Effectiveness of Different Analytical Methods for the Characterization of Propolis: A Case of Study in Northern Italy
Abstract
1. Introduction
2. Results
2.1. Balsam and Moisture Content, Total Phenols, Flavones and Flavonols Content, and Scavenging Activity
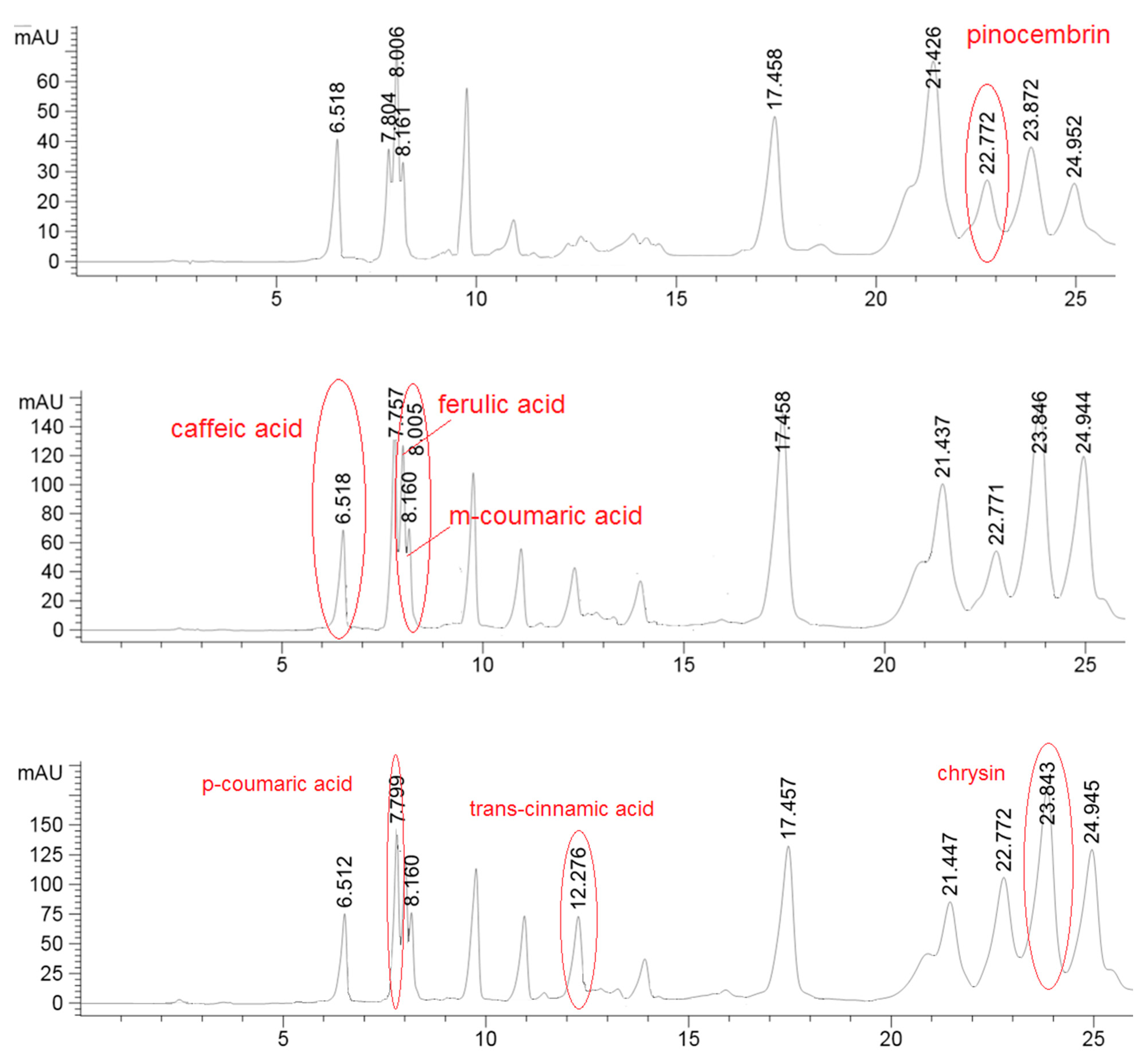
2.2. High-Performance Liquid Chromatography (HPLC) Analysis
2.3. Propolis Volatile Compounds
2.4. Nuclear Magnetic Resonance (NMR)
2.5. HPLC–Q-Exactive-Orbitrap®–MS Analysis
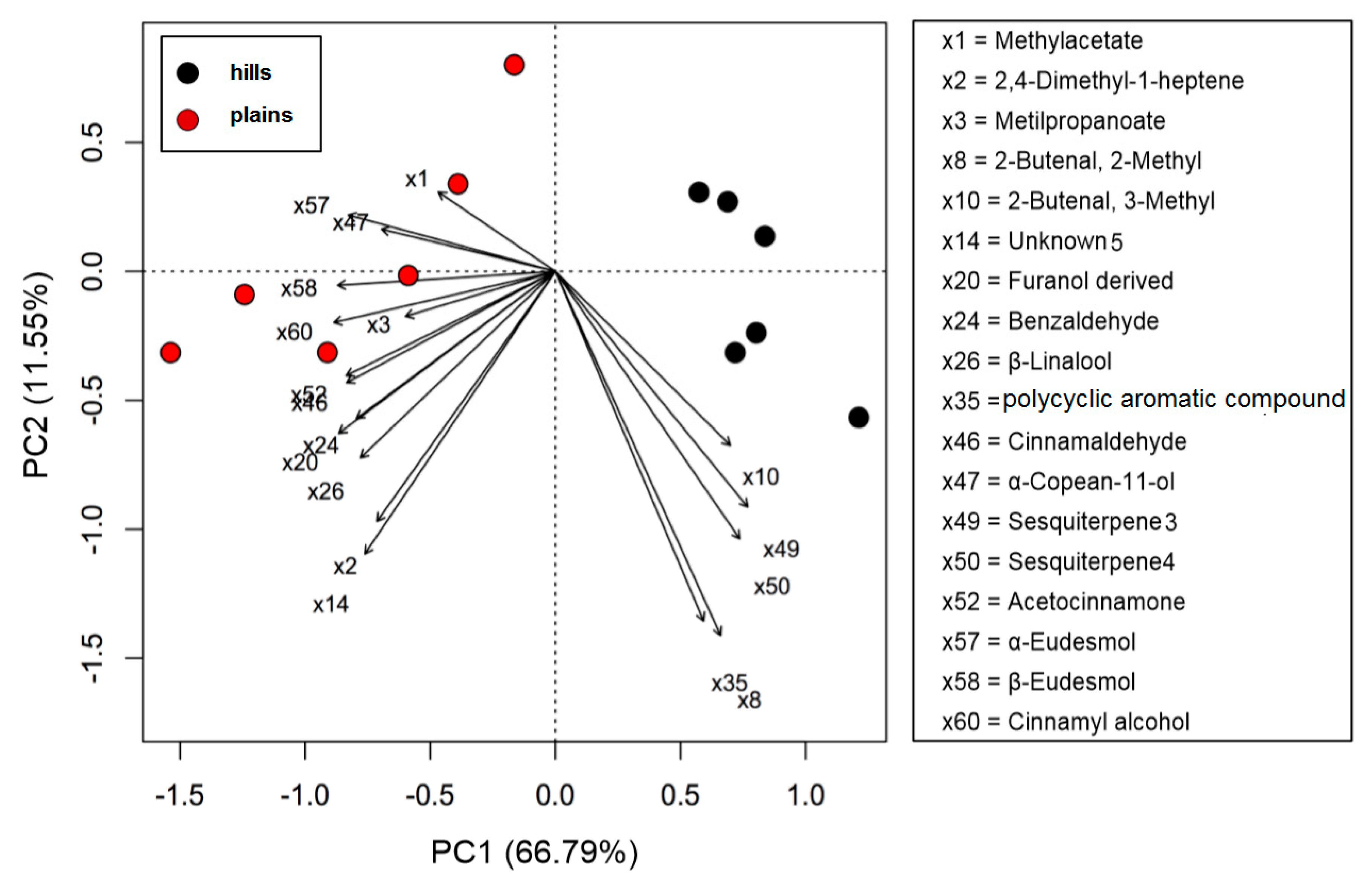
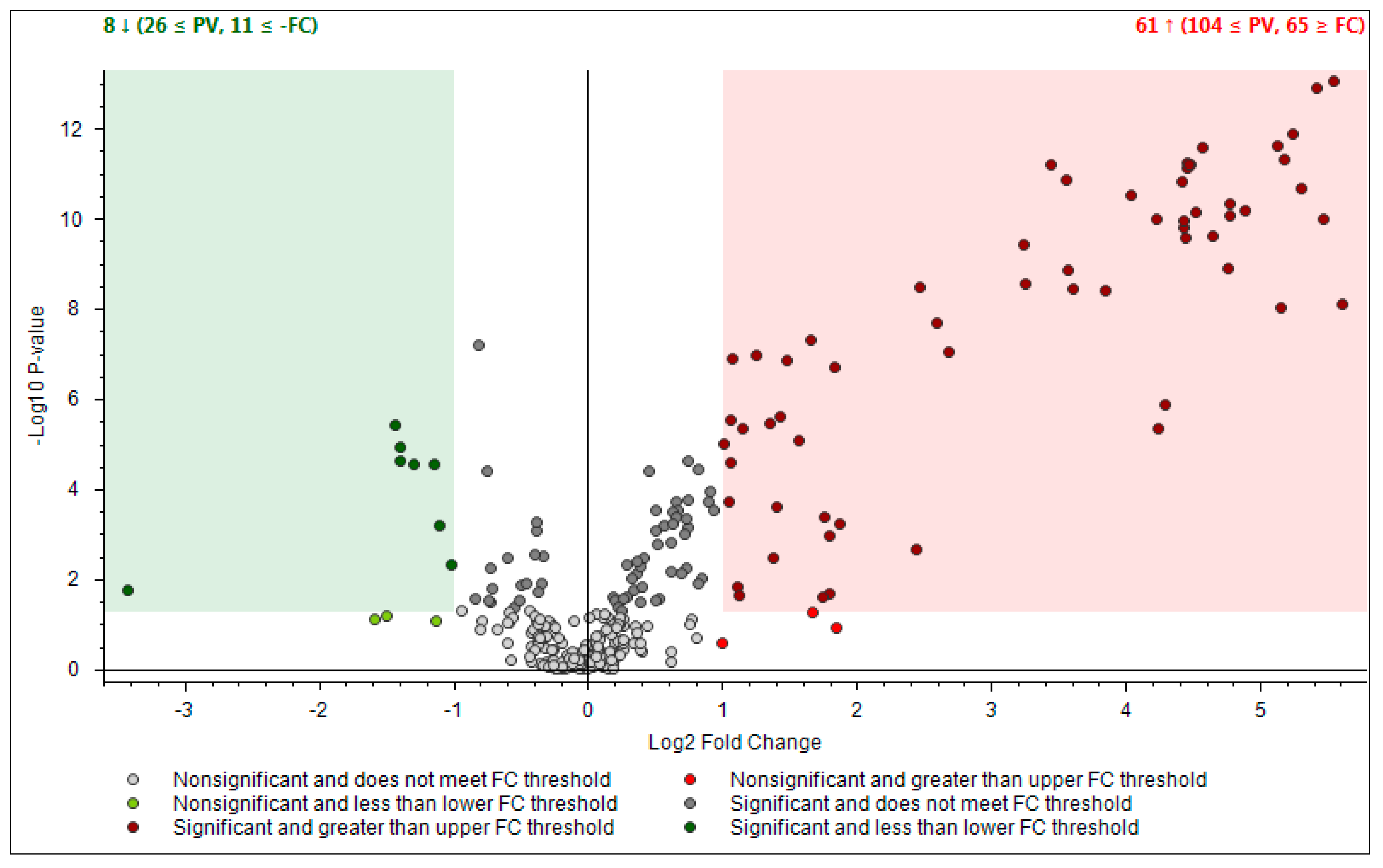
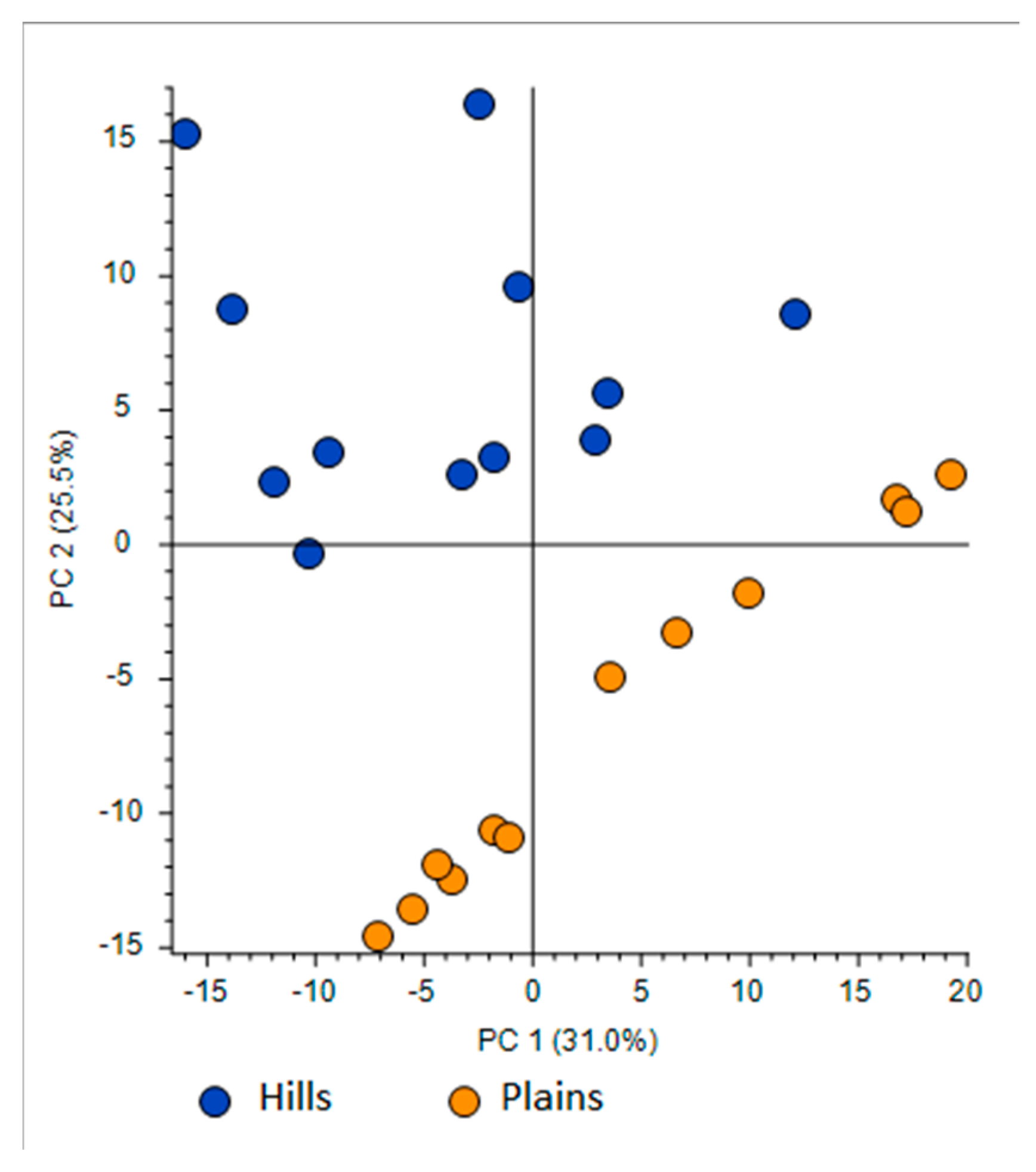
- First one was full scan (FS) at maximum resolution of 140,000 that involved generation of the lists of compounds that are potentially present in the samples (307 candidates). Using Compound Discoverer platform compounds were identified applied workflow that includes RT alignment, blank subtraction, and molecular formula assignment. Also, in FS acquisition mode the additional detection settings were applied: (1) Selecting the unknown peaks with criteria such as mass tolerance (<2 ppm); (2) minimum peak intensity (100,000), 3) integrating isotope and adduct peaks of the same compound into one group to reduce the incidence of false positives. This phase involved also the differential analysis with Volcano Plot (VP) (Figure 5) and principal component analysis (PCA) (Figure 6). PCA clearly distinguished the hills and plains samples, where VP analysis gave more precise response which signals are the main contributors along with the statistical evaluation presented in Table 4.
