NH2-MIL-53(Al) Polymer Monolithic Column for In-Tube Solid-Phase Microextraction Combined with UHPLC-MS/MS for Detection of Trace Sulfonamides in Food Samples
Abstract
1. Introduction
2. Results and Discussion
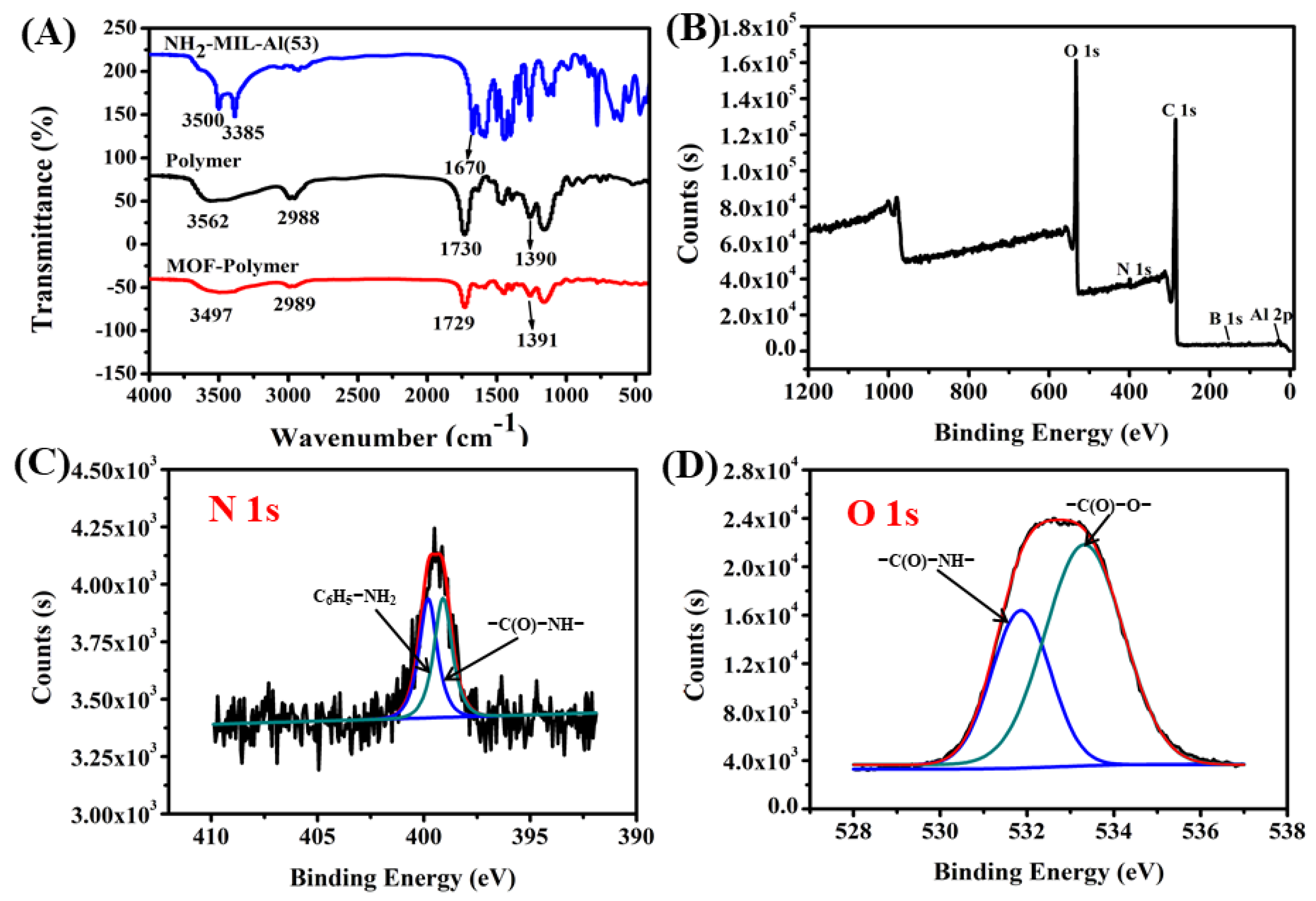
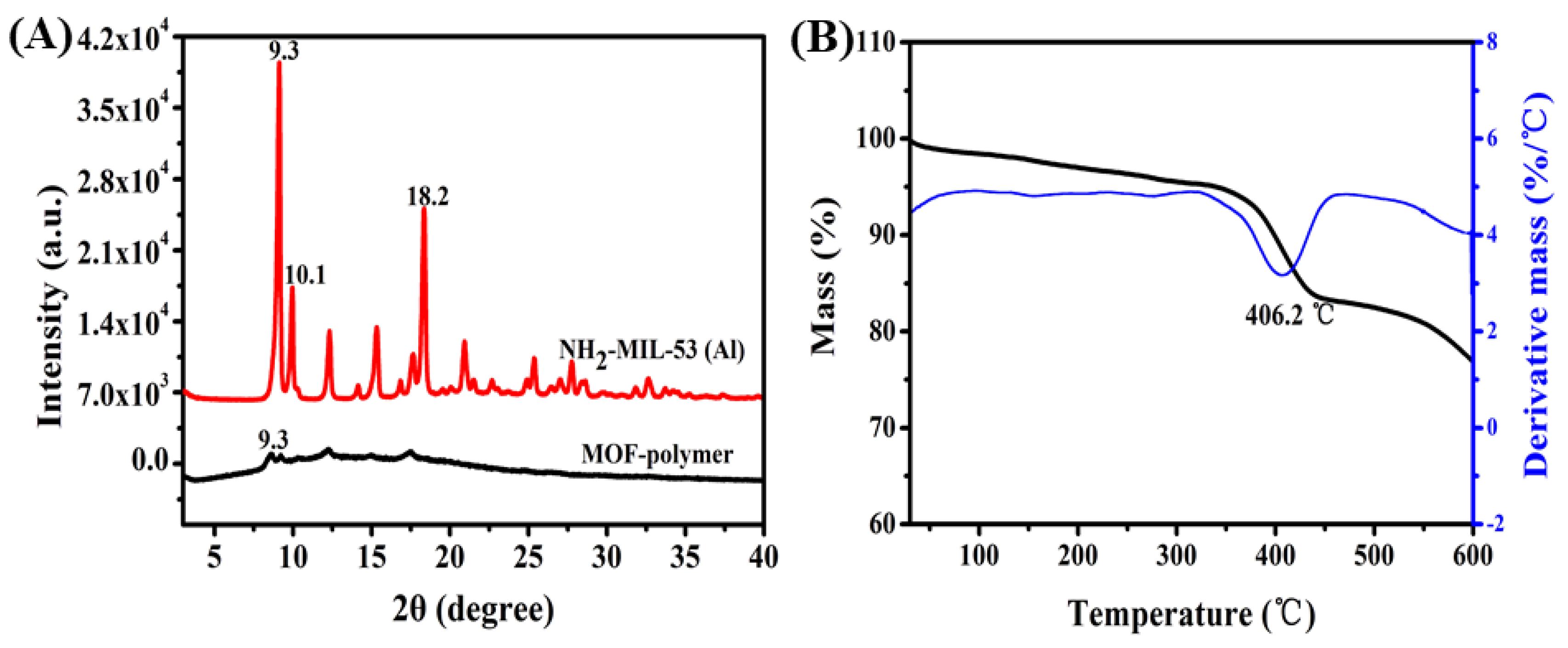
2.1. Characteristics of the MOF-Polymer Monolithic Column
2.2. UHPLC Procedure
2.3. Optimization of Extraction Conditions
2.3.1. Optimization of pH
2.3.2. Optimization of Extraction Flow Rate
2.3.3. Optimization of Clean Volume and Desorption Solvent
2.2.4. Optimization of Desorption Flow Rate and Desorption Volume
2.4. Adsorption Characteristics of the MOF-Polymer Monolithic Column
2.4.1. Extraction Capability and Adsorption Mechanism
2.4.2. Extraction Performance of the MOF-Polymer Monolithic Column
2.5. Application of the MOF-Polymer Monolithic Column
2.5.1. Analytical Method Validation
2.5.2. Sample Analysis
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Instrumentation
3.3. Chromatographic and Spectrometric Conditions
3.4. Preparation of NH2-MIL-53(Al)
3.5. MOF-Polymer Monolithic Column Preparation
3.6. Adsorption Performance of the MOF-Polymer Monolithic Column
3.7. Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Huertas-Pérez, J.F.; Arroyo-Manzanares, N.; Havlíková, L.; Gámiz-Gracia, L.; Solich, P.; García-Campaña, A.M. Method optimization and validation for the determination of eight sulfonamides in chicken muscle and eggs by modified QuEChERS and liquid chromatography with fluorescence detection. J. Pharm. Biomed. Anal. 2016, 124, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Li, K.; Liu, Z.L.; Zhang, H.Q.; Jin, X.Q. Novelty aqueous two-phase extraction system based on ionic liquid for determination of sulfonamides in blood coupled with high-performance liquid chromatography. Microchem. J. 2018, 136, 263–269. [Google Scholar] [CrossRef]
- Li, Y.Y.; Zhu, N.; Chen, T.; Ma, Y.L.; Li, Q. A green cyclodextrin metal-organic framework as solid-phase extraction medium for enrichment of sulfonamides before their HPLC determination. Microchem. J. 2018, 138, 401–407. [Google Scholar] [CrossRef]
- Tetzner, N.F.; Maniero, M.G.; Rodrigues-Silva, C.; Rath, S. On-line solid phase extraction-ultra high performance liquid chromatography-tandem mass spectrometry as a powerful technique for the determination of sulfonamide residues in soils. J. Chromatogr. A 2016, 1452, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.N.M.; Yahaya, N.; Zain, N.N.M.; Lim, V.; Kamaruzaman, S.; Saad, B.; Nishiyama, N.; Yoshida, N.; Hirota, Y. Thiol-functionalized magnetic carbon nanotubes for magnetic micro-solid phase extraction of sulfonamide antibiotics from milks and commercial chicken meat products. Food Chem. 2019, 276, 458–466. [Google Scholar] [CrossRef]
