Honey as Source of Nitrogen Compounds: Aromatic Amino Acids, Free Nucleosides and Their Derivatives
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
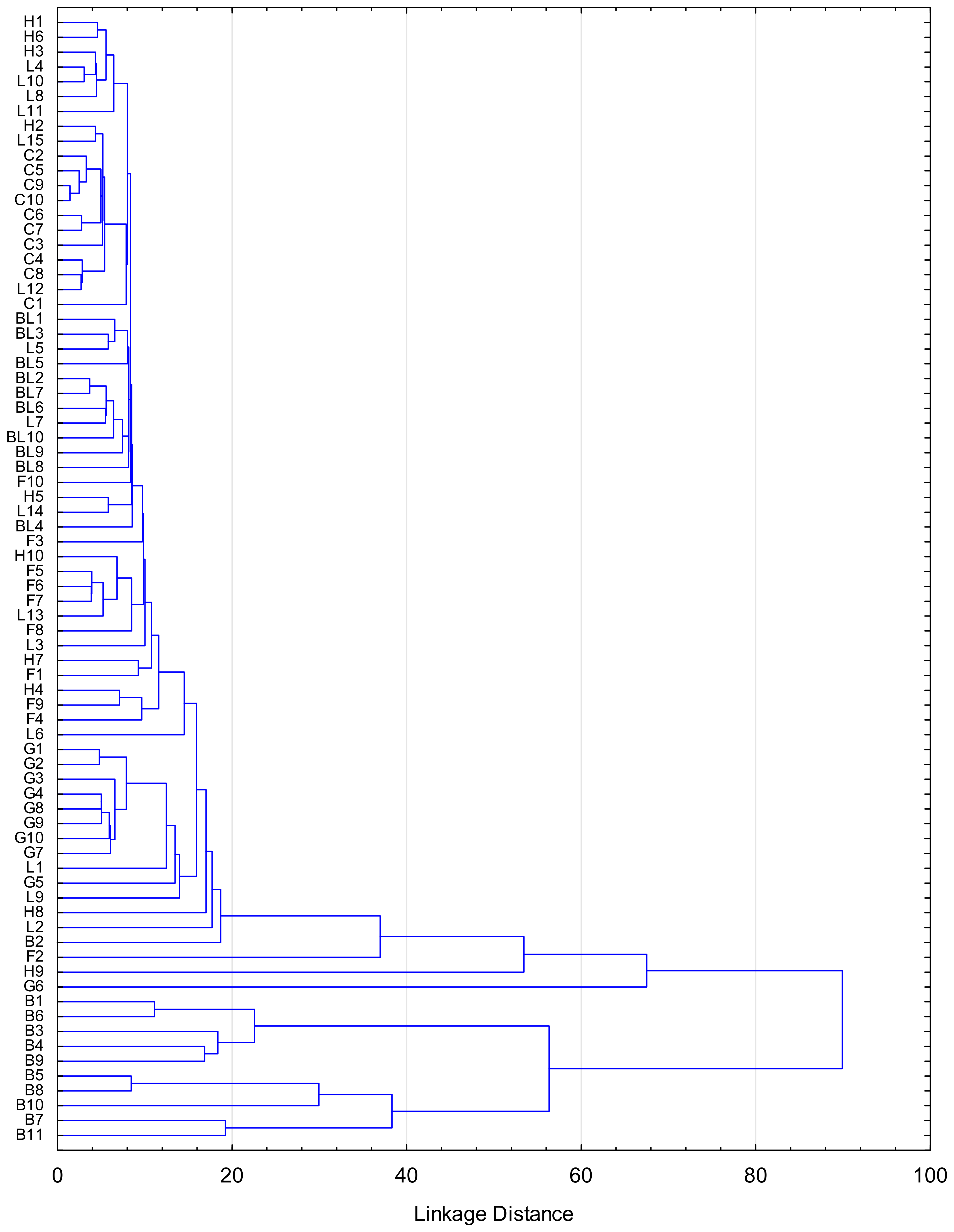
3.1. Honey Samples
3.2. Reagents
3.3. UHPLC-DAD and UHPLC-QqTOF-MS Analysis
3.4. Statistical Analysis
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuś, P.M.; Włodarczyk, M.; Tuberoso, C.I.G. Nitrogen compounds in Phacelia tanacetifolia Benth. honey: First time report on occurrence of (−)-5- epi -lithospermoside, uridine, adenine and xanthine in honey. Food Chem. 2018, 255, 332–339. [Google Scholar] [CrossRef]
- Wang, C.; Song, Z.; Yu, H.; Liu, K.; Ma, X. Adenine: An important drug scaffold for the design of antiviral agents. Acta Pharm. Sin. B 2015, 5, 431–441. [Google Scholar] [CrossRef]
- Dorland, W.A.N. Dorland’s Medical Dictionary, 32nd ed.; Elsevier: Philadelphia, PA, USA, 2007; ISBN 0721631428. [Google Scholar]
- Holguin, S.; Martinez, J.; Chow, C.; Wurtman, R. Dietary uridine enhances the improvement in learning and memory produced by administering DHA to gerbils. FASEB J. 2008, 22, 3938–3946. [Google Scholar] [CrossRef]
- Wurtman, R.J.; Cansev, M.; Ulus, I.H. Synapse formation is enhanced by oral administration of uridine and DHA, the circulating precursors of brain phosphatides. J. Nutr. Heal. Aging 2009, 13, 189–197. [Google Scholar] [CrossRef]
- de Bruin, N.M.W.J.; Kiliaan, A.J.; de Wilde, M.C.; Broersen, L.M. Combined uridine and choline administration improves cognitive deficits in spontaneously hypertensive rats. Neurobiol. Learn. Mem. 2003, 80, 63–79. [Google Scholar] [CrossRef]
- Wurtman, R.J. A Nutrient combination that can affect synapse formation. Nutrients 2014, 6, 1701–1710. [Google Scholar] [CrossRef]
- Stover, P.J.; Field, M.S. Use of uridine and deoxyuridine to treat folate-responsive pathologies. U.S. Patent US9579337B2, 28 February 2017. [Google Scholar]
- Goldberg, H.; Mibielli, M.A.; Nunes, C.P.; Goldberg, S.W.; Buchman, L.; Mezitis, S.G.; Rzetelna, H.; Oliveira, L.; Geller, M.; Wajnsztajn, F. A double-blind, randomized, comparative study of the use of a combination of uridine triphosphate trisodium, cytidine monophosphate disodium, and hydroxocobalamin, versus isolated treatment with hydroxocobalamin, in patients presenting with compress. J. Pain Res. 2017, 10, 397–404. [Google Scholar] [CrossRef]
