magnetic resonance studies of [15N]azoles: 1-phenylpyrazole and the tautomerically mobile 3-methyl-1-phenylpyrazolin-5-one. J. Chem. Soc. Perkin Trans. 1977, 8, 1024–1027. [Google Scholar] [CrossRef]
- Elguero, J.; Martíanez, A.; Singh, S.P.; Grover, M.; Tarar, L.S.A. 1H and 13C NMR study of the structure and tautomerism of 4-pyrazolylpyrazolinones. J. Heterocycl. Chem. 1990, 27, 865–870. [Google Scholar] [CrossRef]
- Evans, N.A.; Whelan, J.; Johns, R.B. Tautomerism in the 5-pyrazolone series: 1(H)-5-pyrazolones and indazolones. Tetrahedron 1965, 21, 3351–3361. [Google Scholar] [CrossRef]
- Amarasekara, A.S.; Owereh, O.; Lyssenko, K.A.; Timofeeva, T.V. Structural tautomerism of 4-acylpyrazolone Schiff bases and crystal structure of 5-methyl-2-phenyl-4-{1-[(pyridin-2-ylmethyl)-amino]-ethylidene}-2,4-dihydro-pyrazol-3-one. J. Struct. Chem. 2009, 50, 1159–1165. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef]
- Hájková, A.; Barek, J.; Vyskočil, V. Electrochemical DNA biosensor for detection of DNA damage induced by hydroxyl radicals. Bioelectrochemistry 2017, 116, 1–9. [Google Scholar] [CrossRef]
- Asadi, N.S.; Heidari, M.M.; Khatami, M. Protective effect of Berberis vulgaris on Fenton reaction-induced DNA cleavage. Avicenna J. Phytomed. 2019, 9, 213–220. [Google Scholar]
- Markovic’, V.; Eric´, S.; Stanojkovic´, T.; Gligorijevic´, N.; Aran-delovic´, S.; Todorovic´, N.; Trifunovic´, S.; Manojlovic´, N.; Jelic´, R.; Joksovic´, M.D. Antiproliferative activity and QSAR studies of a series of new 4-aminomethylidene derivatives of some pyrazol-5-ones. Bioorg. Med. Chem. Lett. 2011, 21, 4416–4421. [Google Scholar] [CrossRef] [PubMed]
- Kasiotis, K.M.; Tzanetou, E.N.; Haroutounian, S.A. Pyrazoles as potential anti-angiogenesis agents: A contemporary overview. Front. Chem. 2014, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, H.; Özkan, A.N.; Zora, M. Novel ferrocenyl pyrazoles inhibit breast cancer cell viability via induction of apoptosis and inhibition of PI3K/Akt and ERK1/2 signaling. Chem. Biol. Interact. 2017, 263, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, M.; Lee, J.; Yu, H.; Hah, J.M. Syntheses of phenylpyrazolodiazepin-7-ones as conformationally rigid analogs of aminopyrazole amide scaffold and their antiproliferative effects on cancer cells. Bioorg. Med. Chem. 2011, 22, 6760–6767. [Google Scholar] [CrossRef] [PubMed]
- Pevarello, P.; Brasca, M.G.; Orsini, P.; Traquandi, G.; Longo, A.; Nesi, M.; Orzi, F.; Piutti, C.; Sansonna, P.; Varasi, M. 3-Aminopyrazole inhibitors of CDK2/cyclin A as antitumor agents. 2. Lead optimization. J. Med. Chem. 2005, 8, 2944–2956. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. CSH Perspect Biol. 2009, 1, a001651. [Google Scholar]
- Xia, Y.; Shen, S.; Verma, I.M. NF-κB, an active player in human cancers. Cancer Immunol. Res. 2014, 2, 823–830. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Mattson, M.P. Dietary factors, hormesis and health. Ageing Res. Rev. 2008, 7, 43–48. [Google Scholar] [CrossRef]
- Calabrese, V.; Cornelius, C.; Trovato-Salinaro, A.; Cambria, M.T.; Locascio, M.S.; Rienzo, L.D.; Calabrese, E.J. The hormetic role of dietary antioxidants in free radical-related diseases. Curr. Pharm. 2010, 16, 877–883. [Google Scholar] [CrossRef]
- Maher, J.; Yamamoto, M. The rise of antioxidant signaling-the evolution and hormetic actions of Nrf2. Toxicol. Appl. Pharm. 2010, 244, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Woods, T.L.; Elder, J.T. Differential responses of S100A2 to oxidative stress and increased intracellular calcium in normal, immortalized, and malignant human keratinocytes. J. Invest. Dermatol. 2002, 119, 1196–1201. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tochigi, M.; Inoue, T.; Suzuki-Karasaki, M.; Ochiai, T.; Ra, C.; Suzuki-Karasaki, Y. Hydrogen peroxide induces cell death in human TRAIL-resistant melanoma through intracellular superoxide generation. Int. J. Oncol. 2013, 42, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Y.; Kaneko, K.; Takayama, H.; Kimura, M.; Wong, F.F. New investigation of Vilsmeier-type reaction using pyrazolones with various amides. Tetrahedron Lett. 2011, 52, 3786–3792. [Google Scholar] [CrossRef]
- Wallace, D.J.; Straley, J.M. Synthesis of 5-oxo-2-pyrazoline-4-carboxaldehydes. J. Org. Chem. 1961, 26, 3825–3826. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gediz Erturk, A.; Omerustaoglu, H. Synthesis and Cytotoxic Evaluation of Some Substituted 5-Pyrazolones and Their Urea Derivatives. Molecules 2020, 25, 900. https://doi.org/10.3390/molecules25040900
Gediz Erturk A, Omerustaoglu H. Synthesis and Cytotoxic Evaluation of Some Substituted 5-Pyrazolones and Their Urea Derivatives. Molecules. 2020; 25(4):900. https://doi.org/10.3390/molecules25040900
Chicago/Turabian StyleGediz Erturk, Aliye, and Hilal Omerustaoglu. 2020. "Synthesis and Cytotoxic Evaluation of Some Substituted 5-Pyrazolones and Their Urea Derivatives" Molecules 25, no. 4: 900. https://doi.org/10.3390/molecules25040900
APA StyleGediz Erturk, A., & Omerustaoglu, H. (2020). Synthesis and Cytotoxic Evaluation of Some Substituted 5-Pyrazolones and Their Urea Derivatives. Molecules, 25(4), 900. https://doi.org/10.3390/molecules25040900




