Synthesis, Properties and Antimicrobial Activity of 5-Trifluoromethyl-2-formylphenylboronic Acid
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis, Molecular and Crystal Structure
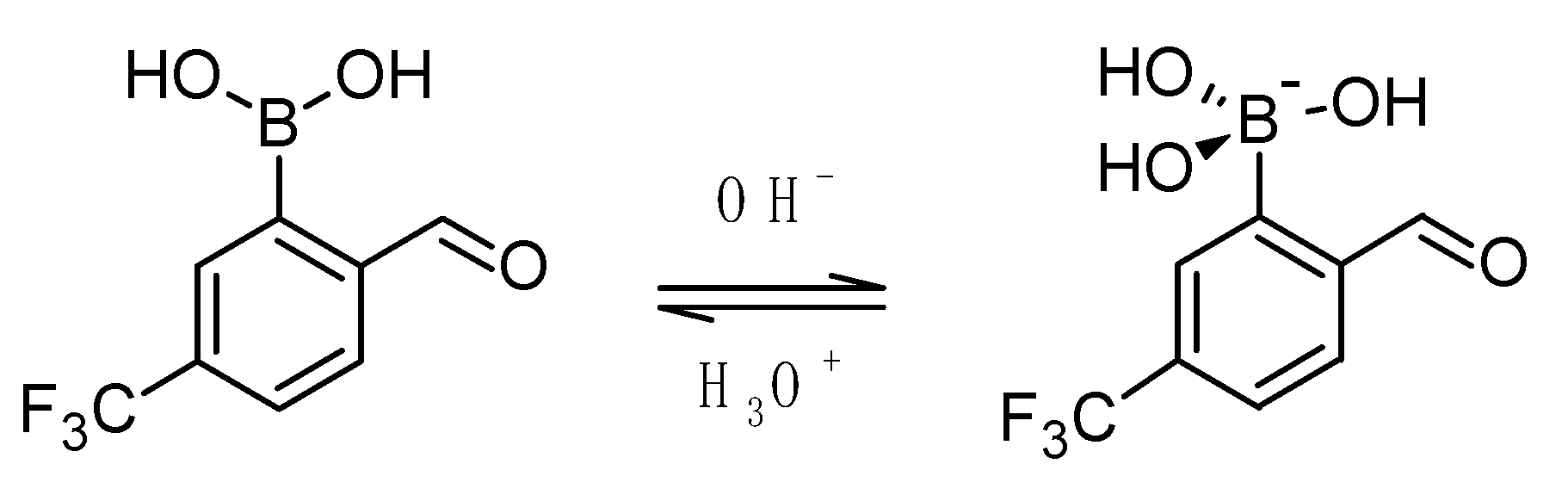
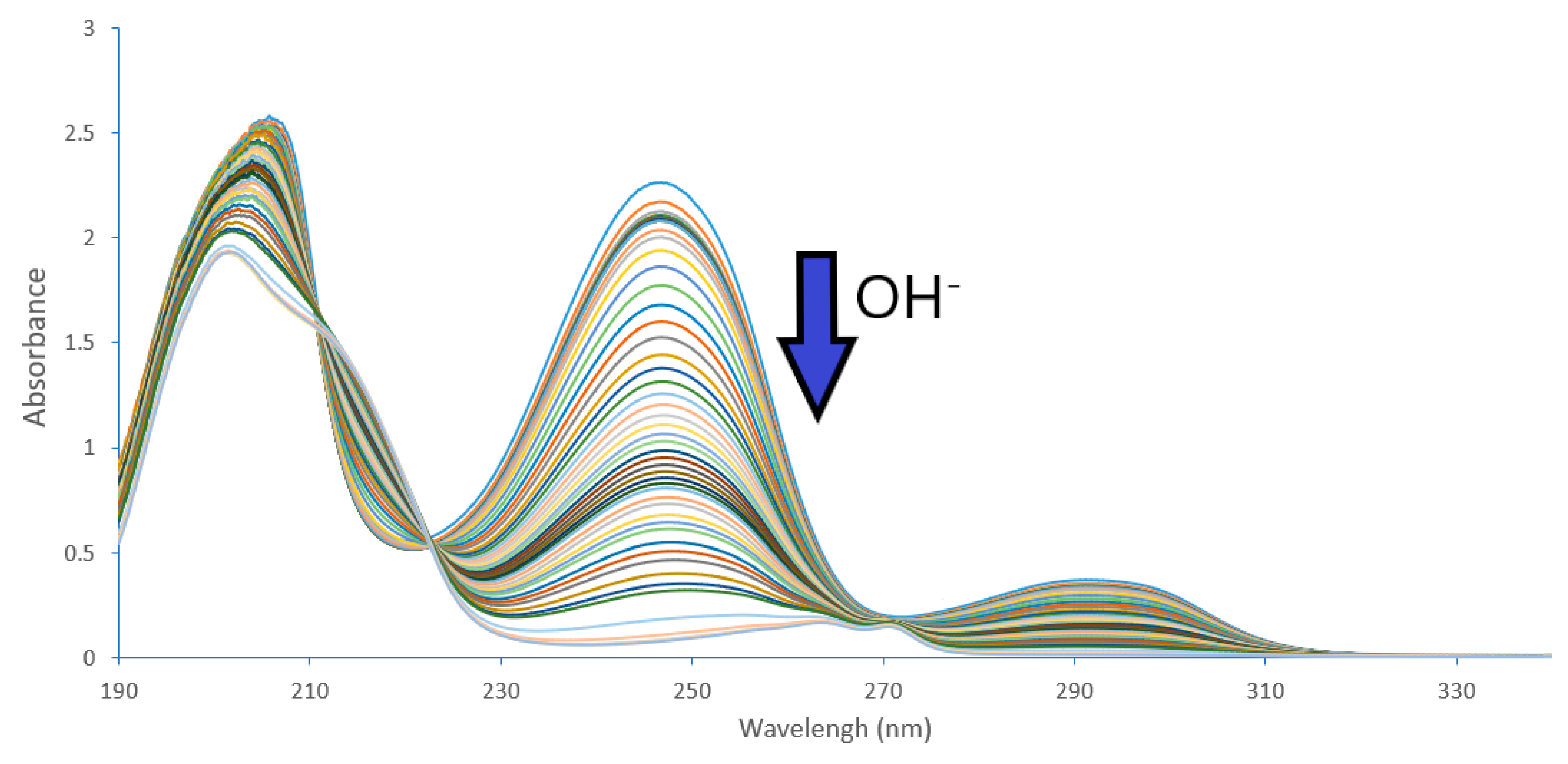
2.2. Acidity Constant Determination
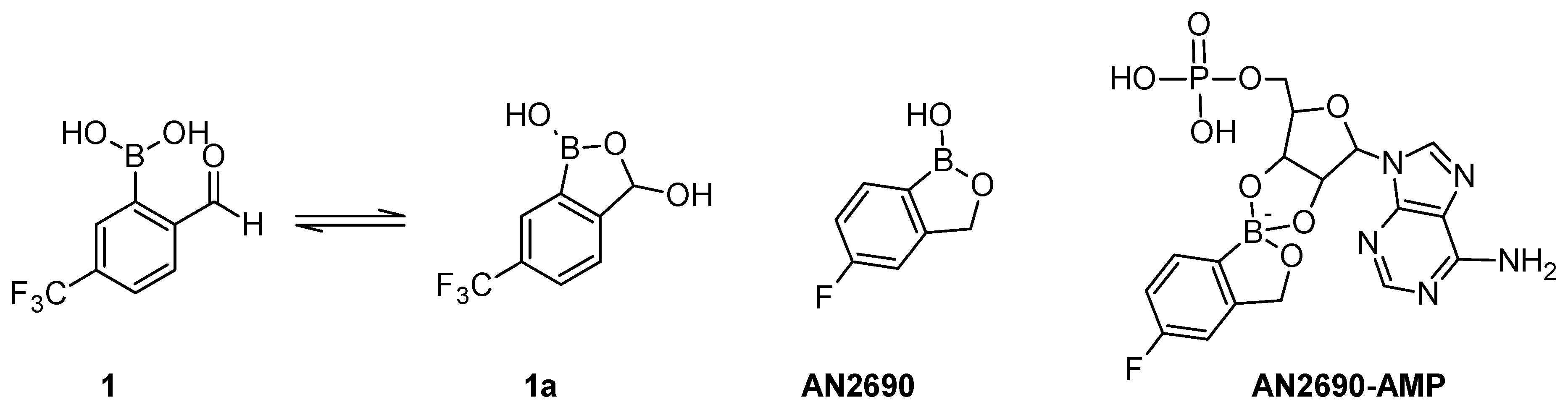
2.3. Spectral Characterization, Cyclization Equilibrium
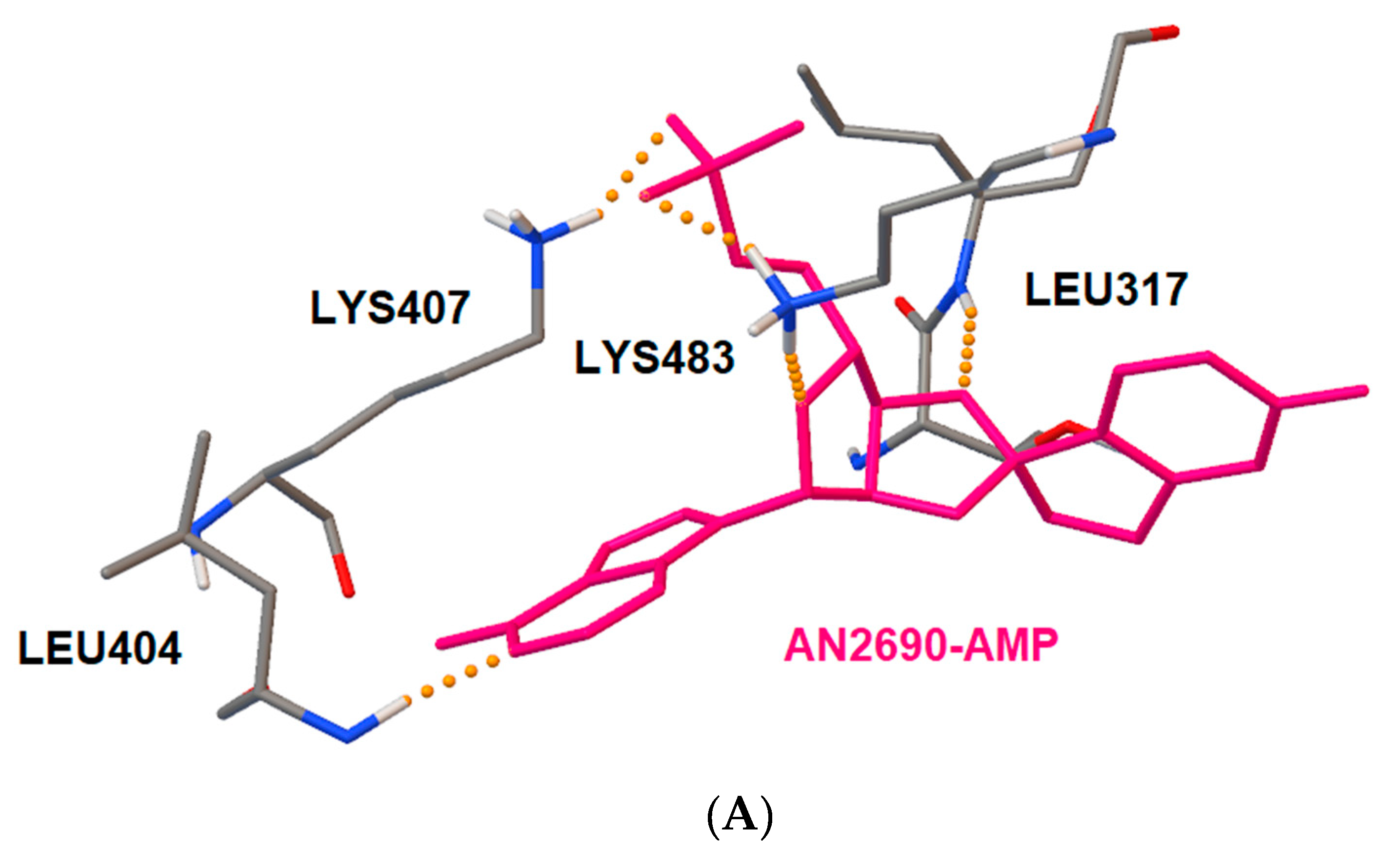
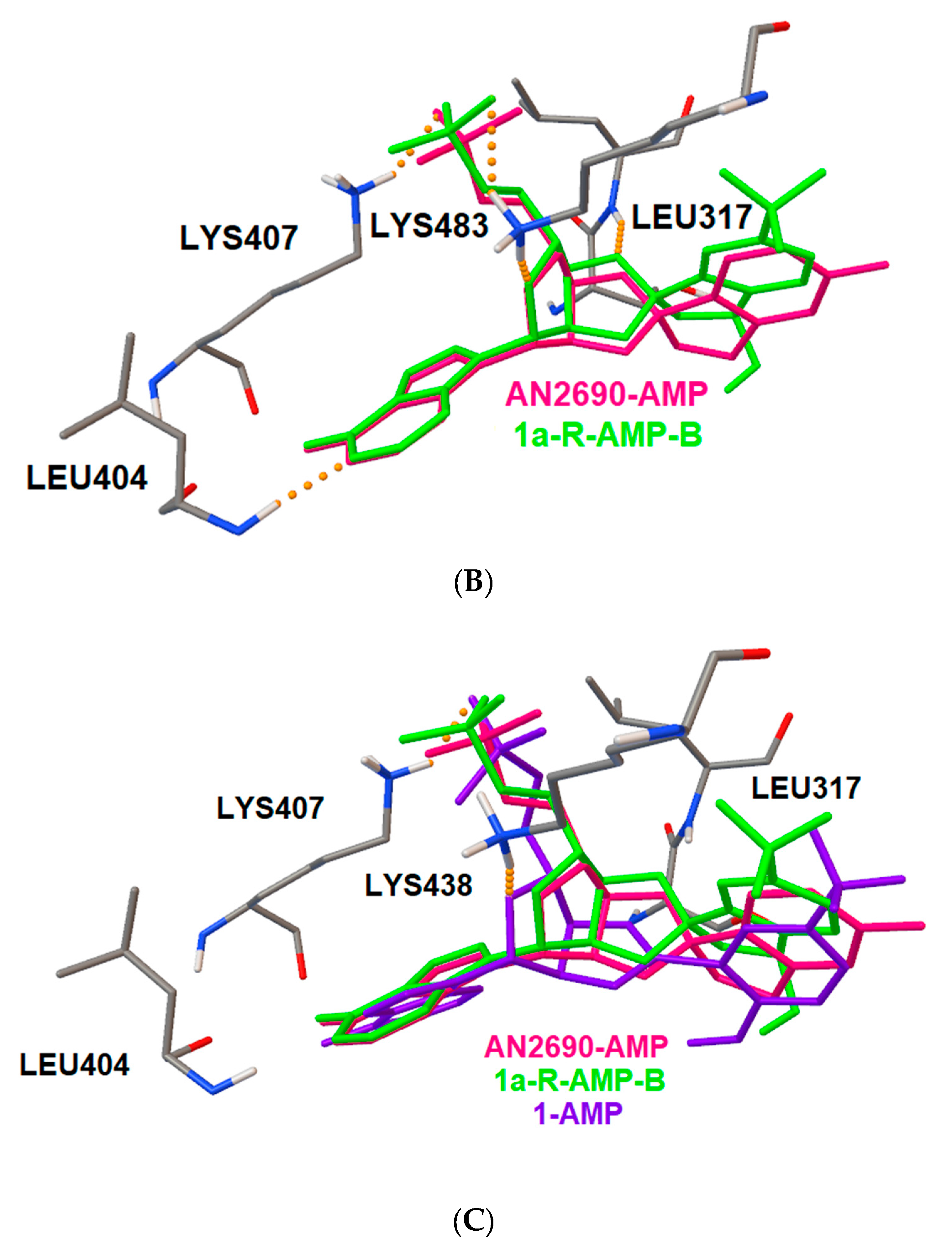
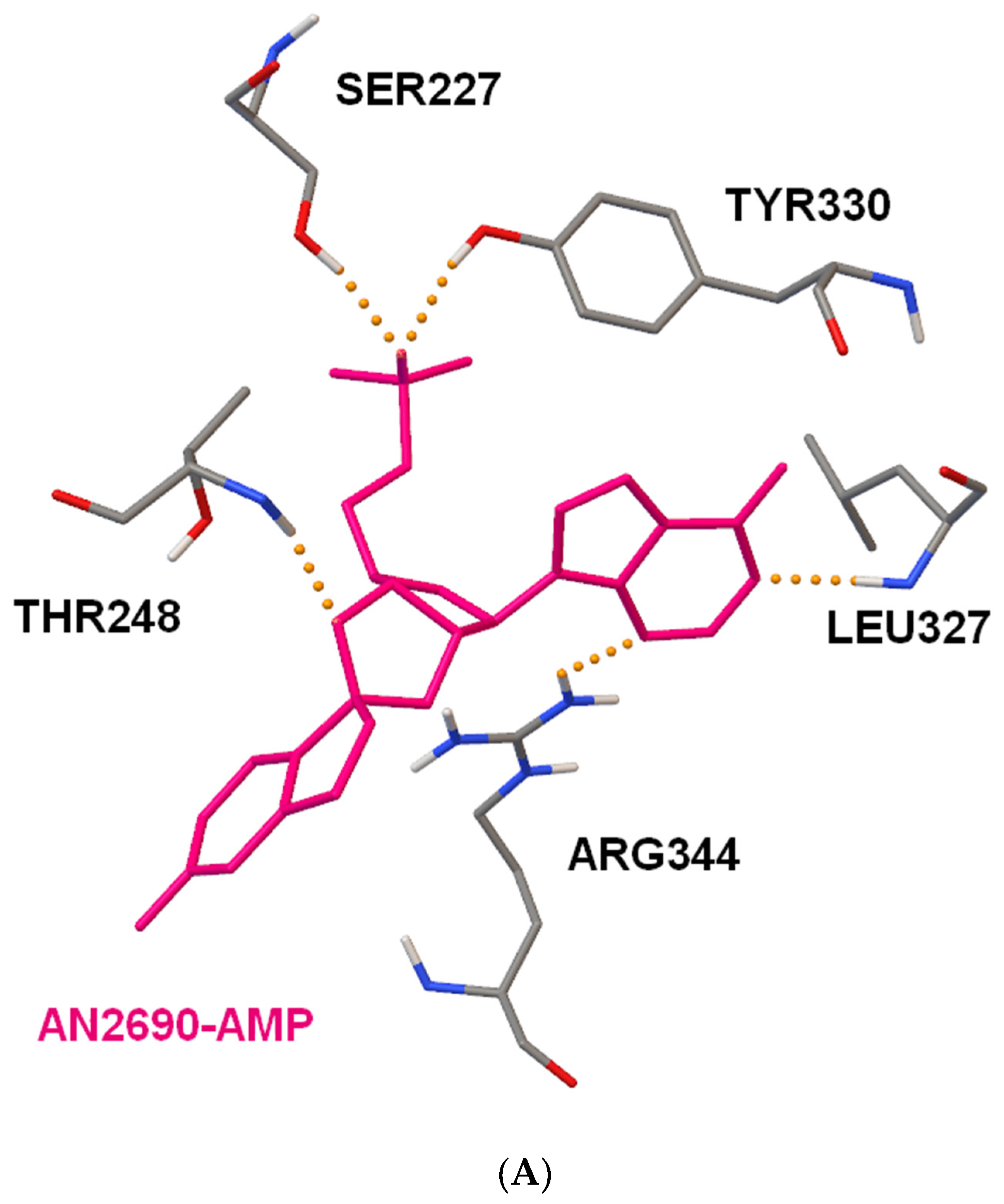
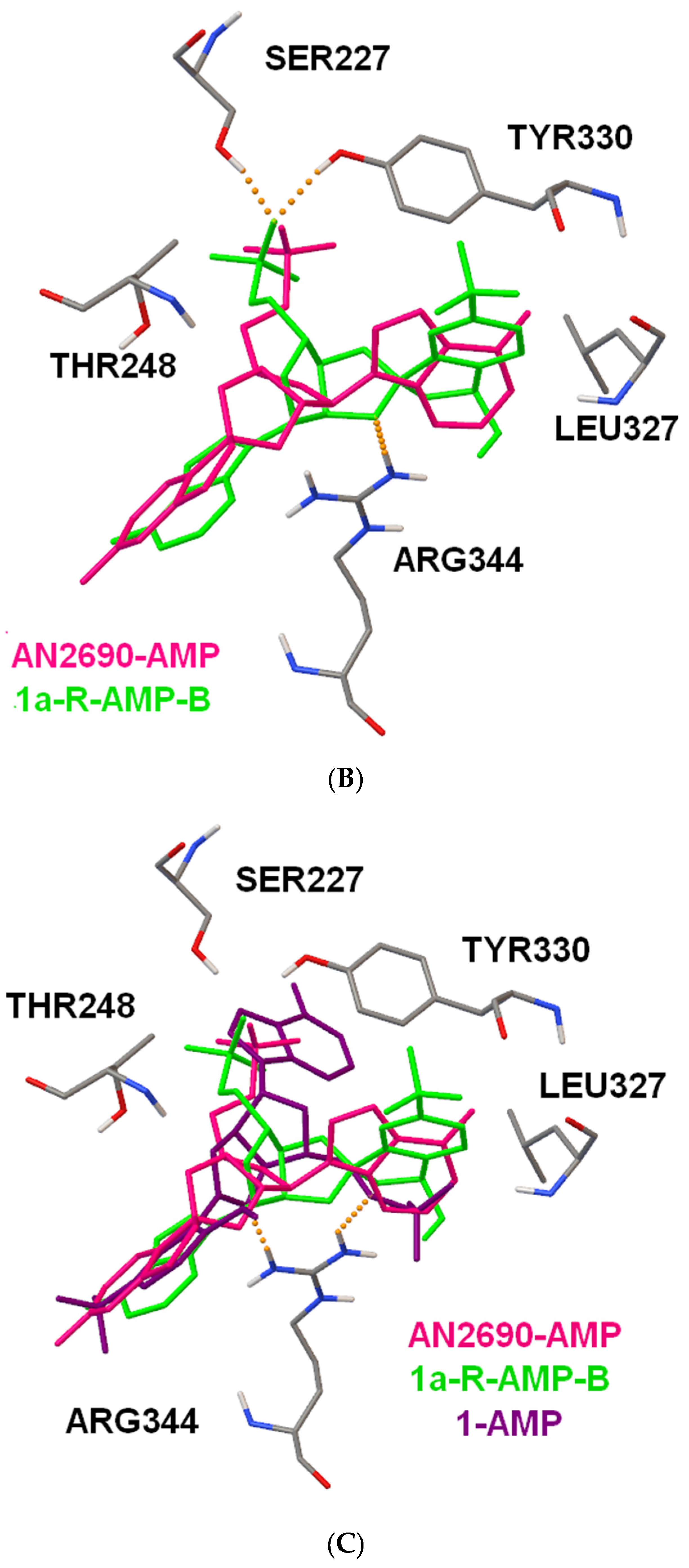
2.4. Docking Studies of Interactions of AN2690 and 1/1a with Candida albicans’ and Eschericha coli LeuRS
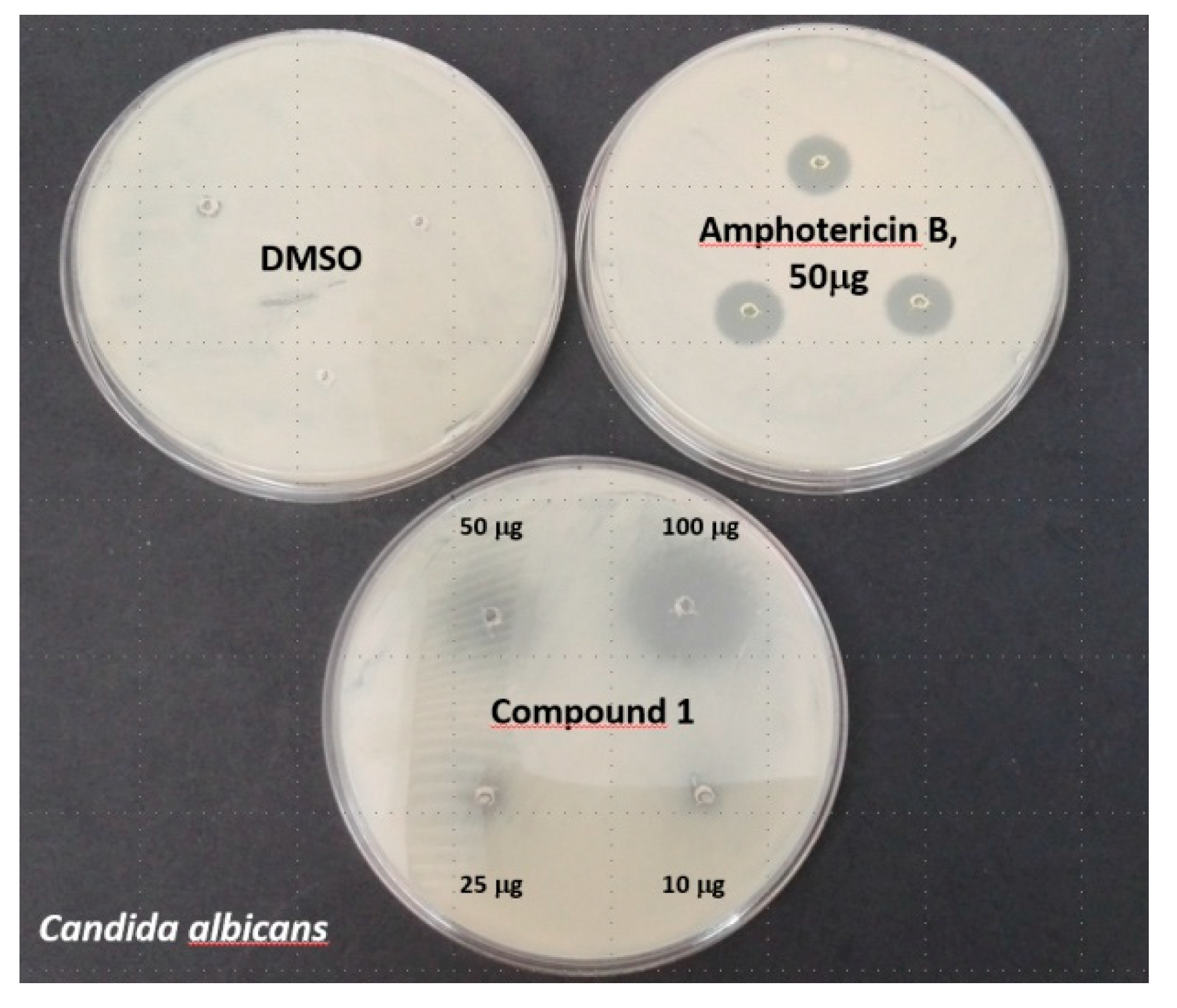
2.5. In Vitro Studies of Antimicrobial Activity of 1
3. Materials and Methods
3.1. Synthesis and Isolation
3.2. Single Crystal X-Ray Diffraction
3.3. Acidity Constant Determination
3.4. NMR Measurements
3.5. Docking Studies
3.6. Microbial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gozdalik, J.T.; Adamczyk-Woźniak, A.; Sporzyński, A. Influence of fluorine substituents on the properties of phenylboronic compounds. Pure Appl. Chem. 2018, 90, 677–702. [Google Scholar] [CrossRef]
- Boron: Sensing, Synthesis and Supramolecular Self-Assembly. Meng, L.; Fossey, J.S.; James, T.D. (Eds.) Monographs in Supramolecular Chemistry; Royal Society of Chemistry: Cambridge, UK, 2015; ISBN 978-1-84973-674-9. [Google Scholar]
- Boronic Acids. Preparation and Applications in Organic Synthesis, Medicine and Materials, 2nd ed.; Hall, D.G., Ed.; WILEY-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Cheng, T.; Li, H.; Ma, Y.; Liu, X.; Zhang, H. Synthesis of boronic-acid-functionalized magnetic attapulgite for selective enrichment of nucleosides. Anal. Bioanal. Chem. 2015, 407, 3525–3529. