Halogenated Metabolites from the Diet of Aplysia dactylomela Rang
Abstract
1. Sea Hare Aplysia dactylomela Rang
2. The Role of Halogenated Metabolites for Aplysia dactylomela Rang
3. Biogenesis of Halogenated Metabolites
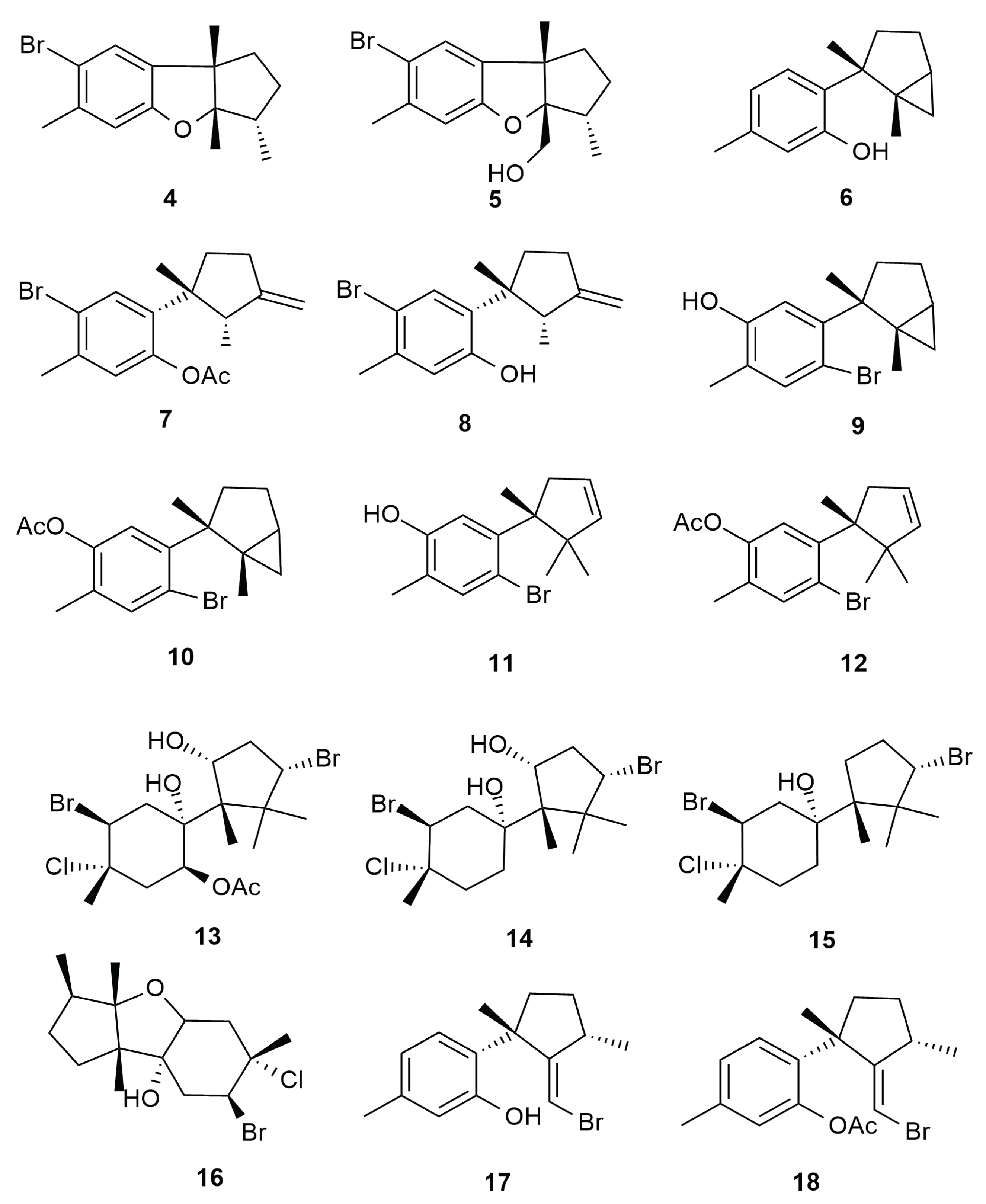
4. Halogenated Secondary Metabolites of Aplysia dactylomela Rang
5. Monoterpenes
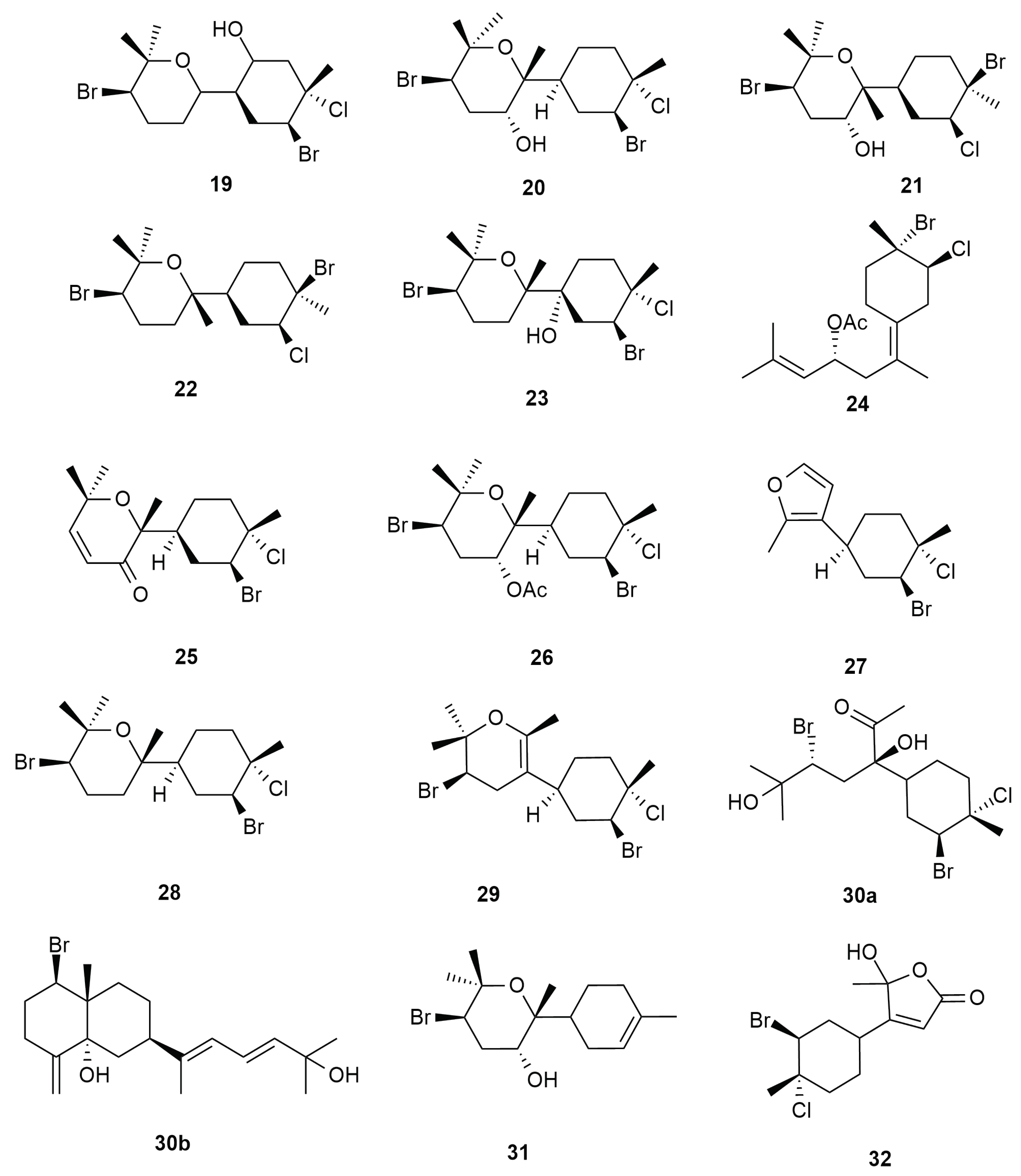
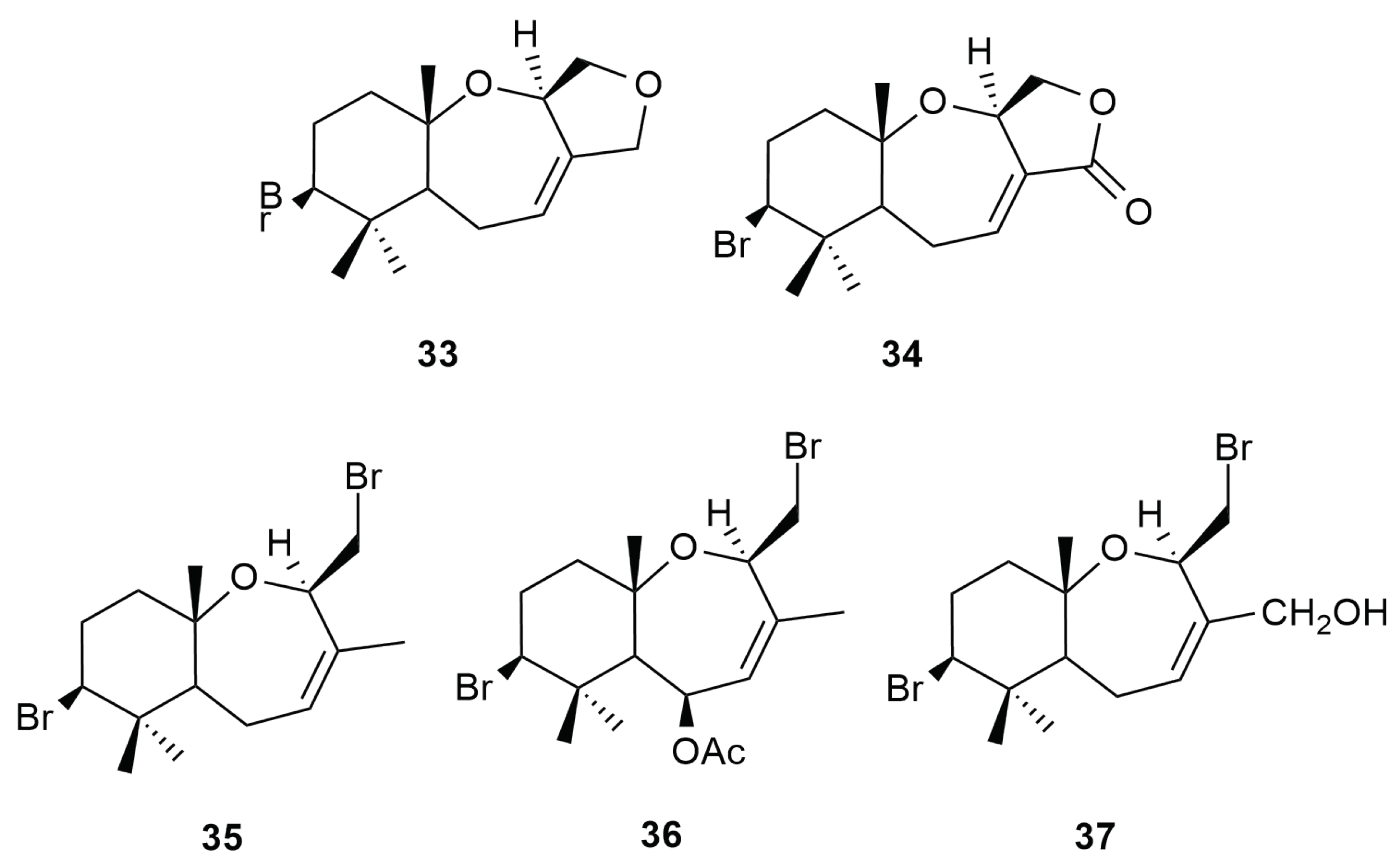
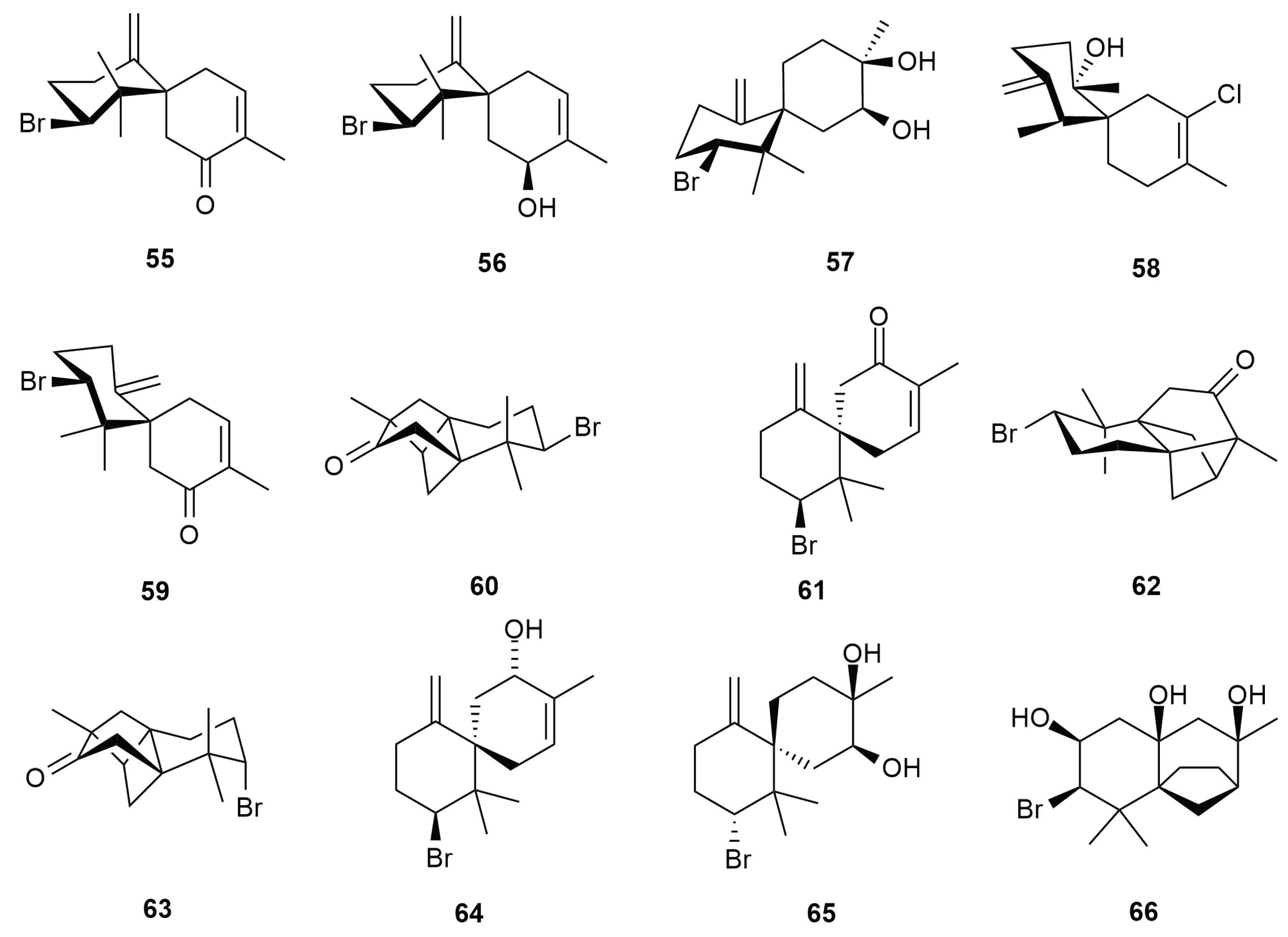
6. Sesquiterpenes
6.1. Cuparane and Laurane Skeleton
6.2. Bisabolene Type Skeleton
6.3. Syndrean Type Skeleton
6.4. Charmigrane Type Skeleton
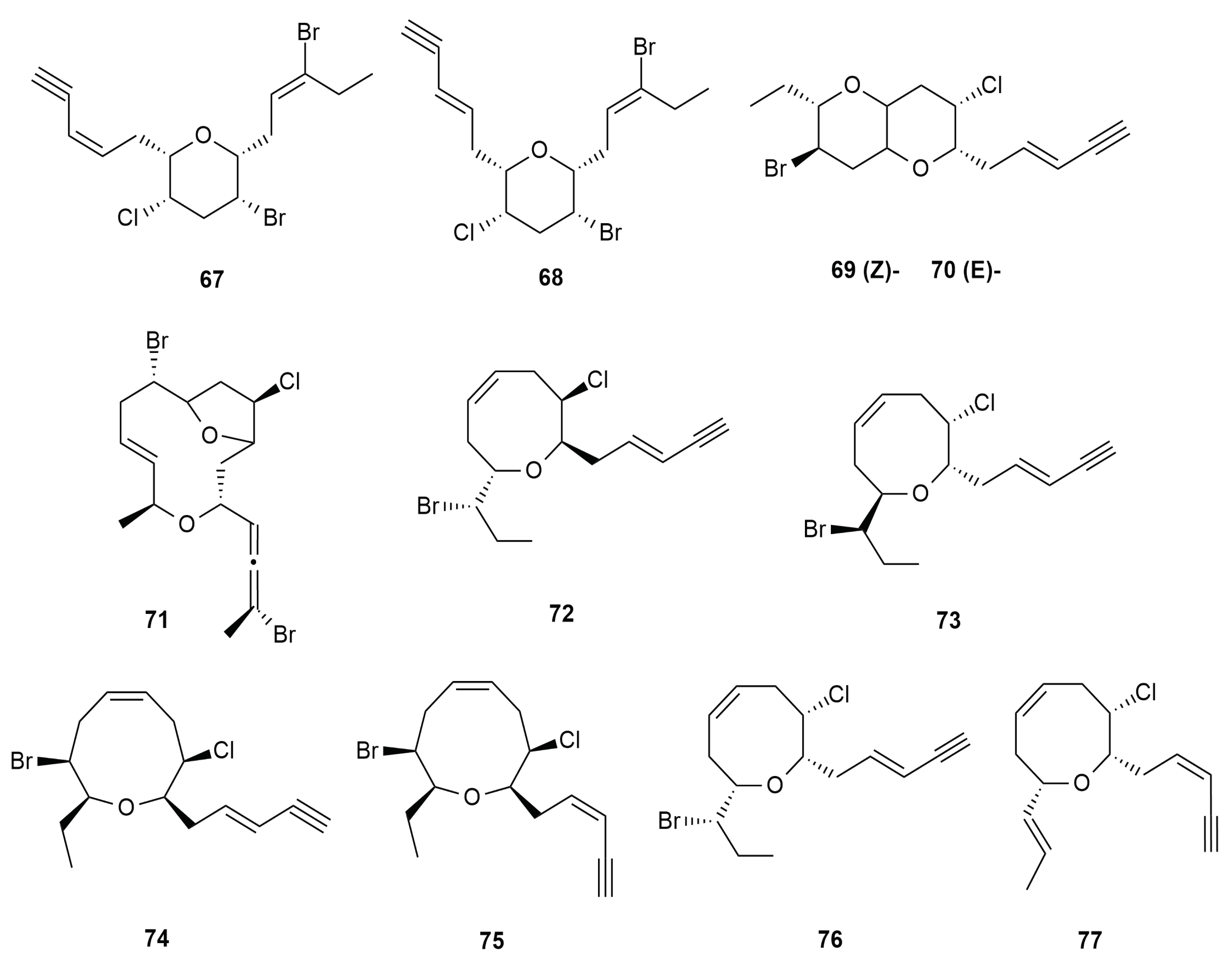
6.5. C-15 Acetogenin Type Skeleton
6.6. Diterpene
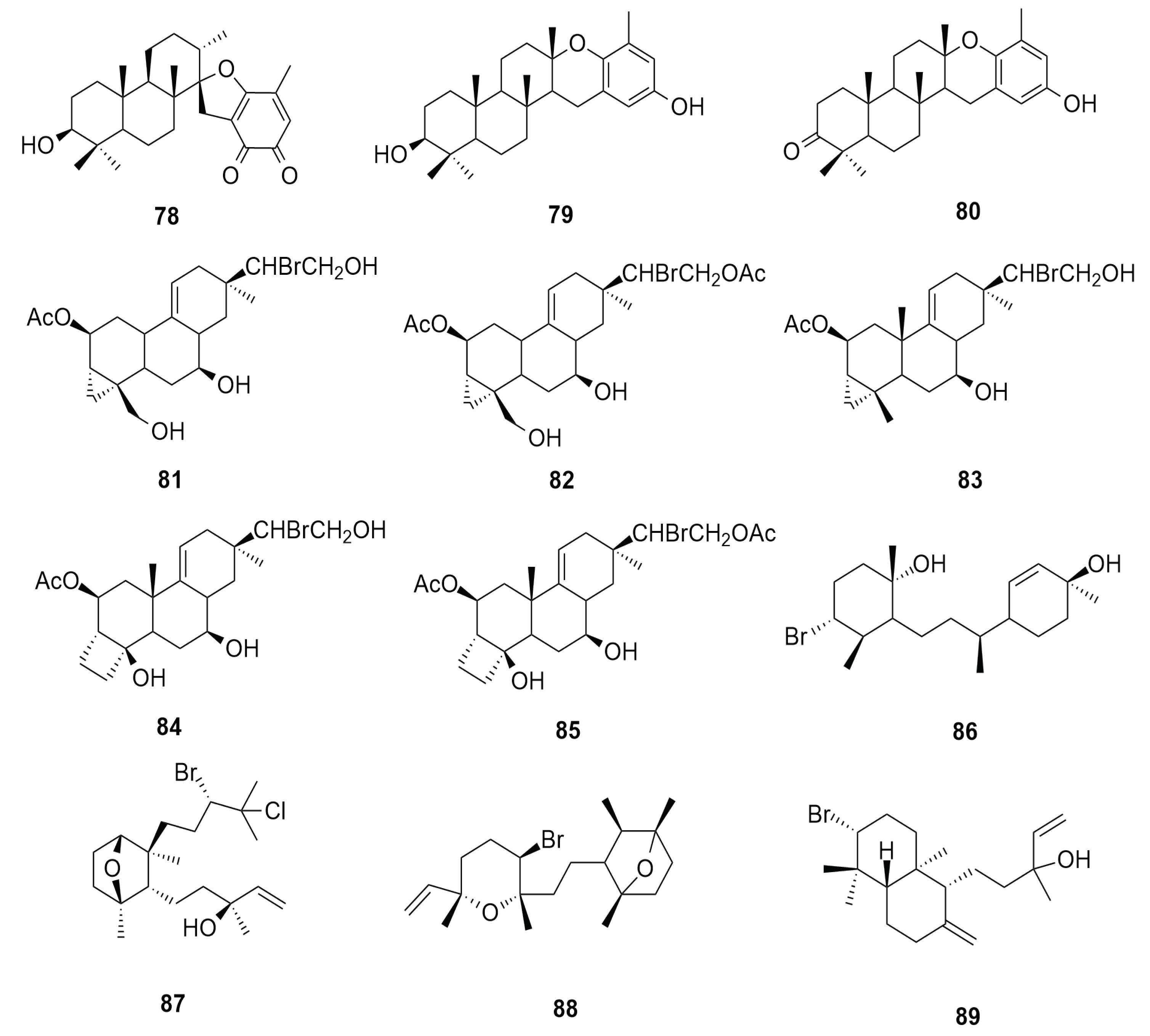
6.7. Triterpene
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pawlik, J.R. Marine invertebrate chemical defences. Chem. Rev. 1993, 93, 1911–1922. [Google Scholar] [CrossRef]
- Grkovic, T.; Appleton, D.R.; Copp, B.R. Chemistry and chemical ecology of some of the common opistobranch molluscs found on the shores of the NE New Zealand. Chem. N. Z. 2005, 69, 12–15. [Google Scholar]
- de Nys, R.; Steinberg, P.D.; Rogers, C.N.; Charlton, T.S.; Duncan, M.V. Quantitative variation of secondary metabolites in the sea hare Aplysia parvula and its host plant, Delisea pulchra. Mar. Ecol. Progress Ser. 1996, 130, 135–146. [Google Scholar] [CrossRef]
- Ginsburg, G.W.; Paul, V.J. Chemical defences in the sea hare Aplysia parvula: Importance of diet and sequestration of algal secondary metabolites. Mar. Ecol. Progress Ser. 2001, 215, 261–274. [Google Scholar] [CrossRef][Green Version]
- Ianora, A.; Boersma, M.; Casotti, R.; Fontana, A.; Harder, J.; Hoffmann, F.; Pavia, H. New trends in marine chemical ecology. Estuaries Coasts 2006, 29, 531–551. [Google Scholar] [CrossRef]
- Bornancin, L.; Bonnard, I.; Mills, S.C.; Banaigs, B. Chemical mediation as a structuring element in marine gastropod predator-prey interactions. Nat. Prod. Rep. 2017, 34, 644–676. [Google Scholar] [CrossRef]