- Bargańska, Z.; Ślebioda, M.; Namieśnik, J. Determination of antibiotic residues in honey. TrAC Trends Anal. Chem. 2011, 30, 1035–1041. [Google Scholar] [CrossRef]
- Borràs, S.; Companyó, R.; Granados, M.; Guiteras, J.; Pérez-Vendrell, A.M.; Brufau, J.; Medina, M.; Bosch, J. Analysis of antimicrobial agents in animal feed. TrAC Trends Anal. Chem. 2011, 30, 1042–1064. [Google Scholar] [CrossRef]
- Huang, X.J.; Ihsan, A.; Wang, X.; Dai, M.H.; Wang, Y.L.; Su, S.J.; Xue, X.J.; Yuan, Z.H. Long-term dose-dependent response of Mequindox on aldosterone, corticosterone and five steroidogenic enzyme mRNAs in the adrenal of male rats. Toxicol. Lett. 2009, 191, 167–173. [Google Scholar] [CrossRef]
- Shen, Q.; Jin, R.; Xue, J.; Lu, Y.B.; Dai, Z.Y. Analysis of trace levels of sulfonamides in fish tissue using micro-scale pipette tip-matrix solid-phase dispersion and fast liquid chromatography tandem mass spectrometry. Food Chem. 2016, 194, 508–515. [Google Scholar] [CrossRef]
- Dmitrienko, S.G.; Kochuk, E.V.; Apyari, V.V.; Tolmacheva, V.V.; Zolotov, Y.A. Recent advances in sample preparation techniques and methods of sulfonamides detection-A review. Anal. Chim. Acta 2014, 850, 6–25. [Google Scholar] [CrossRef]
- He, X.; Wang, G.N.; Yang, K.; Liu, H.Z.; Wu, X.J.; Wang, J.P. Magnetic graphene dispersive solid phase extraction combining high performance liquid chromatography for determination of fluoroquinolones in foods. Food Chem. 2017, 221, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.N.; Zhang, L.; Song, Y.P.; Liu, J.X.; Wang, J.P. Application of molecularly imprinted polymer based matrix solid phase dispersion for determination of fluoroquinolones, tetracyclines and sulfonamides in meat. J. Chromatogr. B 2017, 1065–1066, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Maggira, M.; Deliyanni, E.A.; Samanidou, V.F. Synthesis of graphene oxide based sponges and their study as sorbents for sample preparation of cow milk prior to HPLC determination of sulfonamides. Molecules 2019, 24, 2086. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Álvarez, M.; Barahona, F.; Turiel, E.; Martín-Esteban, A. Supported liquid membrane-protected molecularly imprinted beads for micro-solid phase extraction of sulfonamides in environmental waters. J. Chromatogr. A 2014, 1357, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Mondal, S.; Xu, J.Q.; Chen, G.S.; Huang, S.M.; Huang, C.H.; Yin, L.; Ouyang, G.F. Solid-phase microextraction of antibiotics from fish muscle by using MIL-101(Cr)NH2-polyacrylonitrile fiber and their identification by liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2019, 1047, 62–70. [Google Scholar] [CrossRef]