- Second type of analysis regards FS-data dependent (FS-DDA) acquisition mode and was performed on the inclusion list of 307 signals extracted from the FS data collection. MS–MS fragmentation performed in FS–DDA modality enabled the putative identification beyond the available standards. This phase comprises molecular formula assignment according to the accurate mass, adduct state, isotopes and fragmentation patterns with selecting best-fit candidates for the non-target peaks after comparison and evaluation with the software-linked MS2 libraries (mzCloud, m/z Valut and ChemSpider). To make the results more reliable especially when the mzCloud did not give any well-defined response the matching results are further filtered and checked with other on-line databases (human Metaboloeme at the first place). In some cases, as we have not found any satisfactory confirmation from existing databases, the tentative deduction of the final structure was performed manually assigning the fragments structure in concordance with available literature [35,36].
3. Discussion
4. Materials and Methods
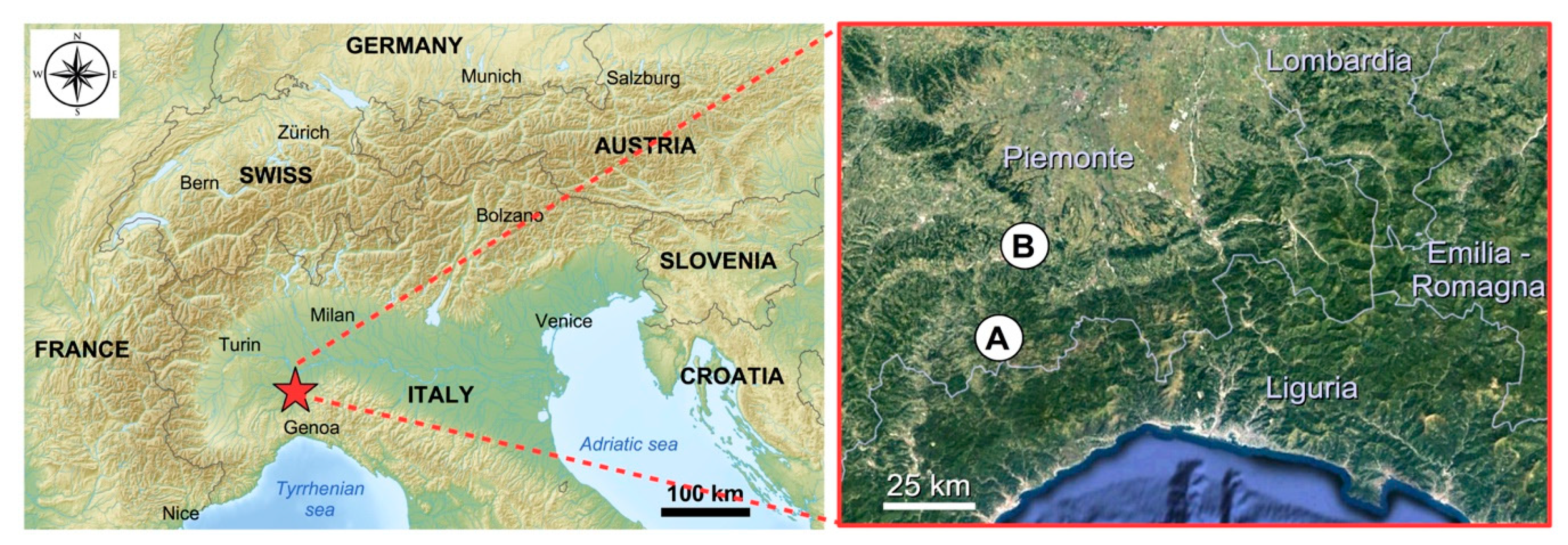
4.1. Sample Collection and Study Area
4.2. Extraction, Balsam and Moisture Content
4.3. Total Phenolic Content, Total Flavones and Flavonols, and Free Radical-Scavenging Activity
4.4. HPLC Analysis
4.5. Solid Phase Microextraction (SPME) and Gas Chromatography Mass Spectrometry (GC–MS) Procedure
4.6. NMR
4.7. HPLC–Q-Exactive-Orbitrap®–MS Analysis: Untargeted Metabolomics Approach
4.8. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical Aspects of Propolis Research in Modern Times. Evid. Complementary Altern. Med. 2013, 11. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food. Agri. 2010, 91, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Propolis—Review. BeeWorld 1979, 60, 59–84. [Google Scholar] [CrossRef]
- Silva, R.O.; Andrade, V.M.; Bullé Rêgo, E.S.; Azevedo Dòria, G.A.; Santos Lima, B.; Silva, F.A.; Araújo, A.A.S.; de Albuquerque, R.L.C., Jr.; Cardoso, J.C.; Gomes, M.Z. Acute and sub-acute oral toxicity of Brazilian red propolis in rats. J. Ethnopharmacol. 2015, 170, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J. Propolis as a Medicine. Are there Scientific Reasons for its Reputation? In Beeswax and Propolis for Pleasure and Profit; Munn, P., Ed.; International Bee Research Association: Cardiff, UK, 1998; p. 10. [Google Scholar]
- Koo, H.; Rosalen, P.L.; Cury, J.A.; Park, Y.K.; Bowen, W.H. Effects of Compounds Found in Propolis on Streptococcus mutans growth and on glucosyltransferase activity. Antimicrob. Agents Chemother. 2002, 46, 1302–1309. [Google Scholar] [CrossRef] [PubMed]
- Silva Cunha, I.B.; Salomão, K.; Shimizu, M.; Bankova, V.S.; Custódio, A.R.; Castro, S.L.; Marcucci, M.C. Antitrypanosomal activity of Brazilian propolis from Apis mellifera. Chem. Pharm. Bull. 2004, 52, 602–604. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Maffia, P.; Pinto, L.; Ianaro, A.; Russo, A.; Capasso, F.; Ialenti, A. Phytochemical compounds involved in the anti-inflammatory effect of propolis extract. Fitoterapia 2002, 73, S53–S63. [Google Scholar] [CrossRef]
- Ahn, M.; Kumazawa, S.; Usui, Y.; Nakamura, J.; Matsuka, M.; Zhu, F.; Nakayama, T. Antioxidant activity and constituents of propolis collected in various areas of China. Food Chem. 2007, 101, 1383–1392. [Google Scholar] [CrossRef]
- Oršolić, N.; Knežević, A.H.; Šver, L.; Terzić, S.; Bašić, I. Immunomodulatory and antimetastatic action of propolis and related polyphenolic compounds. J. Ethnopharmacol. 2004, 94, 307–315. [Google Scholar] [CrossRef]
- Amoros, M.; Lurton, E.; Boustie, J.; Girre, L.; Sauvager, F.; Cormier, M. Comparison of the anti-herpes simplex virus activities of propolis and 3-methyl-but-2-enyl caffeate. J. Nat. Prod. 1994, 57, 644–647. [Google Scholar] [CrossRef]
- Seo, K.W.; Park, M.; Song, Y.J.; Kim, S.J.; Yoon, K.R. The protective effects of propolis on hepatic injury and its mechanism. Phytother. Res. 2003, 17, 250–253. [Google Scholar] [PubMed]
- Ota, C.; Unterkircher, C.; Fantinato, V.; Shimizu, M.T. Antifungal activity of propolis on different species of Candida arten. Mycoses 2001, 44, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Silici, S.; Kutluca, S. Chemical composition and antibacterial activity of propolis collected by three different races of honeybees in the same region. J. Ethnopharmacol. 2005, 99, 69–73. [Google Scholar] [CrossRef]
- Castaldo, S.; Capasso, F. Propolis, an old remedy used in modern medicine. Fitoterapia 2002, 73, S1–S6. [Google Scholar] [CrossRef]
- Stan, L.; Liviu, A.; Dezmirean, D. Quality Criteria for Propolis Standardization. Sci. Pap. Anim. Sci. Biotechnol. 2011, 44, 137–140. [Google Scholar]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Katekhaye, S.; Fearnley, H.; Fearnley, J.; Paradkar, A. Gaps in propolis research: Challenges posed to commercialization and the need for a holistic approach. J. Apic. Res. 2019, 58, 604–616. [Google Scholar] [CrossRef]
