Supramolecular and Liquid Crystalline Contributions to the Assembly of Myofibril
Abstract
1. Introduction
2. Roles of Chemical and Shape Recognition
2.1. Chemical Recognition
2.2. Shape Recognition
2.3. Mixtures
3. Comparison Between Actual and Postulated Assembling Steps
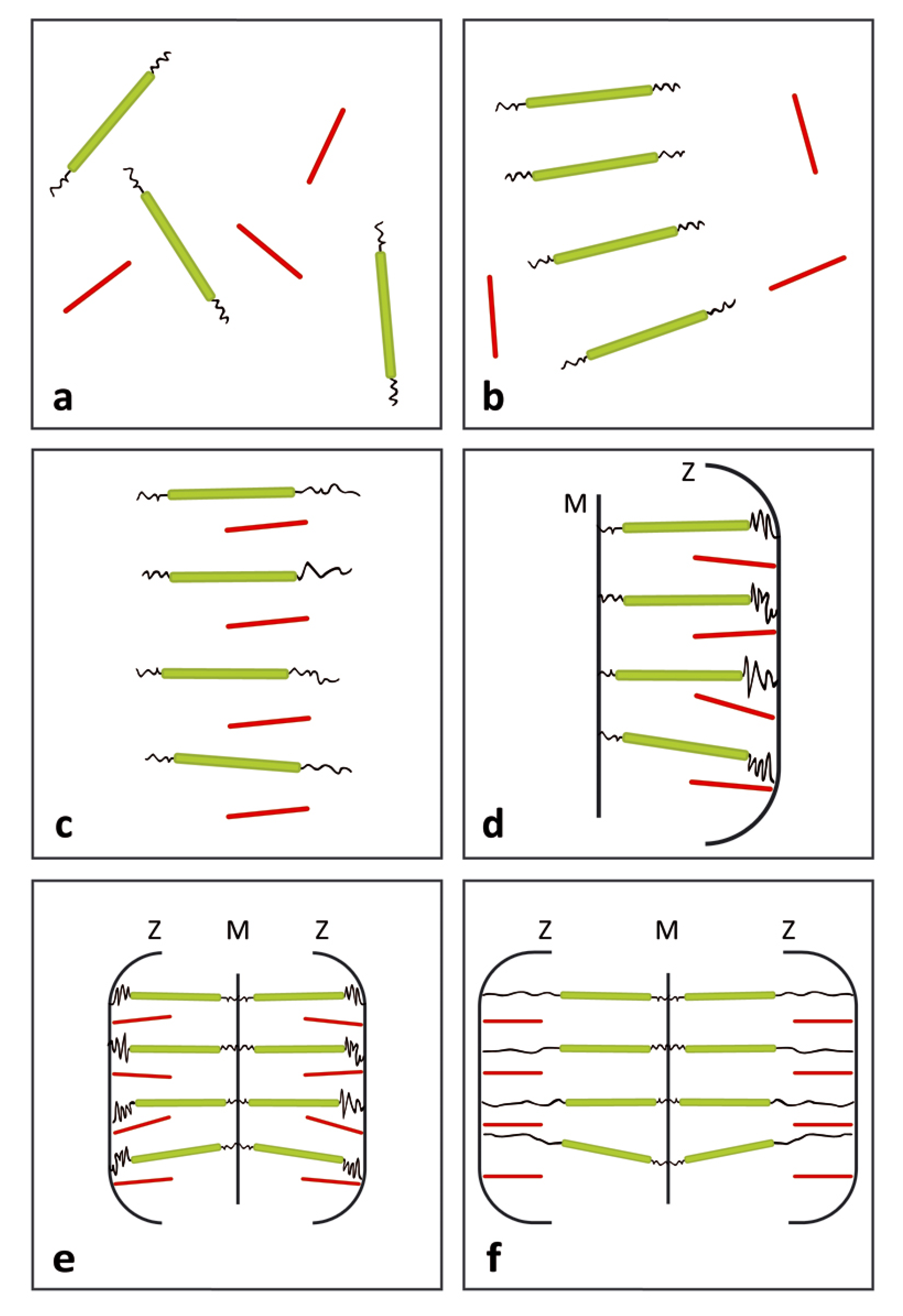
3.1. Formation of the Thin and Thick Filaments
3.2. Development of Liquid Crystallinity
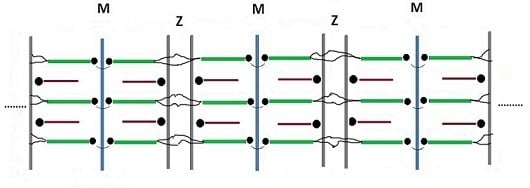
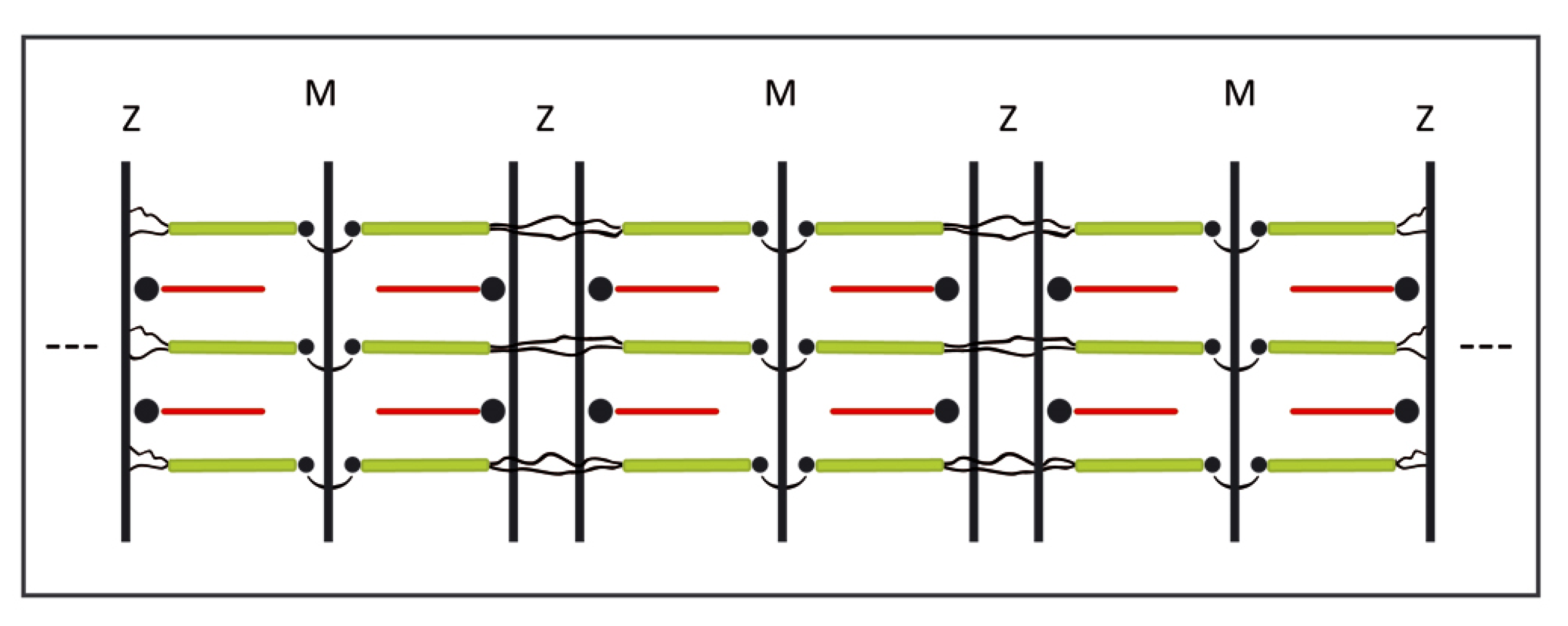
3.3. Assembly of the Sarcomer
3.4. Assembly of the Myofibril
4. Relevance to the Dynamic Function of the Myofiber
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Huxley, H.E. Fifty years of muscle and the sliding filament hypothesis. Eur. J. Biochem. 2014, 271, 1403–1415. [Google Scholar] [CrossRef] [PubMed]
- Hanson, J.; Huxley, H.E. Structural basis of the cross-striations in muscle. Nature 1953, 172, 530–532. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, A.; Crumbliss, A.L. The Assembling and Contraction Mechanisms of Striated Muscles. Front. Chem. 2018, 6, 570. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, A.; Crumbliss, A.L. A supramolecular polymerization approach to the Growth of the Myofibril. Front. Chem. 2019, 7, 487. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.M. Actin, Myosin, and Cell Movement. In The Cell: A Molecular Approach, 6th ed.; Sinauer Associates: Sunderland, MA, USA, 2000; ISBN -10 0-87893-106-6. [Google Scholar]
- Wang, J.; Shaner, N.; Mitta, B.; Zhou, Q.; Chen, J.; Sanger, J.M.; Sanger, J.W. Dynamics of Z-band based proteins in developing skeletal muscle cells. Cell Motil. Cytoskelet. 2005, 61, 34–48. [Google Scholar] [CrossRef]
