Components of Volatile Fractions from Eucalyptus camaldulensis Leaves from Iraqi–Kurdistan and Their Potent Spasmolytic Effects
Abstract
1. Introduction
2. Results and Discussion
2.1. Characteristics of the Volatile Fractions EOS and EOW
2.2. Chemical Analysis of Volatile Fractions (EO)
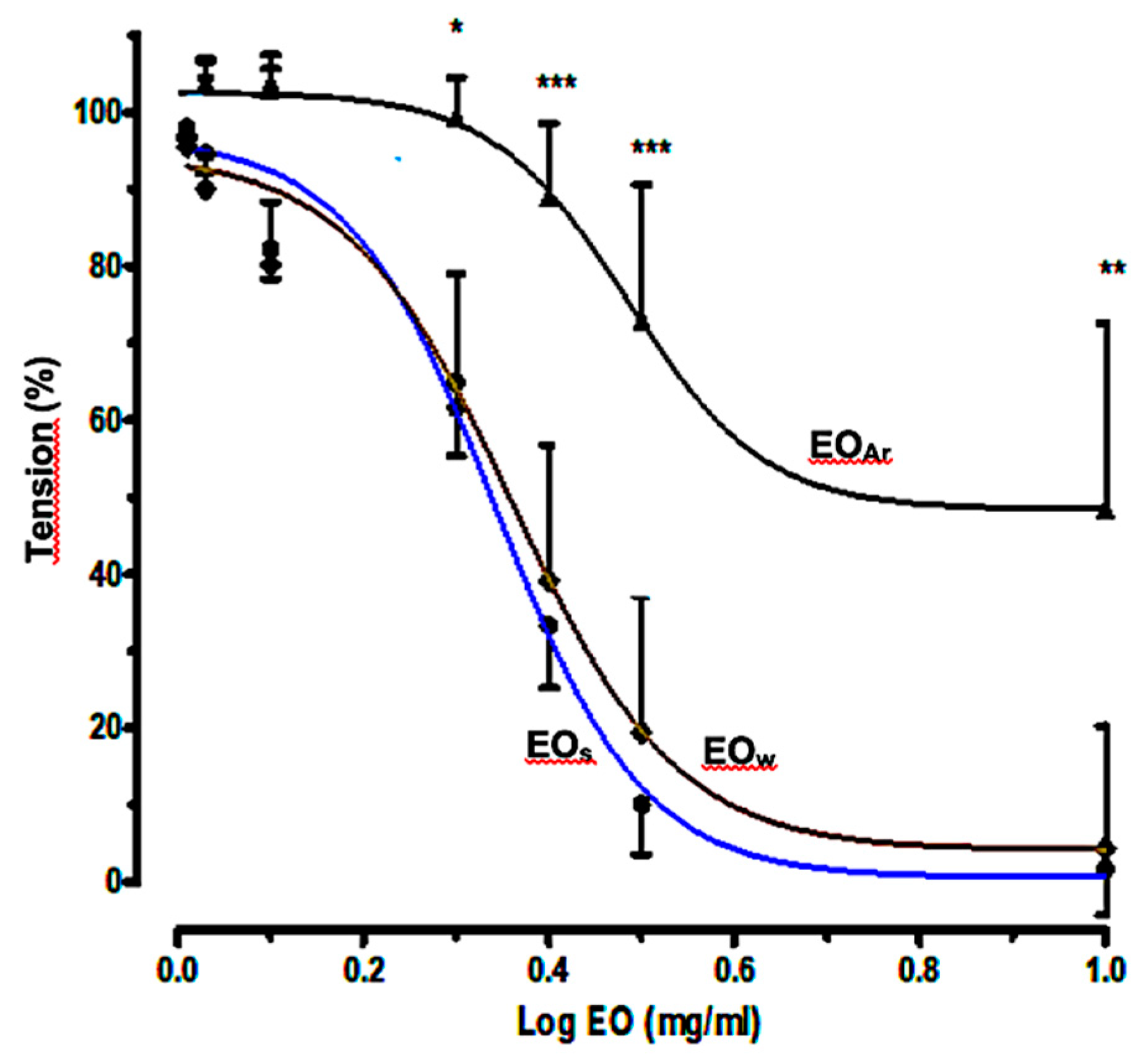
2.3. Relaxant Effects of E. camaldulensis Volatile Fractions on Aortic Rings
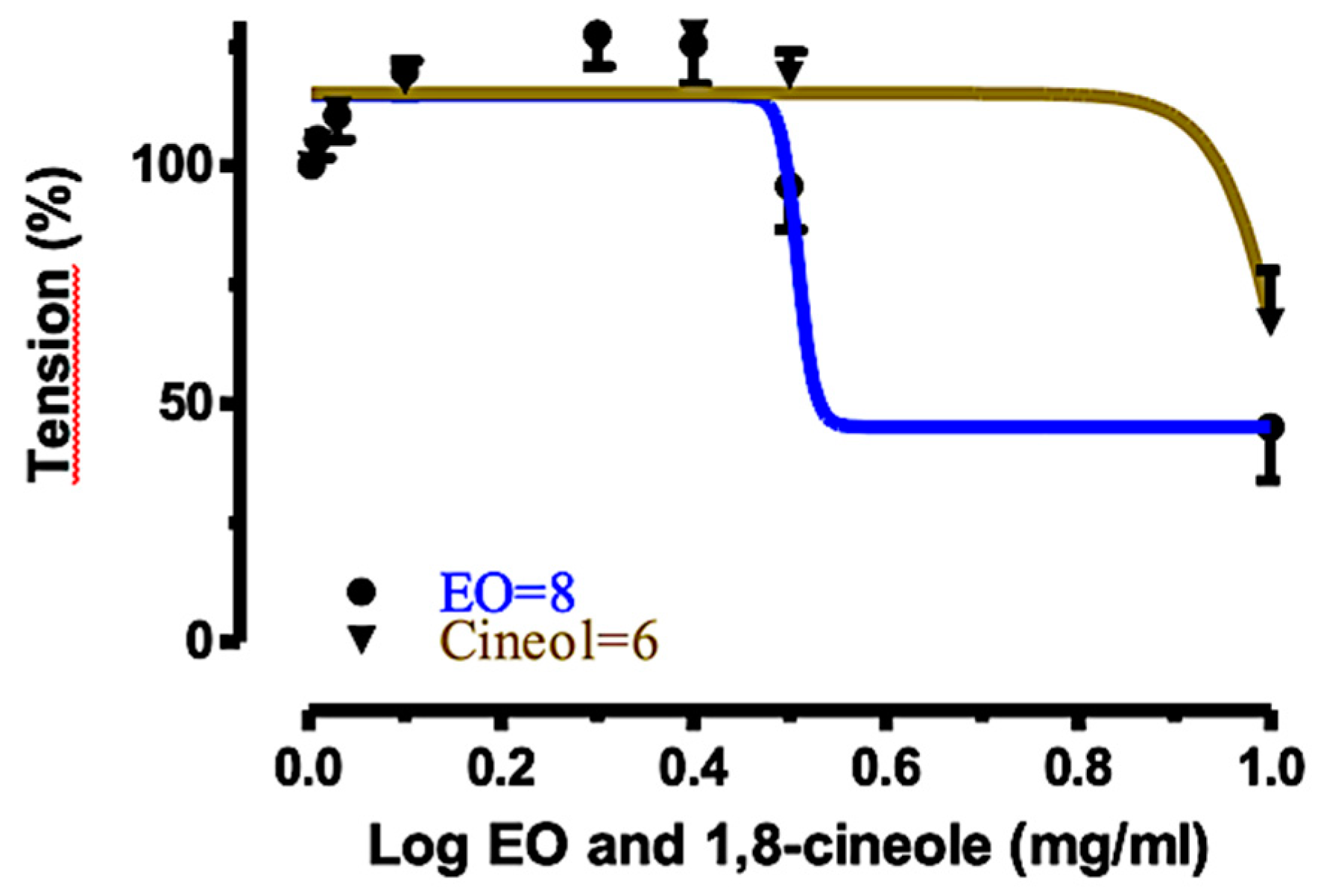
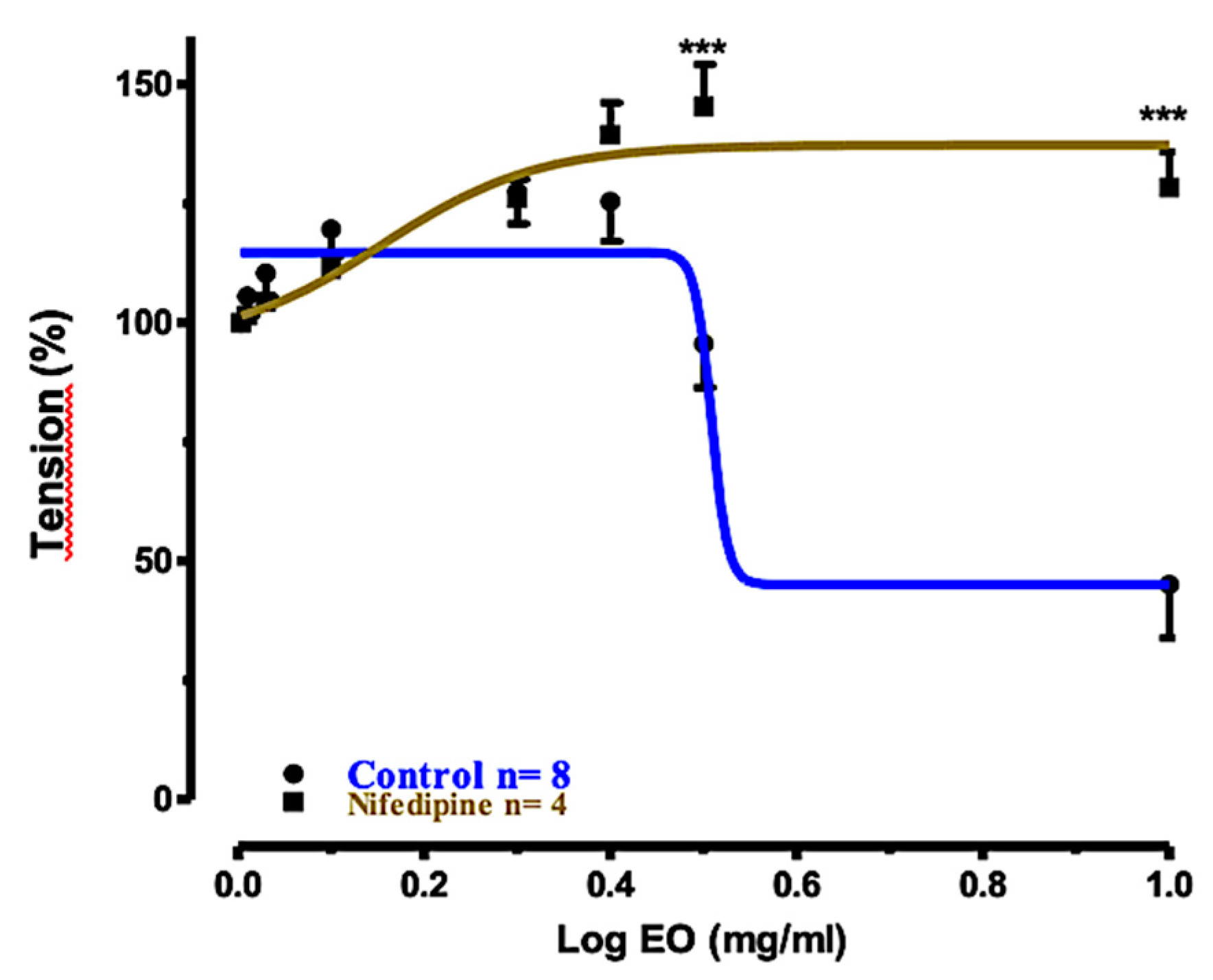
2.4. Relaxant Effects of EOS and 1,8-Cineole (1) on Rat Tracheal Rings
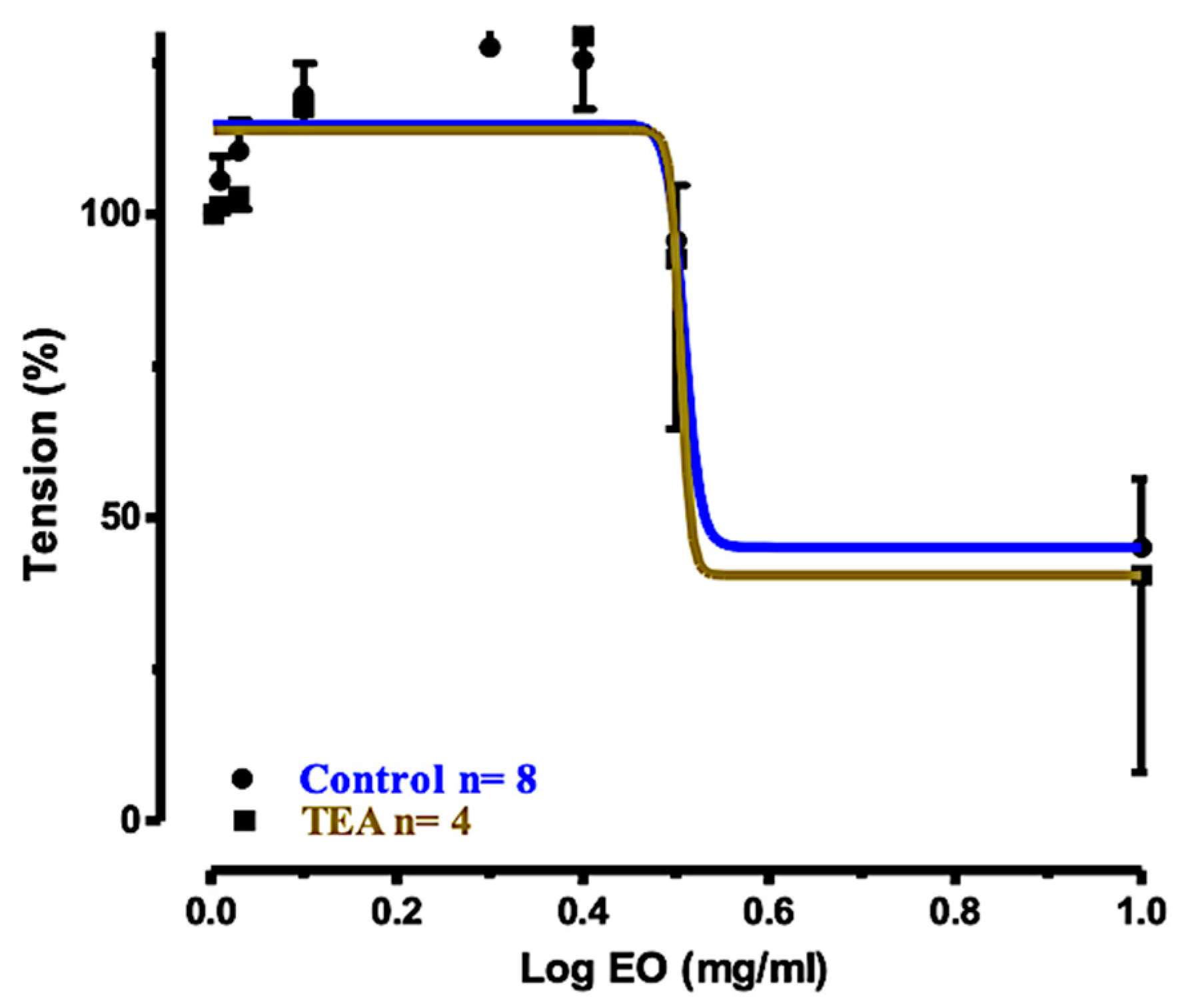
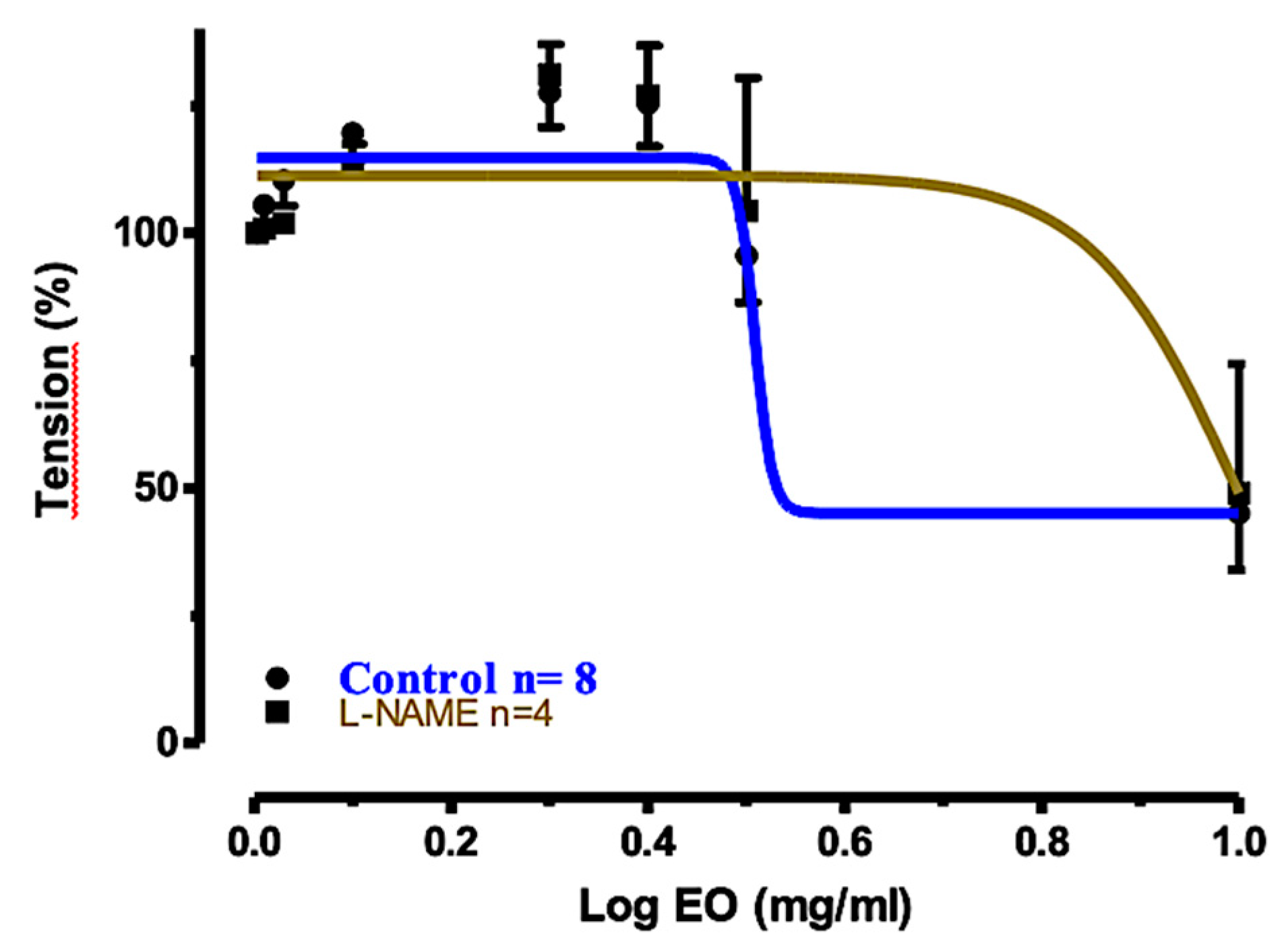
2.5. Relaxation Mechanism
3. Materials and Methods
3.1. Plant Material
3.2. Chemicals
3.3. Isolation of Volatile Fractions EO
3.4. Hydrodistillation
3.5. CO2 Extraction
3.6. GC-FID Analysis
3.7. GC-MS Analysis
3.8. Physiological Activities of Volatile Fractions
3.9. Measurement of Isometric Force with Isolated Thoracic Aortic Rings
3.10. Measurement of Isometric Force with Isolated Tracheal Rings
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shahbaz, S.I. Trees and Shrubs, 1st ed.; University of Duhok: Kurdistan, Iraq, 2010. [Google Scholar]
- Akin, M.; Aktumsek, A.; Nostro, A. Antibacterial activity and composition of the essential oils of Eucalyptus camaldulensis Dehnh. and Myrtus communis L. growing in Northern Cyprus. Afr. J. Biotechnol. 2010, 9, 531–535. [Google Scholar]
- Abd El-Mageed, A.A.; Osman, A.K.; Tawfik, A.Q.; Mohammed, H.A. Chemical composition of the essential oils of four Eucalyptus Species (Myrtaceae) from Egypt. Res. J. Phytochem. 2011, 5, 115–122. [Google Scholar]
- Şahin-Bölükbaşı, S.; Candan, F. Chemical composition and in vitro antioxidant and antidiabetic activities of Eucalyptus camaldulensis Dehnh. essential oil. J. Iran. Chem. Soc. 2010, 7, 216–226. [Google Scholar] [CrossRef]
- Lima, L.M.; Babakhani, B.; Boldaji, S.A.H.; Asadi, M.; Boldaji, R.M. Essential oils composition and antibacterial activities of Eucalyptus camaldulensis Dehn. Int. J. Med. Arom. Plants 2013, 3, 214–219. [Google Scholar]