- Giupponi, L.; Pentimalli, D.; Manzo, A.; Panseri, S.; Giorgi, A. Effectiveness of fine root fingerprinting as a tool to identify plants of the Alps: Results of preliminary study. Plant Biosyst. 2017, 152, 464–473. [Google Scholar] [CrossRef]
- Cottica, S.M.; Sabik, H.; Antoine, C.; Fortin, J.; Graveline, N.; Visentainer, J.V.; Britten, M. Characterization of Canadian propolis fractions obtained from two-step sequential extraction. LWT Food Scie Technol. 2015, 60, 609–614. [Google Scholar] [CrossRef]
- Escriche, I.; Juan-Borrás, M. Standardizing the analysis of phenolic profile in propolis. Food Res. Int. 2018, 106, 834–841. [Google Scholar] [CrossRef]
- Cicco, N.; Lanorte, M.T.; Paraggio, M.; Viggiano, M.; Lattanzio, V. A reproducible, rapid and inexpensive Folin–Ciocalteu micro-method in determining phenolics of plant methanol extracts. Microchem. J. 2009, 91, 107–110. [Google Scholar] [CrossRef]
- Castro, C.; Mura, F.; Valenzuela, G.; Figueroa, C.; Salinas, R.; Zuñiga, M.C.; Torres, J.L.; Fuguet, E.; Delporte, C. Identification of phenolic compounds by HPLC-ESI-MS/MS and antioxidant activity from Chilean propolis. Food Res. Int. 2014, 64, 873–879. [Google Scholar] [CrossRef]
- Popova, M.; Bankova, V.; Bogdanov, S.; Tsvetkova, I.; Naydenski, C.; Marcazzan, G.L.; Sabatini, A.G. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie 2007, 38, 306–311. [Google Scholar] [CrossRef]
- Anđelković, B.; Vujisić, L.; Vučković, I.; Tešević, V.; Vajs, V.; Gođevac, D. Metabolomics study of Populus type propolis. J. Pharm. Biomed. 2017, 135, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Luo, L.; Chen, B.; Fu, Y.; Chaz Du, P. Recent development of chemical components in propolis. Front. Biol. China 2009, 4, 385. [Google Scholar] [CrossRef]
- Graça, M.M.; Dulce, A.M. Is propolis safe as an alternative medicine? J. Pharm. Bioallied Sci. 2011, 3, 479–495. [Google Scholar]
- Pellati, F.; Prencipe, F.P.; Benvenuti, S. Headspace solid-phase microextraction-gas chromatography–mass spectrometry characterization of propolis volatile compounds. J Pharm. Biomed. 2013, 84, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Jelen´, H.H.; Majchera, M.; Dziadas, M. Microextraction techniques in the analysis of food flavor compounds: A review. Anal. Chim. Acta 2012, 738, 13–26. [Google Scholar] [CrossRef]
- Plutowska, B.; Chmiel, T.; Dymerski, T.; Wardencki, W. A headspace solid phase microextraction method development and its application in the determination of volatiles in honeys by gas chromatography. Food Chem. 2011, 126, 1288–1298. [Google Scholar] [CrossRef]
- Belliardo, F.; Bicchi, C.; Cordero, C.; Liberto, E.; Rubiolo, P.; Sgorbini, B. Headspacesolid-phase microextraction in the analysis of the volatile fraction of aromatic and medicinal plants. J. Chromatogr. Sci. 2006, 44, 416–429. [Google Scholar] [CrossRef]
- Petri, G.; Lemberkovics, E.; Foldvari, F. Examination of Differences Between Propolis (Bee Glue) Produced from Different Flora Environment. In Flavors and Fragrances: A World Perspective; Lawrence, B.M., Mookherjee, B.D., Willis, B.J., Eds.; Elsevier Science Publishers B.V.: Amsterdam, The Netherlands, 1988; pp. 439–446. [Google Scholar]
- Miguel, M.M.; Nunes, S.; Cruz, C.; Duarte, J.; Antunes, M.D.; Cavaco, A.M.; Mendes, M.D.; Lima, A.S.; Pedro, L.G.; Barroso, J.G.; et al. Propolis volatiles characterization from acaricide-treated and -untreated beehives maintained at Algarve (Portugal). Nat. Prod. Res. Ifirst 2012, 1–7. [Google Scholar]
- Papotti, G.; Bertelli, D.; Plessi, M.; Rossi, M.C. Use of HR-NMR to classify propolis obtained using different harvesting methods. Int. J. Food Sci. Tech. 2010, 1610–1618. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance Liquid Chromatography and Mass Spectrometry (UHPLC–LTQ/Orbitrap/MS/MS) Study of Phenolic Profile of Serbian Poplar Type Propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Saftić, L.; Peršurić, Z.; Fornal, E.; Pavlešić, T.; Pavelić, S.K. Targeted and untargeted LC-MS polyphenolic profiling and chemometric analysis of propolis from different regions of Croatia. J. Pharm. Biomed. 2019, 165, 162–172. [Google Scholar] [CrossRef]
- Papotti, G.; Bertelli, D.; Bortolotti, L.; Plessi, M. Chemical and Functional Characterization of Italian Propolis Obtained by Different Harvesting Methods. J. Agric. Food Chem. 2012, 60, 2852–2862. [Google Scholar] [CrossRef]
- Borčić, I.; Radonić, A.; Grzunov, K. Comparison of the volatile constituents of propolis gathered in different regions of Croatia. lavour Fragr. J. 1996, 11, 311–313. [Google Scholar]
- Jerković, I.; Mastelić, J. Volatile compounds from leaf-buds of Popolus nigra L. (Salicaceae). Phytochem 2003, 63, 109–113. [Google Scholar] [CrossRef]
- Salatino, A.; Fernandes-Silva, C.C.; Righi, A.A.; Salatino, M.L.F. Propolis research and the chemistry of plant products. Nat. Prod. Rep. 2011, 28, 925–936. [Google Scholar] [CrossRef]
