Carbon-Supported Raney Nickel Catalyst for Acetone Hydrogenation with High Selectivity
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cui, X.; Li, W.; Ryabchuk, P.; Junge, K.; Beller, M. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts. Nat. Catal. 2018, 1, 385–397. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: Catalysis beyond electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, L. Handbook of Industrial Catalysts; Springer Science and Business Media LLC: New York, NY, USA, 2011; pp. 4–10. [Google Scholar]
- Yang, Z.; Chen, W.; Zheng, J.; Yang, Z.; Zhang, N.; Zhong, C.-J.; Chen, B.H. Efficient low-temperature hydrogenation of acetone on bimetallic Pt-Ru/C catalyst. J. Catal. 2018, 363, 52–62. [Google Scholar] [CrossRef]
- Rahman, A.; S-Al-Deyab, S. A review on reduction of acetone to isopropanol with Ni nano superactive, heterogeneous catalysts as an environmentally benevolent approach. Appl. Catal. A: Gen. 2014, 469, 517–523. [Google Scholar] [CrossRef]
- Ophardt, H.; Loos, V.; Hoogers, G.; Lang, A. Direct isopropanol fuel cell. U.S. Patent 10,230,120, 12 March 2019. [Google Scholar]
- Park, H.; Ou, H.-H.; Kang, U.; Choi, J.; Hoffmann, M.R. Photocatalytic conversion of carbon dioxide to methane on TiO2/CdS in aqueous isopropanol solution. Catal. Today 2016, 266, 153–159. [Google Scholar] [CrossRef]
- Balouch, A.; Umar, A.A.; Shah, A.A.; Salleh, M.M.; Oyama, M. Efficient Heterogeneous Catalytic Hydrogenation of Acetone to Isopropanol on Semihollow and Porous Palladium Nanocatalyst. ACS Appl. Mater. Interfaces 2013, 5, 9843–9849. [Google Scholar] [CrossRef]
- Jiang, H.; Lu, S.; Zhang, X.; Dai, W.; Qiao, J. Polymer-Supported Raney Nickel Catalysts for Sustainable Reduction Reactions. Molecules 2016, 21, 833. [Google Scholar] [CrossRef]
- Rao, P.V.R.; Kumar, V.P.; Rao, G.S.; Chary, K.V.R. Vapor phase selective hydrogenation of acetone to methyl isobutyl ketone (MIBK) over Ni/CeO2 catalysts. Catal. Sci. Technol. 2012, 2, 1665. [Google Scholar] [CrossRef]
- Witsuthammakul, A.; Sooknoi, T. Selective hydrodeoxygenation of bio-oil derived products: Ketones to olefins. Catal. Sci. Technol. 2015, 5, 3639–3648. [Google Scholar] [CrossRef]