- Nasiri, M.; Ahmadzadeh, H.; Amiri, A. Sample preparation and extraction methods for pesticides in aquatic environments: A review. TrAC Trends Anal. Chem. 2020, 123, 115772. [Google Scholar] [CrossRef]
- Queiroz, M.E.C.; Souza, I.D.D.; Marchioni, C. Current advances and applications of in-tube solid-phase microextraction. TrAC Trends Anal. Chem. 2019, 111, 261–278. [Google Scholar] [CrossRef]
- Nie, H.Y.; Chen, Y.; Lü, C.C.; Liu, Z. Efficient selection of glycoprotein-binding DNA aptamers via boronate affinity monolithic capillary. Anal. Chem. 2013, 85, 8277–8283. [Google Scholar] [CrossRef]
- Li, H.Y.; Liu, Z. Recent advances in monolithic column-based boronate-affinity chromatography. TrAC Trends Anal. Chem. 2012, 37, 148–161. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, H.Y.; Liu, Y.C.; Liu, Z. A benzoboroxole-functionalized monolithic column for the selective enrichment and separation of cis-diol containing biomolecules. Chem. Commun. 2012, 48, 4115–4117. [Google Scholar] [CrossRef]
- Mei, M.; Huang, X.J.; Luo, Q.; Yuan, D.X. Magnetism-enhanced monolith-based in-tube solid phase microextraction. Anal. Chem. 2016, 88, 1900–1907. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.L.; Jia, S.; Li, G.K.; Hu, Y.L. Organic building block based microporous network SNW-1 coating fabricated by multilayer interbridging strategy for efficient enrichment of trace volatiles. Anal. Chem. 2015, 87, 3373–3381. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Wang, C.; Wu, Q.H.; Li, Z.; Zang, X.H.; Wang, Z. Metal-organic framework derived magnetic nanoporous carbon: Novel adsorbent for magnetic solid-phase extraction. Anal. Chem. 2014, 86, 12199–12205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, J.; Du, T.; Zhang, W.T.; Zhu, W.X.; Yang, C.Y.; Yue, T.L.; Sun, J.; Li, T.; Wang, J.L. NH2-MIL-53(Al) metal-organic framework as the smart platform for simultaneous high-performance detection and removal of Hg2+. Inorg. Chem. 2019, 58, 12573–12581. [Google Scholar] [CrossRef] [PubMed]
- Abedini, R.; Omidkhah, M.; Dorosti, F. Highly permeable poly(4-methyl-1-pentyne)/NH2-MIL 53 (Al) mixed matrix membrane for CO2/CH4 separation. RSC Adv. 2014, 4, 36522–36537. [Google Scholar] [CrossRef]