- Blasi, C. La Vegetazione d’Italia; Palombi & Partner: Roma, Italy, 2010. [Google Scholar]
- Bankova, V.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: Chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 8–28. [Google Scholar] [CrossRef]
- Yang, C.; Luo, L.; Zhang, H.; Yang, X.; Lv, Y.; Song, H. Common aroma-active components of propolis from 23 regions of China. J. Sci. Food. Agric. 2010, 90, 1268–1282. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Dong, J.; Li, J. Analysis of volatile compounds of propolis by solidphase microextraction combined with GC-MS. Sci. Technol. Food Ind. 2008, 5, 57–59. [Google Scholar]
- Cheng, H.; Qin, Z.H.; Hu, X.S.; Wu, J.H. Analysis of volatile compounds of propolis and poplar tree gum by SPME/DHS-GC-MS. J. Food Saf. Qual. 2012, 3, 1–9. [Google Scholar]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid GC/MS determination of botanical precursors of Eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Popravko, S.A.; Sokolov, I.V.; Torgov, I.V. New natural phenolic triglycerides. Chem. Nat. Compd. 1982, 18, 153–157. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Bogdanov, S.; Sabatini, A.G. Chemical Composition of European Propolis: Expected and Unexpected Results. Zeitschrift für Naturforschung C 2014, 57, 530–533. [Google Scholar] [CrossRef]
- Landolt, E.; Bäumler, B.; Erhardt, A.; Klötzli, F.; Lämmler, W.; Urmi, E. Flora Indicativa. Ecological Indicator Values and Biological Attributes of the Flora of Switzerland and the Alps; Haupt Verlag: Bern, Switzerland; Stuttgart, Germany; Vienna, Austria, 2010. [Google Scholar]
- Cislaghi, A.; Giupponi, L.; Tamburini, A.; Giorgi, A.; Bischetti, G.B. The effects of mountain grazing abandonment on plant community, forage value and soil properties: Observations and field measurements in an alpine area. Catena 2019, 181, 104086. [Google Scholar] [CrossRef]
- Radmila, P.; Panseri, S.; Giupponi, L.; Leoni, V.; Citti, C.; Cattaneo, C.; Cavaletto, M.; Giorgi, A. Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps. Front Plant Sci. 2019, 10, 1265. [Google Scholar]
- Giorgi, A.; Bononi, M.; Tateo, F.; Cocucci, M. Yarrow (Achillea millefolium L.) growth at different altitudes in central italian alps: Biomass yield, oil content and quality. J. Herbs Spices Med. Plants 2005, 11, 47–58. [Google Scholar] [CrossRef]
- Bankova, V.; Popova, M.; Trusheva, B. Plant Sources of Propolis: An Update from a Chemist’s Point of View. Nat. Prod. Commun. 2006. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid. Based Complementary Altern. Med. 2015, 2015, 29. [Google Scholar] [CrossRef] [PubMed]
- Bankova, V. Chemical diversity of propolis and the problem of standardization. J. Ethnopharmacol. 2005, 100, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C. How do Bees Prevent Hive Infections? The Antimicrobial Properties of Propolis. In Current Research, Technology and Education Topics in Applied Microbiology and Microbial Biotechnology; Mendez-Vilas, A., Ed.; Microbiology Book Series—Number 2; Researchgate: Berlin, Germany, 2010; Volume 1, pp. 481–493. [Google Scholar]
- Blasi, C.; Capotorti, G.; Copiz, R.; Guida, D.; Mollo, B.; Smiraglia, D.; Zavattero, L. Classification and mapping of the ecoregions of Italy. Plant Biosyst. 2014, 148, 1255–1345. [Google Scholar] [CrossRef]
- Rivas-Martínez, S.; Penas, A.; Díaz, T.E. Biogeographic Map of Europe. 2004. Available online: www.globalbioclimatics.org/form/maps.htm (accessed on 13 March 2019).
- Giupponi, L.; Corti, C.; Manfredi, P. Onopordum acanthium subsp. acanthium in una ex-discarica della Pianura Padana (Piacenza). Ital. Bot. 2013, 45, 213–219. [Google Scholar]
- Giupponi, L.; Corti, C.; Manfredi, P.; Cassinari, C. Application of the floristic-vegetational indexes system for the evaluation of the environmental quality of a semi-natural area of the Po Valley (Piacenza, Italy). Plant Sociol. 2013, 50, 47–56. [Google Scholar]
- Giupponi, L.; Corti, C.; Manfredi, P. The vegetation of the Borgotrebbia landfill (Piacenza, Italy): Phytosociological and ecological characteristics. Plant Biosyst. 2015, 149, 865–874. [Google Scholar] [CrossRef]
- Siniscalco, C.; Bouvet, L. Le Serie di Vegetazione della Regione Piemonte. In La vegetazione d’Italia; Blasi, C., Ed.; Palombi & Partner: Roma, Italy, 2010; pp. 17–37. [Google Scholar]
- Miguel, M.G.; Nunes, S.; Dandlen, S.A.; Cavaco, A.M.; Antunes, M.D. Phenols and antioxidant activity of hydro-alcoholic extracts of propolis from Algarve, South of Portugal. Food Chem. Toxicol. 2010, 48, 3418–3420. [Google Scholar] [CrossRef] [PubMed]
- Marinova, G.V.; Batcharov, V. Evaluation of the methods for determination of the free radical scavenging activity by DPPH. Bulg. J. Agric. Sci. 2011, 17, 11–24. [Google Scholar]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sayeeda, Z. HMDB 4.0—The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Wien, R Foundation for Statistical Computing. 2018. Available online: http://www.r-project.org (accessed on 1 October 2019).