- Ji, Y.; Wu, Y.; Zhao, G.; Wang, D.; Liu, L.; He, W.; Li, Y. Porous bimetallic Pt-Fe nanocatalysts for highly efficient hydrogenation of acetone. Nano Res. 2015, 8, 2706–2713. [Google Scholar] [CrossRef]
- Zhou, Y.; Duan, H.; Lin, B.; Han, K.; Wei, J. Supported TiO2–Pd bifunctional catalysts for the one-pot synthesis of methyl isobutyl ketone from acetone: Modulation of the acid and base property of loaded TiO2 by support. React. Kinet. Mech. Catal. 2018, 125, 303–317. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, P.; Yang, L.; Zhou, Y.; Zhong, H. An efficient bifunctional catalyst of TiO2 coating and supported Pd on cordierite for one-pot synthesis of MIBK from acetone. Catal. Commun. 2015, 71, 61–64. [Google Scholar] [CrossRef]
- Ma, R.; Li, Y.; Wu, G.; He, Y.; Feng, J.; Zhao, Y.; Li, D. Fabrication of Pd-based metal-acid-alkali multifunctional catalysts for one-pot synthesis of MIBK. Chin. J. Catal. 2018, 39, 1384–1394. [Google Scholar] [CrossRef]
- Narayanan, S.; Unnikrishnan, R. Selective hydrogenation of acetone to methyl isobutyl ketone (MIBK) over co-precipitated Ni/Al2O3 catalysts. Appl. Catal. A: Gen. 1996, 145, 231–236. [Google Scholar] [CrossRef]
- Yang, S.-M.; Wu, Y.M. One step synthesis of methyl isobutyl ketone over palladium supported on AlPO4-11 and SAPO-11. Appl. Catal. A: Gen. 2000, 192, 211–220. [Google Scholar] [CrossRef]
- Shen, Y.; Yi, J.; Yan, Y.; Liu, D.; Fan, L.; Li, S. Hydrogenation and Condensation of Acetone over Ni/MgO–Al2O3 Prepared from Hydrotalcite Precursors. J. Chem. Eng. Jpn. 2016, 49, 656–662. [Google Scholar] [CrossRef]
- Bagabas, A.A.; Mokhtar, M.; Akhmedov, V.M.; Narasimharao, K.; Basahel, S.N.; Al-Rabiah, A. Ru–C–ZnO Composite Catalysts for the Synthesis of Methyl Isobutyl Ketone via Single Step Gas Phase Acetone Self-Condensation. Catal. Lett. 2014, 144, 1278–1288. [Google Scholar] [CrossRef]
- Tasis, D.; Tagmatarchis, N.; Bianco, A.; Prato, M. Chemistry of carbon nanotubes. Chem. Rev. 2006, 106, 1105–1136. [Google Scholar] [CrossRef]
- Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar] [CrossRef]