- Sefidkon, F.; Bahmanzadegan, A.; Assareh, M.H.; Abravesh, Z. Seasonal variation in volatile oil of Eucalyptus species in Iran. J. Herbs Spices Med. Plants (IJHSMP) 2009, 15, 106–120. [Google Scholar] [CrossRef]
- Gakuubi, M.M. Steam distillation extraction and chemical composition of essential oils of Toddalia asiatica L. and Eucalyptus camaldulensis Dehnh. J. Pharmacogn. Phytochem. 2016, 5, 99–104. [Google Scholar]
- Grbović, S.; Orčić, D.; Couladis, M.; Jovin, E.; Bugarin, D.; Balog, K.; Mimica-Dukić, N. Variation of essential oil composition of Eucalyptus camaldulensis (Myrtaceae) from the Montengero coastline. Acta Period. Technol. 2010, 41, 151–158. [Google Scholar] [CrossRef]
- Knezevic, P.; Aleksic, V.; Simin, N.; Svircev, E.; Petrovic, A.; Mimica-Dukic, N. Antimicrobial activity of Eucalyptus camaldulensis essential oils and their interactions with conventional antimicrobial agents against multi-drug resistant Acinetobacter baumannii. J. Ethnopharmacol. 2016, 178, 125–136. [Google Scholar] [CrossRef]
- Ashraf, M.; Ali, Q.; Anwar, F.; Hussain, A.I. Composition of leaf essential oil of Eucalyptus camaldulensis. Asian J. Chem. 2010, 22, 1779–1786. [Google Scholar]
- El-Ghorab, A.H.; El-Massry, K.F.; Anjum, F.M.; Shahwarb, M.K.; Shibamoto, T. The chemical composition and antioxidant activity of essential oil of Pakistani Eucalyptus camaldulensis leaves. J. Essent Oil-Bear. Plants 2009, 12, 262–272. [Google Scholar] [CrossRef]
- Ndiaye, E.H.B.; Diop, M.B.; Gueye, M.T.; Ndiaye, I.; Diop, S.M.; Fauconnier, M.-L.; Lognay, G. Characterization of essential oils and hydrosols from Senegalese Eucalyptus camaldulensis Dehnh. J. Essent. Oil Res. 2018, 30, 131–141. [Google Scholar] [CrossRef]
- Akin-Osanaiye, B.C.; Agbaji, A.S.; Dakare, M.A. Antimicrobial Activity of Oils and Extracts of Cymbopogon citratus (Lemon Grass), Eucalyptus citriodora and Eucalyptus camaldulensis. J. Med. Sc. 2007, 4, 694–697. [Google Scholar] [CrossRef]