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Jori, G.; Martini, D.; Sotti, G. Boron neutron capture therapy and 18F-labelled borophenylalanine positron emission tomography: A critical and clinical overview of theliterature. Appl. Radiat. Isot. 2013, 74, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Borys, K.M.; Sporzyński, A. Recent Developments in the Chemistry and Biological Applications of Benzoxaboroles. Chem. Rev. 2015, 115, 5224–5247. [Google Scholar] [CrossRef] [PubMed]
- Madura, I.D.; Adamczyk-Woźniak, A.; Sporzyński, A. Diversified self-association through O–H∙∙∙O hydrogen bonds in crystals of formylphenylboronic acid isomers. J. Mol. Struct. 2015, 1083, 204–211. [Google Scholar] [CrossRef]
- Luliński, S.; Madura, I.; Serwatowski, J.; Szatyłowicz, H.; Zachara, J. A tautomeric equilibrium between functionalized 2-formylphenylboronic acids and corresponding 1,3-dihydro-1,3-dihydroxybenzo[c][2,1]oxaboroles. New J. Chem. 2007, 31, 144–154. [Google Scholar] [CrossRef]
- Borys, K.M.; Wieczorek, D.; Pecura, K.; Lipok, J.; Adamczyk-Woźniak, A. Antifungal activity and tautomeric cyclization equilibria of formylphenylboronic acids. Bioorg. Chem. 2019, 91, 103081. [Google Scholar] [CrossRef]
- Kowalska, K.; Adamczyk-Woźniak, A.; Gajowiec, P.; Gierczyk, B.; Kaczorowska, E.; Popenda, Ł.; Schroeder, G.; Sikorski, A.; Sporzyński, A. Fluoro-substituted 2-formylphenylboronic acids: Structures, properties and tautomeric equilibria. J. Fluor. Chem. 2016, 187, 1–8. [Google Scholar] [CrossRef]
- Das, S.; Alexeev, V.L.; Sharma, A.C.; Geib, S.J.; Asher, S.A. Synthesis and crystal structure of 4-amino-3-fluorophenylboronic acid. Tetrahedron Lett. 2003, 44, 7719–7722. [Google Scholar] [CrossRef]