| Propolis Composition and Activity | Hills | Plains | Statistical Evaluation | |||
|---|---|---|---|---|---|---|
| Average ± SD | Average ± SD | t-Value | DF | p-Value | Sign. | |
| Balsam content (% w/w) | 75.92 ± 4.92 | 63.94 ± 12.86 | 2.131 | 6.433 | 0.074 | ns |
| Moisture content (%) | 0.74 ± 0.38 | 0.69 ± 0.52 | 0.195 | 9.233 | 0.850 | ns |
| total Phenols (mg GAE/g) | 242.42 ± 11.67 | 236.32 ± 40.92 | 0.351 | 5.808 | 0.738 | ns |
| Total Flavones and Flavonols (mg QE/g) | 32.14 ± 4.38 | 26.91 ± 4.31 | 2.082 | 9.998 | 0.064 | ns |
| DPPH radical scavenging activity (%) | 45.01 ± 1.39 | 46.44 ± 0.96 | −2.082 | 8.855 | 0.068 | ns |
| Phenolic Acids/Flavonoids | Hills | Plains | Statistical Evaluation |
|---|---|---|---|
| Average ± SD | Average ± SD | p-Value | |
| caffeic acid | 4.37 ± 0.53 | 4.21 ± 0.80 | ns |
| p-coumaric acid | 6.97 ± 2.12 | 1.40 ± 0.37 | 0.0001 |
| ferulic acid | 7.41 ± 2.22 | 1.64 ± 0.30 | 0.0013 |
| m-coumaric acid | 3.72 ± 0.27 | 2.87 ± 0.66 | 0.0150 |
| trans-cinnamic acid | 3.42 ± 0.21 | 4.48 ± 1.01 | 0.0428 |
| pinocembrin | 19.06 ± 6.27 | 17.90 ± 4.20 | ns |
| chrysin | 33.62 ± 3.49 | 35.64 ± 12.71 | ns |
| RT a | Compounds | Hills | Plains | t-Value | DF | p-Value | Signif. Code | ||
|---|---|---|---|---|---|---|---|---|---|
| Meanb ± SD c | % d | Mean b ± SD c | % d | ||||||
| 2.33 | methyl-acetate | 22.85 ± 5.11 | 5.52 | 34.23 ± 4.85 | 6.75 | −3.9538 | 9.9717 | 0.002729 | ** |
| 2.93 | 2,4−dimethyl-1-heptene | 6.55 ± 0.83 | 1.58 | 10.41 ± 3.25 | 2.06 | −2.8173 | 5.6514 | 0.03251 | * |
| 3.23 | methyl-propanoate | 0.69 ± 0.16 | 0.17 | 1.10 ± 0.28 | 0.22 | −3.1264 | 8.1668 | 0.01373 | * |
| 6.00 | α-pinene | 0.55 ± 0.31 | 0.13 | 0.87 ± 0.46 | 0.17 | −1.448 | 8.7216 | 0.1826 | ns |
| 7.23 | 3-buten-2-ol, 2-methyl- | 1.33 ± 0.41 | 0.32 | 1.30 ± 0.36 | 0.26 | 0.14423 | 9.793 | 0.8882 | ns |
| 7.77 | camphene | 1.92 ± 0.55 | 0.46 | 3.53 ± 1.81 | 0.70 | −2.0744 | 5.9194 | 0.08402 | ns |
| 8.98 | esanal | 2.96 ± 0.73 | 0.71 | 3.86 ± 1.59 | 0.76 | −1.2607 | 7.039 | 0.2476 | ns |
| 9.24 | 2-butenal, 2-methyl- | 1.15 ± 0.32 | 0.28 | 0.67 ± 0.13 | 0.13 | 3.4176 | 6.6211 | 0.01217 | * |
| 11.03 | unknown_1 | 4.49 ± 0.52 | 1.08 | 4.40 ± 1.33 | 0.87 | 0.15257 | 6.4759 | 0.8834 | ns |
| 14.34 | 2-butenal, 3-methyl- | 3.76 ± 0.85 | 0.91 | 2.68 ± 0.46 | 0.53 | 2.7426 | 7.6617 | 0.0264 | * |
| 14.76 | unknown_2 | 7.04 ± 2.87 | 1.70 | 6.09 ± 1.75 | 1.20 | 0.69089 | 8.2804 | 0.5085 | ns |
| 16.70 | unknown_3 | 19.67 ± 3.08 | 4.75 | 15.30 ± 6.80 | 3.02 | 1.4333 | 6.9764 | 0.195 | ns |
| 16.95 | unknown_4 | 5.71 ± 2.21 | 1.38 | 5.48 ± 1.72 | 1.08 | 0.20213 | 9.4253 | 0.8433 | ns |
| 19.04 | unknown_5 | 8.61 ± 1.21 | 2.08 | 12.20 ± 2.99 | 2.41 | −2.7322 | 6.5943 | 0.03101 | * |
| 20.98 | nonanal | 1.92 ± 0.49 | 0.46 | 2.18 ± 0.35 | 0.43 | −1.0457 | 9.071 | 0.3228 | ns |
| 21.32 | benzene, 1-methoxy-2-methyl- | 0.34 ± 0.12 | 0.08 | 0.93 ± 0.84 | 0.18 | −1.7233 | 5.2184 | 0.143 | ns |
| 21.43 | tetradecane | 0.53 ± 0.13 | 0.13 | 0.65 ± 0.23 | 0.13 | −1.1373 | 7.9121 | 0.2887 | ns |
| 21.74 | 2-octenal | 0.50 ± 0.19 | 0.12 | 0.53 ± 0.20 | 0.10 | −0.22144 | 9.9454 | 0.8292 | ns |
| 22.17 | acetic acid | 45.28 ± 6.83 | 10.93 | 57.80 ± 17.65 | 11.42 | −1.6215 | 6.465 | 0.1525 | ns |
| 22.76 | terpene_1 | 0.73 ± 0.26 | 0.18 | 2.46 ± 1.02 | 0.49 | −4.0402 | 5.6519 | 0.007701 | ** |
| 22.82 | trans-linalool oxide | 1.74 ± 0.50 | 0.42 | 1.61 ± 0.44 | 0.32 | 0.46595 | 9.8211 | 0.6514 | ns |
| 23.33 | α-copaene | 2.29 ± 0.94 | 0.55 | 1.98 ± 0.50 | 0.39 | 0.69802 | 7.6553 | 0.5058 | ns |
| 23.61 | (+)-camphor | 1.44 ± 0.30 | 0.35 | 2.89 ± 1.62 | 0.57 | −2.1594 | 5.3419 | 0.07974 | ns |
| 23.84 | benzaldehyde | 11.11 ± 2.69 | 2.68 | 18.05 ± 4.59 | 3.57 | −3.1941 | 8.0829 | 0.01255 | * |
| 24.23 | propanoic acid | 6.34 ± 1.30 | 1.53 | 9.04 ± 4.21 | 1.79 | −1.5016 | 5.938 | 0.1844 | ns |
| 24.66 | β-linalool | 3.06 ± 1.34 | 0.74 | 6.70 ± 3.19 | 1.32 | −2.5733 | 6.7075 | 0.03821 | * |
| 24.91 | 2-methyl-propanoic acid | 19.88 ± 3.52 | 4.80 | 27.67 ± 11.59 | 5.47 | −1.5762 | 5.9158 | 0.1668 | ns |
| 25.69 | sesquiterpene_1 | 0.44 ± 0.08 | 0.11 | 0.42 ± 0.09 | 0.08 | 0.49589 | 9.7629 | 0.6309 | ns |
| 25.99 | β-cyclocitral | 3.80 ± 1.16 | 0.92 | 3.37 ± 1.41 | 0.67 | 0.57503 | 9.6507 | 0.5784 | ns |
| 26.17 | unknown_6 | 16.14 ± 1.92 | 3.90 | 15.86 ± 4.33 | 3.13 | 0.14412 | 6.8858 | 0.8895 | ns |
| 27.03 | 2-methyl-butanoic acid | 20.72 ± 3.90 | 5.00 | 28.79 ± 11.02 | 5.69 | −1.6923 | 6.2338 | 0.1397 | ns |
| 27.35 | 2-butenoic acid | 5.39 ± 1.52 | 1.30 | 10.50 ± 4.88 | 2.08 | −2.4501 | 5.9572 | 0.05 | ns |
| 27.51 | sesquiterpene_2 | 2.38 ± 0.82 | 0.57 | 1.72 ± 0.57 | 0.34 | 1.6229 | 8.9525 | 0.1392 | ns |
| 28.19 | benzyl-acetate | 8.76 ± 2.10 | 2.12 | 8.09 ± 2.47 | 1.60 | 0.506 | 9.7442 | 0.6241 | ns |
| 28.29 | polycyclic aromatic compound | 3.55 ± 0.54 | 0.86 | 2.69 ± 0.59 | 0.53 | 2.6531 | 9.9141 | 0.02435 | * |
| 28.84 | δ-cadinene | 13.63 ± 3.88 | 3.29 | 12.45 ± 3.63 | 2.46 | 0.54221 | 9.9557 | 0.5996 | ns |
| 28.99 | unknown_7 | 3.25 ± 0.61 | 0.79 | 4.26 ± 1.89 | 0.84 | −1.2451 | 6.0414 | 0.2592 | ns |