- Aleksic Sabo, V.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crop Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Chevallier, A. The Encyclopedia of Medicinal Plants: An Excellent Guide to Over 500 of the More Well-Known Medicinal Herbs from around the World; Dorling Kindersley: London, UK, 1966. [Google Scholar]
- Begum, S.; Farhat, F.; Sultana, I.; Siddiqui, B.S.; Shaheen, F.; Gilani, A.H. Spasmolytic constituents from Eucalyptus camaldulensis var. obtusa leaves. J. Nat. Prod. 2000, 63, 1265–1268. [Google Scholar] [CrossRef]
- Al-Habib, O.A.M.; Kheder, A.D.; Vidari, G.; Gilardoni, G. Relaxant effects of essential oils of Eucaluptus camaldulensis on aortic rings in male albino rats. Sci. J. Univ. Zakho 2013, 1, 139–146. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- NIST Chemistry WebBook, SRD 69. 2018. Available online: https://doi.org/10.18434/T4D303 (accessed on 30 December 2019).
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J Chromatogr. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Wiley Registry, 11th ed.; NIST 2017 Mass Spectral Library; Wiley: New York, NY, USA; NIST: Gaithersburg, MD, USA, 2017; ISBN 978-1-119-41223-6.
- Moudachirou, M.; Gbénou, J.D.; Chalchat, J.C.; Chabard, J.L.; Lartigue, C. Chemical composition of essential oils of Eucalyptus from Bénin: Eucalyptus citriodora and E. camaldulensis. Influence of location, harvest time, storage of plants and time of steam distillation. J. Essent. Oil Res. 1999, 11, 109–118. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Porto, D.L.; Menezes, C.P.; Antunes, A.A.; Silva, D.F.; De Sousa, D.P.; Nakao, L.S.; Braga, V.A.; Medeiros, I.A. Unravelling the cardiovascular effects induced by alpha-terpineol: A role for the nitric oxide-cGMP pathway. Clin. Exp. Pharmacol. Physiol. 2010, 37, 811–816. [Google Scholar] [CrossRef]
- Perez-Hernandez, N.; Ponce-Monter, H.; Ortiza, M.I.; Carino-Cortes, R.; Joseph-Nathan, P. Structure-activity relationships of aromadendranes in uterus-relaxant activity. Z. Naturforsch. 2009, 64C, 840–846. [Google Scholar] [CrossRef]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.M.; Lima, F.C.; Lahlou, S.; Magalhães, P.J.C.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers, carvacrol and thymol, on rat isolated aorta. Fund. Clin. Pharmacol. 2010, 24, 341350. [Google Scholar] [CrossRef]