- Kamio, M.; Grimes, T.V.; Hutchins, M.H.; van Dam, R.; Derby, C.D. The purple pigment aplysioviolin in sea hare ink deters predatory blue crabs through their chemical senses. Anim. Behav. 2010, 80, 89–100. [Google Scholar] [CrossRef]
- Hay, M.E. Marine chemical ecology: What’s known and what’s next? J. Exp. Biol. Ecol. 1996, 200, 103–104. [Google Scholar] [CrossRef]
- Vairappan, C.S.; Lee, T.K. Halogenated secondary metabolites from sea hare Aplysia Dactylomela. Malays. J. Sci. 2005, 24, 17–22. [Google Scholar]
- Vairappan, C.S.; Anangdan, S.P.; Lee, T.K. Additional halogenated secondary metabolites from the sea hare Aplysia dactylomela. Chem. Rev. 2007, 26, 57–64. [Google Scholar]
- Vairappan, C.S.; Anangdan, S.P.; Matsunaga, S. Diet-derived halogenated metabolite from the sea hare Aplysia parvula. Malays. J. Sci. 2009, 28, 269–273. [Google Scholar] [CrossRef]
- Manzo, E.; Gavagnin, M.; Bifulco, G.; Cimino, P.; Di Micco, S.; Ciavatta, M.L.; Guo, Y.-W.; Cimino, G. Aplysiols A and B, squalene-derived polyethers from the mantle of the sea hare Aplysia dactylomela. Tetrahedron 2007, 63, 9970–9978. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Tolstikov, A.G.; Tolstikov, G.A. Natural halogenated non-terpenic C15-acetogenins of sea organisms. Chem. Sustain. Dev. 2003, 11, 329–339. [Google Scholar]
- Wessels, M.; Konig, G.M.; Wright, A.D. New natural product isolation and comparison of the secondary metabolite content of three distinct samples of the sea hare Aplysia dactylomela from Tenerife. J. Natural Prod. 2000, 63, 920–928. [Google Scholar] [CrossRef]
- Avila, C. Natural products of opisthobranch molluscs: A biological review. Oceanogr. Mar. Biol. Annu. Rev. 1995, 33, 487–559. [Google Scholar]
- Pereira, R.B.; Andrade, P.B.; Valentao, P. Chemical diversity and biological properties of secondary metabolites from sea hares of Aplysia genus. Mar. Drugs 2016, 14, 39. [Google Scholar] [CrossRef]
- Palaniveloo, K.; Vairappan, C.S. Chemical relationship between red algae genus Laurencia and sea hare (Aplysia dactylomela Rang) in the North Borneo Island. Chem. Rev. 2014, 26, 1199–1205. [Google Scholar] [CrossRef]