- Wang, L.; Geist, J.; Grogan, A.; Hu, L.R.; Kontrogianni-Konstantopoulo, A. Thick filament protein network, functions, and disease association. Compr. Physiol. 2018, 8, 631–709. [Google Scholar] [CrossRef]
- Pizon, V.; Iakovenko, A.; van der Ven, P.F.M.; Kelly, R.; Fatu, C.; Fürst, D.O.; Karsenti, E.; Gautel, M. Transient association of titin and myosin with microtubules in nascent myofibrils directed by the MURF2 RING-finger protein. J. Cell Sci. 2003, 115, 4469–4482. [Google Scholar] [CrossRef]
- Gregorio, C.G.; Antin, P.B. To the heart of myofibril assembly. Trends Cell Biol. 2000, 10, 355–362. [Google Scholar] [CrossRef]
- Ciferri, A. (Ed.) Supramolecular Polymers, 2nd ed.; CRC Press: Baton Rouge, FL, USA, 2005. [Google Scholar] [CrossRef]
- Ciferri, A. Supramolecular polymers. Macromol. Rapid Commun. 2002, 23, 511–529. [Google Scholar] [CrossRef]
- Van der Schoot, P. Theory of supramolecular polymerization. In Supramolecular Polymers, 2nd ed.; Ciferri, A., Ed.; CRC Press: Baton Rouge, FL, USA, 2005; pp. 77–106. [Google Scholar] [CrossRef]
- Ciferri, A. Ionic Mixed Interactions and Hofmeister Effects. In Ionic Interactions in Natural and Synthetic Macromolecules; Ciferri, A., Perico, A., Eds.; Wiley: New York, NY, USA, 2012; pp. 167–209. [Google Scholar] [CrossRef]
- Yang, J.X.; Janmey, P.A. The polyelectrolyte nature of F-actin and the mechanism of actin bundle formation. J. Biol. Chem. 1996, 271, 8556–8563. [Google Scholar] [CrossRef]
- Manning, G.S. Molecular theory of polyelectrolyte solutions. Q. Rev. Biophys. 1978, 11, 179–245. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Sheng, P. Generalized Onsager theory of liquid crystals. Phys. Rev. E 2013, 88, 062501. [Google Scholar] [CrossRef] [PubMed]