- Coelho-de-Souza, L.N.; Leal-Cardoso, J.H.; de Abreu Matos, F.J.; Lahlou, S.; Magalhães, P.J. Relaxant effects of the essential oil of Eucalyptus tereticornis and its main constituent 1,8-cineole on guinea-pig tracheal smooth muscle. Planta Med. 2005, 71, 1173–1175. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, N.R.F.; de Refosco, R.M.C.; Vasconcelos, E.C.F.; Kerntopf, M.R.; Santos, C.F.; Batista, F.J.A.; De Sousa, C.M.; Fonteles, M.C. 1,8-Cineole induces relaxation in rat and guinea-pig airway smooth muscle. J. Pharm. Pharmacol. 2009, 61, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Pinto, N.V.; Assreuy, A.M.S.; Coelho-de-Souza, A.N.; Ceccatto, V.M.; Magalhães, P.J.C.; Lahlou, S.; Leal-Cardoso, J.H. Endothelium-dependent vasorelaxant effects of the essential oil from aerial parts of Alpinia zerumbet and its main constituent 1,8-cineole in rats. Phytomedicine 2009, 16, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Soares, M.C.M.S.; Damiani, C.E.N.; Moreira, C.M.; Stefanon, I.; Vassallo, D.V. Eucalyptol, an essential oil, reduces contractile activity in rat cardiac muscle. Braz. J. Med. Biol. Res. 2005, 38, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Juergens, U.R. Anti-inflammatory properties of the monoterpene 18-cineole: Current evidence for co-medication in inflammatory airway diseases. Drug Res. 2014, 64, 638–646. [Google Scholar]
- Santos, F.A.; Rao, V.S. Antiinflammatory and antinociceptive effects of 1,8-cineole a terpenoid oxide present in many plant essential oils. Phytother Res. 2000, 14, 240–244. [Google Scholar] [CrossRef]
- Hardel, D.K.; Sahoo, L. A review on phytochemical and pharmacological of Eucalyptus globulus: A multipurpose tree. Int. J. Res. Ayurveda Pharm. 2011, 2, 1527–1530. [Google Scholar]
- Santos, M.R.V.; Moreira, F.V.; Fraga, B.I.S.P.O.; De Sousa, D.O.; Bonjardim, L.R.; Quintans-Junior, L.J. Cardiovascular effects of monoterpenes: A review. Rev. Bras. Pharmacogn. 2011, 21, 764–771. [Google Scholar] [CrossRef]