- Conxita, A.; Paul, V.J. Chemical ecology of the nudibranch Glossodoris pallida: Is the location of diet-derived metabolites important for defense? Mar. Ecol. Progress Ser. 1997, 150, 171–180. [Google Scholar]
- Cabrita, M.T.; Vale, C.; Rauter, A.P. Halogenated compounds form Marine Algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef]
- Stengel, D.B.; Connan, S.; Popper, Z.A. Algal chemodiversity and bioactivity: Sources of natural variability and implications for commercial application. Biotechnol. Adv. 2011, 29, 483–501. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural Products: A continuing source of novel drug leads. Mar. Ecol. Program Ser. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Paul, N.A.; de Nys, R.; Steinberg, P.D. Seaweed–herbivore interactions at a small scale: Direct tests of feeding deterrence by filamentous algae. Mar. Ecol. Program Ser. 2006, 323, 1–9. [Google Scholar] [CrossRef]
- Perveen, S. Introductory Chapter: Terpenes and Terpenoids. In Terpenes and Terpenoids; Perveen, S., Al-Taweel, A., Eds.; IntechOpen: London, UK, 2018; pp. 1–13. [Google Scholar]
- Singh, B.; Sharma, R.A. Plant terpenes: Defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech 2015, 5, 129–151. [Google Scholar] [CrossRef]
- Carter-Franklin, J.N.; Butler, A. Vanadium bromoperoxidase-catalysed biosynthesis of halogenated marine natural products. J. Am. Chem. Soc. 2004, 26, 15060–15066. [Google Scholar] [CrossRef]
- Hopwood, M.J.; Rapp, I.; Schlosser, C.; Achterberg, E.P. Hydrogen peroxide in deep waters from the Mediterranean Sea, South Atlantic and South Pacific Oceans. Sci. Rep. 2017, 7, 43436. [Google Scholar] [CrossRef]
- Butler, A.; Carter-Franklin, J.N. The role of vanadium bromoperoxidase in the biosynthesis of halogenated marine natural products. Nat. Prod. Rep. 2004, 21, 180–188. [Google Scholar] [CrossRef]
- Rogers, C.N.; de Nys, R.; Charlton, T.S.; Steinberg, P.D. Dynamics of algal secondary metabolites in two species of sea hare. J. Chem. Ecol. 2000, 26, 721–744. [Google Scholar] [CrossRef]