- Mihaylov, M.; Chakarova, K.; Andonova, S.; Drenchev, N.; Ivanova, E.; Sabetghadam, A.; Seoane, B.; Gascon, J.; Kapteijn, F.; Hadjiivanov, K. Adsorption forms of CO2 on MIL-53(Al) and NH2-MIL-53(Al) as revealed by FTIR spectroscopy. J. Phys. Chem. C 2016, 120, 23584–23595. [Google Scholar] [CrossRef]
- Jadhav, M.R.; Pudale, A.; Raut, P.; Utture, S.; Shabeer, T.P.A.; Banerjee, K. A unified approach for high-throughput quantitative analysis of the residues of multi-class veterinary drugs and pesticides in bovine milk using LC-MS/MS and GC-MS/MS. Food Chem. 2019, 272, 292–305. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, W.Q.; Ding, Y.Q.; Zhu, B.Q.; Fan, Z.Y.; Luo, J.W. Rapid determination of sulfonamides in chicken using two-dimensional online cleanup mode with three columns coupled to liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2019, 1114-1115, 110–118. [Google Scholar] [CrossRef]
- Zhang, C.J.; Luo, X.L.; Wei, T.F.; Hu, Y.F.; Li, G.K.; Zhang, Z.M. Novel acylhydrazone bond dynamic covalent polymer gel monolithic column online coupling to high-performance liquid chromatography for analysis of sulfonamides and fluorescent whitening agents in food. J. Chromatogr. A 2017, 1519, 28–37. [Google Scholar] [CrossRef]
- Song, Y.P.; Zhang, L.; Wang, G.N.; Liu, J.X.; Liu, J.; Wang, J.P. Dual-dummy-template molecularly imprinted polymer combining ultra performance liquid chromatography for determination of fluoroquinolones and sulfonamides in pork and chicken muscle. Food Control 2017, 82, 233–242. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, W.J.; Liu, Y.W.; Xue, J.M.; Zhang, S.Q.; Li, Z.J. A simple, sensitive, and reliable method for the simultaneous determination of multiple antibiotics in vegetables through SPE-HPLC-MS/MS. Molecules 2018, 23, 1953. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Sun, L.; Yang, H.Y.; Ma, Y.L.; Deng, X.Y.; Peng, C.; Zheng, C.; Dong, L.Y.; Wang, X.H. Preparation of phenyl-boronic acid polymeric monolith by initiator-free ring-opening polymerization for microextraction of sulfonamides prior to their determination by ultra-performance liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2019, 1609, 460510. [Google Scholar] [CrossRef] [PubMed]
- Ahnfeldt, T.; Gunzelmann, D.; Loiseau, T.; Hirsemann, D.; Senker, J.; Fe’rey, G.; Stock, N. Synthesis and modification of a functionalized 3D open-framework structure with MIL-53 topology. Inorg. Chem. 2009, 48, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.L.; Li, G.K.; Hu, Y.F. In-tube solid-phase microextraction based on NH2-MIL-53(Al)-polymer monolithic column for online coupling with high-performance liquid chromatography for directly sensitive analysis of estrogens in human urine. Talanta 2017, 165, 377–383. [Google Scholar] [CrossRef]