- Hirota, K.; Hashiba, E.; Yoshioka, H.; Kabara, S.; Matsuki, A. Effects of three different L-type Ca2+ entry blockers on airway constriction induced by muscarinic receptor stimulation. Br. J. Anaesth. 2003, 90, 671–675. [Google Scholar] [CrossRef][Green Version]
- Ahmed, F.; Foster, R.W.; Small, R.C. Some effects of nifedipine in guinea-pig isolated trachealis. Br. J. Pharmacol. 1988, 84, 861–869. [Google Scholar] [CrossRef]
- Honkanen, E.; Karvonen, P. Isolation of volatile flavour compounds from fats and oils by vacuum carbon dioxide distillation. Acta Chem. Scand. 1966, 20, 2626–2627. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.; Matos, F.J. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ghalem, B.R.; Mohamed, B. Antibacterial activity of essential oil of north west Algerian Eucalyptus camaldulensis against Escherichia coli and Staphylococcus aureus. J. Coast. Life Med. 2014, 2, 799–804. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
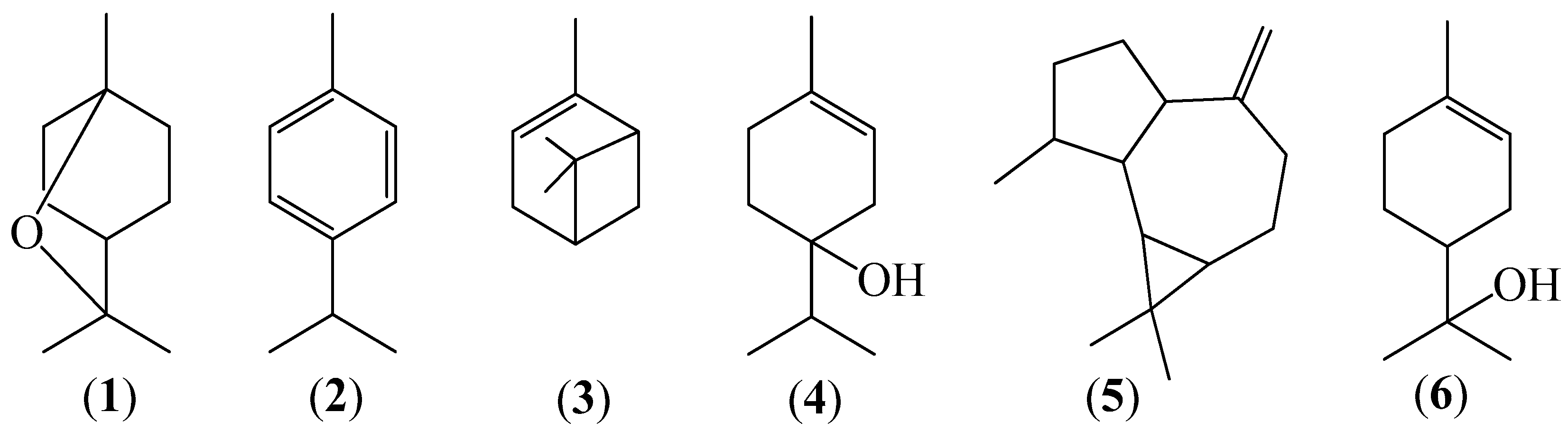
| Component | RIb | LRIc | EOS d | EOW d | EOA | EOAr | EOTot e | EOD |
|---|---|---|---|---|---|---|---|---|
| Isovaleric acid f | 848 | 842 | ‒ | ‒ | ‒ | 0.23 | < 0.01 | ‒ |
| Isopentyl acetate | 869 | 870 | 0.07 | 0.07 | ‒ | ‒ | 0.05 | ‒ |
| Undetermined | 919 | 0.06 | 0.02 | ‒ | ‒ | 0.01 | ‒ | |
| α-Pinene g (3) | 932 | 925 | 4.71 | 3.75 | ‒ | ‒ | 2.73 | 1.89 |
| 4-Methylvaleric acid f | 949 | 946 | ‒ | ‒ | ‒ | 0.01 | <0.01 | ‒ |
| Hexanoic acid f | 990 | 993 | ‒ | ‒ | ‒ | 0.01 | <0.01 | ‒ |
| p-Cymene (2) | 1020 | 1024 | 6.70 | 6.55 | 0.16 | ‒ | 4.80 | 3.44 |
| 1,8-Cineole g (1) | 1026 | 1031 | 62.70 | 59.09 | 53.41 | 10.14 | 55.83 | 89.25 |
| Phenylacetaldehyde f,g | 1047 | 1048 | ‒ | ‒ | 0.11 | 0.06 | 0.03 | ‒ |
| p-Mentha-3,8-diene | 1068 | 1065 | 2.44 | 2.28 | ‒ | ‒ | 1.66 | 1.32 |
| p-Cymenene | 1089 | 1090 | 0.15 | 0.17 | 0.13 | 0.13 | 0.16 | ‒ |
| Isopentyl isovalerate | 1102 | 1107 | 0.28 | 0.38 | ‒ | ‒ | 0.28 | 0.15 |
| endo-Fenchol | 1114 | 1110 | 0.14 | 0.21 | ‒ | ‒ | 0.15 | ‒ |
| cis-p-Menth-2-en-1-ol | 1118 | 1120 | ‒ | ‒ | 0.16 | 0.22 | 0.05 | ‒ |