- Alexeev, V.L.; Sharma, A.C.; Goponenko, A.V.; Das, S.; Lednev, I.K.; Wilcox, C.S.; Finegold, D.N.; Asher, S.A. High ionic strength glucose-sensing photonic crystal. Anal. Chem. 2003, 75, 2316–2323. [Google Scholar] [CrossRef]
- Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; WILEY-VCH: Darmstadt, Germany, 2013; ISBN 9783527603930. [Google Scholar]
- Purser, S.; Moore, P.R.; Swallow, S.; Gouverneur, V. Fluorine in medicinal chemistry. Chem. Soc. Rev. 2008, 37, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tian, J.; Liu, Y.; Tao, C.; Zhu, H.; Zhang, A.; Xua, D.; Zhao, B. Decarboxylative Umpolung of conjugated enals to β-carbanions for intramolecular nucleophilic addition to an aldehyde. Org. Chem. Front. 2017, 4, 1586–1589. [Google Scholar] [CrossRef]
- Etter, M.C.; MacDonald, J.C.; Bernstein, J. Graph-set analysis of hydrogen-bond patterns in organic crystals. Acta Crystallogr. Sect. B Struct. Sci. 1990, 46, 256–262. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel tools for visualizing and exploring intermolecular interactions in molecular crystals. Acta Crystallogr. Sect. B Struct. Sci. 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Matsumura, T.; Takao, N.; Yamagishi, H.; Takahashi, M.; Iwatsuki, S.; Ishihara, K. Fast trigonal/tetragonal interconversion followed by slow chelate-ring closure in the complexation of boronic acids. Inorg. Chim. Acta 2005, 358, 3355–3361. [Google Scholar] [CrossRef]
- Westmark, P.R.; Gardiner, S.J.; Smith, B.D. Selective monosaccharide transport through lipid bilayers using boronic acid carriers. J. Am. Chem. Soc. 1996, 118, 11093–11100. [Google Scholar] [CrossRef]