- Gao, M.J.; Fu, Q.F.; Wang, M.; Zhang, K.L.; Zeng, J.; Wang, L.J.; Xia, Z.N.; Gao, D. Facile synthesis of porous covalent organic frameworks for the effective extraction of nitroaromatic compounds from water samples. Anal. Chim. Acta 2019, 1084, 21–32. [Google Scholar] [CrossRef]
- Rosa, M.A.; Faria, H.D.D.; Carvalho, D.T.; Figueiredo, E.C. Biological sample preparation by using restricted-access nanoparticles prepared from bovine serum albumin: Application to liquid application to liquid chromatographic determination of β-blockers. Microchim. Acta 2019, 186, 647. [Google Scholar] [CrossRef]
- Zhou, L.J.; Hu, Y.L.; Li, G.K. Conjugated microporous polymers with built-in magnetic nanoparticles for excellent enrichment of trace hydroxylated polycyclic aromatic hydrocarbons in human urine. Anal. Chem. 2016, 88, 6930–6938. [Google Scholar] [CrossRef]
- Zhang, Q.C.; Li, G.K.; Xiao, X.H. Acrylamide-modified graphene for online micro-solid-phase extraction coupled to high-performance liquid chromatography for sensitive analysis of heterocyclic amines in food samples. Talanta 2015, 131, 127–135. [Google Scholar] [CrossRef]
Sample Availability: Samples of the SAs compounds are available from the authors. |




| Analytes | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qmax (mg/m) | KL (L/mg) | R2 | KF (L/mg) | n | R2 | |
| SDZ | 334.22 | 5.05 | 0.80306 | 11.63 | 0.55 | 0.88174 |
| STZ | 1284.03 | 5.14 | 0.76070 | 9.05 | 0.70 | 0.91181 |
| SMI | 874.13 | 5.55 | 0.69402 | 25.63 | 0.64 | 0.84180 |
| SMZ | 366.57 | 6.58 | 0.71091 | 17.06 | 0.52 | 0.88569 |
| SMM | 520.29 | 15.64 | 0.90646 | 26.44 | 0.69 | 0.98678 |
| SMX | 78.31 | 20.66 | 0.78464 | 59.14 | 0.40 | 0.82074 |
| SIZ | 86.36 | 10.62 | 0.72585 | 31.42 | 0.40 | 0.79187 |
| SDM | 355.87 | 66.23 | 0.95141 | 114.11 | 0.67 | 0.99618 |
| Analytes | Regression Equation | R2 | Linear Range (µg/L) | LOD a (ng/L) | LOQ a (ng/L) | RSD b (%) |
|---|---|---|---|---|---|---|
| SDZ | y = 0.2911x − 0.000883 | 0.9998 | 0.015–25.0 | 4.7 | 14.2 | 4.7 |
| STZ | y = 0.3201x + 0.0243 | 0.9954 | 0.015–25.0 | 3.1 | 9.35 | 4.4 |
| SMI | y = 0.3595x + 0.0303 | 0.9989 | 0.015–25.0 | 1.7 | 5.30 | 6.1 |
| SMZ | y = 0.3441x + 0.0207 | 0.9997 | 0.015–25.0 | 2.4 | 7.42 | 4.8 |
| SMM | y = 0.3448x − 0.00505 | 0.9994 | 0.015–25.0 | 3.5 | 10.9 | 4.2 |