| α-Campholenal | 1122 | 1122 | 0.05 | 0.18 | 0.36 | 0.6 | 0.24 | ‒ |
| trans-Pinocarveol | 1135 | 1138 | 0.94 | 0.95 | ‒ | 3.55 | 0.83 | ‒ |
| trans-p-Menth-2-en-1-ol | 1136 | 1140 | 0.13 | 0.03 | ‒ | 0.16 | 0.03 | ‒ |
| Sabina ketone | 1154 | 1151 | 0.05 | 0.04 | 1.93 | 0.03 | 0.48 | ‒ |
| Pinocarvone | 1160 | 1162 | 0.62 | ‒ | ‒ | ‒ | ‒ | ‒ |
| Umbellulone | 1167 | 1162 | ‒ | 0.31 | 0.23 | 0.01 | 0.28 | ‒ |
| Borneolg | 1165 | 1166 | 0.09 | 0.47 | 1.47 | 0.68 | ‒ | |
| Terpinen-4-ol g (4) | 1174 | 1177 | 3.86 | 3.90 | 6.96 | 2.70 | 4.56 | 0.39 |
| Cryptone | 1183 | 1184 | 1.34 | 1.10 | 6.69 | 13.92 | 2.90 | ‒ |
| p-Cymen-8-ol | 1179 | 1187 | ‒ | ‒ | ‒ | 13.48 | 0.53 | ‒ |
| α-Terpineol g (6) | 1186 | 1189 | 2.85 | 2.94 | 6.70 | 17.26 | 4.38 | 0.09 |
| Undetermined | 1213 | 0.25 | 0.25 | ‒ | 0.01 | 0.18 | ‒ | |
| trans-Carveol g | 1215 | 1219 | 0.14 | 0.15 | 0.65 | 1.02 | 0.30 | ‒ |
| cis-Carveol g | 1229 | 1222 | ‒ | ‒ | 0.91 | 2.07 | 0.29 | ‒ |
| cis-p-Mentha-1(7),8-dien-2-ol | 1227 | 1227 | ‒ | 0.19 | 1.21 | 2.12 | 0.50 | ‒ |
| (E)-Ocimenone | 1235 | 1231 | 0.19 | 1.73 | 3.81 | 0.55 | ‒ | |
| Cuminaldehyde | 1238 | 1238 | 0.11 | 0.12 | 0.32 | 0.39 | 0.18 | ‒ |
| Carvone g + carvotanacetone | 1239-1244 | 1244-1246 | ‒ | ‒ | 0.32 | 0.55 | 0.10 | ‒ |
| Piperitone g | 1249 a,1254 d | 1254 | 0.11 | 0.11 | 0.47 | 0.92 | 0.23 | ‒ |
| p-Menth-1-en-7-al | 1273 | 1274 | 0.73 | 0.68 | 1.67 | 3.41 | 1.02 | ‒ |
| α-Terpinen-7-al | 1283 | 1283 | ‒ | ‒ | ‒ | 0.01 | tr | ‒ |
| Thymol g | 1289 | 1289 | 0.49 | 0.58 | 1.78 | 2.52 | 0.93 | ‒ |
| p-Cymen-7-ol | 1289 | 1292 | ‒ | ‒ | 0.24 | 0.49 | 0.07 | ‒ |
| Carvacrol g | 1298 | 1302 | 0.39 | 0.44 | 1.15 | 2.73 | 0.69 | ‒ |
| 3-Oxo-p-menth-1-en-7-al | 1330 | 1335 | ‒ | ‒ | 0.35 | 0.39 | 0.10 | ‒ |
| Undetermined | 1334 | 0.10 | 0.09 | 0.59 | 0.79 | 0.23 | ‒ | |
| Undetermined | 1353 | ‒ | 0.17 | 0.11 | 0.04 | ‒ | ||
| Isoledene | 1374 | 1376 | ‒ | 0.09 | ‒ | ‒ | 0.06 | ‒ |
| α-Copaene | 1374 | 1378 | ‒ | 0.08 | ‒ | ‒ | 0.07 | ‒ |
| β-Elemene | 1389 | 1394 | ‒ | 0.03 | ‒ | ‒ | 0.02 | ‒ |
| Undetermined | 1419 | ‒ | ‒ | 0.13 | 0.06 | 0.03 | ‒ | |