- Neumann, C.S.; Fujimori, D.G.; Walsh, C.T. Halogenation Strategies In Natural Product Biosynthesis. Chem. Biol. 2008, 15, 99–109. [Google Scholar] [CrossRef]
- Gribble, G.W. Natural Organohalogens:A New Frontier for Medicinal Agents? J. Chem. Educ. 2004, 81, 1441–1449. [Google Scholar] [CrossRef]
- Blunt, J.W.; Copp, B.R.; Hu, W.P.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2010, 26, 170–244. [Google Scholar] [CrossRef]
- Kutateladze, A.G.; Reddy, D.S. High-Throughput in Silico Structure Validation and Revision of Halogenated Natural Products Is Enabled by Parametric Corrections to DFT-Computed 13C NMR Chemical Shifts and Spin Spin Coupling Constants. J. Org. Chem. 2017, 82, 3368–3381. [Google Scholar] [CrossRef] [PubMed]
- Mynderse, J.S.; Faulkner, D.J. Polyhalogenated monoterpenes from the red alga Plocamium cartilagineum. Tetrahedron 1975, 31, 1963–1967. [Google Scholar] [CrossRef]
- Al-Massarani, S.M. Phytochemical and Biological Properties of Sesquiterpene Constituents From the Marine Red Seaweed Laurencia: A Review? Nat. Prod. Chem. Res. 2014, 2, 1–13. [Google Scholar]
- Yamamura, S.; Hirata, Y. Structures of aplysin and aplysinol, naturally occurring bromo-compound. Tetrahedron 1963, 19, 1485–1496. [Google Scholar] [CrossRef]
- Irie, T.; Suzuki, M.; Kurosawa, E.; Masamune, T. Laurinterol and debromolaurinterol, constituents from Laurencia intermedia. Tetrahedron Lett. 1966, 7, 1837–1840. [Google Scholar] [CrossRef]
- Irie, T.; Suzuki, M.; Hayakawa, Y. Isolation of aplysin, debromoaplysin and aplysinol from Laurencia okamurai Yamada. Bull. Chem. Soc. Jpn. 1969, 42, 843–844. [Google Scholar] [CrossRef]
- McPhail, K.L.; Davies-Coleman, M.T.; Copley, R.C.B.; Eggleston, D.S. New halogenated sesquiterpenes from South African specimens of the circumtropical sea hare Aplysia dactylomela. J. Nat. Prod. 1999, 62, 1618–1623. [Google Scholar] [CrossRef]