- Zizzo, M.G.; Caldara, G.; Bellanca, A.; Nuzzo, D.; Di, M.; Rosa, C. Preventive effects of guanosine on intestinal inflammation in 2,4-dinitrobenzene sulfonic acid (DNBS)-induced colitis in rats. Inflammopharmacology 2019, 27, 349–359. [Google Scholar] [CrossRef]
- Lanznaster, D.; Dal-Cim, T.; Tetsadê, C.B.; Piermartiri, C.I.T. Guanosine: A Neuromodulator with therapeutic potential in brain disorders. Aging Dis. 2016, 7, 657–679. [Google Scholar] [CrossRef]
- Dal-cim, T.; Poluceno, G.G.; Lanznaster, D.; de Oliveira, K.A.; Nedel, C.B.; Tasca, C.I. Guanosine prevents oxidative damage and glutamate uptake impairment induced by oxygen / glucose deprivation in cortical astrocyte cultures: Involvement of A 1 and A 2A adenosine receptors and PI3K, MEK, and PKC pathways. Purinergic Signal. 2019, 15, 465–476. [Google Scholar] [CrossRef]
- Rosa, P.B.; Bettio, L.E.B.; Neis, V.B.; Moretti, M.; Werle, I.; Leal, R.B.; Rodrigues, A.L.S. The antidepressant-like effect of guanosine is dependent on GSK-3 β inhibition and activation of MAPK / ERK and Nrf2 / heme oxygenase-1 signaling pathways. Purinergic Signal. 2019, 2, 491–504. [Google Scholar] [CrossRef]
- Dobrachinski, F.; Gerbatin, R.R.; Sartori, G.; Golombieski, R.M.; Antoniazzi, A.; Nogueira, C.W.; Royes, L.F.; Fighera, M.R.; Porciúncula, L.O.; Cunha, R.A.; et al. Guanosine attenuates behavioral deficits after traumatic brain injury by modulation of adenosinergic receptors. Mol. Neurobiol. 2019, 56, 3145–3158. [Google Scholar] [CrossRef]
- Rahman, M.M.; Gan, S.H.; Khalil, I. Neurological effects of honey: Current and future prospects. Evidence-Based Complement. Altern. Med. 2014, 2014, 958721. [Google Scholar] [CrossRef]
- Goudgaon, N.M.; Schinazi, R.F. Activity of acyclic 6-(phenylselenenyl)pyrimidine nucleosides against human immunodeficiency viruses in primary lymphocytes. J. Med. Chem. 1991, 34, 3305–3309. [Google Scholar] [CrossRef]
- Clercq, E. De Nucleosides, nucleotides and nucleic acids guanosine analogues as anti-herpesvirus agents. Nucleotides and Nucleic Acids 2000, 19, 1531–1541. [Google Scholar] [CrossRef]
- Mayer, A.; Slezak, V.; Takac, P.; Olejnik, J.; Majtan, J. Treatment of non-healing leg ulcers with honeydew honey. J. Tissue Viability 2014, 23, 94–97. [Google Scholar] [CrossRef]