| 29.07 | unknown_8 | 2.51 ± 1.40 | 0.61 | 3.46 ± 1.23 | 0.68 | −1.2557 | 9.8345 | 0.2343 | ns |
| 29.25 | unknown_9 | 1.50 ± 0.25 | 0.36 | 1.72 ± 0.59 | 0.34 | −0.85269 | 6.6761 | 0.4234 | ns |
| 29.55 | pentanoic acid, 4-methyl- | 1.42 ± 0.56 | 0.34 | 1.27 ± 0.45 | 0.25 | 0.50394 | 9.5007 | 0.6258 | ns |
| 29.81 | unknown_10 | 1.24 ± 0.51 | 0.30 | 1.57 ± 0.45 | 0.31 | −1.1763 | 9.8542 | 0.2671 | ns |
| 30.18 | calamenene | 4.97 ± 1.45 | 1.20 | 4.01 ± 1.39 | 0.79 | 1.1844 | 9.9827 | 0.2637 | ns |
| 30.28 | α-methyl crotonoic acid | 43.69 ± 6.73 | 10.55 | 59.28 ± 16.62 | 11.72 | −2.1297 | 6.5967 | 0.07309 | ns |
| 30.90 | benzyl-alcohol | 48.04 ± 10.13 | 11.60 | 38.98 ± 10.75 | 7.70 | 1.5022 | 9.9647 | 0.1641 | ns |
| 31.54 | phenethyl-alcohol | 24.41 ± 3.79 | 5.90 | 31.67 ± 7.52 | 6.26 | −2.1124 | 7.3894 | 0.07045 | ns |
| 32.81 | cinnamaldehyde | 4.98 ± 0.89 | 1.20 | 9.57 ± 2.63 | 1.89 | −4.0482 | 6.1243 | 0.006458 | ** |
| 32.88 | α-copaen-11-ol | 0.74 ± 0.48 | 0.18 | 1.50 ± 0.48 | 0.30 | −2.7538 | 9.9998 | 0.02034 | * |
| 33.04 | octanoic acid | 0.38 ± 0.11 | 0.09 | 0.54 ± 0.20 | 0.11 | −1.7018 | 7.941 | 0.1275 | ns |
| 33.08 | sesquiterpene_3 | 1.16 ± 0.43 | 0.28 | 0.22 ± 0.07 | 0.04 | 5.3763 | 5.2743 | 0.002547 | ** |
| 33.15 | sesquiterpene_4 | 1.69 ± 0.50 | 0.41 | 0.62 ± 0.22 | 0.12 | 4.8115 | 6.8895 | 0.002028 | ** |
| 33.39 | guaiol | 0.39 ± 0.46 | 0.09 | 0.37 ± 0.12 | 0.07 | 0.12043 | 5.6469 | 0.9083 | ns |
| 33.56 | acetocinnamone | 1.14 ± 0.21 | 0.27 | 2.22 ± 0.74 | 0.44 | −3.4706 | 5.8256 | 0.01394 | * |
| 34.11 | unknown_11 | 0.78 ± 0.28 | 0.19 | 0.88 ± 0.23 | 0.17 | −0.70911 | 9.6282 | 0.4951 | ns |
| 34.22 | Sesquiterpene_5 | 3.04 ± 1.68 | 0.73 | 3.26 ± 0.55 | 0.64 | −0.30368 | 6.0741 | 0.7715 | ns |
| 34.30 | Sesquiterpene_6 | 1.19 ± 0.68 | 0.29 | 1.91 ± 0.58 | 0.38 | −1.965 | 9.7885 | 0.07841 | ns |
| 34.40 | Sesquiterpene_7 | 1.88 ± 0.99 | 0.45 | 1.97 ± 0.30 | 0.39 | −0.21625 | 5.9323 | 0.836 | ns |
| 34.79 | α-eudesmol | 2.05 ± 0.93 | 0.49 | 4.49 ± 0.72 | 0.89 | −5.105 | 9.399 | 0.00056 | ** |
| 34.90 | β-eudesmol | 4.21 ± 0.68 | 1.02 | 8.42 ± 1.32 | 1.66 | −6.9394 | 7.5091 | 0.000161 | ** |
| 35.16 | α-Copaen-11-ol | 0.52 ± 0.47 | 0.13 | 0.79 ± 0.34 | 0.16 | −1.1297 | 9.1588 | 0.2873 | ns |
| 35.60 | cinnamyl alcohol | 1.64 ± 0.35 | 0.40 | 5.39 ± 1.90 | 1.06 | −4.7549 | 5.3445 | 0.004271 | ** |
| Compounds | Ret. Time | Formula | Exact Mass | Differential Analysis * |
|---|---|---|---|---|
| Phenolic acids and their derivatives | ||||
| p-hydroxybenzoic acid | 5.48 | C7H8O4 | 137.0244 | ns |
| cinnamic acid isomer | 5.87 | C9H8O2 | 147.0452 | up-regulated in hills |
| Salicylic acid | 9.92 | C7H8O4 | 137.0244 | ns |
| Caffeic acid | 10.52 | C9H8O4 | 179.035 | ns |
| m-Coumaric acid | 11.03 | C9H8O3 | 163.0401 | up-regulated in hills |
| p-Coumaric acid | 12.61 | C9H8O3 | 163.0401 | up-regulated in hills |
| Cinnamic acid | 13.12 | C9H8O2 | 147.0452 | up-regulated in plains |
| Ferulic acid | 13.43 | C10H10O4 | 193.0506 | up-regulated in hills |
| Cinnamic acid isomer | 13.81 | C9H8O2 | 147.0452 | up-regulated in hills |
| Isoferulic acid | 15.22 | C10H10O4 | 193.0506 | ns |
| Cinnamic acid isomer | 16.15 | C9H8O2 | 147.0452 | up-regulated in hills |
| p-Coumaroylquinic acid | 17.12 | C16H18O8 | 337.0922 | ns |
| Chlorogenic acid | 21.05 | C16H18O9 | 353.0876 | ns |
| Benzyl-caffeate | 21.17 | C16H13O4 | 269.0819 | ns |
| Prenyl-caffeate | 21.7 | C14H16O4 | 247.0976 | up-regulated in hills |
| Caffeic acid phenethyl-ester (CAPE) | 21.93 | C17H16O4 | 283.0976 | ns |
| p-Coumaric acid prenyl-ester | 22.08 | C14H16O3 | 231.1027 | up-regulated in hills |
| Coniferyl-ferulate isomer | 22.61 | C20H20O6 | 355.1187 | ns |
| Caffeic acid cinnamyl-ester | 22.93 | C18H16O4 | 295.0976 | up-regulated in plains |
| Dupunin (Prenylated phenyl-propanoic acid) | 23.01 | C14H16O3 | 231.1027 | ns |
| Coniferyl ferulate | 25.51 | C20H20O6 | 355.1187 | ns |
| Capillartemisin A (Prenylated phenyl-propanoic acid) | 25.74 | C18H24O4 | 315.16 | up-regulated in hills |
| Phenolic glycerides | ||||
| Caffeoyl-glycerol | 10.01 | C12H14O6 | 253.0713 | highly up-regulated in hills |
| Dicaffeoyl-acetyl-glycerol | 19.14 | C23H21O10 | 457.1142 | highly up-regulated in hills |
| Diferuloyl-glycerol | 19.33 | C23H24O9 | 443.1349 | highly up-regulated in hills |
| acetyl-caffeoyl-feruloyl-glycerol | 20.09 | C24H24O10 | 471.1298 | highly up-regulated in hills |
| Coumaroyl-caffeoyl-acetyl-glycerol | 20.21 | C23H22O9 | 441.1185 | highly up-regulated in hills |
| Acetyl-coumaroyl-feruloyl-glycerol | 21.17 | C24H24O9 | 455.1347 | highly up-regulated in hills |
| Di-p-coumaroyl-acetyl-glycerol | 21.28 | C26H22O8 | 425.1242 | highly up-regulated in hills |
| Coumaroyl-acetyl-glycerol | 24.22 | C18H16O3 | 279.086 | highly up-regulated in hills |
| Coumarins | ||||
| 4-Ethyl-7-hydroxy-3-(p-methoxyphenyl)-coumarin | 23.05 | C18H16O4 | 295.0976 | up-regulated in plains |
| Flavanones | ||||
| Pinostrobin | 23.65 | C16H14O4 | 269.0819 | up-regulated in plains |
| Strobopinin | 19.55 | C16H14O4 | 269.0819 | up-regulated in plains |
| Pinocembrin | 21.88 | C15H12O4 | 255.0663 | ns |
| Sakuranetin | 21.46 | C16H14O5 | 285.0768 | ns |