- Siodła, T.; Ozimiński, W.P.; Hoffmann, M.; Koroniak, H.; Krygowski, T.M. Toward a physical interpretation of substituent effects: The case of fluorine and trifluoromethyl groups. J. Org. Chem. 2014, 79, 7321–7331. [Google Scholar] [CrossRef]
- Zarzeczańska, D.; Adamczyk-Woźniak, A.; Kulpa, A.; Ossowski, T.; Sporzyński, A. Fluorinated Boronic Acids: Acidity and Hydrolytic Stability of Fluorinated Phenylboronic Acids. Eur. J. Inorg. Chem. 2017, 2017, 4493–4498. [Google Scholar] [CrossRef]
- Gozdalik, J.T.; Marek, P.H.; Madura, I.D.; Gierczyk, B.; Popenda, Ł.; Schroeder, G.; Adamczyk-Woźniak, A.; Sporzyński, A. Structures and properties of trifluoromethylphenylboronic acids. J. Mol. Struct. 2019, 1180, 237–243. [Google Scholar] [CrossRef]
- Torssell, K.; McClendon, J.H. Chemistry of Arylboric Acids VIII. The Relationship between Physico-chemical Properties and Activity in Plants. Acta Chem. Scand. 1958, 12, 1373–1385. [Google Scholar] [CrossRef]
- Tomsho, J.W.; Pal, A.; Hall, D.G.; Benkovic, S.J. Ring Structure and Aromatic Substituent Effects on the pKa of the Benzoxaborole Pharmacophore. ACS Med. Chem. Lett. 2011, 3, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Cabaj, M.K.; Dominiak, P.M.; Gajowiec, P.; Gierczyk, B.; Lipok, J.; Popenda, Ł.; Schroeder, G.; Tomecka, E.; Urbański, P.; et al. The influence of fluorine position on the properties of fluorobenzoxaboroles. Bioorg. Chem. 2015, 60, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk-Woźniak, A.; Ejsmont, K.; Gierczyk, B.; Kaczorowska, E.; Matuszewska, A.; Schroeder, G.; Sporzyński, A.; Zarychta, B. Novel 2,6-disubstituted phenylboronic compounds: Synthesis, crystal structures, solution behaviour and reactivity. J. Organomet. Chem. 2015, 788, 36–41. [Google Scholar] [CrossRef]