- Al-Waili, N.S. Topical honey application vs. acyclovir for the treatment of recurrent herpes simplex lesions. Med. Sci. Monit. 2004, 10, MT94–MT98. [Google Scholar]
- Chen, H.; Jin, L.; Chang, Q.; Peng, T.; Hu, X.; Fan, C.; Pang, G.; Wang, W. Discrimination of botanical origins for Chinese honey according to free amino acids content by high-performance liquid chromatography with fluorescence detection with chemometric approaches. J. Sci. Food Agric. 2017, 97, 2042–2049. [Google Scholar] [CrossRef]
- Janiszewska, K.; Aniołowska, M.; Nowakowski, P. Free amino acids content of honeys from Poland. Polish J. Food Nutr. Sci. 2012, 62, 85–89. [Google Scholar] [CrossRef]
- Kečkeš, J.; Trifković, J.; Andrić, F.; Jovetić, M.; Tešić, Z.; Milojković-Opsenica, D. Amino acids profile of Serbian unifloral honeys. J. Sci. Food Agric. 2013, 93, 3368–3376. [Google Scholar] [CrossRef]
- Shen, S.; Wang, J.; Chen, X.; Liu, T.; Zhuo, Q.; Zhang, S. Evaluation of cellular antioxidant components of honeys using UPLC-MS / MS and HPLC-FLD based on the quantitative composition-activity relationship. Food Chem. 2019, 293, 169–177. [Google Scholar] [CrossRef]
- Zieliński, Ł.; Deja, S.; Jasicka-Misiak, I.; Kafarski, P. Chemometrics as a tool of origin determination of Polish monofloral and multifloral honeys. J. Agric. Food Chem. 2014, 62, 2973–2981. [Google Scholar] [CrossRef]
- Kňazovická, V.; Gábor, M.; Miluchová, M.; Bobko, M.; Medo, J. Diversity of bacteria in Slovak and foreign honey, with assessment of its physico-chemical quality and counts of cultivable microorganisms. J. Microbiol. Biotechnol. Food Sci. 2019, 9, 414–421. [Google Scholar] [CrossRef]
- Mach, J. Uridine ribohydrolase and the balance between nucleotide degradation and salvage. Plant Cell 2009, 21, 699. [Google Scholar] [CrossRef][Green Version]
- Nepi, M. New perspectives in nectar evolution and ecology: Simple alimentary reward or a complex multiorganism interaction? Acta Agrobot. 2017, 70, 1704. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of melissopalynology by International Commission for Bee Botany of IUBS. Bee World 1970, 51, 125–138. [Google Scholar] [CrossRef]
- EMEA ICH Topic Q2 (R1) Validation of analytical procedures: Text and methodology. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500002662.pdf. (accessed on 14 February 2020).