- Flory, P.J. Molecular Theory of Liquid Crystals Polymers I. Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1984; Volume 59. [Google Scholar] [CrossRef]
- Odijk, T. Ordered phases of elongated micelles. Curr. Opin. Colloid Interface Sci. 1996, 1, 337–340. [Google Scholar] [CrossRef]
- Nyrkova, I.A.; Khokhlov, A.R. Liquid-crystalline ordering in polyelectrolyte Solutions. Biofizika (Biophys. USSR) 1986, 31, 771–780. [Google Scholar] [CrossRef]
- Sasaki, S.; Nigao, M.; Gotoch, M.; Uematsjuu, I. Compatibility and mesophase separation in blends of α-helical polyglutamates. J. Polym. Sci. Polym. Phys. Ed. 1988, 26, 637–648. [Google Scholar] [CrossRef]
- Maissa, P.; Sixou, P. Mixtures of liquid crystalline polymers. Liq. Cryst. 1989, 5, 1861–1869. [Google Scholar] [CrossRef]
- Ciferri, A. (Ed.) Phase behavior of rigid and semirigid mesogens. In Liquid Crystallinity in Polymers; VCH Verlagesellschaff: Weinheim, Germany, 1991; pp. 541–544. ISBN 0-89573-771-X. [Google Scholar]
- Jerôme, B. Surface effects and anchoring in liquid crystals. Rep. Prog. Phys. 1991, 54, 391–452. [Google Scholar] [CrossRef]
- Ishiwata, S.; Miyazaki, M.; Sato, K.; Nakagome, K.; Shintani, S.A.; Kobirumaki-Shimozawa, F.; Fukuda, N.; Suzuki, K.; Takagi, J.; Shimamoto, Y.; et al. Dynamic properties of bio-motile systems with a liquid crystalline structure. Mol. Cryst. Liq. Cryst. 2017, 647, 127–150. [Google Scholar] [CrossRef]
- Liversage, A.D.; Holmes, D.; Knight, P.J.; Tskhovrebova, L.; Trinick, J. Titin and the sarcomer symmetry paradox. J. Mol. Biol. 2000, 305, 401–409. [Google Scholar] [CrossRef]