| Undetermined | 1434 | ‒ | ‒ | 0.17 | 0.05 | 0.04 | ‒ | |
| α-Gurjunene | 1409 | 1412 | ‒ | 0.03 | ‒ | ‒ | 0.02 | ‒ |
| β-Gurjunene | 1433 | 1432 | ‒ | 0.04 | ‒ | ‒ | 0.03 | ‒ |
| Aromadendrene (5) | 1439 | 1443 | 3.08 | 3.97 | 0.3 | 0.15 | 2.97 | 0.47 |
| 9-epi-(E)-Caryophyllene | 1464 | 1465 | 1.99 | 2.57 | 0.17 | 0.02 | 1.91 | ‒ |
| γ-Gurjunene | 1475 | 1476 | ‒ | 0.06 | ‒ | ‒ | 0.04 | ‒ |
| γ-Muurolene | 1478 | 1480 | ‒ | 0.43 | ‒ | ‒ | 0.31 | ‒ |
| 2-Phenylethyl 3-methylbutanoate | 1490 | 1493 | 0.16 | 0.72 | 0.29 | 0.27 | 0.60 | ‒ |
| Viridiflorene | 1496 | 1499 | ‒ | 0.28 | ‒ | ‒ | 0.20 | ‒ |
| Undetermined | 1520 | 1534 | ‒ | ‒ | 0.10 | 0.22 | 0.03 | ‒ |
| α-Muurolene | 1500 | 1504 | ‒ | 0.28 | ‒ | ‒ | 0.20 | 0.42 |
| γ-Cadinene | 1513 | 1518 | ‒ | 0.07 | ‒ | ‒ | 0.05 | ‒ |
| Palustrol | 1567 | 1566 | 0.29 | 0.60 | 0.13 | 0.22 | 0.48 | ‒ |
| Spathulenol | 1577 | 1584 | 1.65 | 1.72 | 2.57 | 5.74 | 2.08 | 2.58 |
| Globulol | 1590 | 1590 | 1.65 | 1.96 | 0.18 | 0.29 | 1.48 | ‒ |
| Viridiflorol | 1592 | 1598 | ‒ | 0.48 | ‒ | ‒ | 0.35 | ‒ |
| Isoaromadendrene epoxide f | 1612 | 1610 | 0.37 | ‒ | 0.20 | 0.23 | 0.05 | ‒ |
| Undetermined | 1630 | 0.21 | 0.45 | 0.13 | 0.19 | 0.36 | ‒ | |
| Occidenol | 1676 | 1670 | ‒ | ‒ | 0.32 | 0.57 | 0.10 | ‒ |
| Undetermined | 1680 | ‒ | ‒ | 0.11 | 0.18 | 0.03 | ‒ | |
| Nootkatol | 1714 | 1721 | ‒ | ‒ | 0.02 | 0.01 | tr | ‒ |
| Undetermined | 1727 | ‒ | ‒ | 0.74 | 1.64 | 0.24 | ‒ | |
| Undetermined | 1751 | ‒ | ‒ | 0.68 | 1.53 | 0.22 | ‒ | |
| 14-Oxy-α-muurolene | 1767 | 1766 | 0.10 | 0.10 | ‒ | ‒ | 0.07 | ‒ |
| Undetermined | 1774 | ‒ | ‒ | 0.37 | 0.63 | 0.11 | ‒ | |
| Undetermined | 1855 | ‒ | ‒ | 0.05 | 0.12 | 0.02 | ‒ | |
| 11,12-Dihydroxyvalencene | 1914 | 1908 | ‒ | ‒ | 0.20 | 0.55 | 0.07 | ‒ |
| Monoterpene hydrocarbons (4) h | 14.00 (4) | 12.75 (4) | 0.29 (2) | 0.13 (1) | 9.35 (4) | 6.65 (3) | ||
| Oxygenated monoterpenoids (28) h | 74.93 (18) | 71.49 (18) | 88.71 (22) | 82.50 (25) | 75.90 (27) | 89.73 (3) | ||
| Sesquiterpene hydrocarbons (12) h | 5.07 (2) | 7.93 (12) | 0.47 (2) | 0.17 (2) | 5.88 (12) | 0.89 (2) | ||