| SMX | y = 0.3666x + 0.00663 | 0.9967 | 0.015–25.0 | 1.3 | 4.15 | 3.7 |
| SIZ | y = 0.3617x + 0.0395 | 0.9972 | 0.015–25.0 | 2.3 | 6.96 | 2.2 |
| SDM | y = 0.3137x + 0.0167 | 0.9996 | 0.015–25.0 | 3.8 | 11.7 | 2.1 |
| Sample | Analytes | Concentration (µg/Kg) | RSD (%) | Spiked Concentration (µg/Kg) | |||
|---|---|---|---|---|---|---|---|
| 0.50 (µg/Kg) | 5.0 (µg/Kg) | ||||||
| Recovery (%) | RSD (%) | Recovery (%) | RSD (%) | ||||
| Chicken | SDZ | 0.0951 | 9.3 | 85.7 | 7.3 | 92.7 | 2.5 |
| STZ | 0.0507 | 8.3 | 115 | 4.2 | 103 | 3.6 | |
| SMI | 0.747 | 6.2 | 110 | 6.0 | 107 | 5.2 | |
| SMZ | 0.620 | 7.4 | 108 | 5.4 | 93.6 | 3.8 | |
| SMM | 0.145 | 7.2 | 98.8 | 2.7 | 107 | 4.1 | |
| SMX | N.Q. | -- | 91.9 | 5.5 | 97.3 | 2.8 | |
| SIZ | 0.147 | 6.5 | 88.1 | 4.3 | 96.1 | 4.6 | |
| SDM | 1.81 | 3.9 | 112 | 3.9 | 104 | 5.7 | |
| Fish | SDZ | 0.494 | 7.8 | 96.8 | 6.6 | 101 | 4.7 |
| STZ | 0.228 | 5.6 | 89.9 | 5.6 | 105 | 3.6 | |
| SMI | 3.09 | 6.2 | 95.8 | 5.1 | 95.3 | 5.1 | |
| SMZ | 4.28 | 4.8 | 89.0 | 4.0 | 96.0 | 3.1 | |
| SMM | 1.23 | 7.2 | 86.7 | 4.3 | 96.6 | 3.8 | |
| SMX | 0.554 | 6.2 | 86.8 | 5.2 | 89.5 | 2.0 | |
| SIZ | 1.22 | 8.3 | 102 | 6.3 | 113 | 6.2 | |
| SDM | 7.89 | 4.0 | 104 | 3.9 | 85.6 | 3.2 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.-C.; Xia, G.-P.; Liang, J.-Y.; Zhang, X.-L.; Jiang, L.; Zheng, Y.-G.; Wang, X.-Y. NH2-MIL-53(Al) Polymer Monolithic Column for In-Tube Solid-Phase Microextraction Combined with UHPLC-MS/MS for Detection of Trace Sulfonamides in Food Samples. Molecules 2020, 25, 897. https://doi.org/10.3390/molecules25040897
Zhang Q-C, Xia G-P, Liang J-Y, Zhang X-L, Jiang L, Zheng Y-G, Wang X-Y. NH2-MIL-53(Al) Polymer Monolithic Column for In-Tube Solid-Phase Microextraction Combined with UHPLC-MS/MS for Detection of Trace Sulfonamides in Food Samples. Molecules. 2020; 25(4):897. https://doi.org/10.3390/molecules25040897
Chicago/Turabian StyleZhang, Qian-Chun, Guang-Ping Xia, Jun-Yi Liang, Xiao-Lan Zhang, Li Jiang, Yu-Guo Zheng, and Xing-Yi Wang. 2020. "NH2-MIL-53(Al) Polymer Monolithic Column for In-Tube Solid-Phase Microextraction Combined with UHPLC-MS/MS for Detection of Trace Sulfonamides in Food Samples" Molecules 25, no. 4: 897. https://doi.org/10.3390/molecules25040897
APA StyleZhang, Q.-C., Xia, G.-P., Liang, J.-Y., Zhang, X.-L., Jiang, L., Zheng, Y.-G., & Wang, X.-Y. (2020). NH2-MIL-53(Al) Polymer Monolithic Column for In-Tube Solid-Phase Microextraction Combined with UHPLC-MS/MS for Detection of Trace Sulfonamides in Food Samples. Molecules, 25(4), 897. https://doi.org/10.3390/molecules25040897