- Tornell, L.; Andersson, K.; Sjostrom, M. The relationship between mechanical stress and myofibrillar organization in heart Purkinje fibres. J. Mol. Cell. Cardiol. 1976, 8, 689–692. [Google Scholar] [CrossRef]
- Mitchinson, T.J.; Cramer, L.P. Actin-based cell motility and cell locomotion. Cell 1996, 4, 371–379. [Google Scholar] [CrossRef]
- Pertici, I.; Bongini, L.; Melli, L.; Bianchi, G.; Salvi, L.; Falorsi, G.; Squarci, C.; Bozó, T.; Cojoc, D.; Kellermayer, M.S.Z.; et al. A myosin II nanomachine mimicking the striated muscle. Nat. Commun. 2018, 9, 3532. [Google Scholar] [CrossRef] [PubMed]
- Stokes, D.L. Calcium transport across the sarcoplasmic reticulum. Structure and function of Ca2+-ATPase and the ryanodine receptor. Eur. J. Biochem. 2000, 267, 5274–5279. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciferri, A.; Crumbliss, A.L. Supramolecular and Liquid Crystalline Contributions to the Assembly of Myofibril. Molecules 2020, 25, 862. https://doi.org/10.3390/molecules25040862
Ciferri A, Crumbliss AL. Supramolecular and Liquid Crystalline Contributions to the Assembly of Myofibril. Molecules. 2020; 25(4):862. https://doi.org/10.3390/molecules25040862
Chicago/Turabian StyleCiferri, Alberto, and Alvin L. Crumbliss. 2020. "Supramolecular and Liquid Crystalline Contributions to the Assembly of Myofibril" Molecules 25, no. 4: 862. https://doi.org/10.3390/molecules25040862
APA StyleCiferri, A., & Crumbliss, A. L. (2020). Supramolecular and Liquid Crystalline Contributions to the Assembly of Myofibril. Molecules, 25(4), 862. https://doi.org/10.3390/molecules25040862