- Georgakilas, V.; Perman, J.A.; Tuček, J.; Zbořil, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115, 4744–4822. [Google Scholar] [CrossRef]
- Shen, K.; Chen, X.; Chen, J.; Li, Y. Development of MOF-Derived Carbon-Based Nanomaterials for Efficient Catalysis. ACS Catal. 2016, 6, 5887–5903. [Google Scholar] [CrossRef]
- Bianco, A.; Chen, Y.; Chen, Y.; Ghoshal, D.; Hurt, R.H.; Kim, Y.A.; Koratkar, N.; Meunier, V.; Terrones, M. A carbon science perspective in 2018: Current achievements and future challenges. Carbon 2018, 132, 785–801. [Google Scholar] [CrossRef]
- Liu, X.; Conte, M.; Elias, D.; Lu, L.; Morgan, D.J.; Freakley, S.J.; Johnston, P.; Kiely, C.J.; Hutchings, G.J. Investigation of the active species in the carbon-supported gold catalyst for acetylene hydrochlorination. Catal. Sci. Technol. 2016, 6, 5144–5153. [Google Scholar] [CrossRef]
- Contreras-Mora, J.; Banerjee, R.; Bolton, B.K.; Valentin, J.; Monnier, J.R.; Williams, C.T. Characterization and Evaluation of Carbon-Supported Noble Metals for the Hydrodeoxygenation of Acetic Acid. Org. Process. Res. Dev. 2018, 22, 1628–1635. [Google Scholar] [CrossRef]
- Perez, R.F.; Soares, O.S.; De Farias, A.M.D.; Pereira, M.F.R.; Fraga, M.A. Conversion of hemicellulose-derived pentoses over noble metal supported on 1D multiwalled carbon nanotubes. Appl. Catal. B: Environ. 2018, 232, 101–107. [Google Scholar] [CrossRef]
- Qin, Q.; Heil, T.; Antonietti, M.; Oschatz, M. Single-Site Gold Catalysts on Hierarchical N-Doped Porous Noble Carbon for Enhanced Electrochemical Reduction of Nitrogen. Small Methods 2018, 2, 1800202. [Google Scholar] [CrossRef]
- Du, H.; Zhao, C.X.; Lin, J.; Guo, J.; Wang, B.; Hu, Z.; Shao, Q.; Pan, D.; Wujcik, E.K.; Guo, Z. Carbon Nanomaterials in Direct Liquid Fuel Cells. Chem. Rec. 2018, 18, 1365–1372. [Google Scholar] [CrossRef]
- Singhania, A.; Bhaskarwar, A.N. Performance of Activated-Carbon-Supported Ni, Co, and Ni-Co Catalysts for Hydrogen Iodide Decomposition in a Thermochemical Water-Splitting Sulfur-Iodine Cycle. Energy Technol. 2018, 6, 1104–1111. [Google Scholar] [CrossRef]