- Dolbier, W.R., Jr. Guide to Fluorine NMR for Organic Chemists, 2nd ed.; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Rock, F.L.; Mao, W.; Yaremchuk, A.; Tukalo, M.; Crepin, T.; Zhou, H.; Zhang, Y.-K.; Hernandez, V.; Akama, T.; Baker, S.J.; et al. An Antifungal Agent Inhibits an Aminoacyl-tRNA Synthetase by Trapping tRNA in the Editing Site. Science 2007, 316, 1759–1761. [Google Scholar] [CrossRef]
- Palencia, A.; Crepin, T.; Vu, M.T.; Lincecum, T.L., Jr.; Martinis, S.A.; Cusack, S. Structural Dynamics of the Aminoacylation and Proofreading Functional Cycle of Bacterial Leucyl-tRNA Synthetase. Nat. Struct. Mol. Biol. 2012, 19, 677. [Google Scholar] [CrossRef]
- Gualerzi, C.O.; Brandi, L.; Fabbretti, A.; Pon, C.L. Antibiotics: Targets, Mechanism and Resistance; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Kim, S. (Ed.) Aminoacyl-tRNA Synthetases in Biology and Medicine. In Topics in Current Chemistry; Springer Verlag: Berlin Heidelberg, Germany, 2014; p. 312. [Google Scholar]
- CrysAlisPro 1.171.38.46, Rigaku; Oxford Diffraction: Abingdon-on-Thames, UK, 2015.
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Farrugia, L.J. Ortep-3 for Windows-a version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Crystallogr. 1997, 30, 565. [Google Scholar] [CrossRef]
- Burley, S.K.; Berman, H.M.; Bhikadiya, Ch.; Bi, Ch.; Chen, L.; Di Costanzo, L.; Christie, C.; Dalenberg, K.; Duarte, J.M.; Dutta, S.; et al. RCSB Protein Data Bank: biological macromolecular structures enabling research and education in fundamental biology, biomedicine, biotechnology and energy. Nucleic Acids Res. 2019, 47, D464–D474. Available online: www.rcsb.org (accessed on 11 December 