- Appleton, D.R.; Babcock, R.C.; Copp, B.R. Novel tryptophan-derived and bioactive metabolites from the sea hare Aplysia dactylomela. Tetrahedron 2001, 57, 10181–10189. [Google Scholar] [CrossRef]
- Blunt, J.W.; Lake, R.J.; Munro, M.H.G. Sesquiterpenes from the marine red alga Laurencia distichophylla. Phytochemistry 1984, 23, 1951–1954. [Google Scholar] [CrossRef]
- Ichiba, T.; Higa, T. New cuparene-derived sesquiterpenes with unprecedented oxygenation patterns from the sea hare Aplysia dactylomela. J. Organ. Chem. 1986, 51, 3364–3366. [Google Scholar] [CrossRef]
- Hollenbaek, K.H.; Schmitz, F.J.; Hossain, M.B.; van der Helm, D. Marine natural products: Deodactol, antineoplastic sesquiterpenoid from the sea hare Aplysia dactylomela. Tetrahedron 1979, 35, 541–545. [Google Scholar] [CrossRef]
- Gonzalez, A.G.; Martin, J.D.; Perez, C.; Ramirez, M.A.; Ravelo, F. Total synthesis of 8-desoxy-isocaespitol, a new polyhalogenated sesquiterpene from Laurencia caespitosa. Tetrahedron Lett. 1980, 21, 187–188. [Google Scholar] [CrossRef]
- Gopichand, Y.; Schmitz, F.J.; Shelly, J.; Rahman, A.; van der Helm, D. Marine natural products: Halogenated acetylenic ethers from the sea hare Aplysia dactylomela. J. Organ. Chem. 1981, 46, 5192–5197. [Google Scholar] [CrossRef]
- Brito, I.; Dias, T.; Diaz-Marrero, A.R.; Darias, J.; Cueto, M. Aplysiadiol from Aplysia dactylomela suggested a key intermediate for a unified biogenesis of regular and irregular marine algal bisabolene-type metabolites. Tetrahedron 2006, 62, 9655–9660. [Google Scholar] [CrossRef]
- Ojika, M.; Yoshida, Y.; Okumura, M.; Ieda, S.; Yamada, K. Aplysiadiol, a new brominated diterpene from the marine mollusc Aplysia kurodai. J. Nat. Prod. 1990, 53, 1619–1622. [Google Scholar] [CrossRef]
- Pettit, G.R.; Herald, C.L.; Allen, M.S.; von Dreele, R.B.; Vanell, L.D.; Kao, J.P.Y.; Blake, W. Antineoplastic agents. 48. The isolation and structure of Aplysistatin. J. Am. Chem. Soc. 1976, 99, 262–263. [Google Scholar] [CrossRef]
- Tanaka, A.; Suzuki, M.; Yamashita, K. Total synthesis of (+)-Palisadin A and (+)-12-hydroxypalisadin B. Agric. Biol. Chem. 1986, 50, 1069–1071. [Google Scholar]