| Oxygenated sesquiterpenoids (9) h | 4.06 (4) | 4.86 (5) | 3.62 (7) | 7.61 (7) | 4.68 (9) | 2.58 (1) | ||
| Others (7) h | 0.51 (3) | 1.17 (3) | 0.40 (2) | 0.58 (5) | 0.96 (7) | 0.15 (1) | ||
| Undetermined (13) h | 0.62 (4) | 0.81 (4) | 3.24 (11) | 5.53 (12) | 1.54 (13) | 0.00 (0) | ||
| Total % h | 98.57 (31) | 98.20 (42) | 93.49 (35) | 90.99 (40) | 96.77 (59) | 100 (10) | ||
| EOS | EOW | EOAr | |
|---|---|---|---|
| Log EC50 | 0.3384 | 0.3689 | 0.4852 |
| 95% CI for Log EC50 | 0.2974–0.3795 | 0.2627–0.4571 | 0.3621–0.6084 |
| Relaxation (%) ± SEM | 95.66 ± 0.198 | 88.17 ± 11.4 | 72.09 ± 10.61 |
| Treatment of Tracheal Rings | Control | Preincubated with TEA (1 mM) | Preincubated with Nifedizpine (30 μM) | Preincubated with L-NAME (0.3 mM) |
|---|---|---|---|---|
| Log EC50 | 0.508 | 0.505 | 0.156 | 1.014 |
| 95% CI for Log EC50 | (Very wide) | (Very wide) | –0.0466 to 0.3577 | (Very wide) |
| Relaxation (%) ± SEM | 79.44% ± 9.101 | 73.68% ± 10.39 | −26.42% ± 10.79 | 45.41% ± 10.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kheder, D.A.; Al-Habib, O.A.M.; Gilardoni, G.; Vidari, G. Components of Volatile Fractions from Eucalyptus camaldulensis Leaves from Iraqi–Kurdistan and Their Potent Spasmolytic Effects. Molecules 2020, 25, 804. https://doi.org/10.3390/molecules25040804
Kheder DA, Al-Habib OAM, Gilardoni G, Vidari G. Components of Volatile Fractions from Eucalyptus camaldulensis Leaves from Iraqi–Kurdistan and Their Potent Spasmolytic Effects. Molecules. 2020; 25(4):804. https://doi.org/10.3390/molecules25040804
Chicago/Turabian StyleKheder, Dlzar A., Omar A. M. Al-Habib, Gianluca Gilardoni, and Giovanni Vidari. 2020. "Components of Volatile Fractions from Eucalyptus camaldulensis Leaves from Iraqi–Kurdistan and Their Potent Spasmolytic Effects" Molecules 25, no. 4: 804. https://doi.org/10.3390/molecules25040804
APA StyleKheder, D. A., Al-Habib, O. A. M., Gilardoni, G., & Vidari, G. (2020). Components of Volatile Fractions from Eucalyptus camaldulensis Leaves from Iraqi–Kurdistan and Their Potent Spasmolytic Effects. Molecules, 25(4), 804. https://doi.org/10.3390/molecules25040804