2019). [CrossRef] [PubMed]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. Autodock4 and AutoDockTools4: automated docking with selective receptor flexiblity. J. Comput. Chem. 2009, 16, 2785–2791. Available online: http://autodock.scripps.edu/ (accessed on 11 December 2019). [CrossRef] [PubMed]

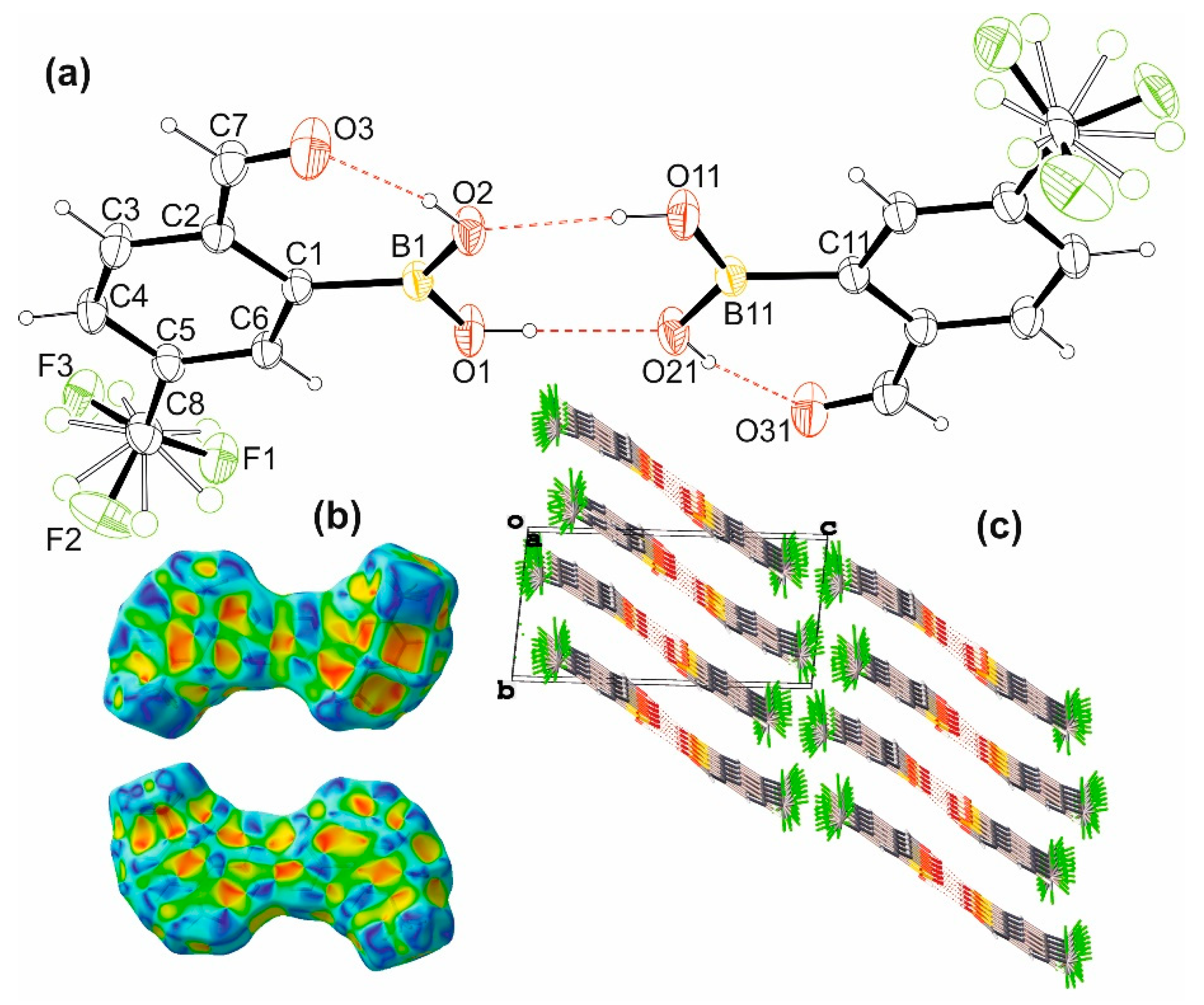
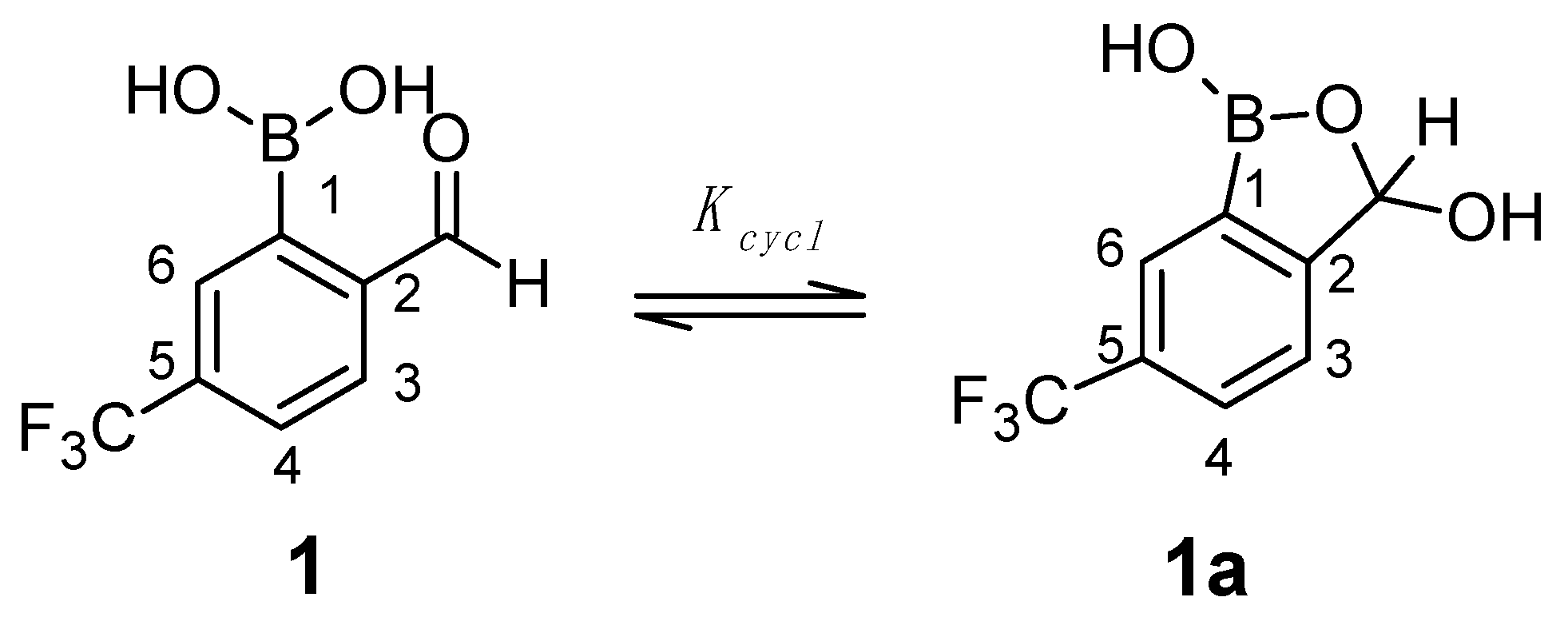
| H∙∙∙A | D∙∙∙A | D-H∙∙∙A | |
|---|---|---|---|
| Intramolecular | |||
| O2‒H2…O3 | 1.82(1) | 2.619(1) | 164(2) |
| O21‒H21…O31 | 1.81(1) | 2.605(1) | 164(2) |
| Intermolecular | |||
| O1‒H1…O21 | 1.95(1) | 2.774(1) | 177(2) |
| O11‒H11…O2 | 1.97(1) | 2.779(1) | 174(2) |
| C7‒H7…O1 i | 2.61 | 3.254(2) | 127 |
| C71‒H71…O11 ii | 2.56 | 3.220(2) | 128 |
| General Formula | Substituent | pKa Value | Reference |
|---|---|---|---|
 | - | 8.86 | [10] |
| 3-F | 7.50 ± 0.02 | [22] | |
| 3-CF3 | 7.85 ± 0.05 | [23] | |
| 2-CHO | 7.31 | [24] | |
| 2-CHO, 5-F | 6.72 ± 0.03 | [10] | |
| 2-CHO, 5-CF3 (1) | 5.67 ± 0.01 | This work |
| 1H NMR | 11B | 19F | ||||||
|---|---|---|---|---|---|---|---|---|
| Solvent (Form) | B(OH)2 | CHO | H3 | H4 | H6 | |||
| OH | H | |||||||
| CDCl3 (1) | 7.13 (s) | 10.04 (m) | 8.54 (m) | 7.97 (m) | 8.06 (d) 3JH5 = 7.8 | 27 (s) | −63.44 | |
| C6D6 (1) | 7.04 (s) | 9.04 (s) | 8.55 (m) | 7.20 (m) | 6.73 (d) 3JH5 = 7.8 | 27 (s) | −63.29 | |
| (CD3)2CO (1) | 7.84 (s) | 10.40 (s) | 8.10 (m) | 7.93 (m) | 8.15 (m) | 29 (s) | −62.94 | |
| (CD3)2CO (1a) | 8.50 (s) | 6.38 (d) 3JH = 7.9 | 6.22 (d) 3JOH = 8.1 | 8.01 (m) | 7.85 (m) | 7.70 (m) | 31 (s) | −61.98 |
| (CD3)2SO (1) | 8.47 (s) | 10.24 (s) | 7.89 (m) | 7.91 (m) | 8.07 (m) | 30 (s) | −61.67 | |
| (CD3)2SO (1a) | 9.57 (s) | 7.23 (d) 3JH = 8.1 | 6.25 (d) 3JOH = 8.1 | 8.01 (m) | 7.85 (m) | 7.64 (m) | 30 (s) | −60.73 |