- Paul, V.J.; Fenical, W. Palisadin A, B, and related monocyclofarnesol derived sesquiterpenoids from the red marine alga Laurencia cf palisada. Tetrahedron Lett. 1980, 21, 2787–2790. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Gopichand, Y.; Michaud, D.P.; Prasad, S.; Remaley, S. Recent developments in research on metabolites from Caribbean marine invertebrates. Pure Appl. Chem. 1981, 53, 853–865. [Google Scholar] [CrossRef][Green Version]
- Dias, T.; Brito, I.; Moujir, L.; Paiz, N.; Darias, J.; Cueto, M. Cytotoxic sesquiterpenes from Aplysia dactylomela. J. Nat. Prod. 2005, 68, 1677–1679. [Google Scholar] [CrossRef]
- Kimura, J.; Kamada, N.; Tsujimoto, Y. Fourteen chamigrane derivatives from a red alga, Laurencia nidifica. Bull. Chem. Soc. Jpn. 1999, 72, 289–292. [Google Scholar] [CrossRef]
- Kaiser, C.R.; Pitombo, L.F.; Pinto, A.C. Complete 1H and 13C NMR assignments of chamigranes from Aplysia dactylomela. Magn. Resonance Chem. 2001, 39, 147–149. [Google Scholar] [CrossRef]
- Shubina, L.K.; Fedorov, S.N.; Kalinovskiy, A.I.; Dmitrenok, A.S.; Jin, J.O.; Song, M.G.; Kwak, J.Y.; Stonik, V.A. Four new chamigrane sesquiterpenoids from the opistobranch mollusk Aplysia dactylomela. Rus. Chem. Bull. 2007, 56, 2109–2114. [Google Scholar] [CrossRef]
- Lyakhova, E.G.; Fedorov, S.N.; Shubina, L.K.; Radchenko, O.S.; Kalinocsky, A.I.; Dvitrenok, P.S.; Stonik, V.A. Chemical properties of marine terpenoids. 1. Some reactions of (6S,10R)-10-bromo-3,11,11-trimethyl-7-methylidenespiro[5,5]undec-2-en-4-one, a sesquiterpenoid from the sea hare Aplysia dactylomela. Rus. Chem. Bull. 2003, 52, 1022–1026. [Google Scholar] [CrossRef]
- Gerwick, W.H.; Whatley, G. Aplysia sea hare assimilation of secondary metabolites from brown seaweed, Stypopodium zonale. J. Chem. Ecol. 1989, 15, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, S.N.; Shubina, L.K.; Kalinovsky, A.I.; Lyakhova, E.G.; Stonik, V.A. Structure and absolute configuration of the new rearranged chamigrane-type sesquiterpenoid from the sea hare Aplysia sp. Tetrahedron Lett. 2000, 41, 1979–1982. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Shubina, L.K.; Bode, A.M.; Stonik, V.A.; Dong, Z. Dactylone inhibits Epidermal Growth Factor–induced transformation and phenotype expression of human cancer cells and induces G1-S arrest and apoptosis. Cancer Res. 2007, 67, 5914–5920. [Google Scholar] [CrossRef]
- Fedorov, S.N.; Radchenko, O.S.; Shubina, L.K.; Kalinovsky, A.I.; Gerasimenko, A.V.; Popov, D.Y.; Stonik, V.A. Aplydactone, a new sesquiterpenoid with an unprecedented carbon skeleton from the sea hare Aplysia dactylomela, and its cargill-like rearrangement. J. Am. Chem. Soc. 2001, 123, 504–505. [Google Scholar] [CrossRef]
- Matsuura, B.S.; Koelle, P.; Trauner, D.; de Vivie-Riedle, R.; Meier, R. Unravelling photochemical relationships among natural products from Aplysia dactylomela. ACS Cent. Sci. 2017, 3, 39–46. [Google Scholar] [CrossRef]
- Díaz-Marrero, A.R.; de La Rosa, J.M.; Brito, I.; Darias, J.; Cueto, M. Dactylomelatriol, a biogenetically intriguing omphalane-derived marine sesquiterpene. J. Nat. Prod. 2012, 75, 115–118. [Google Scholar] [CrossRef]
- Wanke, T.; Philippus, A.C.; Zatelli, G.A.; Vieira, L.F.O.; Lhullier, C.; Falkenberg, M. C15 acetogenins from the Laurencia complex: 50 years of research—An overview. Rev. Bras. Farmacogn. 2015, 25, 569–587. [Google Scholar] [CrossRef]
- Vanderah, D.J.; Schmitz, F.J. Marine natural products: Isodactylyne, a halogenated acetylenic ether from the sea hare Aplysia dactylomela. J. Organ. Chem. 1976, 41, 3480–3481. [Google Scholar] [CrossRef]
- Ciavatta, M.L.; Gavagnin, M.; Puliti, R.; Cimino, G.; Martinez, E.; Ortea, J.; Mattia, C.A. Dactylallene: A novel dietary C15 bromoallene from the Atlantic anaspidean mollusk Aplysia dactylomela. Tetrahedron 1997, 53, 17343–17350. [Google Scholar] [CrossRef]
- Manzo, E.; Ciavatta, M.L.; Gavagnin, M.; Puliti, R.; Mollo, E.; Guo, Y.-W.; Mattia, C.A.; Mazzarella, L.; Cimino, G. Structure and absolute stereochemistry of novel C15-halogenated acetogenins from the anaspidean mollusk Aplysia dactylomela. Tetrahedron 2005, 61, 7456–7460. [Google Scholar] [CrossRef]