Sample Availability: Samples of the compounds are not available from the authors. |


| Compound | tR (min) | UVmax (nm) | [M + H]+ | Formula | Error (ppm) | |
|---|---|---|---|---|---|---|
| 1 | Adenine | 1.17 | 263 | 136.0621 | C5H5N5 | 1.62 |
| 2 | Uridine | 1.72 | 262 | 245.0764 | C9H12N2O6 | 3.93 |
| 3 | Xanthine | 1.78 | 268 | 153.0405 | C5H4N4O2 | 4.91 |
| 4 | Tyrosine | 2.21 | 224, 275 | 182.0812 | C9H11NO3 | 2.75 |
| 5 | Guanosine | 3.10 | 254, 274 | 284.0989 | C10H13N5O5 | 2.09 |
| 6 | Phenylalanine | 3.41 | 258 | 166.0864 | C9H11NO2 | 2.41 |
| Adenine (mg/kg) | Xanthine (mg/kg) | Uridine (mg/kg) | Tyrosine (mg/kg) | Guanosine (mg/kg) | Phenylalanine (mg/kg) | |
|---|---|---|---|---|---|---|
| Heather | 11.8a ± 2.9 | nd | 30.7bc ± 9.9 | 35.8a ± 28.9 | 3.4ab ± 1.1 | 17.1abc ± 4.0 |
| (n = 10) | ||||||
| Buckwheat | 11.6a ± 4.4 | nd | 40.2cd ± 13.6 | 263.9b ± 91.6 | nd* | 34.6c ± 17.1 |
| (n = 10) | ||||||
| Black locust | 11.1a ± 1.5 | 3.3b ± 0.8 | 51.2d ± 7.8 | 12.1a ± 5.2 | 2.6a ± 0.3 | 11.3ab ± 5.5 |
| (n = 10) | ||||||
| Goldenrod | 14.0ab ± 2.7 | 1.2a ± 0.6 | 24.6ab ± 2.7 | 44.9a ± 25.1 | 2.5a ± 1.3 | 64.1d ± 20.3 |
| (n = 10) | ||||||
| Canola | 8.9a ± 1.4 | 3.0b ± 0.6 | 17.5a ± 3.9 | 7.8a ± 3.3 | 2.0a ± 0.7 | 9.5a ± 3.6 |
| (n = 10) | ||||||
| Fir | nd* | nd | 32.5bc ± 12.8 | 31.6a ± 17.9 | nd* | 18.1abc ± 13.3 |
| (n = 10) | ||||||
| Linden | 18.4b ± 6.5 | 1.7a ± 0.9 | 28.6ab ± 10.6 | 21.8a ± 12.3 | 4.1b ± 1.5 | 28.4bc ± 20.6 |
| (n = 15) | ||||||
| LOD | 0.1 | 0.2 | 0.3 | 0.4 | 0.1 | 0.3 |
| LOQ | 0.4 | 0.6 | 1.0 | 1.1 | 0.3 | 0.8 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuś, P.M. Honey as Source of Nitrogen Compounds: Aromatic Amino Acids, Free Nucleosides and Their Derivatives. Molecules 2020, 25, 847. https://doi.org/10.3390/molecules25040847
Kuś PM. Honey as Source of Nitrogen Compounds: Aromatic Amino Acids, Free Nucleosides and Their Derivatives. Molecules. 2020; 25(4):847. https://doi.org/10.3390/molecules25040847
Chicago/Turabian StyleKuś, Piotr M. 2020. "Honey as Source of Nitrogen Compounds: Aromatic Amino Acids, Free Nucleosides and Their Derivatives" Molecules 25, no. 4: 847. https://doi.org/10.3390/molecules25040847
APA StyleKuś, P. M. (2020). Honey as Source of Nitrogen Compounds: Aromatic Amino Acids, Free Nucleosides and Their Derivatives. Molecules, 25(4), 847. https://doi.org/10.3390/molecules25040847