| Solvent | Cyclization Constant | 1H/19F NMR Shift of the Cyclic Form (1a) | 1H/19F NMR Shift of The Opened Form (1) |
|---|---|---|---|
| (CD3)2SO | 0.59 ± 0.01 | 6.25/−60.73 | 10.24/−61.67 |
| (CD3)2CO | 0.24 ± 0.02 | 6.22/−61.98 | 10.40/−62.94 |
| D2O | 0.26 ± 0.03 * | 6.36/−62.22 | 10.06/−63.26 |
| Form | CHO/CH-OH | CF3 | C1 | C2 | C3 | C4 | C5 | C6 |
|---|---|---|---|---|---|---|---|---|
| 1 | 193.82 (s) | 124.5 (q) 1JF = 270.5 | 140.8 (bs) | 142.1 (d) 4JF = 1.1 | 129.5 (m) | 125.7 (m) | 132.2 (q) 2JF = 31.5 | 129.9 (s) |
| 1a | 97.06 (s) | Not Observed | Not Observed | 158.9 (s) | 126.8 (q) 3JF = 3.7 | 127.8 (m) | Not Observed | 123.8 (s) |
| Ligand | The Lowest Binding Energy [kcal/mol] | Number of Structures | Mean Binding Energy [kcal/mol] | Inhibition Constant | Number of Hydrogen Bonds | |
|---|---|---|---|---|---|---|
| Candida albicans | AN2690-AMP | −11.98 | 17 | −10.80 | 1.93 nM | 5 |
| 1a-R-AMP-A | −10.77 | 2 | −10.21 | 12.77 nM | 4 | |
| 1a-R-AMP-B | −11.83 | 3 | −11.66 | 2.12 nM | 5 | |
| 1a-S-AMP-A | −10.75 | 19 | −10.42 | 13.08 nM | 3 | |
| 1a-S-AMP-B | −11.59 | 4 | −10.94 | 3.17 nM | 4 | |
| 1-AMP | −11.05 | 2 | −10.77 | 7.95 nM | 2 | |
| Escherichia coli | AN2690-AMP | −8.27 | 22 | −7.55 | 865.19 nM | 5 |
| 1a-R-AMP-A | −7.54 | 21 | −6.90 | 2.99 μM | 2 | |
| 1a-R-AMP-B | −8.15 | 2 | −7.81 | 1.09 μM | 3 | |
| 1a-S-AMP-A | −7.54 | 12 | −6.98 | 2.96 μM | 2 | |
| 1a-S-AMP-B | −7.72 | 4 | −7.45 | 2.21 μM | 3 | |
| 1-AMP | −8.23 | 4 | −7.75 | 924.99 nM | 2 |
| 10 µg | 25 µg | 50 µg | 100 µg | AN2690 (50 µg) | Antibiotic (50 µg) | |
|---|---|---|---|---|---|---|
| Candida albicans | 0 | 9 | 13 ± 2 | 17 ± 3 (8 ± 1) | (53) | (10 ± 1) * |