- Schmitz, F.J.; Michaud, D.P.; Schmidt, P.G. Marine natural products: Parguerol, deoxyparguerol, and isoparguerol. New brominated diterpenes with modified pimarane skeletons from the sea hare Aplysia dactylomela. J. Am. Chem. Soc. 1982, 104, 6415–6423. [Google Scholar] [CrossRef]
- Huang, X.C.; Kumar, P.; Anreddy, N.; Xiao, X.; Yang, D.H.; Chen, Z.S. P-gp inhibitory activity from marine sponges, tunicates and algae. In Handbook of Anticancer Drugs from Marine Origin; Se-Kwon, K., Ed.; Springer: Berlin, Germany, 2018; pp. 593–619. [Google Scholar]
- Schmitz, F.J.; Hollenbaek, K.H. Marine natural products: 14-bromoobtus-1-ene-3,11-diol, a new diterpenoid from the sea hare Aplysia dactylomela. J. Organ. Chem. 1979, 44, 2445–2447. [Google Scholar] [CrossRef]
- Vera, B.; Rodriguez, A.D.; Aviles, E.; Ishikawa, Y. Aplysqualenols A and B: Squalene-derived polyethers with antitumoral and antiviral activity from the Carribean sea slug Aplysia dactylomela. Eur. J. Organ. Chem. 2009, 31, 5327–5336. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palaniveloo, K.; Rizman-Idid, M.; Nagappan, T.; Abdul Razak, S. Halogenated Metabolites from the Diet of Aplysia dactylomela Rang. Molecules 2020, 25, 815. https://doi.org/10.3390/molecules25040815
Palaniveloo K, Rizman-Idid M, Nagappan T, Abdul Razak S. Halogenated Metabolites from the Diet of Aplysia dactylomela Rang. Molecules. 2020; 25(4):815. https://doi.org/10.3390/molecules25040815
Chicago/Turabian StylePalaniveloo, Kishneth, Mohammed Rizman-Idid, Thilahgavani Nagappan, and Shariza Abdul Razak. 2020. "Halogenated Metabolites from the Diet of Aplysia dactylomela Rang" Molecules 25, no. 4: 815. https://doi.org/10.3390/molecules25040815
APA StylePalaniveloo, K., Rizman-Idid, M., Nagappan, T., & Abdul Razak, S. (2020). Halogenated Metabolites from the Diet of Aplysia dactylomela Rang. Molecules, 25(4), 815. https://doi.org/10.3390/molecules25040815