| 3′,5,7-Trihydroxy-4’-methoxyflavanone | 19.15 | C16H14O6 | 301.0718 | up-regulated in hills |
| Hesperetin | 20.01 | C16H14O6 | 301.0718 | up-regulated in hills |
| Chalcones | ||||
| Pinostrobin-chalcone | 19.62 | C16H14O4 | 269.0819 | up-regulated in hills |
| 4-Hydroxy-4′-methoxychalcone | 23.02 | C16H14O3 | 253.087 | highly up-regulated in hills |
| Flavonols | ||||
| Quercetin | 18.2 | C5H10O7 | 301.0354 | ns |
| Quercetin-3-O-methyl-ether | 19.01 | C16H12O7 | 315.051 | up-regulated in hills |
| Rhamnetin | 20.16 | C16H12O7 | 315.051 | up-regulated in hills |
| Kaempferol | 19.86 | C15H10O6 | 285.0405 | up-regulated in hills |
| Isorhamnetin | 21.24 | C16H12O7 | 315.051 | up-regulated in hills |
| Kaempferide | 22.93 | C16H12O6 | 299.0563 | up-regulated in hills |
| Bis-methylated quercetin | 21.79 | C17H14O7 | 329.0667 | ns |
| Galangin | 23.87 | C15H10O5 | 269.0455 | ns |
| Rhamnocitrin | 23.18 | C16H12O6 | 299.0563 | up-regulated in hills |
| Flavanonols | ||||
| Pinobanksin | 18.05 | C15H12O5 | 271.0613 | ns |
| Pinobanksin-5-methyl-ether | 17.59 | C16H14O5 | 285.0768 | ns |
| Pinobanksin-5-methylether-3-O-acetate | 20.2 | C18H16O6 | 327.0869 | up-regulated in hills |
| Pinobanksin-3-O-propionate | 22.78 | C18H16O6 | 327.0869 | up-regulated in hills |
| Pinobanksin-3-O-butyrate | 24.02 | C19H18O6 | 341.103 | ns |
| Pinobanksin-3-O-acetate | 21.43 | C17H14O6 | 313.0712 | ns |
| Aromadendrin | 14.85 | C15H12O6 | 287.0651 | ns |
| Isoflavones | ||||
| Genistein | 19.08 | C15H10O5 | 269.0542 | ns |
| Formononetin glucoside | 18.28 | C22H22O9 | 429.1191 | highly up-regulated in hills |
| Formononetin (biochanin B) | 20.8 | C16H12O4 | 267.0663 | ns |
| Hispiludin | 20.52 | C16H12O6 | 299.0563 | up-regulated in hills |
| Flavones | ||||
| Apigetrin (Apigenin-7-O-glucoside) | 18.01 | C21H20O10 | 431.0983 | highly up-regulated in hills |
| Apigenin | 20.44 | C15H10O5 | 269.0542 | up-regulated in hills |
| Dihydroxyflavone | 21.43 | C15H10O4 | 253.0506 | ns |
| Chrysin | 22.04 | C15H10O4 | 253.0506 | ns |
| Methoxy-chrysin | 23.01 | C16H12O5 | 283.0612 | ns |
| Tricin | 20.59 | C17H14O7 | 329.0667 | ns |
| Chrysoeriol | 20.52 | C16H12O6 | 299.0563 | up-regulated in hills |
| Terpenoids | ||||
| Ursolic acid | 29.65 | C30H48O3 | 455.3531 | highly up-regulated in hills |
| trans,trans-Abscisic acid | 16.58 | C15H20O4 | 263.1289 | up-regulated in plains |
| cis,trans-Abscisic acid | 23.66 | C15H20O4 | 263.1289 | up-regulated in plains |
| Unknowns | ||||
| Unknown 1 (phenylacetaldehide or isomer) | 12.51 | C8H8O | 119.0502 | highly up-regulated in hills |
| Unknown 2 (p-coumaric derivate) | 14.97 | C19H20O6 | 359.11 | highly up-regulated in hills |
| Unknown 3 (ferulic acid derivative) | 15.01 | C20H22O8 | 389.124 | highly up-regulated in hills |
| Unknown 4 | 18.28 | C27H42O4 | 429.1191 | highly up-regulated in hills |
| Unknown 5 | 19.05 | C17H16O6 | 315.0851 | highly up-regulated in hills |
| Unknown 6 | 19.11 | / | 597.1007 | highly up-regulated in hills |
| Unknown 8 (p-coumaric acid derivative) | 20.05 | C28H27O7 | 475.1762 | ns |
| Unknown 9 (Chrysin derivate) | 21.09 | / | 639.1112 | highly up-regulated in hills |
| Unknown 10 (p-Hydroxybenzoic acid derivative) | 21.15 | C28H27O7 | 475.1762 | ns |
| Unknown 11 | 21.19 | / | 461.1007 | highly up-regulated in hills |
| Unknown 12 | 22.29 | C20H37O9 | 421.2441 | highly up-regulated in hills |
| Unknown 13 (flavone ester of caffeic acid) | 22.78 | / | 565.1509 | up-regulated in plains |
| Unknown 14 (Chrysin derivate) | 23.44 | C25H22O6 | 417.135 | highly up-regulated in hills |
| Unknown 15 (Chrysin derivate) | 24 | C25H22O6 | 417.135 | highly up-regulated in hills |
| Unknown 16 | 25.17 | / | 413.1963 | highly up-regulated in hills |
| Unknown 17 | 26.5 | C18H30O3 | 293.212 | highly up-regulated in hills |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlovic, R.; Borgonovo, G.; Leoni, V.; Giupponi, L.; Ceciliani, G.; Sala, S.; Bassoli, A.; Giorgi, A. Effectiveness of Different Analytical Methods for the Characterization of Propolis: A Case of Study in Northern Italy. Molecules 2020, 25, 504. https://doi.org/10.3390/molecules25030504
Pavlovic R, Borgonovo G, Leoni V, Giupponi L, Ceciliani G, Sala S, Bassoli A, Giorgi A. Effectiveness of Different Analytical Methods for the Characterization of Propolis: A Case of Study in Northern Italy. Molecules. 2020; 25(3):504. https://doi.org/10.3390/molecules25030504
Chicago/Turabian StylePavlovic, Radmila, Gigliola Borgonovo, Valeria Leoni, Luca Giupponi, Giulia Ceciliani, Stefano Sala, Angela Bassoli, and Annamaria Giorgi. 2020. "Effectiveness of Different Analytical Methods for the Characterization of Propolis: A Case of Study in Northern Italy" Molecules 25, no. 3: 504. https://doi.org/10.3390/molecules25030504
APA StylePavlovic, R., Borgonovo, G., Leoni, V., Giupponi, L., Ceciliani, G., Sala, S., Bassoli, A., & Giorgi, A. (2020). Effectiveness of Different Analytical Methods for the Characterization of Propolis: A Case of Study in Northern Italy. Molecules, 25(3), 504. https://doi.org/10.3390/molecules25030504