| Aspergillus niger | 0 | 8 ± 1 | 13 ± 2 | 26 ± 4 (5 ± 1) | (62) | (9 ± 1) * |
| Escherichia coli | 2 ± 1 | 5 ± 1 | 7 ± 1 | 9 ± 1 | (22) | (16 ± 1) # |
| Bacillus cereus | 12 | 15 ± 1 | 18 | 19 ± 1 | (14) | (21 ± 1) # |
| MIC [µg/mL] | |||
|---|---|---|---|
| 1 | AN 2690 | Antibiotic | |
| Candida albicans | 250 | 2 | ≤ 1 * |
| Aspergillus niger | 32 | ≤ 1 | - |
| Escherichia coli | 125 | 7.8 | 2 # |
| Bacillus cereus | 8 | 62.5 | 4 # |
| * Amphotericin B, # Streptomycin | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamczyk-Woźniak, A.; Gozdalik, J.T.; Wieczorek, D.; Madura, I.D.; Kaczorowska, E.; Brzezińska, E.; Sporzyński, A.; Lipok, J. Synthesis, Properties and Antimicrobial Activity of 5-Trifluoromethyl-2-formylphenylboronic Acid. Molecules 2020, 25, 799. https://doi.org/10.3390/molecules25040799
Adamczyk-Woźniak A, Gozdalik JT, Wieczorek D, Madura ID, Kaczorowska E, Brzezińska E, Sporzyński A, Lipok J. Synthesis, Properties and Antimicrobial Activity of 5-Trifluoromethyl-2-formylphenylboronic Acid. Molecules. 2020; 25(4):799. https://doi.org/10.3390/molecules25040799
Chicago/Turabian StyleAdamczyk-Woźniak, Agnieszka, Jan T. Gozdalik, Dorota Wieczorek, Izabela D. Madura, Ewa Kaczorowska, Ewa Brzezińska, Andrzej Sporzyński, and Jacek Lipok. 2020. "Synthesis, Properties and Antimicrobial Activity of 5-Trifluoromethyl-2-formylphenylboronic Acid" Molecules 25, no. 4: 799. https://doi.org/10.3390/molecules25040799
APA StyleAdamczyk-Woźniak, A., Gozdalik, J. T., Wieczorek, D., Madura, I. D., Kaczorowska, E., Brzezińska, E., Sporzyński, A., & Lipok, J. (2020). Synthesis, Properties and Antimicrobial Activity of 5-Trifluoromethyl-2-formylphenylboronic Acid. Molecules, 25(4), 799. https://doi.org/10.3390/molecules25040799