- Mironenko, R.M.; Belskaya, O.B.; Likholobov, V.A. Approaches to the synthesis of Pd/C catalysts with controllable activity and selectivity in hydrogenation reactions. Catal. Today 2019. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Zhao, X.; Yao, T.; Chen, W.; You, R.; Zhao, C.; Wu, G.; Wang, J.; Huang, W. Regulation of coordination number over single Co sites: Triggering the efficient electroreduction of CO2. Angew. Chem. Int. Ed. 2018, 57, 1944–1948. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Qi, G.; Li, B.; Song, Z.; Jiang, H.; Zhang, X.; Qiao, J. A novel N-doped porous carbon microsphere composed of hollow carbon nanospheres. Carbon 2016, 96, 864–870. [Google Scholar] [CrossRef]
- Shi, M.-M.; Bao, D.; Li, S.-J.; Wulan, B.-R.; Yan, J.-M.; Jiang, Q. Anchoring PdCu Amorphous Nanocluster on Graphene for Electrochemical Reduction of N2to NH3under Ambient Conditions in Aqueous Solution. Adv. Energy Mater. 2018, 8, 1800124. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |
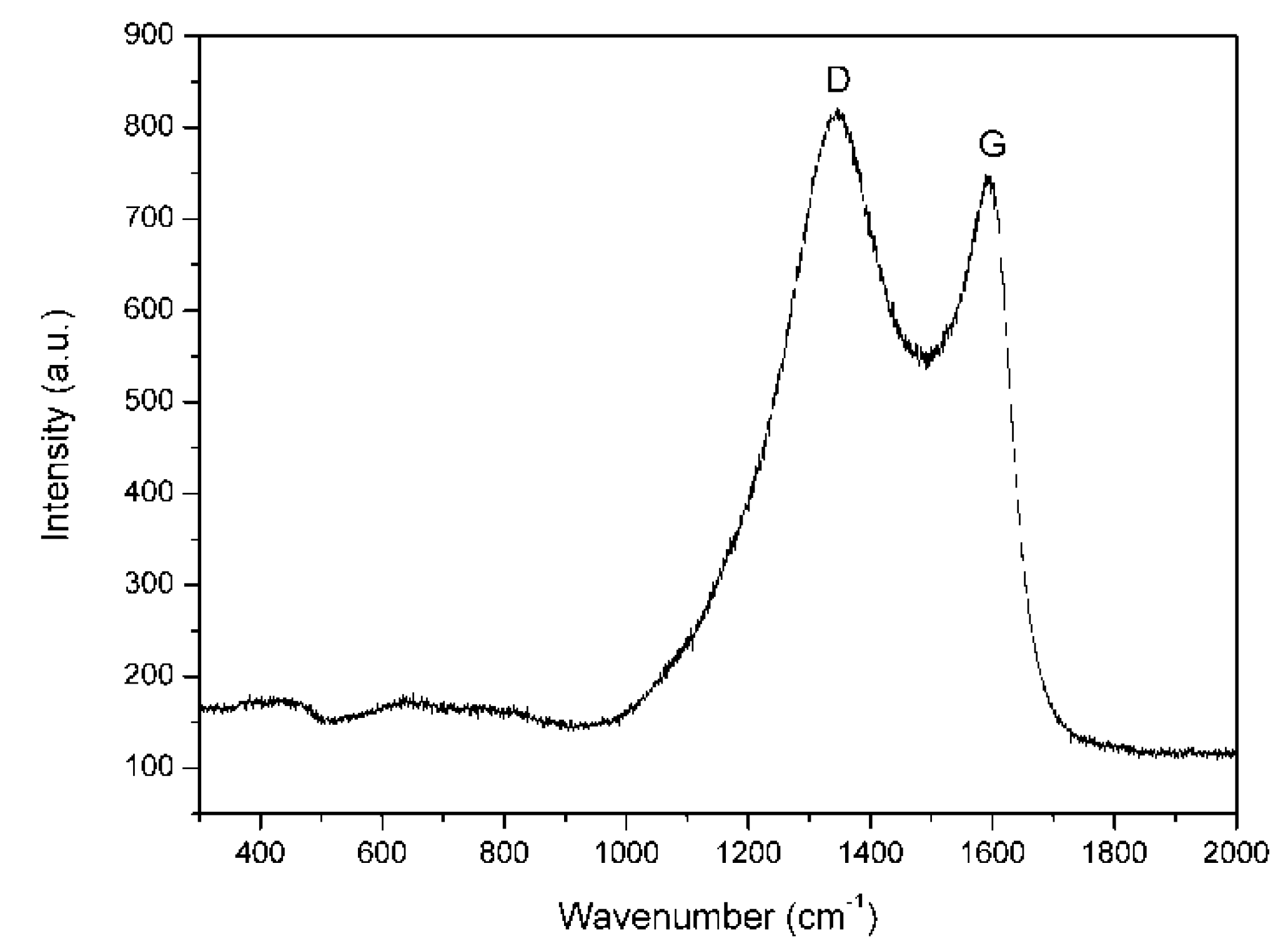
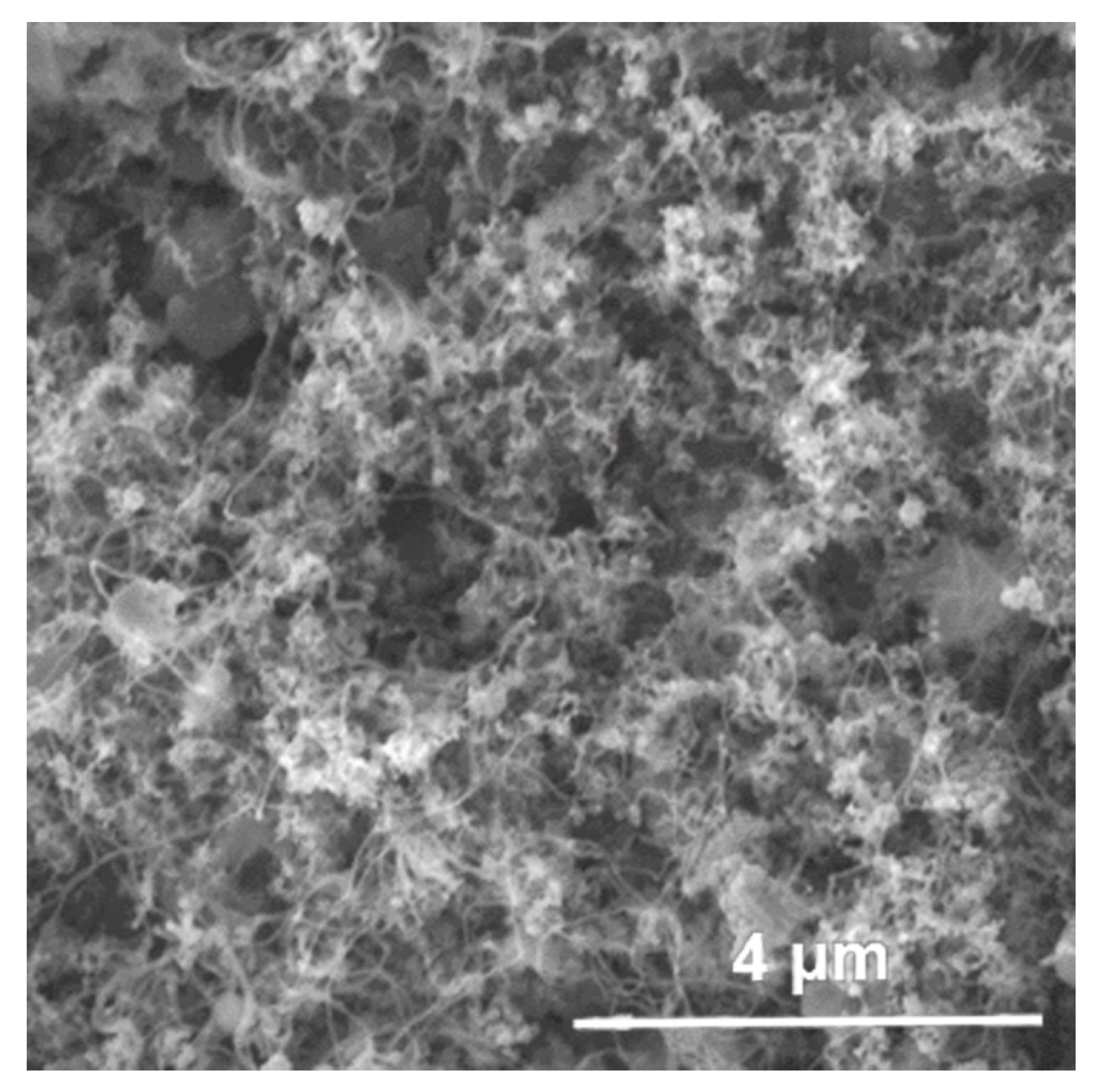

| Catalyst | Temperature (°C) | Conversion of AC (%) | MIBC (wt%) |
|---|---|---|---|
| Ni/Al2O3 a | 80 | 94.3 | 0.3 |
| 100 | 99.9 | 1.9 | |
| 120 | 99.9 | 2.6 | |
| 150 | 99.9 | 5.6 | |
| Raney Ni/C b | 80 | 76.3 | 0 |
| 100 | 93.4 | 0.006 | |
| 120 | 99.9 | 0.02 | |
| 150 | 99.9 | 0.05 | |
| Resin + Raney Ni/C c | 100 | 99.9 | 15 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, S.; Wu, J.; Peng, H.; Chen, Y. Carbon-Supported Raney Nickel Catalyst for Acetone Hydrogenation with High Selectivity. Molecules 2020, 25, 803. https://doi.org/10.3390/molecules25040803
Lu S, Wu J, Peng H, Chen Y. Carbon-Supported Raney Nickel Catalyst for Acetone Hydrogenation with High Selectivity. Molecules. 2020; 25(4):803. https://doi.org/10.3390/molecules25040803
Chicago/Turabian StyleLu, Shuliang, Jiajia Wu, Hui Peng, and Yong Chen. 2020. "Carbon-Supported Raney Nickel Catalyst for Acetone Hydrogenation with High Selectivity" Molecules 25, no. 4: 803. https://doi.org/10.3390/molecules25040803
APA StyleLu, S., Wu, J., Peng, H., & Chen, Y. (2020). Carbon-Supported Raney Nickel Catalyst for Acetone Hydrogenation with High Selectivity. Molecules, 25(4), 803. https://doi.org/10.3390/molecules25040803





